Abstract
Plants need to cope with complex environments throughout their life cycle. Abiotic stresses, including drought, cold, salt and heat, can cause a reduction in plant growth and loss of crop yield. Plants sensing stress signals and adapting to adverse environments are fundamental biological problems. We review the stress sensors in stress sensing and the responses, and then discuss ionic stress signaling and the responses. During ionic stress, the calcineurin B-like proteins (CBL) and CBL-interacting protein kinases (CBL−CIPK) complex is identified as a primary element of the calcium sensor for perceiving environmental signals. The CBL−CIPK system shows specificity and variety in its response to different stresses. Obtaining a deeper understanding of stress signaling and the responses will mitigate or solve crop yield crises in extreme environments with fast-growing populations.
Keywords: abiotic stress, ionic stress, sensing, response, CBL−CIPK
1. Introduction
Plants cannot escape adverse environmental conditions [1]. Due to the constantly changing environment, abiotic stress is the main factor affecting crop growth and productivity, as plants are sessile organisms. Abiotic stress includes soil salinity, drought and extreme temperature conditions (cold or hot) [2]. Anthropogenic activities directly affect global climate change. Identifying the mechanisms through which plants counteract abiotic stress and maintain their growth and survival holds significance for plants in coping with global climate change [3,4,5].
There are inherent physical, morphological and molecular limitations to the plant’s ability to respond to different abiotic stresses [6]. Although plants differ physically, morphologically and molecularly between wild-type and modern cultivars in terms of their stress sensing ability, their stress sensing mechanisms are still poorly understood. To overcome this limitation, an initial step in decoding the process involves stress sensor signals that are produced by the plant cell. Studies on calcineurin B-like proteins (CBLs) and their targets, CBL-interacting protein kinases (CIPKs), generally known as the CBL−CIPK network, provide useful models for understanding the mechanisms of stress signal transduction in plants [2].
Cold stress can rigidify the plasma membrane and rearrange the cytoskeleton so calcium can pass through calcium channels into the cytoplasm [7]. The calcium signal is then decoded by a calcium-binding protein, which leads to downstream actions that deliver the cold signal [7]. COLD1, a quantitative trait locus gene identified in rice that encodes a regulator of G-protein signaling localized on the plasma membrane and endoplasmic reticulum, was found to cause the G-protein α subunit to initiate the guanosine triphosphatase (GTPase) activity of regulator of G-protein activate 1 (RGA1) [8]. COLD1/RGA1 represents a potential calcium permeable channel, as a lack of COLD1 affects calcium (Ca2+) signaling with cold stress in rice [7,8]. Phytochromes (Phy), plant photoreceptors, regulate photomorphogenesis, and PhyB, the primary photoreceptor, controls plant growth in Arabidopsis, both in cold and high temperature (HT) stress [7,9,10]. Under normal and HT conditions, phytochrome interacting factor 4 (PIF4), a basic helix-loop-helix transcription factor, forms part of the central regulatory hub mediating the diurnal growth of plants [9]. It was proposed that the phyB−PIF4 signaling module plays an important role in balancing plant growth and defenses during the response to HT stress [11]. PIF4 can integrate brassinosteroid (BR) and gibberellin (GA) signaling. Evidence has shown that PIF4 directly binds to the promoters of DWARF4 (DWF4) and BRASSINOSTEROID-6-OXIDASE2 (BR6ox2), and then promotes the expression of these BR biosynthesis genes [12].
Soil salinization is a worldwide problem that seriously threatens agriculture as it restricts the growth and yield of crops [13]. Salt stress includes two types: osmotic stress and ionic stress. Rice is known to be a salt-sensitive crop and the annotations of the rice genome sequence enable the study of the functional genomics of the salt stress response [14]. Oryza sativa Salt tolerance activation 2 (OsSta2) was identified and studied in rice. Under salt stress, OsSta2-Ox (overexpression) plants were more tolerant to osmotic stress and could maintain a much healthier growth pattern than wild type (WT) seedlings in response to mannitol treatment, which indicates that OsSta2 may respond to salt and drought stress [15]. Studies have found that some RNA functions are also involved in crop salt tolerance mechanisms [16,17,18,19]. With RNA-seq and sRNA-seq, there were 2574 mRNAs and 76 miRNAs, respectively, that were differentially expressed in citrus root under salt and dehydration treatments [17]. In maize, eight novel miRNAs and their targets, in response to salinity stress, have been identified. A total of 37 potential new miRNAs were selected based on the same criteria in response to salt stress [16]. The interaction between miRNAs and their targets may also play important roles in response to salinity.
Multiple Mitogen-activated protein kinases (MAPKs) have been observed, which can respond to abiotic stimuli such as salt, drought, cold, heat and wounding, as well as to growth and developmental signals [20]. Abscisic acid (ABA) has been shown to modulate the transcription, protein accumulation and kinase activity of several MAPK signaling components [20]. The ABA-signaling pathway is central to drought and salt stress responses in plants [2,21]. The interactions of CIPK1, CBL1 and CBL9 form different complexes that seem to work in distinct processes, including drought-responsive gene expression and ABA responses.
Therefore, understanding the physiological and molecular aspects of plant stress sensing functions and improving plant stress resistance are critical for agricultural growth and productivity.
2. Sensing of Organellar Stress
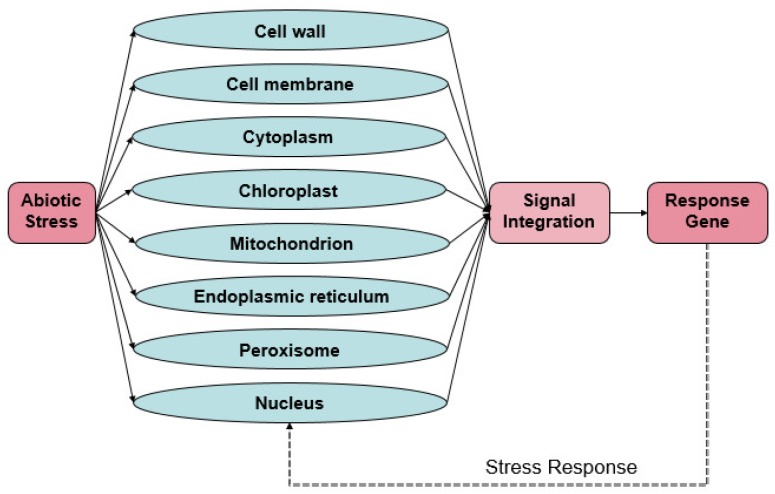
Plant cells are capable of sensing various environmental signals in response to different abiotic stress conditions. The plant then demonstrates variations in gene expression, metabolism and physiology [2]. Research has revealed abiotic stress signaling perception in plants. Sensors that recognize the initial stress signal initiate cascades that transmit the signal to different organelles and activate transcription factors (TFs) that induce the expression of a particular set of genes. A single sensor can regulate the branches of the signal cascade, which is triggered by one aspect of the stress condition (Figure 1).
Figure 1.
Stress sensing and signaling; a model of the response to abiotic stresses in different organelles. The signal generated by organelles can cause gene expression and cellular activities, which can restore cellular homeostasis under abiotic stress. Arrows indicate activation and signal transduction, and dashed lines indicate response.
Stress-sensing frequently occurs on the cell surface or at the cell membrane [22]. From there, the signal can be relayed to different organelles. Protein folding in the endoplasmic reticulum (ER), referred to as ER stress, is widely recognized as an important cellular response to stress, which can be caused by both biotic and abiotic stress [2,23]. ER membrane-associated transcription factors, basic leucine zipper (bZIP) proteins, are important for restoring ER homeostasis [24]. The bZIP transcription factors (TFs) constitute one of the largest families in plants with nearly 15,498 genes from 166 species [25].
Based on the plant transcription factor database, different kinds and numbers of TFs are found in different plant species, which are identified as loci and classified into different families (Table 1). For instance, the gene annotation from TAIR10 is used to identify transcription factors (TFs) of Arabidopsis thaliana. According to the family assignment rules, 2296 TFs (1717 loci) have been identified and classified into 58 families [25].
Table 1.
Different transcription factors (TFs) and loci in different plant species.
| Taxonomic Group | Latin Name | Species | TFs | Loci | Families |
|---|---|---|---|---|---|
| Monocots | Oryza sativa subsp. japonica | Rice | 2408 | 1862 | 56 |
| Oryza sativa subsp. indica | 1891 | 1891 | 56 | ||
| Sorghum bicolor | Sorghum | 2654 | 1859 | 56 | |
| Triticum aestivum | Wheat | 3606 | 3606 | 56 | |
| Zea mays | Maize | 3308 | 2289 | 56 | |
| Eudicots | Arabidopsis thaliana | Arabidopsis | 2296 | 1717 | 58 |
| Nicotiana tabacum | Tobacco | 5176 | 3625 | 57 | |
| Solanum lycopersicum | Tomato | 1845 | 1845 | 58 | |
| Solanum tuberosum | Potato | 2405 | 1736 | 56 |
The production of reactive oxygen species (ROS), including superoxide anion, hydrogen peroxide (H2O2), hydroxyl radicals and singlet oxygen, mainly affects chloroplasts [26,27]. As the major cellular component for photosynthesis, chloroplasts are highly exposed to ROS damage since photosynthesis is a source of ROS, especially H2O2 [28]. Like chloroplasts, mitochondria and peroxisomes can generate retrograde signals. They produce ROS and many metabolites that may affect calcium signaling, some of which may serve as retrograde signals [29,30,31].
The primary cell wall of the plant is composed of cellulose fibrils, which are connected by hemicellulose tethers and embedded in a pectin gel [22]. The pectins are often modified when exposed to drought stress. By comparing the cell wall changes of two different drought-resistant wheat varieties under stress, an increase in the pectin polymers rhamnogalacturonan I and II (RGI and RGII) side chains was found to probably be due to the hydrogel formation of pectin, which limited the damage to the cells [32]. The cell wall also contains phenolics, enzymes, proteins, and Ca2+. Salt, drought, and other osmotic stress treatments can lead to the accumulation of ROS in the cell wall [22]. The dirigent (DIR) proteins in plants modulate the cell wall metabolism during abiotic and biotic stress [33]. Some proteins in the cellulose synthase complex help connect the complex to microtubules for plant growth during salt stress [34]. Members of different receptor-like kinases perceive the stress, which includes a large family of integral plasma membrane proteins. These kinases, upon perceiving stress, can transmit their signal into the cell [35]. Constitutive expression of receptor-like protein kinase 1 (RPK1) can cause upregulation of a number of stress-induced genes and enhance abiotic stress tolerance in Arabidopsis [36].
As well, the changes in organellar gene expression (OGE) in response to developmental and environmental changes, and perturbations of OGE homeostasis, regularly result in the activation of tolerance responses [37].
3. Ionic Stress Signaling
High salinity stress causes an ion homeostasis imbalance inside the plant cell. Plant cells produce an ionic stress signal. The salinity stress signal is then perceived by a receptor or salt sensor present at the plasma membrane of the cell, which is generally regulated by the coordinated action of various salt overly sensitive (SOS) pathways and ion pumps, and its downstream interacting partners, which ultimately results in the efflux of excess ions
3.1. Sodium
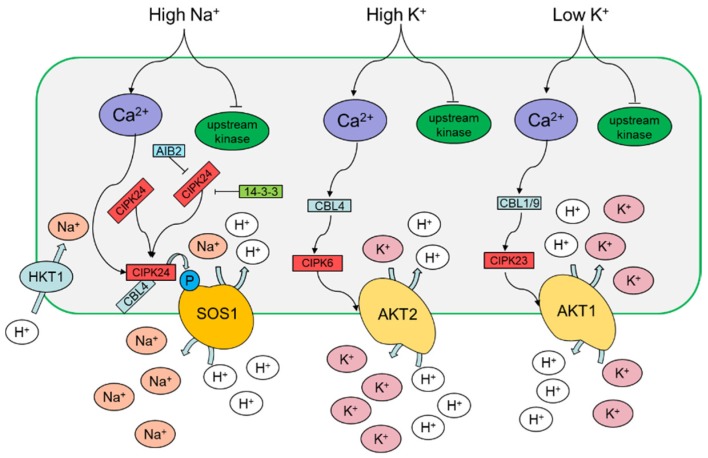
Detrimental ionic stress, especially salt stress, including chloride (Cl−), carbonate (CO32−) and sulfate (SO42−) salts of sodium (Na+), magnesium (Mg2+) or calcium (Ca2+), is harmful to plants and occurs in different geographical areas around the world [38]. Na+ has a deleterious effect when it accumulates in cells and certain tissues, directly interfering with cellular functions. Plants offset the initial osmotic components of salt stress by adjusting the osmotic gradient, although the accumulation of Na+ can lead to toxic effects in the long-term [39]. Na+ affects the hydration shell of other molecules, causes damage to the cell wall, disturbs the K+/Na+ ratio of cells by several mechanisms and impairs plant physiology [40,41,42]. Plants use a calcium-dependent protein kinase pathway, known as the SOS pathway, for salt stress signaling and Na+ tolerance [21]. Until recently, a sensor or receptor of Na+ has not been identified in plants [43,44]. In plants, the Ca2+ sensor relay proteins do not have any kinase activity (Figure 2); they interact with sensor responder proteins to modulate downstream reactions after binding to Ca2+. The name CBL has been widely used to refer to a family of EF-hand calcium-binding proteins [45].
Figure 2.
The abiotic stress of high sodium, low potassium and high potassium stress. Different colors indicate different pathways. AKT1: Arabidopsis K+ transporter 1, AKT2: Arabidopsis K+ transporter 2, HKT1: high-affinity potassium transporter 1, SOS1: salt overly sensitive. Arrows indicate activation, and bars indicate inhibition.
In the plant SOS pathway, SOS3 (CBL4), an EF-hand calcium-binding protein, senses the cytosolic calcium signal. SOS3 subsequently interacts with, and activates, the CBL-interacting protein kinase SOS2 (CIPK24) [46,47]. Despite the ability of SOS3 to bind Ca2+, the in vitro formation and activation of SOS3-SOS2 complexes was not enhanced by increasing Ca2+ concentrations [48]. The activated SOS2 phosphorylates and activates SOS1, a Na+/H+ antiporter at the plasma membrane [2,21]. Dysfunction in any of the SOS genes increases the sensitivity of the mutant plants to salt stress. The sos1 mutants appeared to be the most salt-sensitive, followed by sos2 mutants exhibiting intermediate salt sensitivity, with sos3 mutants being the least salt sensitive [49].
SOS1 and high-affinity potassium transporter 1 (HKT1) have antagonistic functions. The HKT protein family has been shown to be critical for salinity tolerance in plants [50]. HKT1 in Arabidopsis is a Na+ importer, expressed in parenchyma cells and other cells in the vascular system throughout the plant [51,52]. In the roots, HKT1 unloads Na+ from the xylem in order to limit the amount of Na+. In the leaves, HKT1 was thought to be able to load Na+ into the phloem and recycle it back to the root [53]. A mutation of HKT1 caused obvious sensitivity to salt stress, but inhibited the salt sensitivity of SOS mutants in the culture medium [53].
Under salt stress, OsHKT1;1, a Na+ transporter expressed in the vascular tissue of rice shoots, enhances Na+ ion exclusion from the cell [54,55]. Mostly in monocot plants, HKT1 was found to function in the vascular tissue of roots and shoots, and regulate Na+ ion movement from root to shoot. In salt-tolerant landraces, unlike OsHKT1;1, the OsHKT1;4 gene more efficiently contributes to Na+ exclusion from leaf blades at leaf sheaths, compared with the japonica rice species [56]. In japonica rice cultivars, OsHKT1;4 down-regulates Na+ ion concentration in leaves by regulating Na+ ion movements from stem to leaves at the vegetative stage, but has no impact on lowering the high Na+ ion concentration directly from leaves [57,58]. A study showed that mutants with an overexpression of OsHKT1;4 are more sensitive to salt than WT plants [59]. OsHKT1;5, known as the SKC1 gene, was determined to be the salt-tolerant determinant gene through quantitative trait loci (QTL) analysis [60]. OsHKT1;5-dependent translocation of Na+ in roots, leaf sheaths, and stems is a key mechanism of salt tolerance throughout the growth stages of rice and Arabidopsis. AtHKT1 was expressed mainly in the phloem tissues and was proposed to function in the recirculation of Na+. The function of OsHKT1;5 is crucial, including the protection of the next generation seeds and vital leaf blades under salt stress [61].
SOS3 has an N-terminal myristoylation signal peptide crucial for SOS3 function in salt stress. SOS3-like calcium-binding proteins are phosphorylated by interacting with SOS2-like protein kinases, and phosphorylation appears important for the activation of kinases by calcium-binding proteins [62]. 14-3-3 proteins, regulatory factors, are highly conserved in eukaryotes and function in almost every aspect of plant growth and development. 14-3-3 proteins interact with SOS2 and can repress SOS2 activity under non-stress conditions, but sodium can reduce the interaction between 14-3-3 proteins and SOS2 to activate an SOS pathway for salt tolerance [63].
SOS2, a large family of protein kinases, has two similar kinases in yeast and mammals: sucrose nonfermenting 1 (SNF1) and AMP-activated protein kinases (AMPKs). In plants, these proteins are generally referred to as SNF1-related kinases (SnRKs), which play a major role in regulating gene expression in plant cells [64]. In several plants, members of the SnRK subfamily have been identified [65,66]. The first identified plant SnRK is the rice homolog of SNF1-encoded protein-serine/threonine kinase (RKIN1), which was found in rye endosperm [67]. According to its amino acid sequences, and based on the evolutionary relationships, the SnRK family has been divided into three subfamilies: SnRK1, SnRK2, and SnRK3 [68]. The SnRK1 subfamily has a highly conserved (ca. 62–64% amino acid identity) N-terminal catalytic domain that has three members [68,69]. Through gain of function experiments, the SnRK1 subfamily was shown to complement yeast snf1-defective mutants. This suggested that the SnRK1 subfamily is involved in glucose signaling and transcription regulation [70]. Compared to the members of the SnRK1 subfamily, the SnRK2 subfamily has a conserved (ca. 42–46% amino acid identity) N-terminal catalytic domain, and has relatively short C-terminal conserved domains. In rice, the SnRK2 subfamily has 10 members, OsSAPK1−OsSAPK10 [71], and SnRK2 subfamily proteins that are essential to both osmotic stress responses and ABA signaling [72,73].
When the Ca2+ concentration changes, plants use a calcium effector protein to sense this signal, and then manage the external stimulation by regulating the expression of the plant stress gene. All these effector proteins have an EF-hand domain, defined by its helix-loop-helix secondary structure, to bind Ca2+ [74]. SnRK3s are rare protein kinases in plants, called calcineurin B-like calcium sensor-interacting protein kinases (CIPK or PKS), and SnRK3s interact with one or more members of the family of SOS3-like calcium-binding proteins (SCaBPs or CBLs) through a common motif known as NAF/FISL in the N-terminal regulatory region of the kinases [48,75].
3.2. Potassium
Potassium (K+) is the most commonly found cation in living plant cells. It is involved in many aspects of plant growth and development. It can affect all aspects of crop production and tolerance to various abiotic stresses [76]. Thus, the maintenance of K+ ion transporters and channels across the plasma membrane is essential for proper K+ homeostasis in plants. The large number of possible CBL−CIPK combinations suggests that the Ca2+−SOS3−SOS2 signaling pathway is widely used in plants. The maintenance of a high cytosolic K+/Na+ ratio is essential for plant salt tolerance. During low potassium (K+) stress, which presumably triggers a cytosolic calcium signal, the CBL1/CBL9−CIPK23 module activates the affinity K+ transporter (AKT) for K+ uptake (Figure 2) [77]. AKT1 is one of the most important K+ transporters in Arabidopsis. It mediates continuous growth by absorption of K+ by plant roots through various exogenous K+ concentrations and promotes high affinity K+ uptake in the low-K+ concentration range [78]. The results of cipk23, cbl1/cbl9, and akt1 mutants showed similar reduced growth and chlorotic leaves under low K+ conditions [78,79,80]. Instead of mutant experiments, AKT1 overexpressed (OE) in Arabidopsis did not show any significant improvement in growth when they were grown in low K+ conditions, whereas At/PeCBL1, AtCBL9, and AtCIPK23 OE plants demonstrated a comparative tolerance compared to control plants under the same conditions [81,82]. In rice, the inward K+ channel, OsAKT1, functions in K+ uptake in rice roots, whose activity is regulated by OsCBL1 and OsCIPK23 [83]. The CBL−CIPK network is involved in the negative regulation of AKT1 activity. AtCBL10 is thought to be directly bound to AKT1 in competition with AtCIPK23, so AtCIPK23 stops binding and activating AKT1 [84]. OsAKT1 is expressed in almost all rice tissues and organs, which contributes considerably to K+ uptake in a wide range of external K+ concentrations. OsAKT1 impacts stomatal conductance during osmotic stress, which is important for water stress [76].
AKT2 is another K+ transporter involved in moving K+ across the plasma membrane [85,86,87]. The CBL4−CIPK6 complex controls the plasma membrane targeting of the Arabidopsis K+ channel AKT2 (Figure 2) [88]. The (de)phosphorylation network regulates the functional switch from influx to efflux. In Arabidopsis, protein phosphatase 2C (AtPP2CA) dephosphorylation was found to be able to repress the ability of AKT2 to move K+ out of the cell [89]. The knockout mutant akt2 reduced K+ dependence of the phloem and affected sugar loading into the phloem [87]. The single knockout mutants cipk6 and cbl4 reduced growth and delayed bolting, which is similar to the akt2 mutant [85,86,88]. Moreover, CBL2 and CBL3 may function in the regulation of K+ transport between the vacuole and cytoplasm [90].
The H+-ATPases are important components in the initial sensing in plant responses to K+ deficiency [91]. The plant K+ transporters are mainly derived from several gene families, including KUP/HAK/KT, HKT, NHX, and CHX [92]. OsHAK1, OsHAK2 and OsHAK5, as K+ transporters, play important roles in K+ acquisition and distribution in rice [93]. The over-expression of OsHAK1 significantly improved salinity tolerance and drought tolerance [93,94,95]. The rice OsHAK2 protein is sensitive to extracellular Na+ and transports Na+ more effectively than K+ [96]. The over-expression of OsHAK5 in rice plants increased the shoot [K+]/[Na+] ratio and enhanced the salt tolerance of the plant. OsHAK5 plays an important role in the process of obtaining potassium from roots that are faced with low exogenous potassium and upward migration of potassium, transporting potassium from roots to aboveground parts in K-deficient rice plants [97].
3.3. Nitrate
Nitrogen is important for crop production and plant growth, and is highly regulated and coordinated with other transport and metabolic pathways [98,99]. Plants mainly uptake NO3− as the nitrogen source [100]. There are two systems for nitrate uptake: the high-affinity system and the low-affinity system for nitrate uptake [101,102]. The high-affinity system is induced in nitrate deficit, while the low-affinity system is used for the primary nitrate uptake and response under a sufficient situation [101,102]. In plants, there are three nitrate transporter families: nitrate transporter 1 (NRT1), nitrate transporter 2 (NRT2) and chloride channel (CLC). The first identified nitrate transporter was NRT1, or chlorate resistant 1 (CHL1), which is a dose-dependent master controller of multiple signaling mechanisms, capable of responding to a wide range of soil nitrate levels [103,104]. There are 53 AtNRT1, 7 AtNRT2 and 7 AtCLC that have been identified in Arabidopsis [103,105,106]. Among them, AtNRT2.1 and AtNRT2.2 are engaged in high-affinity uptake. AtNRT1.1 is involved in both high- and low-affinity uptake of NO3−, whereas AtNRT1.2 works in low-affinity uptake [107,108]. In rice, up to 250-fold increases in the induction of gene expression after nitrate resupply were observed for the high-affinity transporters OsNRT2.1 and OsNRT2.2 [109].
When faced with a lack of nitrate, AtCBL19-AtCIPK23 compounds are responsible for NRT1.1 phosphorylation, which improves the high binding affinity and transport capacity, in order to increase the absorption of nitrate [110]. AtCIPK8 may participate in response to high concentrations of nitrate by perceiving and activating the low affinity nitrate reaction [111]. Experiments on the cipk8 mutant showed that AtCIPK8 participates in the long-term process of nitrate-regulating root growth, and the response to primary nitrate produced a positive effect [111].
3.4. Phosphorus
Phosphorus is an essential nutrient for plant growth and development that accounts for about 0.2% of a plant’s dry weight [112]. It is also a constituent of nucleic acids and membrane phospholipids. High efficiency P nutrition in plants is related to many factors, such as root morphology and root exudates [113]. Plants can absorb Pi from the soil as an inorganic orthophosphate ion, but the availability is strictly limited by reactions of inorganic and organic phosphates with soil constituents [114].
The CBL−CIPK system is also involved during the response to low Pi in Brassica napus [115]. Yeast two-hybrid analysis revealed that BnCIPK6 is able to interact with Arabidopsis CBL1, CBL2, CBL3 and CBL9. BnCBL1 and BnCIPK6 were upregulated in Pi deficiency treatment and both proteins interacted with each other in yeast two-hybrid screens and a split-yellow fluorescent protein (YFP) system. This means that BnCBL1 and BnCIPK6 regulate the processes involved in the plant’s response to Pi deficiency [115]. Overexpression of either BnCBL1 or BnCIPK6 can enhance growth and biomass production under low Pi stress in Arabidopsis [115].
3.5. Magnesium
Although plants rely on a sufficient supply of Mg2+ for normal growth and development, excessive Mg2+ accumulation often causes toxicity to plant cells [116]. Two CBL proteins, CBL2 and CBL3, act as key regulators for plant growth under high-Mg conditions [117]. The cbl2 cbl3 double-mutant plants retained a lower Mg content than wild-type plants under either normal or high-Mg conditions, suggesting that CBL2 and CBL3 may be required for vacuolar Mg2+ sequestration [117]. Four CIPKs; CIPK3, CIPK9, CIPK23 and CIPK26, act as functionally overlapping components downstream of CBL2/3 in the signaling pathway that facilitates Mg2+ homeostasis [117]. This Mg2+ partitioning process in the vacuole, controlled by the CBL−CIPK pathway, may represent a general mechanism underlying detoxification [118].
3.6. Calcium
Calcium acts as a critical messenger in the adaptation and developmental processes of plants. Generally, Ca2+ transmits the stress signal through a downstream pathway by binding to protein sensors called CBL, which interact with CIPKs, a specific group of protein kinases [75]. In plants, the protein CBL family represents a unique group of calcium sensors and helps to decode calcium transients by specifically interacting with, and regulating, a family of protein kinases (CIPKs). The unique feature of the cbl10 mutant, despite being more sensitive to salt, is that it accumulates significantly less salt than the wild-type [119]. Different CBLs and their target kinases have been shown to function in stress responses. CBL4 (SOS3), and its interacting kinase CIPK24 (SOS2), together with SOS1, constitute a pathway that may function in Na+ exclusion from the cytoplasm [2].
Ca2+-dependent pathways always play critical roles in salt stress responses [120]. Ca2+ is the second most important messenger in terms of abiotic stress responses, such as salinity tolerance [121]. In the case of Na+ ion exclusion from the cytoplasm, Ca2+ sensor protein CBL4 (SOS3) interacts with the protein kinase CIPK24 (SOS2) and another Na+/H+ exchanger pathway (SOS1) at the plasma membrane [122]. The Ca2+-stress signaling system is complex; the Ca2+ signaling process is activated with the presence of a Ca2+ sensor and their target proteins.
4. Discussion
Plants are exposed to various adverse stress conditions during their growth and development processes. The stress sensor identification remains a vital part of abiotic stress research. Through mutant analysis and the CRISPR/CAS9 approach, an increasing number of genes that are relative to stress sensors will be studied deeply.
The discovery of the CBL and CIPK network represents an example of a significantly diverged Ca2+-decoding model for stress sensing that is distinct in plants. It is a key signaling system in various stress signaling pathways in plants [123]. Different CBL proteins have been reported to interact with different CIPK proteins, and the types of this interaction determine the network outcome [122]. To date, CBL and CIPK members have been comprehensively analyzed in many species (Table 2). In rice, there are more than 30 OsCIPKs, most of which respond to at least one stress factor among salt, drought and other abiotic stresses. OsCIPK3, OsCIPK12 and OsCIPK15 function as positive regulators of cold, drought and salt stress tolerance, respectively [124]. OsCIPK3 was shown to negatively regulate salt stress tolerance in rice [125]. During the seed germination and seedling stage, OsCIPK31/OsCK1 was associated with abiotic stress responses, which can lead to the differential expression of various stress-responsive genes [126]. OsCIPK14 and OsCIPK15 play an important role in the defense signaling pathway triggered by microbes in cultured rice cells [127].
Table 2.
Calcineurin B-like proteins (CBL) and CBL-interacting protein kinase (CIPK) members in different species.
Many genes for putative CIPK proteins were found in the Arabidopsis genome. In previous studies, the exchange of sodium (Na+), potassium (K+) and nitrate (NO3−) ion transportation across the plasma membrane and tonoplast in Arabidopsis was demonstrated to be regulated by the CBL−CIPK pathway [133]. cDNA cloning and sequencing have been confirmed in at least 25 CIPK genes. The interaction of CIPK24/SOS2 and CBL4/SOS3 was identified by a genetic screen that highlighted a role in salt tolerance in Arabidopsis [136]. In this connection, studies have established that CBL4/SOS3−CIPK24/SOS2 directly regulates the downstream component SOS1, a putative Na+/H+ antiporter that interacts with the Na+ ion detoxification process [137], as well as low-K-tolerant mutants
CIPK23, a critical K-nutrition determinant in Arabidopsis, was identified in a forward genetic screen [78]. CBL10, in a salt tolerant pathway, was mainly expressed and functioned in the shoots and leaves, unlike CBL4, another salt tolerant pathway, that only worked in the roots. The CBL4 (SOS) pathway interacted with CIPK24 (SOS2) protein kinase to export salt from the plasma membrane [119]. These studies support the idea that CBL−CIPK networks play important roles in abiotic stress sensing and regulating ionic balances.
During evolution, plants adopt different morphological and physiological changes in response to different abiotic stresses. Research has revealed that CBL−CIPK networks are integral in enabling plants to respond to abiotic stress and coordinate essential defense mechanisms [133]. By better understanding the CBL−CIPKs stress-sensing pathway, the ionic stress response mechanism of plants should be easily revealed. Previously, most of the research in CBL and CIPK focused on identifying the interactions between CBLs and CIPKs, the location of their interaction and the phenotypic analysis of CBL or CIPK mutants exposed to different abiotic stresses [80]. Further experiments have extended the analysis of CBL−CIPK interactions to the entire family of CBLs and a large fraction of the CIPK family in an effort to determine functional pairs of CBLs and CIPKs [120]. However, determining the downstream targets is essential to gaining a complete understanding of the CIPK and CBL signaling network.
Acknowledgments
This work was supported by National Science-technology Support Projects (2015BAD01B02-02) and National Natural Science Foundation of China (31461143014 and 31601277).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Manik S.M., Shi S., Mao J., Dong L., Su Y., Wang Q., Liu H. The calcium sensor CBL-CIPK is involved in plant’s response to abiotic stresses. Int. J. Genom. 2015;2015:493191. doi: 10.1155/2015/493191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wani S.H., Kumar V., Shriram V., Sah S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop. J. 2016;4:162–176. doi: 10.1016/j.cj.2016.01.010. [DOI] [Google Scholar]
- 4.Srivastava A.K., Penna S., Nguyen D.V., Tran L.S. Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit. Rev. Biotechnol. 2016;36:389–398. doi: 10.3109/07388551.2014.973367. [DOI] [PubMed] [Google Scholar]
- 5.Latif F., Ullah F., Mehmood S., Khattak A., Khan A.U., Khan S., Husain I. Effects of salicylic acid on growth and accumulation of Phenolics in Zea mays L. under drought stress. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015;66:325–332. [Google Scholar]
- 6.Gorji A.H., Hajianfar R., Rostamforody B., Production P. Effects of cold and other abiotic stress on plants. Int. J. Agron. 2013:3597–3604. [Google Scholar]
- 7.Guo X., Liu D., Chong K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018;60:745–756. doi: 10.1111/jipb.12706. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y., Dai X., Xu Y., Luo W., Zheng X., Zeng D., Pan Y., Lin X., Liu H., Zhang D., et al. COLD1 confers chilling tolerance in rice. Cell. 2015;162:1209–1221. doi: 10.1016/j.cell.2015.01.046. [DOI] [PubMed] [Google Scholar]
- 9.Li B., Gao K., Ren H., Tang W. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018;60:757–779. doi: 10.1111/jipb.12701. [DOI] [PubMed] [Google Scholar]
- 10.Jung J.H., Domijan M., Klose C., Biswas S., Ezer D., Gao M., Khattak A.K., Box M.S., Charoensawan V., Cortijo S. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi: 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- 11.Gangappa S.N., Berriri S., Kumar S.V. PIF4 coordinates thermosensory growth and immunity in Arabidopsis. Curr. Biol. 2017;27:243–249. doi: 10.1016/j.cub.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Z., Yuan T., Tarkowská D., Kim J., Nam H.G., Novák O., He K., Gou X., Li J. Brassinosteroid biosynthesis is modulated via a transcription factor cascade of COG1, PIF4, and PIF5. Plant Physiol. 2017;174:1260–1273. doi: 10.1104/pp.16.01778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar M. Crop plants and abiotic stresses. J. Biomol. Res. Ther. 2014;03:e125. doi: 10.4172/2167-7956.1000e125. [DOI] [Google Scholar]
- 14.Kumar K., Kumar M., Kim S.R., Ryu H., Cho Y.G. Insights into genomics of salt stress response in rice. Rice. 2013;6:1–15. doi: 10.1186/1939-8433-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar M., Choi J., An G., Kim S.R. Ectopic expression of OSSTA2 enhances salt stress tolerance in rice. Front. Plant Sci. 2017;8:316. doi: 10.3389/fpls.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu R., Zhang M., Zhao Y., He X., Ding C., Wang S., Feng Y., Song X., Li P., Wang B. Identification of salt tolerance-related microRNAs and their targets in maize (Zea mays L.) using high-throughput sequencing and degradome analysis. Front. Plant Sci. 2017;8:864. doi: 10.3389/fpls.2017.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie R., Zhang J., Ma Y., Pan X., Dong C., Pang S., He S., Deng L., Yi S., Zheng Y., et al. Combined analysis of mRNA and miRNA identifies dehydration and salinity responsive key molecular players in citrus roots. Sci. Rep. 2017;7:42094. doi: 10.1038/srep42094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q., Yan S., Yang T., Zhang S., Chen Y.Q., Liu B. Small RNAs in regulating temperature stress response in plants. J. Integr. Plant Biol. 2017;59:774–791. doi: 10.1111/jipb.12571. [DOI] [PubMed] [Google Scholar]
- 19.Huang J., Yang M., Zhang X. The function of small RNAs in plant biotic stress response. J. Integr. Plant Biol. 2016;58:312–327. doi: 10.1111/jipb.12463. [DOI] [PubMed] [Google Scholar]
- 20.De Zelicourt A., Colcombet J., Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant. Sci. 2016;21:677–685. doi: 10.1016/j.tplants.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant. Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenhaken R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014;5:771. doi: 10.3389/fpls.2014.00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J.X., Howell S.H. Managing the protein folding demands in the endoplasmic reticulum of plants. New Phytol. 2016;211:418–428. doi: 10.1111/nph.13915. [DOI] [PubMed] [Google Scholar]
- 24.Banerjee A., Roychoudhury A. Abscisic-acid-dependent basic leucine zipper (BZIP) transcription factors in plant abiotic stress. Protoplasma. 2017;254:3–16. doi: 10.1007/s00709-015-0920-4. [DOI] [PubMed] [Google Scholar]
- 25.Jin J., Tian F., Yang D.C., Meng Y.Q., Kong L., Luo J., Gao G. PlantTFDB 4.0: Toward a central HUB for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017;45:D1040–D1045. doi: 10.1093/nar/gkw982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mignolet-Spruyt L., Xu E., Idanheimo N., Hoeberichts F.A., Muhlenbock P., Brosche M., van Breusegem F., Kangasjarvi J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016;67:3831–3844. doi: 10.1093/jxb/erw080. [DOI] [PubMed] [Google Scholar]
- 27.Bobik K., Burch-Smith T.M. Chloroplast signaling within, between and beyond cells. Front. Plant Sci. 2015;6:781. doi: 10.3389/fpls.2015.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye W., Hu S., Wu L., Ge C., Cui Y., Chen P., Wang X., Xu J., Ren D., Dong G., et al. White stripe leaf 12 (WSL12), encoding a nucleoside diphosphate kinase 2 (OsNDPK2), regulates chloroplast development and abiotic stress response in rice (Oryza sativa L.) Mol. Breed. 2016;36:57. doi: 10.1007/s11032-016-0479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng S., de Clercq I., Van Aken O., Law S.R., Ivanova A., Willems P., Giraud E., van Breusegem F., Whelan J. Anterograde and retrograde regulation of nuclear genes encoding mitochondrial proteins during growth, development, and stress. Mol. Plant. 2014;7:1075–1093. doi: 10.1093/mp/ssu037. [DOI] [PubMed] [Google Scholar]
- 30.Huang S., van Aken O., Schwarzlander M., Belt K., Millar A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016;171:1551–1559. doi: 10.1104/pp.16.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You J., Chan Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015;6:1092. doi: 10.3389/fpls.2015.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leucci M.R., Lenucci M.S., Piro G., Dalessandro G. Water stress and cell wall polysaccharides in the apical root zone of wheat cultivars varying in drought tolerance. J. Plant Physiol. 2008;165:1168–1180. doi: 10.1016/j.jplph.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Paniagua C., Bilkova A., Jackson P., Dabravolski S., Riber W., Didi V., Houser J., Gigli-Bisceglia N., Wimmerova M., Budinska E., et al. Dirigent proteins in plants: Modulating cell wall metabolism during abiotic and biotic stress exposure. J. Exp. Bot. 2017;68:3287–3301. doi: 10.1093/jxb/erx141. [DOI] [PubMed] [Google Scholar]
- 34.Endler A., Kesten C., Schneider R., Zhang Y., Ivakov A., Froehlich A., Funke N., Persson S. A mechanism for sustained cellulose synthesis during salt stress. Cell. 2015;162:1353–1364. doi: 10.1016/j.cell.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., Tran L.S. Sensing the environment: Key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 2013;64:445–458. doi: 10.1093/jxb/ers354. [DOI] [PubMed] [Google Scholar]
- 36.Osakabe Y., Mizuno S., Tanaka H., Maruyama K., Osakabe K., Todaka D., Fujita Y., Kobayashi M., Shinozaki K., Yamaguchi-Shinozaki K. Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis. J. Biol. Chem. 2010;285:9190–9201. doi: 10.1074/jbc.M109.051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leister D., Wang L., Kleine T. Organellar gene expression and acclimation of plants to environmental stress. Front. Plant Sci. 2017;8:387. doi: 10.3389/fpls.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koster P., Wallrad L., Edel K.H., Faisal M., Alatar A.A., Kudla J. The battle of two ions: Ca2+ signalling against Na+ stress. Plant Biol. 2018 doi: 10.1111/plb.12704. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Aragon R., Rodriguez-Navarro A. Nitrate-dependent shoot sodium accumulation and osmotic functions of sodium in Arabidopsis under saline conditions. Plant J. 2017;91:208–219. doi: 10.1111/tpj.13556. [DOI] [PubMed] [Google Scholar]
- 40.Munns R., Tester M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 41.Julkowska M.M., Testerink C. Tuning plant signaling and growth to survive salt. Trends Plant. Sci. 2015;20:586–594. doi: 10.1016/j.tplants.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Shabala S., Wu H., Bose J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015;241:109–119. doi: 10.1016/j.plantsci.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y., Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. N. Phytol. 2018;217:523–539. doi: 10.1111/nph.14920. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y., Guo Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018;60:796–804. doi: 10.1111/jipb.12689. [DOI] [PubMed] [Google Scholar]
- 45.Yu Q., An L., Li W. The CBL-CIPK network mediates different signaling pathways in plants. Plant Cell Rep. 2014;33:203–214. doi: 10.1007/s00299-013-1507-1. [DOI] [PubMed] [Google Scholar]
- 46.Halfter U., Ishitani M., Zhu J.K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA. 2000;97:3735–3740. doi: 10.1073/pnas.97.7.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J., Ishitani M., Halfter U., Kim C.S., Zhu J.K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA. 2000;97:3730–3734. doi: 10.1073/pnas.97.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Y., Halfter U., Ishitani M., Zhu J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell. 2001;13:1383–1399. doi: 10.1105/tpc.13.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J.K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000;124:941–948. doi: 10.1104/pp.124.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waters S., Gilliham M., Hrmova M. Plant high-affinity potassium (HKT) transporters involved in salinity tolerance: Structural insights to probe differences in ion selectivity. Int. J. Mol. Sci. 2013;14:7660–7680. doi: 10.3390/ijms14047660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rus A., Lee B.H., Munoz-Mayor A., Sharkhuu A., Miura K., Zhu J.K., Bressan R.A., Hasegawa P.M. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004;136:2500–2511. doi: 10.1104/pp.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunarpi, Horie T., Motoda J., Kubo M., Yang H., Yoda K., Horie R., Chan W.Y., Leung H.Y., Hattori K., et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 53.Maser P., Eckelman B.R., Horie T., Fairbairn D.J., Kubo M., Yamagami M., Yamaguchi K., Nishimura M., Uozumi N., Robertson W. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 2002;531:157–161. doi: 10.1016/S0014-5793(02)03488-9. [DOI] [PubMed] [Google Scholar]
- 54.Wang R., Jing W., Xiao L., Jin Y., Shen L., Zhang W. The rice high-affinity potassium transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol. 2015;168:1076–1090. doi: 10.1104/pp.15.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell M.T., Bandillo N., Al Shiblawi F.R.A., Sharma S., Liu K., Du Q., Schmitz A.J., Zhang C., Very A.A., Lorenz A.J., et al. Allelic variants of OsHKT1;1 underlie the divergence between indica and japonica subspecies of rice (Oryza sativa) for root sodium content. PLoS Genet. 2017;13:e1006823. doi: 10.1371/journal.pgen.1006823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cotsaftis O., Plett D., Shirley N., Tester M., Hrmova M. A two-staged model of Na+ exclusion in rice explained by 3D modeling of HKT transporters and alternative splicing. PLoS ONE. 2012;7:e39865. doi: 10.1371/journal.pone.0039865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K., Yamaji N., Costa A., Okuma E., Kobayashi N.I., Kashiwagi T., Katsuhara M., Wang C., Tanoi K., Murata Y., et al. OsHKT1;4-mediated Na+ transport in stems contributes to Na+ exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol. 2016;16:22. doi: 10.1186/s12870-016-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wangsawang T., Chuamnakthong S., Kohnishi E., Sripichitt P., Sreewongchai T., Ueda A. A salinity-tolerant japonica cultivar has Na+ exclusion mechanism at leaf sheaths through the function of a Na+ transporter OsHKT1;4 under salinity stress. J. Agron. Crop. Sci. 2018;204:274–284. doi: 10.1111/jac.12264. [DOI] [Google Scholar]
- 59.Oda Y., Kobayashi N.I., Tanoi K., Ma J.F., Itou Y., Katsuhara M., Itou T., Horie T. T-DNA tagging-based gain-of-function of OsHKT1;4 reinforces na exclusion from leaves and stems but triggers Na toxicity in roots of rice under salt stress. Int. J. Mol. Sci. 2018;19:235. doi: 10.3390/ijms19010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren Z.H., Gao J.P., Li L.G., Cai X.L., Huang W., Chao D.Y., Zhu M.Z., Wang Z.Y., Luan S., Lin H.X. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi N.I., Yamaji N., Yamamoto H., Okubo K., Ueno H., Costa A., Tanoi K., Matsumura H., Fujii-Kashino M., Horiuchi T., et al. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J. 2017;91:657–670. doi: 10.1111/tpj.13595. [DOI] [PubMed] [Google Scholar]
- 62.Du W., Lin H., Chen S., Wu Y., Zhang J., Fuglsang A.T., Palmgren M.G., Wu W., Guo Y. Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol. 2011;156:2235–2243. doi: 10.1104/pp.111.173377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou H., Lin H., Chen S., Becker K., Yang Y., Zhao J., Kudla J., Schumaker K.S., Guo Y. Inhibition of the Arabidopsis salt overly sensitive pathway by 14–3–3 proteins. Plant Cell. 2014;26:1166–1182. doi: 10.1105/tpc.113.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y.L., Wang J., Chen X., Wang Z.X., Wu J.W. Crystal structure of the kinase and UBA domains of SnRK reveals a distinct UBA binding mode in the AMPK family. Biochem. Biophys. Res. Commun. 2018;495:1–6. doi: 10.1016/j.bbrc.2017.10.105. [DOI] [PubMed] [Google Scholar]
- 65.Carraro D.M., Lambais M.R., Carrer H. In silico characterization and expression analyses of sugarcane putative sucrose non-fermenting-1 (SNF1) related kinases. Genet. Mol. Biol. 2001;24:35–41. doi: 10.1590/S1415-47572001000100006. [DOI] [Google Scholar]
- 66.Dong X.F., Cui N., Wang L., Zhao X.C., Qu B., Li T.L., Zhang G.L. The SnRK protein kinase family and the function of SnRK1 protein kinase. Int. J. Agric. Biol. 2012;14:575–579. [Google Scholar]
- 67.Alderson A., Sabelli P.A., Dickinson J.R., Cole D., Richardson M., Kreis M., Shewry P.R., Halford N.G. Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cdna. Proc. Natl. Acad. Sci. USA. 1991;88:8602–8605. doi: 10.1073/pnas.88.19.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halford N.G., Hardie D.G. SNF1-related protein kinases: Global regulators of carbon metabolism in plants? Plant Mol. Biol. 1998;37:735–748. doi: 10.1023/A:1006024231305. [DOI] [PubMed] [Google Scholar]
- 69.Crozet P., Margalha L., Confraria A., Rodrigues A., Martinho C., Adamo M., Elias C.A., Baena-Gonzalez E. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front. Plant Sci. 2014;5:190. doi: 10.3389/fpls.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muranaka T., Banno H., Machida T. Characterization of tobacco protein kinase NPK5, a homolog of saccharomyces cerevisiae SNF1 that constitutively activates expression of the glucose-repressible SUC2 gene for a secreted invertase of S. cerevisiae. Mol. Cell Biol. 1994;14:2958–2965. doi: 10.1128/MCB.14.5.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saha J., Chatterjee C., Sengupta A., Gupta K., Gupta B. Genome-wide analysis and evolutionary study of sucrose non-fermenting 1-related protein kinase 2 (SnRK2) gene family members in Arabidopsis and Oryza. Comput. Biol. Chem. 2014;49:59–70. doi: 10.1016/j.compbiolchem.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Fujii H., Verslues P.E., Zhu J.K. Arabidopsis decuple mutant reveals the importance of SnRK2 kinases in osmotic stress responses in vivo. Proc. Natl. Acad. Sci. USA. 2011;108:1717–1722. doi: 10.1073/pnas.1018367108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshida T., Mogami J., Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant. Biol. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Gifford J.L., Walsh M.P., Vogel H.J. Structures and metal-ion-binding properties of the Ca2+-binding helix-loop-helix EF-hand motifs. Biochem. J. 2007;405:199–221. doi: 10.1042/BJ20070255. [DOI] [PubMed] [Google Scholar]
- 75.Lin H., Du W., Yang Y., Schumaker K.S., Guo Y. A calcium-independent activation of the Arabidopsis SOS2-like protein kinase24 by its interacting SOS3-like calcium binding protein1. Plant Physiol. 2014;164:2197–2206. doi: 10.1104/pp.113.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad I., Mian A., Maathuis F.J. Overexpression of the rice AKT1 potassium channel affects potassium nutrition and rice drought tolerance. J. Exp. Bot. 2016;67:2689–2698. doi: 10.1093/jxb/erw103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams E., Shin R. Transport, signaling, and homeostasis of potassium and sodium in plants. J. Integr. Plant Biol. 2014;56:231–249. doi: 10.1111/jipb.12159. [DOI] [PubMed] [Google Scholar]
- 78.Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 79.Cheong Y.H., Pandey G.K., Grant J.J., Batistic O., Li L., Kim B.G., Lee S.C., Kudla J., Luan S. Two calcineurin B-like calcium sensors, interacting with protein kinase CIPK23, regulate leaf transpiration and root potassium uptake in Arabidopsis. Plant J. 2007;52:223–239. doi: 10.1111/j.1365-313X.2007.03236.x. [DOI] [PubMed] [Google Scholar]
- 80.Thoday-Kennedy E.L., Jacobs A.K., Roy S.J. The role of the CBL–CIPK calcium signalling network in regulating ion transport in response to abiotic stress. Plant Growth Regul. 2015;76:3–12. doi: 10.1007/s10725-015-0034-1. [DOI] [Google Scholar]
- 81.Pilot G., Gaymard F., Mouline K., Chérel I., Sentenac H. Regulated expression of Arabidopsis shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003;51:773–787. doi: 10.1023/A:1022597102282. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H., Lv F., Han X., Xia X., Yin W. The calcium sensor PeCBL1, interacting with PeCIPK24/25 and PeCIPK26, regulates Na+/K+ homeostasis in Populus euphratica. Plant Cell Rep. 2013;32:611–621. doi: 10.1007/s00299-013-1394-5. [DOI] [PubMed] [Google Scholar]
- 83.Li J., Long Y., Qi G.N., Li J., Xu Z.J., Wu W.H., Wang Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell. 2014;26:3387–3402. doi: 10.1105/tpc.114.123455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ren X.L., Qi G.N., Feng H.Q., Zhao S., Zhao S.S., Wang Y., Wu W.H. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J. 2013;74:258–266. doi: 10.1111/tpj.12123. [DOI] [PubMed] [Google Scholar]
- 85.Gajdanowicz P., Michard E., Sandmann M., Rocha M., Correa L.G., Ramirez-Aguilar S.J., Gomez-Porras J.L., Gonzalez W., Thibaud J.B., van Dongen J.T., et al. Potassium K+ gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA. 2011;108:864–869. doi: 10.1073/pnas.1009777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandmann M., Skłodowski K., Gajdanowicz P., Michard E., Rocha M., Gomez-Porras J.L., González W., Corrêa L.G.G., Ramírez-Aguilar S.J., Cuin T.A., et al. The K+ battery-regulating Arabidopsis K+ channel AKT2 is under the control of multiple post-translational steps. Plant Signal. Behav. 2011;6:558–562. doi: 10.4161/psb.6.4.14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deeken R., Geiger D., Fromm J., Koroleva O., Ache P., Langenfeld-Heyser R., Sauer N., May S.T., Hedrich R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta. 2002;216:334–344. doi: 10.1007/s00425-002-0895-1. [DOI] [PubMed] [Google Scholar]
- 88.Held K., Pascaud F., Eckert C., Gajdanowicz P., Hashimoto K., Corratge-Faillie C., Offenborn J.N., Lacombe B., Dreyer I., Thibaud J.B., et al. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 2011;21:1116–1130. doi: 10.1038/cr.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chérel I.E.M., Platet N., Mouline K., Alcon C., Sentenac H., Thibaud J.B. Physical and functional interaction of the Arabidopsis K+ channel AKT2 and phosphatase AtPP2CA. Plant Cell Online. 2002;14:1133–1146. doi: 10.1105/tpc.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu L.L., Ren H.M., Chen L.Q., Wang Y., Wu W.H. A protein kinase, CIPK9 interacts with calcium sensor CBL3 and regulates K+ homeostasis under low-K+ stress in Arabidopsis. Plant Physiol. 2013;161:266–277. doi: 10.1104/pp.112.206896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmgren M.G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:817. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 92.Wang Y., Wu W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013;64:451–476. doi: 10.1146/annurev-arplant-050312-120153. [DOI] [PubMed] [Google Scholar]
- 93.Chen G., Hu Q., Luo L., Yang T., Zhang S., Hu Y., Yu L., Xu G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015;38:2747–2765. doi: 10.1111/pce.12585. [DOI] [PubMed] [Google Scholar]
- 94.Chen G., Liu C., Gao Z., Zhang Y., Jiang H., Zhu L., Ren D., Yu L., Xu G., Qian Q. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017;8:1885. doi: 10.3389/fpls.2017.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen G., Liu C., Gao Z., Zhang Y., Zhang A., Zhu L., Hu J., Ren D., Yu L., Xu G., et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a Japonica rice cultivar. Front. Plant Sci. 2017;8:2216. doi: 10.3389/fpls.2017.02216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horie T., Sugawara M., Okada T., Taira K., Kaothien-Nakayama P., Katsuhara M., Shinmyo A., Nakayama H. Rice sodium-insensitive potassium transporter, OsHAK5, confers increased salt tolerance in tobacco BY2 cells. J. Biosci. Bioeng. 2011;111:346–356. doi: 10.1016/j.jbiosc.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 97.Yang T., Zhang S., Hu Y., Wu F., Hu Q., Chen G., Cai J., Wu T., Moran N., Yu L., et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 2014;166:945–959. doi: 10.1104/pp.114.246520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Taghavi T., Folta K.M. A comparison of wild and cultivated strawberries for nitrogen uptake and reduction. Horticult. Environ. Biotechnol. 2014;55:196–206. doi: 10.1007/s13580-014-0190-7. [DOI] [Google Scholar]
- 99.Bouguyon E., Gojon A., Nacry P. Nitrate sensing and signaling in plants. Semin. Cell Dev. Biol. 2012;23:648–654. doi: 10.1016/j.semcdb.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Crawford N.M. Nitrate: Nutrient and signal for plant growth. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Forde B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002;53:203–224. doi: 10.1146/annurev.arplant.53.100301.135256. [DOI] [PubMed] [Google Scholar]
- 102.Krouk G., Crawford N.M., Coruzzi G.M., Tsay Y.F. Nitrate signaling: Adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010;13:266–273. doi: 10.1016/j.pbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 103.Tsay Y.F., Schroeder J.I., Feldmann K.A., Crawford N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993;72:705–713. doi: 10.1016/0092-8674(93)90399-B. [DOI] [PubMed] [Google Scholar]
- 104.Guan P. Dancing with hormones: A current perspective of nitrate signaling and regulation in Arabidopsis. Front. Plant Sci. 2017;8:1697. doi: 10.3389/fpls.2017.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Angeli A., Monachello D., Ephritikhine G., Frachisse J.M., Thomine S., Gambale F., Barbier-Brygoo H. CLC-mediated anion transport in plant cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:195–201. doi: 10.1098/rstb.2008.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forde B.G. Nitrate transporters in plants: Structure, function and regulation. Biochim. Biophys. Acta. 2000;1465:219–235. doi: 10.1016/S0005-2736(00)00140-1. [DOI] [PubMed] [Google Scholar]
- 107.Liu K., Tsay Y., Huang C. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li W., Wang Y., Okamoto M., Crawford N.M., Siddiqi M.Y., Glass A.D. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007;143:425–433. doi: 10.1104/pp.106.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Araújo O.J.L., Pinto M.S., Sperandio M.V.L., Santos L.A., Stark E.M.L.M., Fernandes M.S., Santos A.M.D., Souza S.R.D. Expression of the genes OsNRT1.1, OsNRT2.1, OsNRT2.2, and kinetics of nitrate uptake in genetically contrasting rice varieties. Am. J. Plant Sci. 2015;06:306–314. doi: 10.4236/ajps.2015.62035. [DOI] [Google Scholar]
- 110.Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 111.Hu H.C., Wang Y.Y., Tsay Y.F. AtCIPK8, a CBL-interacting protein kinase, regulates the low-affinity phase of the primary nitrate response. Plant J. 2009;57:264–278. doi: 10.1111/j.1365-313X.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 112.Schachtman D.P., Reid R.J., Ayling S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998;116:447–453. doi: 10.1104/pp.116.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rausch C., Bucher M. Molecular mechanisms of phosphate transport in plants. Planta. 2002;216:23–37. doi: 10.1007/s00425-002-0921-3. [DOI] [PubMed] [Google Scholar]
- 114.Bieleski R.L. Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant Physiol. 1973;24:225–252. doi: 10.1146/annurev.pp.24.060173.001301. [DOI] [Google Scholar]
- 115.Chen L., Ren F., Zhou L., Wang Q.Q., Zhong H., Li X.B. The Brassica napus Calcineurin B-like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J. Exp. Bot. 2012;63:6211–6222. doi: 10.1093/jxb/ers273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Niu Y., Chen P., Zhang Y., Wang Z., Hu S., Jin G., Tang C., Guo L. Natural variation among Arabidopsis thaliana accessions in tolerance to high magnesium supply. Sci. Rep. 2018;8:13640. doi: 10.1038/s41598-018-31950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang R.J., Zhao F.G., Garcia V.J., Kleist T.J., Yang L., Zhang H.X., Luan S. Tonoplast CBL-CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2015;112:3134–3139. doi: 10.1073/pnas.1420944112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao C., Zhao Q., Jiang L. Vacuoles protect plants from high magnesium stress. Proc. Natl. Acad. Sci. USA. 2015;112:2931–2932. doi: 10.1073/pnas.1501318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim B.G., Waadt R., Cheong Y.H., Pandey G.K., Dominguez-Solis J.R., SchãLtke S., Lee S.C., Kudla J., Luan S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007;52:473–484. doi: 10.1111/j.1365-313X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- 120.Luan S., Kudla J., Rodriguez-Concepcion M., Yalovsky S., Gruissem W. Calmodulins and calcineurin B–like proteins. Plant Cell. 2002;14:S389–S400. doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanders D., Pelloux J., Brownlee C., Harper J.F. Calcium at the crossroads of signaling. Plant Cell. 2002;14:S401. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu J.K. Regulation of ion homeostasis under salt stress. Curr Opin. Plant Biol. 2003;6:441–445. doi: 10.1016/S1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 123.Yin X., Wang Q., Chen Q., Xiang N., Yang Y., Yang Y. Genome-wide identification and functional analysis of the calcineurin B-like protein and calcineurin B-like protein-interacting protein kinase gene families in turnip (Brassica rapa var. rapa) Front. Plant Sci. 2017;8:1191. doi: 10.3389/fpls.2017.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiang Y., Huang Y., Xiong L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007;144:1416. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rao X.L., Zhang X.H., Li R.J., Shi H.T., Lu Y.T. A calcium sensor-interacting protein kinase negatively regulates salt stress tolerance in rice (Oryza sativa) Funct. Plant Biol. 2011;38:441–450. doi: 10.1071/FP10205. [DOI] [PubMed] [Google Scholar]
- 126.Piao H., Xuan Y.H., Suhyun P., Byoungil J., Soonju P., Sunghan P., Chulmin K., Jin H., Wang G.K., Kim M. OsCIPK31, a CBL-interacting protein kinase is involved in germination and seedling growth under abiotic stress conditions in rice plants. Mol. Cells. 2010;30:19–27. doi: 10.1007/s10059-010-0084-1. [DOI] [PubMed] [Google Scholar]
- 127.Kurusu T., Hamada J., Nokajima H., Kitagawa Y., Kiyoduka M., Takahashi A., Hanamata S., Ohno R., Hayashi T., Okada K., et al. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010;153:678–692. doi: 10.1104/pp.109.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kolukisaoglu U. Calcium sensors and their interacting protein kinases: Genomics of the Arabidopsis and rice CBL-CIPK signaling networks. Plant Physiol. 2004;134:43–58. doi: 10.1104/pp.103.033068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H., Yang B., Liu W.Z., Li H., Wang L., Wang B., Deng M., Liang W., Deyholos M.K., Jiang Y.Q. Identification and characterization of CBL and CIPK gene families in canola ( Brassica napus L.) BMC Plant Biol. 2014;14:8. doi: 10.1186/1471-2229-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun T., Wang Y., Wang M., Li T., Zhou Y., Wang X., Wei S., He G., Yang G. Identification and comprehensive analyses of the CBL and CIPK gene families in wheat (Triticum aestivum L.) BMC Plant Biol. 2015;15:269. doi: 10.1186/s12870-015-0657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen X.F., Zhi-Min G.U., Feng L., Bo-Jun M.A., Zhang H.S. Molecular analysis of rice CIPKs involved in both biotic and abiotic stress responses. Rice Sci. 2011;18:1–9. doi: 10.1016/S1672-6308(11)60001-2. [DOI] [Google Scholar]
- 132.Yu Y., Xia X., Yin W., Zhang H. Comparative genomic analysis of CIPK gene family in Arabidopsis and Populus. Plant Growth Regul. 2007;52:101–110. doi: 10.1007/s10725-007-9165-3. [DOI] [Google Scholar]
- 133.Zhang H., Yin W., Xia X. Calcineurin B-like family in Populus: Comparative genome analysis and expression pattern under cold, drought and salt stress treatment. Plant Growth Regul. 2008;56:129–140. doi: 10.1007/s10725-008-9293-4. [DOI] [Google Scholar]
- 134.Jing L., Jiang M.M., Li R., Yang L., Chen H.Y. Identification and characterization of CBL and CIPK gene families in eggplant (Solanum melongena L.) Mol. Genet. Genom. 2016;291:1769–1781. doi: 10.1007/s00438-016-1218-8. [DOI] [PubMed] [Google Scholar]
- 135.Niu L., Dong B., Song Z., Meng D., Fu Y. Genome-wide identification and characterization of CIPK family and analysis responses to various stresses in apple (Malus domestica) Int. J. Mol. Sci. 2018;19:2131. doi: 10.3390/ijms19072131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chinnusamy V., Schumaker K., Zhu J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- 137.Shi H., Ishitani M., Kim C., Zhu J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]