Abstract
Babies have the most stable tears and people with dry eye have the least stable tears. Meibum may contribute to tear film stability, so in this study, the hydrocarbon chain conformation and rheology of meibum from babies was studied for the first time. Infrared spectroscopy was used to measure lipid phase transitions. Rheology was measured using Langmuir film technology. Meibum from 25 donors 1 to 13 years old was compared with meibum from 18 donors 13 to 25 years old. The phase transition temperature and lipid order (stiffness) increased with increasing age from 1 to 25 years. The increase in meibum lipid order from 1 to 25 years of age may contribute to the instability of the tear film with age and contribute to films with a higher reciprocal compressibility modulus that are not as compressible and not as viscoelastic. Changes in the lipid phase transition parameters of meibum lipid with dry eye are an exacerbation of the changes observed with age. The lower reciprocal compressibility moduli of meibum films from children and babies compared with meibum from adults reiterates higher stability in their films which spread better, resist deformation, and facilitates their ability to be quickly restored after blinking.
Keywords: Langmuir trough, meibum, spectroscopy, tear film, tear film lipid layer
1. Introduction
Among many functions, tears keep the cornea hydrated. The tear film on the surface of the cornea is spread upon blinking. If the eye were to remain open, the tear film “breaks up” resulting in dry regions on the surface of the cornea. Chemical and temperature receptors on the surface of the cornea signal the brain to cause a blink spontaneously before drying occurs [1]. Infants have an unusually stable tear film [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16]. The spontaneous blink rate of infants increases by 250% from birth to the first year of age, from about two to five blinks per minute [4]. The blink rate only doubles to about 10 blinks per minute from one year to 25 years of age, and only increases by 10% by 60 years of age [3,4,5,9,10,11,12,13,14,15,16,17,18]. A similar trend is observed for tear break up time measured by fluorescence spectroscopy [2,6,7,8]. Thus, the major changes in tear film stability occur from birth to 25 years of age. Tear film stability could be related to meibum composition and tear film lipid structure. Compared with adults, infants have less meibum on the lid reservoir [19], meibum that is more saturated [20,21,22], more ordered [22,23], and contains more proteins [20]. Saturation is the relative amount of single C-C bonds whereas unsaturation is the relative amount of C=C double bonds. When lipids are stiff and ordered like butter or lard, the lipid chain carbons are arranged in a trans conformation and the hydrocarbon chains are straight enabling them to pack closely together maximizing Van der Waal’s interactions. When lipids are fluid like olive oil, gauche rotamers cause bends in the hydrocarbon chains minimizing how close the chains can pack.
We found that saturation increased the phase transition temperature of human meibum by over 20 °C, a relatively high amount [24,25]. The phase transition temperature is the temperature at which half of the lipid molecules undergo a change from an ordered gel phase to a disordered liquid crystalline phase. By breaking lipid–lipid interactions with increasing temperature, the strength of the lipid–lipid interactions can be calculated. With increasing temperature, many lipids undergo a phase transition from an ordered phase called the gel phase to a disordered phase called the liquid crystalline phase. The amount of casual lipid on the eye lid decreases with increasing meibum lipid order, phase transition temperature, and age [26]. Surface pressure area studies suggest that a saturated meibum film is quite molecularly ordered (stiff molecular arrangement) and elastic (molecules are able to rearrange during compression and expansion) compared with native meibum films which are more fluid.
In this in vitro study, using Langmuir trough technology, we tested if the surface properties of meibum from infants were different from that of adults. We used infrared spectroscopy to measure phase transition parameters to assess meibum lipid–lipid interaction strength and the conformation of meibum from infants and children 1 to 12 years-old and adolescents 13 to 25 years old.
2. Results
Donors were divided into two cohorts, young, <13 years-old (Cy) and older, and 13 to 25 years-old (Co). The demographics of the meibum donors are provided in Table 1. The contribution of the one Asian in the Cy cohort and three three Asians in the Co cohort was negligible to the phase transition parameters measured as removing them changed the reported average Tm (temperature of melting), order, and cooperativity by only 0.05 ± 1.9%. Three samples from Caucasians representing Cy were used for the Langmuir trough study: a female, 8 years 5 months-old and males 1 year 8 months-old and 6 months-old.
Table 1.
Donor demographics and phase transition parameters.
| Parameter | Cy, My | Co, Mo | p |
|---|---|---|---|
| Average Age (year) | 3.9 (0.5) | 20.0 (0.8) | >0.05 |
| Age Range (year) | 1 to 12 | 13 to 25 | |
| Gender (% male) | 76 | 78 | |
| Race (%) | C (88), B (8), A (4) | C (72), B (5.5) A (17) ? (5.5) | |
| Tm | 27 (1) | 31.0 (0.8) | 0.028 * |
| Cooperativity (Hill Coefficient) | 7.3 (0.6) | 7.8 (0.6) | >0.05 |
| Order 36.0 °C (% trans) | 32 (1) | 37 (2) | <0.02 * |
| Order 33.4 °C (% trans) | 37 (1) | 42 (2) | 0.008 * |
| Δ enthalpy (kcal/mol) | 141 (8) | 142 (9) | >0.05 |
| Δ entropy (kcal/mol/degree) | 0.45 (0.02) | 0.48 (0.03) | >0.05 |
| Magnitude (cm−1) | 4.2 (0.1) | 4.0 (0.2) | >0.05 |
| Minimum Frequency (cm−1) | 2849.60 (0.04) | 2849.63 (0.08) | >0.05 |
| Maximum Frequency (cm−1) | 2853.77 (0.09) | 2853.7 (0.2) | >0.05 |
| Δ Order 33.4 °C–36.0 °C (% trans) | 4.8 (0.7) | 5.1 (0.3) | >0.05 |
| Number of Participants | 25 | 18 |
Cy = younger cohort, Co = older cohort, My = meibum from Cy, Mo = meibum from Co, C = Caucasian, B = Black, A = Asian, ? = race unknown. (Standard Deviation). * = significant difference (p < 0.05).
2.1. Phase Transition Parameters by Infrared Spectroscopy
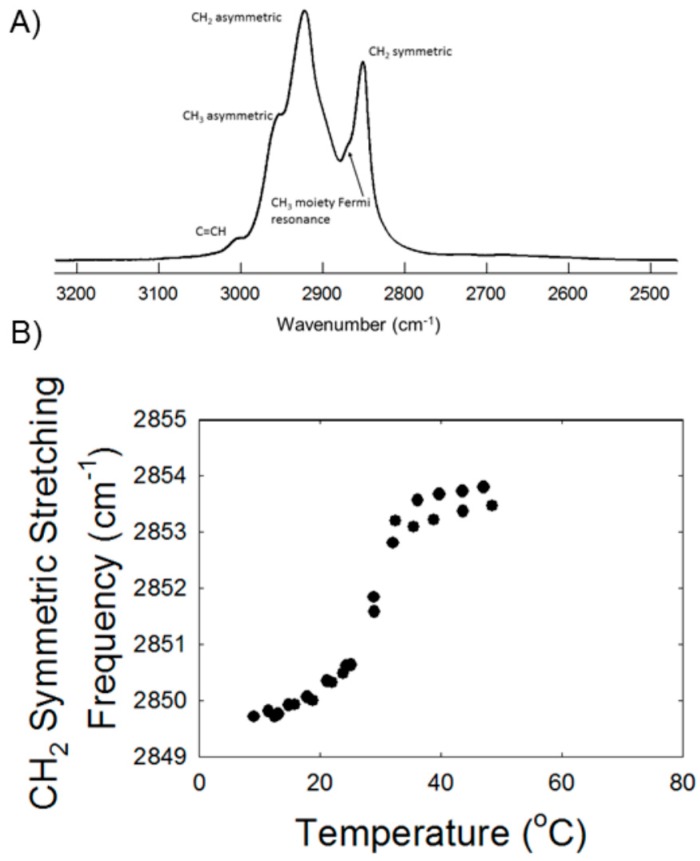
Lipid phase transitions were measured for meibum from the young cohort (My) and meibum from the older cohort (Mo). The frequency of the symmetric CH2 stretching band near 2850 cm−1 (sym) was used to estimate the trans to gauche rotamer content of the hydrocarbon chains (Figure 1A) [23], and it increased with an increase in temperature (Figure 1B) concurrent with a decrease in intensity [23,27]. The more gauche rotamers, the higher the value for sym. Two of the phase transition parameters measured in the current study, the minimum and maximum vibrational frequency of the C–H symmetric stretch (sym), correspond to the most ordered and disordered states of hydrocarbon chains, respectively. The relative cooperativity of the phase transition describes how the order of a lipid influences that of neighboring lipids. Broad phase transitions have a relatively smaller absolute value of the cooperativity. Lipid order was calculated at 33.4 °C, the temperature at the surface of the eye, and at 36 °C, the temperature of the eye lid [28]. The phase transition parameters are listed in Table 1. The phase transition temperature and lipid order at 33.4 °C and 36 °C, increased significantly with age (from Pearson’s coefficient) for the samples studied, p = 0.031. r = 0.342; p = 0.032, r = 0.34; p = 0.013, r = 0.40, respectively. The same parameters were lower for Cy compared with Co (Table 1). All of the other phase transition parameters were not significantly different p > 0.05 for Cy compared with Co.
Figure 1.
(A) Typical infrared carbon-hydrogen stretching region for meibum from a 2-year-old Caucasian male. (B) Typical phase transition of meibum from a four-year-old Caucasian female. The larger the CH stretching frequency the larger the disorder (fluidity) of the lipid.
2.2. Langmuir Trough Study
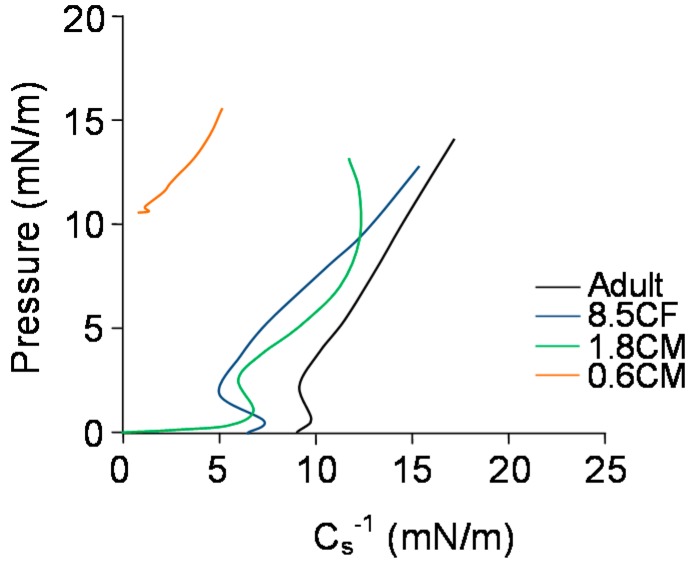
Parameters measured for the Langmuir trough study are listed in Table 2. Meibum samples upon spreading on AT (artificial tear) solution at maximum area showed a zero baseline spreading pressure, except the infant sample, 0.6CM. The lift-off area, the surface area at which pressure rise over baseline was first observed upon compression, for the adult sample was ~47 cm2, while for children samples, 8.5CF and 1.8CM, it was higher, ~68 cm2. All pressure-area isotherms showed a slow and continuous increase in pressure upon compression, typical of Meibomian films (Figure 2). The maximum surface pressures at the highest compression (Πmax) were 14 ± 1 mN/m. The rheology of the infant sample, 0.6CM, was different from the other meibum samples. Its baseline spreading pressure was high, ~10 mN/m, and the pressure upon compression increased slowly over a wide surface area demonstrating a very high compressibility (Figure 2).
Table 2.
Reciprocal compressibility moduli and inflections for meibum films with maximal reciprocal compressibility moduli () and the surface pressure (Πmax) at which they are achieved.
| Sample * | Cs−1 at Lift-Off (mN/m) | Surface Area Range for Inflection (cm2) | Pressure Range for Inflection (mN/m) | Πmax (mN/m) | |
|---|---|---|---|---|---|
| Adult | 9 | 47–38 | 0–2 | 17 | 14 |
| 8.5CF | 6 | 68–49 | 0–2 | 15 | 13 |
| 1.8CM | 0 | 66–43; 19–15 | 0–3, 10–13 | 12 | 13 |
| 0.6CM | Near zero | 90–72 | 10–11 | 5 | 15 |
* Numbers represent age (years, months); C = Caucasian; M = male; F = female.
Figure 2.
Pressure-area isotherms of meibum films of infants, children, and adults at 35 °C at the air-artificial tear interface.
The reciprocal compressibility modulus, Cs−1, calculated from the pressure area isotherms, gave information on the compressibility and physical state of the meibum films. A plot of Cs−1 with surface area (Figure 3) showed that Cs−1 for the adult sample had an inflection just after lift-off of 47–38 cm2. The pressure then increased steadily and assumed a maximum value of 17 mN/m at the highest compression. Sample 8.5CF started with a lower Cs−1 compared with the adult sample at lift-off, showed a longer infection, 68–49 cm2, and assumed a maximum value of 15 mN/m at the highest compression. Sample 1.8CM started with zero Cs−1 at the lift-off, showed two long inflections at 66–43 cm2 and 19–15 cm2, and assumed a maximum value of 12 mN/m at the highest compression. Sample 0.6CM started with a Cs−1 near zero at the lift-off, increased extremely slowly with compression and assumed a maximum value of 5 mN/m at the highest compression.
Figure 3.
Reciprocal compressibility modulus as a function of surface area for meibum films of infants, children, and adults at 35 °C at the air-artificial tear interface.
A plot of Cs−1 with surface pressure (Figure 4) showed that for the adult meibum sample, Cs−1 had an inflection after lift-off at low pressures (0–2 mN/m) and the maximal Cs−1 (17 mN/m) was achieved at Πmax of 14 mN/m. Sample 8.5CF showed a lower Cs−1 at lift off, had a longer inflection at similar low pressures and its maximal Cs−1 (15 mN/m) was achieved at a Πmax of 13 mN/m. Sample 1.8CM with a zero Cs−1 at lift off and two long inflections at two pressure ranges (lower 0–3 m and higher 10–13 mN/m) achieved its maximal Cs−1 (12 mN/m) at a Πmax of 13 mN/m. Sample 0.6CM with near zero Cs−1 at lift off and one inflection at higher pressures (10–11 mN/m) achieved its maximal Cs−1 (5 mN/m) at a Πmax of 15 mN/m.
Figure 4.
Reciprocal compressibility modulus as a function of surface pressure for meibum films of infants, children, and adults at 35 °C at the air-artificial tear interface.
3. Discussion
There is a paucity of data on the physical characteristics of meibum from children and babies below eight years of age. Meibum from babies less than four years old have never been studied in detail and the 21 samples below six years of age examined in this study are the highest number studied in this age group to date.
We observed an increase in the lipid order (stiffness) and the phase transition temperature with age between one and 25 years of age. The tandem increase would be expected as lipid order and the phase transition temperature are directly related [23]. The observed increase in lipid order with age below 25 years of age was not expected as above 25 years of age, lipid order has been shown to decreases slightly but significantly [24,29] The slight decrease in hydrocarbon chain order above 25 years of age does not contribute much to lipid stability because as stated in the Introduction, there is very little change in tear stability above 25 years of age. The increase in lipid order with age below 25 years of age measured in the current study was not in agreement with a study of seven meibum samples three to six years of age [25], which showed that the lipid order of the cohort was much higher than that reported for other ages. The discrepancy was due to two samples, three and four years of age that had an order of 62 and 67%, respectively, much higher than the order of any meibum sample and more than two standard deviation units above the order of aged matched controls. The increase in lipid order and phase transition temperature with age between one and 25 years of age, observed in the current study, may contribute to the observed decrease in tear film stability with age as the largest change in tear film stability occurs below 20 years of age (see Introduction). Correlation does not necessitate cause, but one may speculate that more ordered lipids could cause the tear film lipid layer to become too viscous, a state where deformations become irreversible. Furthermore, too much lipid order may cause lipids on the surface of tears to aggregate into “islands” (lateral phase separated) keeping them from spreading. In support of this idea, lipids from donors with dry eye symptoms and an unstable tear film due to meibomian gland dysfunction (MGD) have the most ordered lipid measured, 47% ordered [23]. Thus, in context to the increase in lipid order with age below 25 years of age and the high order of meibum from donors with MGD, the increase in lipid order with MGD is an exacerbation of aging and could contribute to tear film instability. Too much lipid order may also keep meibum from flowing out of the meibomian glands. We calculated from the current study that the 2- to 3-degree higher temperature of the Meibomian glands compared with the tear film surface [28] would cause the lipids in the meibomian gland to be about 16% less ordered than the lipids on the surface of tears. The difference in order could facilitate the flow of meibum out of the Meibomian glands and facilitate the positive effects of ordered lipids on the surface of tears.
An increase in lipid order driven by saturation causes changes in the rheology of human meibum surface films, resulting in stiffer, more elastic films with lower lift off, increased maximum surface area, and an increase in the maximum compressibility modulus [24,30]. In the current study, except for no difference in the maximum surface area, the differences in the rheology of Cy compared with Co could be explained by the differences in lipid order between the cohorts [24,30]. As unsaturation has been shown to increase with age [25], one would expect a decrease in lipid order with age, as observed in meibum from donors above 25 years of age, but opposite for meibum from donors below 25 years of age [31]. Other factors that could influence lipid order include cholesteryl esters, hydrocarbon chain length [23], proteins [20,32], and hydrocarbon chain branching. Antieso branching rather than iso branching would be expected to fluidize hydrocarbon chains [33].
Pressure-area isotherms of all meibum samples indicate the existence of a liquid expanded state of interfacial Meibomian films, but a closer look at the nature and variations of reciprocal compressibility modulus within different samples of different ages conveys further information regarding their characteristics and functions that is useful in explaining higher stability in the tear films of children and infants.
The common features of all meibum samples were that they all were highly compressible without any collapse. Lack of collapse and very low molecular area (~10 Å2, if an apparent mean molecular weight of about 720 was assigned to meibum [34,35,36,37,38]) at maximum compression is indicative of multilayer formation which happens successively with gradual increase in thickness along the course of film compression. This concurs with the multi-molecular thick tear film lipid layer (about 100 nm or 20 molecules thick) typically dominated by nonpolar components like wax esters and cholesterol esters [39]. Low reciprocal compressibility modulus of all meibum samples correspond to a liquid expanded state [40]. The multilayered structure of meibum facilitates the quick rearrangement of molecules during blinking allowing the tear film lipid layer to withstand the high pressures of blinking. The non-linear dependence of surface pressure on reciprocal compressibility modulus is indicative of the existence of a multicomponent viscoelastic film of Meibomian lipids [41]. The fact that meibum is viscoelastic and its molecular structure governs its viscoelasticity has been substantiated by rheological studies [42].
In comparison with adult meibum, the meibum samples from children and babies had a lower reciprocal compressibility modulus both at low compression and at the highest compression. A highly compressible and reversible multilayered structure may be responsible for the observed low values. Such low reciprocal compressibility modulus accompanied with multilayered organization allows meibum films of children and babies to quickly reorganize and recover from deformation. This makes meibum films of children and babies more stable (more tolerant to high shear stress of blinking) than that of adults. An extremely low value of reciprocal compressibility modulus for meibum from babies shows it is exceptionally stable.
Compared with Mo, My had a flatter isotherm at high-film areas and higher surface activity, the surface pressure = 10 mN/m at 100% area prior the start of compression. Proteins could account for this observation as a protein enriched layer, but not lipids, is known to form highly compressible films [43]. Proteins are found in meibum [20,43,44,45,46,47] and the meibum protein profile changes dramatically with age [20]. However, the amount of protein measured in the current samples was not measured. Compared with adult meibum, the increased surface activity of an equivalent amount of My suggests an elevated concentration of surfactant molecular species in My.
The rheology of the infant sample, 0.6CM, was different from the other meibum samples. Given the small sample size and variability inherent in human meibum samples, it would be premature to speculate on the physiological significance of the difference. The Introduction cites studies that show major differences occur in tear film stability between the age of 0.6 years and two years. Future studies may or may not find the difference to be meaningful. Contamination from the trough are unlikely to contribute to the observed difference as cleaning of the trough, as stated in the Methods Section, was thorough. The rheology experiments were repeated three times to ensure reproducibility of the data.
Exceptionally high stability of the interfacial films of meibum from babies can help in explaining low blink rates in infants in comparison with adults. Very low blink rate in infants (<4/min verses 15–30/min in adults) [16,17,18] has been associated with a thicker lipid layer and greater ability to withstand repeated compression and expansion [2,48], thereby showing high elastic resistance to dilational deformation. This can also be correlated to a study where a longer time was needed to stabilize a less viscous tear film, triggering blinking in human subjects [49]. Therefore, adult films requiring more time to stabilize will trigger quicker blinking resulting in higher blink rate while a stable film, like the one in infants, will result in a lower blink rate.
The values of reciprocal compressibility modulus of meibum samples observed in our study are representative of physiological values because we used a physiologically relevant temperature, 35 °C, pH, 7.4, and salt composition (artificial tears). The variations in other studies are likely to be due to different experimental conditions used such as a different meibum source (bovine), lower temperatures, 25 °C or 28 °C, and a non-physiological subphase of saline [36,41] or tris-buffered saline [50]. In particular, temperature is a major factor that influences the behavior of meibum [37]. As temperature rises, lipid films show lower reciprocal compressibility because higher temperature results in more fluid films. Very high reciprocal compressibility modulus observed for bovine meibum [36] or a mixture of synthetic lipids [51] in comparison with human meibum, and variations due to other experimental conditions, highlights the need for having a consensus for conducting experiments under standard conditions, ideally close to physiological conditions [37], across various laboratories to allow effective comparisons. If the composition and physical properties of meibum from people with a more unstable tear film such as adults and people suffering from dry eye could be made to have the physical properties of meibum of babies and infants with a more stable tear film, one could hope that the signs and symptoms of dry eye could be ameliorated.
4. Materials and Methods
4.1. Collection and Processing of Human Meibum
Written, informed consent was obtained from all donors or their guardians. Human meibum samples were collected from participants recruited from the Kentucky Lions Eye Center, Louisville Kentucky. Meibomian gland orifices of the participants showed no evidence of keratinization or plugging with turbid or thickened secretions, and no dilated blood vessels were observed on the eyelid margin. The participants did not recall having dry eye symptoms. Protocols and procedures were reviewed by the University of Louisville Institutional Review Board (code: 11.0319, 19 July 2018). All procedures were in accord with the Declaration of Helsinki. Meibomian glands were expressed by lightly compressing the eyelids with strict attention to avoid touching the eyelid margin during expression. All four eyelids were expressed, and approximately 0.5 mg of meibum was collected per individual. The expressate was collected with a platinum spatula and dissolved in a vial of chloroform. None of the samples were pooled.
4.2. Infrared Spectroscopic Study
Meibum was collected and lipid phase transitions were measured as described previously [21]. Curves were fit using Sigma plot 10 software (Systat Software, Inc., Chicago, IL, USA) and the confidence levels were obtained from a critical value table of the Pearson product–moment correlation coefficient. Averages were compared using the Student’s t test. A value of p < 0.05 was considered statistically significant. Data are reported as the mean plus or minus the standard error.
4.3. Langmuir Trough Study
Surface pressure-area profiles of meibum samples were recorded using a computer-controlled single-barrier Langmuir Teflon trough (Nima 102M; Nima Technology Ltd., Coventry, UK) with a surface area of 15 cm2–90 cm2. The surface pressure was measured by a pressure sensor with a Wilhelmy plate (Whatman, Chr 1 filter paper). The trough was thoroughly cleaned with chloroform before each experiment. It was enclosed in a transparent Perspex cabinet to avoid air currents and airborne contaminants. The temperature of the trough was maintained at 35 °C. The trough was filled with an AT solution [52] which emulated the salt composition of human tears (NaCl 6.6 g/L; KCl 1.7 g/L; NaHCO3 1.4 g/L; CaCl2.2H2O 0.15 g/L; NaH2PO4.H2O 0.1 g/L; MOPS 4.18 g/L; pH 7.4). Purified water (Milli-Q, resistance >18.2 MΩ; Millipore, Billerica, MA, USA) was used for preparing AT solution. The surface of the AT solution was cleaned with a vacuum aspirator until a clean surface was achieved (pressure change <0.02 mN/m when the surface area was compressed and expanded completely). Lipid sample (20 µL of 1 mg/mL) in chloroform was spread drop-wise on the surface of AT solution using a microsyringe (Hamilton Co., Bonaduz, Switzerland) and chloroform was allowed to evaporate for 10 min. The lipid film was compressed and expanded with a barrier speed of 15 cm2/min and changes in pressure (∏) with area (A) were recorded as Π-A isotherms. Experiments were repeated at least three times.
Compressibility (Cs) of interfacial films at a given surface pressure was calculated from the compression part of Π-A isotherm data using the following equation and was expressed in millinewton/meter (mN/m) [40]:
where AΠ is the area at a surface pressure Π. The inverse of Cs was used to determine in-plane (2 dimensional) elasticity modulus, also called reciprocal compressibility modulus or Cs−1 using the following equation and was expressed in millinewton/meter (mN/m):
The physical state of the interfacial films was determined from Cs−1 values as per Davies and Rideal [40] (<100 mN/m refers to liquid-expanded state, 100–250 mN/m to liquid-condensed state, and >250 to solid state of the film).
5. Conclusions
Changes in the lipid phase transition parameters of meibum lipid with dry eye are an exacerbation of the changes observed with age. If lipid order does play a role in tear film stability, and is not just a marker for it, the increase in meibum lipid order from one to 25 years of age may add to the stability of the tear film. Too much order, as observed with meibum from donors with MGD, is likely to decrease tear film stability. The lower reciprocal compressibility moduli of meibum films from children and babies compared with meibum from adults reiterates higher stability in their films which spread better, resist deformation and their ability to be quickly restored after deformation (blinking).
Author Contributions
Conceptualization, D.B.; Methodology, D.B. and P.M.; Formal Analysis, D.B and P.M; Investigation, D.B. and P.M.; Resources, D.B., P.M. and A.R.; Writing—Original Draft Preparation, D.B and P.M.; Writing—Review & Editing, D.B., P.M. and A.R.; Funding Acquisition, D.B.
Funding
This research was funded by National Institute of Health EYO RO126180 (DB) and an unrestricted grant from Research to Prevent Blindness, Inc. New York, NY, USA GN151619B.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Belmonte C., Nichols J.J., Cox S.M., Brock J.A., Begley C.G., Bereiter D.A., Dartt D.A., Galor A., Hamrah P., Ivanusic J.J., et al. TFOS DEWS II pain and sensation report. Ocul. Surf. 2017;15:404–437. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isenberg S.J., Del Signore M., Chen A., Wei J., Guillon J.P. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology. 2003;110:1408–1411. doi: 10.1016/S0161-6420(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 3.Bacher L.F. Factors regulating eye blink rate in young infants. Optom. Vis. Sci. 2010;87:337–343. doi: 10.1097/OPX.0b013e3181d951b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrenson J.G., Birhah R., Murphy P.J. Tear-film lipid layer morphology and corneal sensation in the development of blinking in neonates and infants. J. Anat. 2005;206:265–270. doi: 10.1111/j.1469-7580.2005.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sforza C., Rango M., Galante D., Bresolin N., Ferrario V.F. Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol. Opt. 2008;28:345–353. doi: 10.1111/j.1475-1313.2008.00577.x. [DOI] [PubMed] [Google Scholar]
- 6.Cho P., Yap M. Age, gender, and tear break-up time. Optom. Vis. Sci. 1993;70:828–831. doi: 10.1097/00006324-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Mohidin N., Bay T.C., Yap M. Non-invasive tear break-up time in normal Malays. Clin. Exp. Optom. 2002;85:37–41. doi: 10.1111/j.1444-0938.2002.tb03070.x. [DOI] [PubMed] [Google Scholar]
- 8.Ozdemir M., Temizdemir H. Age- and gender-related tear function changes in normal population. Eye (Lond.) 2010;24:79–83. doi: 10.1038/eye.2009.21. [DOI] [PubMed] [Google Scholar]
- 9.Maissa C., Guillon M. Tear film dynamics and lipid layer characteristics—Effect of age and gender. Contact Lens Anterior Eye. 2010;33:176–182. doi: 10.1016/j.clae.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Sun W.S., Baker R.S., Chuke J.C., Rouholiman B.R., Hasan S.A., Gaza W., Stava M.W., Porter J.D. Age-related changes in human blinks. Passive and active changes in eyelid kinematics. Investig. Ophthalmol. Vis. Sci. 1997;38:92–99. [PubMed] [Google Scholar]
- 11.Lavezzo M.M., Schellini S.A., Padovani C.R., Hirai F.E. Eye blink in newborn and preschool-age children. Acta Ophthalmol. 2008;86:275–278. doi: 10.1111/j.1600-0420.2007.00969.x. [DOI] [PubMed] [Google Scholar]
- 12.Craig J.P., Tomlinson A. Age and gender effects on the normal tear film. Adv. Exp. Med. Biol. 1998;438:411–415. doi: 10.1007/978-1-4615-5359-5_57. [DOI] [PubMed] [Google Scholar]
- 13.Henderson J.W., Prough W.A. Influence of age and sex on flow of tears. Arch. Ophthalmol. 1950;43:224–231. doi: 10.1001/archopht.1950.00910010231004. [DOI] [PubMed] [Google Scholar]
- 14.Guillon M., Maissa C. Tear film evaporation—Effect of age and gender. Contact Lens Anterior Eye. 2010;33:171–175. doi: 10.1016/j.clae.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Cruz A.A., Garcia D.M., Pinto C.T., Cechetti S.P. Spontaneous eyeblink activity. Ocul. Surf. 2011;9:29–41. doi: 10.1016/S1542-0124(11)70007-6. [DOI] [PubMed] [Google Scholar]
- 16.Mantelli F., Tiberi E., Micera A., Lambiase A., Visintini F., Bonini S. MUC5AC overexpression in tear film of neonates. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:1377–1381. doi: 10.1007/s00417-007-0602-9. [DOI] [PubMed] [Google Scholar]
- 17.Doughty M.J., Naase T. Further analysis of the human spontaneous eye blink rate by a cluster analysis-based approach to categorize individuals with ‘normal’ versus ‘frequent’ eye blink activity. Eye Contact Lens. 2006;32:294–299. doi: 10.1097/01.icl.0000224359.32709.4d. [DOI] [PubMed] [Google Scholar]
- 18.Bentivoglio A.R., Bressman S.B., Cassetta E., Carretta D., Tonali P., Albanese A. Analysis of blink rate patterns in normal subjects. Mov. Disord. 1997;12:1028–1034. doi: 10.1002/mds.870120629. [DOI] [PubMed] [Google Scholar]
- 19.Chew C.K., Hykin P.G., Jansweijer C., Dikstein S., Tiffany J.M., Bron A.J. The casual level of meibomian lipids in humans. Curr. Eye Res. 1993;12:255–259. doi: 10.3109/02713689308999471. [DOI] [PubMed] [Google Scholar]
- 20.Borchman D., Yappert M.C., Foulks G.N. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp. Eye Res. 2010;91:246–256. doi: 10.1016/j.exer.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borchman D., Foulks G.N., Yappert M.C., Milliner S.E. Changes in human meibum lipid composition with age using nuclear magnetic resonance spectroscopy. Investig. Ophthalmol. Vis. Sci. 2012;53:475–482. doi: 10.1167/iovs.11-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borchman D., Foulks G.N., Yappert M.C. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr. Eye Res. 2010;35:778–786. doi: 10.3109/02713683.2010.490895. [DOI] [PubMed] [Google Scholar]
- 23.Borchman D., Foulks G.N., Yappert M.C., Bell J., Wells E., Neravetla S., Greenstone V. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011;52:3805–3817. doi: 10.1167/iovs.10-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mudgil P., Borchman D., Yappert M.C., Duran D., Cox G.W., Smith R.J., Bhola R., Dennis G.R., Whitehall J.S. Lipid order, saturation and surface property relationships: A study of human meibum saturation. Exp. Eye Res. 2013;116:79–85. doi: 10.1016/j.exer.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Sledge S., Henry C., Borchman D., Yappert M.C., Bhola R., Ramasubramanian A., Blackburn R., Austin J., Massey K., Sayied S., et al. Human Meibum Age, Lipid-Lipid Interactions and Lipid Saturation in Meibum from Infants. Int. J. Mol. Sci. 2017;18:1862. doi: 10.3390/ijms18091862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashraf Z., Pasha U., Greenstone V., Akbar J., Apenbrinck E., Foulks G.N., Borchman D. Quantification of human sebum on skin and human meibum on the eye lid margin using Sebutape(R), spectroscopy and chemical analysis. Curr. Eye Res. 2011;36:553–562. doi: 10.3109/02713683.2011.574331. [DOI] [PubMed] [Google Scholar]
- 27.Kota Z., Debreczeny M., Szalontai B. Separable contributions of ordered and disordered lipid fatty acyl chain segments to nuCH2 bands in model and biological membranes: A Fourier transform infrared spectroscopic study. Biospectroscopy. 1999;5:169–178. doi: 10.1002/(SICI)1520-6343(1999)5:3<169::AID-BSPY6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Abreau K., Callan C., Kottaiyan R., Zhang A., Yoon G., Aquavella J.V., Zavislan J., Hindman H.B. Temperatures of the Ocular Surface, Lid, and Periorbital Regions of Sjogren’s, Evaporative, and Aqueous-Deficient Dry Eyes Relative to Normals. Ocul. Surf. 2016;14:64–73. doi: 10.1016/j.jtos.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Borchman D., Foulks G.N., Yappert M.C., Kakar S., Podoll N., Rychwalski P., Schwietz E. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42. doi: 10.1159/000283606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nencheva Y., Ramasubramanian A., Eftimov P., Yokoi N., Borchman D., Georgiev G.A. Effects of Lipid Saturation on the Surface Properties of Human Meibum Films. Int. J. Mol. Sci. 2018;19:2209. doi: 10.3390/ijms19082209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrestha R.K., Borchman D., Foulks G.N., Yappert M.C., Milliner S.E. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using ¹H-NMR spectroscopy. Investig. Ophthalmol. Vis. Sci. 2011;52:7350–7358. doi: 10.1167/iovs.11-7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faheem S., Kim S.H., Nguyen J., Neravetla S., Ball M., Foulks G.N., Yappert M.C., Borchman D. Wax-tear and meibum protein, wax-β-carotene interactions in vitro using infrared spectroscopy. Exp. Eye Res. 2012;100:32–39. doi: 10.1016/j.exer.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvius J.R., McElhaney R.N. Effects of phospholipid acyl chain structure on thermotropic phase properties. 3. Phosphatidylcholines with (−)-anteiso and (+/−)-anteiso acyl chains. Chem. Phys. Lipids. 1980;26:67–77. doi: 10.1016/0009-3084(80)90012-2. [DOI] [Google Scholar]
- 34.Holly F.J. Formation and stability of the tear film. Int. Ophthalmol. Clin. 1973;13:73–96. [PubMed] [Google Scholar]
- 35.Miano F., Calcara M., Millar T.J., Enea V. Insertion of tear proteins into a meibomian lipids film. Colloids Surf. B Biointerfaces. 2005;44:49–55. doi: 10.1016/j.colsurfb.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Georgiev G.A., Kutsarova E., Jordanova A., Krastev R., Lalchev Z. Interactions of Meibomian gland secretion with polar lipids in Langmuir monolayers. Colloids Surf. B Biointerfaces. 2010;78:317–327. doi: 10.1016/j.colsurfb.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 37.Mudgil P., Millar T.J. Surfactant properties of human meibomian lipids. Investig. Ophthalmol. Vis. Sci. 2011;52:1661–1670. doi: 10.1167/iovs.10-5445. [DOI] [PubMed] [Google Scholar]
- 38.Mudgil P., Borchman D., Gerlach D., Yappert M.C. Sebum/Meibum Surface Film Interactions and Phase Transitional Differences. Investig. Ophthalmol. Vis. Sci. 2016;57:2401–2411. doi: 10.1167/iovs.16-19117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bron A.J., Tiffany J.M., Gouveia S.M., Yokoi N., Voon L.W. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004;78:347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Davies J.T., Rideal E.K. Interfacial Phenomena. 2nd ed. Academic Press; New York, NY, USA: 1963. [Google Scholar]
- 41.Tsanova A., Georgiev G.A., Lalchev Z. In Vitro Application of Langmuir Monolayer Model to Study In Vivo Biological Systems. Biotechnol. Biotechnol. Equip. 2012;26(Supp. 1):185–190. doi: 10.5504/50YRTIMB.2011.0034. [DOI] [Google Scholar]
- 42.Kulovesi P., Telenius J., Koivuniemi A., Brezesinski G., Vattulainen I., Holopainen J.M. The impact of lipid composition on the stability of the tear fluid lipid layer. Soft Matter. 2012;8:5826–5834. doi: 10.1039/c2sm25210d. [DOI] [Google Scholar]
- 43.Georgiev G.A., Eftimov P., Yokoi N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp. Eye Res. 2017;163:17–28. doi: 10.1016/j.exer.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Butovich I.A., Lu H., McMahon A., Ketelson H., Senchyna M., Meadows D., Campbell E., Molai M., Linsenbardt E. Biophysical and morphological evaluation of human normal and dry eye meibum using hot stage polarized light microscopy. Investig. Ophthalmol. Vis. Sci. 2014;55:87–101. doi: 10.1167/iovs.13-13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMahon A., Lu H., Butovich I.A. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids. 2013;48:513–525. doi: 10.1007/s11745-013-3755-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong B.L., Hodson S.A., Wigham T., Miller F., Larke J.R. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr. Eye Res. 1991;10:1113–1119. doi: 10.3109/02713689109024128. [DOI] [PubMed] [Google Scholar]
- 47.Tsai P.S., Evans J.E., Green K.M., Sullivan R.M., Schaumberg D.A., Richards S.M., Dana M.R., Sullivan D.A. Proteomic analysis of human meibomian gland secretions. Br. J. Ophthalmol. 2006;90:372–377. doi: 10.1136/bjo.2005.080846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaercher T., Mobius D., Welt R. Biophysical behaviour of the infant Meibomian lipid layer. Int. Ophthalmol. 1994;18:15–19. doi: 10.1007/BF00919408. [DOI] [PubMed] [Google Scholar]
- 49.Tiffany J.M. The viscosity of human tears. Int. Ophthalmol. 1991;15:371–376. doi: 10.1007/BF00137947. [DOI] [PubMed] [Google Scholar]
- 50.Arciniega J.C., Uchiyama E., Butovich I.A. Disruption and destabilization of meibomian lipid films caused by increasing amounts of ceramides and cholesterol. Investig. Ophthalmol. Vis. Sci. 2013;54:1352–1360. doi: 10.1167/iovs.12-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulovesi P., Telenius J., Koivuniemi A., Brezesinski G., Rantamaki A., Viitala T., Puukilainen E., Ritala M., Wiedmer S.K., Vattulainen I., et al. Molecular organization of the tear fluid lipid layer. Biophys. J. 2010;99:2559–2567. doi: 10.1016/j.bpj.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mirejovsky D., Patel A.S., Rodriguez D.D., Hunt T.J. Lipid adsorption onto hydrogel contact lens materials. Advantages of Nile red over oil red O in visualization of lipids. Optom. Vis. Sci. 1991;68:858–864. doi: 10.1097/00006324-199111000-00005. [DOI] [PubMed] [Google Scholar]