Abstract
There is growing evidence that lifestyle choices account for the overall quality of health and life (QoL) reflecting many potential lifestyle risks widely associated with alterations of the reproductive function up to the infertility. This review aims to summarize in a critical fashion the current knowledge about the potential effects of stress and QoL on female reproductive function. A specific literature search up to August 2017 was performed in IBSS, SocINDEX, Institute for Scientific Information, PubMed, Web of Science and Google Scholar. Current review highlights a close relationship in women between stress, QoL and reproductive function, that this association is more likely reported in infertile rather than fertile women, and that a vicious circle makes them to have supported each other. However, a precise cause-effect relationship is still difficult to demonstrate due to conflicting results and the lack of objective measures/instruments of evaluation.
Keywords: Infertility, Lifestyle, Quality of life, Sterility, Stress
Background
The original definition of “stress” was about a non-specific body’s response to demand for change and any stimulus able to trigger it was termed as “stressor” [1, 2]. Despite the actual connotation refers to something negative, the concept of stress should be ascribed to the way by which physiological processes and biological tissues are solicited by stressful stimuli. Thus, from a positive point of view, stress can equally represent the ability of a trained body to reach the best athletic performance or the evolutionary pressure at which humans keep on being subjected through ages.
Based on the two dimensions of duration and course, stressors can be distinguished in five categories: 1) acute time-limited stressors involving laboratory challenges, such as a public speaking, 2) brief naturalistic stressors involving a person confronting a real-life short-term challenge, such as an academic examination, 3) stressful event sequences, such as individual events that give rise to a series of related challenges that it is not known when they will subside, 4) chronic stressors pervading persons’ life and forcing him/her to restructure social identity and roles, such as suffering a traumatic injury leading to physical disability and 5) distant stressors linked to traumatic experiences occurred in the past that yet have the potential to influence people’s life, such as having been sexually assaulted during childhood [3]. This classical classification allowed to clarify how stressful sources may either come from the outside, namely they are generated by the physical environment, job, relationships with others, marital life and all the situations, challenges, difficulties and expectations at which people are faced to daily, or they may be internal factors as well, like the nutritional status, the overall health, fitness levels, and the emotional well-being, that collectively establish the human attitude to respond to, and deal with, external stress-inducing factors.
Unfortunately, there is no consensus in defining and measuring objectively individual body’s stress response but physiological stress can be defined as a wide range of physical responses occurring as a direct effect of a stressor and causing an upset in the homeostasis of the body [4]. The consequence is an immediate disruption of either psychological or physical equilibrium at which the body responds to by stimulating the nervous, endocrine and immune systems and accounting for physical changes with both short- and long-term effects. For example, regular high intensity exercise (i.e. outside stressor) in professional athletes or physically active females may induce menstrual disturbances (i.e. body response to a stressful stimulus or stress) due to the endocrine system adaptation to negative energy balance exercise-dependent (i.e. internal stressor) with the following functional/hypothalamic amenorrhea (i.e. altered physical equilibrium). Along the same lines, the individual perception of one’s life in culture and social contexts in which people live (i.e. outside stressor), also called “quality of life” (QoL) [5], constitutes either a positive or negative stressful stimulus of relevance for reproductive purposes (i.e. altered physical equilibrium) and the fertility potential (i.e. body response to a stressful stimulus or stress effect) [5–8]. Interestingly, studies in cynomolgus monkeys suggest how the energy imbalance and psychosocial stress might interact synergistically at causing a greater impairment of the reproductive axis than single stressor alone [9].
QoL is a broad ranging concept, incorporating in a complex way individuals’ physical health, psychological state, level of independence, social relationships, personal beliefs and their relationships to salient features of the environment [5]. This definition highlights the view that QoL is subjective, multi-dimensional and includes both positive and negative facets of life [5]. At regard, interesting questions are whether QoL-induced stress contributes to or is a consequence of infertility, and whether a cause-effect relationship can be identified [10–13]. From a different perspective, given that deterioration of QoL or low QoL were associated with infertility and that this latter may account per se for significant levels of mainly psychological stressful stimuli [14, 15], it is remains unclear whether infertility induces negative emotional stress (also called “distress” and opposite to the “eustress”, i.e. positive emotional stress) reflecting in poor QoL or whether a poor QoL accounts for chronic distress during lifespan and finally for infertility.
Based on these considerations, the aim of the present paper will be to comprehensively and critically review the available data regarding the influence of stress and QoL on female reproductive function in order to clarify their relationship(s).
Methods
We searched all available articles discussing the relationship between stress, QoL and female infertility alone or in concert. Specifically, stress issue was searched throughout its different stressful stimuli and kindred terms including “distress”, “depression”, “anxiety”, “psychological”, “physical”, “physiological” and “emotional stress” as well as issue on QoL was searched using “motherhood”, “sexual attitude”, “marital life”, “life satisfaction” and “work life”. In current analysis, no restriction was used for the different questionnaires to assess the psychological stress and/or the QoL.
Multiple strategies were used to collect relevant demographic, epidemiological, clinical and experimental studies consulting sociological online libraries (IBSS, SocINDEX), Institute for Scientific Information, PubMed, Web of Science and Google Scholar with no language limitations. Studies collected encompass those published up to August 2017. Additional journal articles were included after hand screening of references of collected bibliography.
Since man and women respond to and perceive differently stressful events related to infertility and QoL, specific studies on stress/QoL and male fertility and/or reproductive function in males were excluded from the analysis [14, 16–18]. On the other hand, studies on couples or male population were partially considered not to exclude whether the quality of partnered relationship contributes in defining women’s QoL.
Stress and infertility
The reasonable association between woman’s stress response and fertility potential made literature to accumulate studies with conflicting results [19–29]. However, there is likewise converging evidence on female body-stress response and hormones involvement [30–32] (Fig. 1).
Fig. 1.
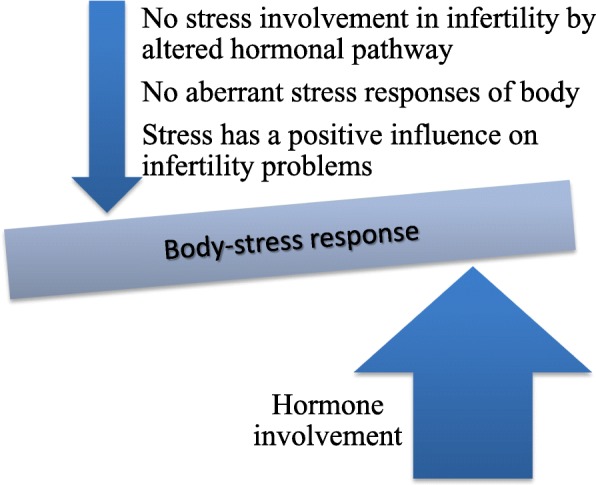
Hormonal involvement in female body-stress response. Since 1967 the majority of studies are in favor of the theory about an aberrant stress response of female body to distress stimuli mediated by hormones changes, whereas only a minority studies provided contradictory statements
Stressful stimuli cause the activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) axis [33]. The hormones secreted by these systems after stressful stimuli result in an abnormal, prolonged and/or excessive stress-induced body’s set-up that can potentially produce long-term neuroendocrine changes, affecting female fertility [34–39]. Biologically, neurons of the hypothalamic paraventricular nucleus of HPA axis release vasopressin and corticotropin-releasing hormone (CRH) to mediate the secretion of adrenocorticotropic hormone (ACTH) from the anterior lobe of the pituitary gland [33, 40]. In turn, ACTH mediates the secretion of cortisol and glucocorticoid hormones by the adrenal cortex [33, 40].
Differentially, the preganglionic sympathetic fibers of SAM axis, in response to environmental stressful stimuli, activate the adrenal medulla to release epinephrine and norepinephrine into the blood [33]. Experimental data showed that statistically significant reductions in the probability of conception across the fertile window during the first cycle attempting pregnancy were observed for women whose salivary concentrations of α-amylase were in the upper quartiles in comparison with women in the lower quartiles [41]. Even if the salivary α-amylase is considered only a surrogate marker of stress and SAM activity, these data seem to confirm the ability of stress to exert its effect on female fecundity through the SAM pathway [41].
All stress-induced hormones from the adrenal cortex and medulla are responsible for several physiological and mental consequences, which cause the individual to fight with or flight from the stressor. Differences in individual responses could be explained by findings from ewes showing that animals with divergent cortisol responses to ACTH exhibit functional differences in the HPA axis due to innate differences in the gene expression/function of HPA molecules [42]. Further results from female cynomolgus monkeys, exposed to mild combined psychosocial and metabolic stress, show a selected and specific (rather than generalized) increased activity in the adrenal framework significantly related to stress-induced reproductive dysfunction [43].
Increased glucocorticoid release/concentrations leads to profound dysfunction of the hypothalamic-pituitary-ovary (HPO) axis [31, 43–47]. Specifically, distress concentrations of glucocorticoids in the bloodstream reach high levels acting directly on hypothalamus altering the physiologic release of gonadotropin releasing hormone (GnRH) [48, 49]. The synthesis and release of gonadotropins from the pituitary are thus indirectly inhibited, even if a direct pituitary effect of glucocorticoid has been also demonstrated [48, 49]. Accordingly, evidences from animal models are available [41]. In sheep model the infusion of cortisol at concentrations comparable to those produced in humans under stress generates a delay in follicular maturation and ovulation by attenuating or blocking the expected increase of estrogens and luteinizing hormone (LH) surge [41].
However, the signaling pathway by which this occurs remain unclear and is further complicated by the recent findings of kisspeptin (KISS1) and gonadotropin-inhibitory hormone (GnIH). These two neuropeptides induce opposite effects on hypothalamic GnRH release being sensitive to high levels of glucocorticoids [32]. KISS1 exerts stimulatory effects on GnRH secretion [50]. In mouse model, corticosterone administration reduced hypothalamic expression of KISS1 during the estradiol-induced LH surge and decreased the activation of KISS1 neurons [51]. Differentially, GnIH neurons inhibit the activity mediated by either GnRH and KISS1 molecules [52]. Experimental data in ewes demonstrated a direct relationship between both acute and chronic stress and inhibiting GnIH effects on hypothalamus [53] up to inhibition of LH release from the pituitary [54].
Consequently, the stressful stimuli on the female adrenal and HPO axis impact more than one physiological event of fertility including ovulation, fertilization and the implantation rate [34, 48], independently of stimulus origin. Anomalies in the LH pulses induce and inhibition of the ovulatory function directly or thought an effect on sex steroid synthesis/secretion in the ovary [45, 55]. This circumstance can be produced by job-induced stress that exerts its effect through increased LH-plasma concentrations in both the follicular and luteal phases of the ovarian cycle [56].
Both in general and infertile population, distress was respectively associated with decreased conception rates and long menstrual cycles (≥35 days) and lower outcomes of reproductive medicine, including oocytes retrieved, fertilization, pregnancy and live birth rates [11, 41, 57–59]. In addition, in infertile women “chronic” lifetime psychosocial stressors were also identified as detriments to ovarian reserve. Specifically, they were predictive of an enhanced likelihood for diminished ovarian reserve [60]. To this regard, a low socioeconomic status aggravated by sources of stress such as undernutrition and financial hardships potentially plays a key role in affecting ovarian reserve [61].
Of note, the distress can act on female fecundity acting on uterine receptivity also independently from ovarian function. Using a mouse implantation model, the distress induced a poorer endometrial receptivity even if the hormone supplementation was administrated [62].
Depression, high active coping, avoidance and expression of emotions may produce the same consequences on female fecundity [58]. Depression is significantly correlated with the alternative manifestation of stress, i.e. anxiety, affecting cortisol release [44] and symptoms are observed in approximately 37% of infertile women [63]. Consistently, both emotions are prevalent in female partners of infertile couples [64] and more common among females suffering from infertility compared to fertile females [65–67]. The role of emotional distress and anxiety is not still understood, but a small body of evidence suggests that the induction of oxidative stress may be the mechanism by which psychosocial stressors affect oocyte quality through impairment of the overall female health [12, 68, 69].
Many women undergoing reproductive medicine report depressive symptoms prior to beginning their treatments, reflecting a prior history of mood/anxiety disorders independent of infertility itself [63]. Of interest, resilience, i.e. psychosocial stress-resistance, in infertile couples acts as a protective factor against infertility-specific distress and impaired QoL [70] probably through its effect on freedom from anxiety [71]. Moreover, data on psychological interventions or counseling interfering with depression and anxiety are reliable to speculate that the less women are physiologically reactive to distressing stimuli the more they potentially become capable of alleviating their negative consequences on reproductive system [38, 44, 72–83]. Nonetheless, albeit these interventions are effective to optimize natural fertility and outcomes of reproductive medicine strong clinical evidences are still lacking [67, 84, 85].
QoL and infertility
Although a variety of patient self-reported outcome (PRO) measures are available to investigate the intriguing aspects on the relationship between QoL and infertility (Table 1), only the two Fertility Quality of Life (FertiQoL) and Fertility Problem Inventory (FPI) questionnaires are recently acknowledged as the best useful tools to address this issue in interventional studies [86]. Specifically, the FertiQoL questionnaire is the most widely applied tool and it was developed to tackle limitations of the FPI and other questionnaires designed for specific subpopulations and therefore unable to be used as generic measures for female infertility [87, 88]. The FertiQoL items capture the key life domains affected by fertility problems, including the emotional, mind-body (cognitive and physical), relational and social domains together with the individual perception of the treatment environment and tolerability [87, 88].
Table 1.
Infertility-related questionnaires exploring patients’ self-reported measures. Questionnaires are characterized by different domains and items and the targeted population
| Questionnaire | Items and domains | Target population |
|---|---|---|
| Infertility Questionnaire | Self-esteem | Infertile patients |
| Blame/guilt | ||
| Sexuality | ||
| Infertility Reaction Scale | Duration of infertility | Infertile couples who enter an ART treatment program |
| Degree of social support effect of infertility on sexual relationship | ||
| Expected likelihood of achieving pregnancy | ||
| Anticipation of stress during treatment | ||
| Self-rating scale of emotional reactions to infertility | ||
| Fertility Problem Inventory | Social concern | Patients seeking for infertility treatment |
| Sexual concern | ||
| Relationship concern | ||
| Need for parenthood | ||
| Rejection of childfree lifestyle | ||
| SCREENIVF | State of anxiety | Women and men undergoing infertility treatment cycle |
| State of depression | ||
| Helplessness | ||
| Lack of acceptance | ||
| Perceived social support | ||
| Fertility Problems Stress Inventory | Depression | Infertile or presumed infertile couples |
| Sexual dissatisfaction | ||
| Self-esteem | ||
| Infertility Feelings Questionnaire | Adults’ cognitive appraisals of infertility | Patients |
| Daily Record-keeping Sheet | Negative emotional reactions | Women about to begin a trial of ART |
| Physical reactions | ||
| Psychologic evaluation test after ART | Emotional reactions | Women submitted to ART |
| Concerns about reproductive technologies | Medical aspects | Women submitted to ART |
| Difficulty with infertility and its treatment | The uncertainty and lack of control | Women undergoing evaluation and treatment of fertility problems |
| Family and social pressures | ||
| Impact on self and spouse | ||
| treatment-induced problems | ||
| treatment-related procedures | ||
| Polycystic Ovary Syndrome Quality of Life | Emotions | Women with PCOS |
| Body hair | ||
| Weight | ||
| Infertility | ||
| Menstrual problems | ||
| Endometriosis Health Profile-30 | Pain | Support group of patients |
| Control | ||
| Powerlessness | ||
| Emotional well-being | ||
| Social support | ||
| Self-image | ||
| Sexual intercourse | ||
| Work | ||
| Relationship with children | ||
| Feelings about the medical profession, treatment, and infertility | ||
| Fertility Quality of Life | Items that assess core and treatment-related quality of life | People with fertility problems |
| Items that assess the overall life | ||
| Items that assess physical health |
Moreover, there is reasonable evidence for adequate linguistic validation of FertiQoL [86] as confirmed by a plethora of data collected from several populations [8, 89–92]. This support that PROs of FertiQoL reliably measures QoL in women facing infertility and prove that infertility significantly reduces female QoL by increasing anxiety and depression levels [6–8, 89–92]. Both conditions belong to the emotional domain independently of the infertility cause and constitute stressful stimuli (namely distress) acting on the HPA and SAM frameworks as previously described.
For women who have ever met the criteria for infertility and perceive a fertility problem, life satisfaction is significantly lower and the association is weaker for employed women. On the contrary, for women with infertility who do not perceive a problem, not being mother is associated with higher life satisfaction [93]. As consequence, if becoming pregnant is a priority that cannot to be voluntary achieved, this denied attempt affects female QoL and identity with long-term effects and significant higher levels of distress compared to voluntary childlessness women [94].
Unsatisfied motherhood may have implications on female QoL for stress related to marital life too, hampering also couple’s attitude towards successful infertility treatments [59]. Consistently, partnered women who give up a strong intention to have children show more depressive symptoms when relinquished fertility intentions occur in the context of declining relationship quality [95, 96] and in the relational domain, female sexual function positively correlates with male partner sexual function [64]. In addition, infertile women are more likely to underestimate the importance of sexual intimacy in marital life [97] and this is consistent with the deleterious effect of the infertility on sexual dysfunction and poor QoL in women [98, 99]. This scenario can constitute a negative event in women’s life with an impact on QoL because it may potentially trigger chronic distress and subsequently reduce the changes of successful infertility treatments [100]. However, this pathway still needs further clarification [101].
QoL can be impaired in case of reproductive illness at which women are faced to during fertile lifespan. For instance, the polycystic ovary syndrome (PCOS) may be a factor favoring the occurrence of mood disorders as there is evidence that infertile women with PCOS experience high psychological distress and difficulties with coping with their condition as well as poor QoL [102–104]. These and other variables including body mass index, woman’s job, menstrual cycle intervals and sexual satisfaction appear to define QoL in women with PCOS [105]. The validated questionnaire for evaluating the impact of PCOS on health-related QoL in affected women revealed that how weight decrease is of relevance for the overall phenotypic spectrum improvement and a relative decrement in psychological distress [106]. Co-morbidities (for example obesity) may impact many patient’ characteristics, such as social and patient perspective reflected in well-being and QoL individual perception [107].
Moreover, QoL argument is of relevance in Eastern [108, 109] and African [110] societies, where social parenthood cognitions as well as community and family pressure consistently interfere with QoL of infertile women due to the cultural importance of bearing children.
Stress, QoL and assisted reproductive technologies (ARTs)
Although the influence of stress and distress (measured as anxiety and depression) on ART outcomes was appeared somewhat limited up to 2011 [84], four years later the European Society of Human Reproduction and Embryology (ESHRE) acknowledged the clinical weight of stress and QoL in female reproduction and prompted to incorporate psychosocial assistance into clinical practice of reproductive medicine [111]. In fact, each specific step of ART treatment seems to be closely related to increased levels of distress [112, 113].
This picture seems to be gender-related [114]. During an ART cycle, women show lower levels of QoL compared to men and the number of ART failures in becoming pregnant influences more women’ QoL rather than men [92, 114, 115]. Before knowing ART outcome, women undergoing a cognitive coping and relaxation in their first in vitro fertilization (IVF) cycle showed improved QoL as compared with patients undergoing routine care [116]. From a different perspective, many ART women may report depressive symptoms prior to beginning their cycle, which likely reflects the impact of repeated, unsuccessful, less invasive forms of treatment, but may reflect also a prior history of mood/anxiety disorders independent of infertility [117]. Interestingly, lower concentrations of norepinephrine and cortisol in serum and follicular fluid on the oocyte retrieval day were found in women whose treatments were successful suggesting that both stress-induced biomarkers may negatively influence the clinical pregnancy rate in IVF treatment [118]. Similar findings whereby stress levels where measured in terms of circulating prolactin and cortisol levels suggest that infertile women have a different personality profile in terms of more suspicion, guilt and hostility as compared to the fertile controls [119]. To this regard, the infertility status or its awareness could influence the hormonal release of prolactin/cortisol. On the other hand, the psychological stress may affect the outcome of IVF treatment since anxiety levels in patients who do not achieve pregnancy are higher than in those who become pregnant [119]. Furthermore, women with successful treatment have lower concentrations of adrenaline at oocyte retrieval and lower concentrations of adrenaline and noradrenaline at embryo-transfer day, compared with unsuccessful women [58]. That data emphasizes the positive relationship between adrenal stress-related biomarkers concentrations and pregnancy and depression [58].
Conclusions
In the current review, we discussed and summarized the literature published over the past years until nowadays concerning the relationship between stress, QoL and female fertility. Much of information stems from cross-sectional and interventional studies in which female population is recruited from clinics of reproductive medicine and kindred registries. Considering that 15% of couples are infertile among general population and a million of couples every year looks for time-consuming and expensive fertility treatment [117], the cohort here argued is not representative of the overall female population. This could reasonably explain some conflicting results cited.
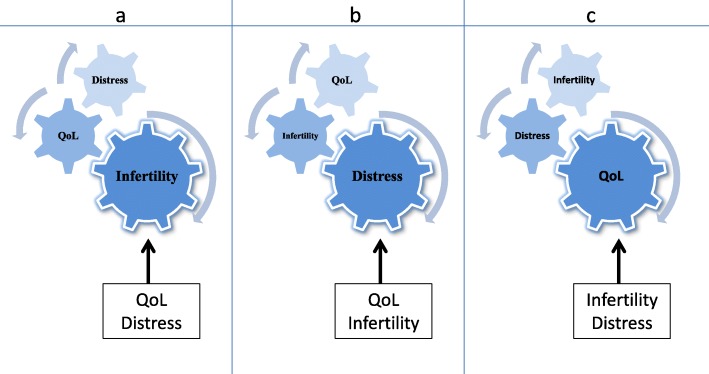
Mood states are manifestations of well-being encompassing psychological condition and life satisfaction. In this perspective, depression and anxiety represent distress-mediated symptoms of infertility that affects more women than men in four aspects of their life: psychological well-being (depending on the presence or absence of distressing stimuli from any source), marital relationship including sexual intimacy, and QoL. Specifically, most women plan their fertility as meticulously as they do career, educational and lifestyle choices waiting for the right moment of motherhood. In the absence of difficulties, achieving motherhood allows women to reach adult status, social identity, to fulfill gender-role and to complete the marriage. On the other hand, the inability to realize these social expectations can constitute a source of stress and strain resulting in QoL deterioration. This consideration joins others in literature [71, 120, 121] that can be collectively represented by the gearwheel mechanism illustrated in Fig. 2.
Fig. 2.
The gearwheel mechanism between infertility, QoL and distress. Depending on which setting a, b or c the mechanism is read into, infertility, distress and QoL can be interchangeably considered the main factor (largest gearwheel) responsible for infertility, QoL and/or distress in females (smallest gearwheels). At their turn, a, b and c mechanisms can be triggered by QoL, distress or infertility (squared boxes), suggesting a mutual and perpetuating effect on female reproductive functions
On one side, stress from any source has more impact on the wives’ live than husbands’, more impact on satisfaction with self and general well-being than on satisfaction with the marriage or health, and affects QoL mostly indirectly through its impact on the marriage factors. Most results address the alteration of hormonal signaling between the HPA and HPO axis as the more likely mechanism by which stress-related molecules negatively modulate female fertility. Going beyond the emotional fences of depression and anxiety leads women to make the decision to reveal information about their infertility with a resulting positive impact in QoL. Accordingly, literature data show that when a direct disclosure of their infertility issues (i.e. face-to-face, clearly, verbally and with the opportunity for an immediate response) is adopted by women, the perceived support quality from social network members is also related to improved QoL supporting towards infertility treatments [122]. Concisely, when the appropriate infertility patient-centered care is not offered, poor QoL is observed among women [71, 123].
On the other side, QoL and lifestyle choices are non-synonymous concepts, albeit some habits of modern life (classified as social lifestyle factors) can interfere with female health and account for reproductive problems. As consequence, the inability of becoming pregnant can be linked to social behaviors worsening female QoL indirectly.
Thus, it is possible to speculate that information on lifestyle habits should be useful to encourage women by clinicians to improve the overall health because positively affects their ability to reproduce. Moreover, handling the topic of stress with accidently childless couples should be included in routinely cares to minimize the effects of modern life on infertility. In addition, managing the baseline stress (chronic distress) prior to infertility treatment appears to have even greater importance than managing the (acute) stress inherent to fertility treatment itself. This hypothesis is in line with the results of two pilot studies exploring the efficacy of integrative approaches demonstrating that ongoing emotional and instrumental supports are both pivotal to the well-being and QoL of infertile women [82, 124].
This is particularly true for ART population for which health-care providers should be aware of offering psychological support to patients, especially women, during all phases of the medical procedures, given the emotional and physical difficulties associated with this experience. The usefulness of this support has been also acknowledged somewhat of importance to contrast psychological discomfort that could lead to premature termination of ART and consequently to reduce pregnancy rate [13]. For this purpose, it should be also considered that until the desire of motherhood does not become a priority in female life, the presence of an eventual baseline acute and/or chronic stress as low QoL determinants can be not a determinant of such a relevance. However, when the need for ART procedures occurs, it becomes difficult to establish whether ART-stress is related to ART cycle itself (acute or procedural stress, due to the timing and experience during which it arises) rather than QoL-stress, i.e. chronic distress accumulated during lifespan.
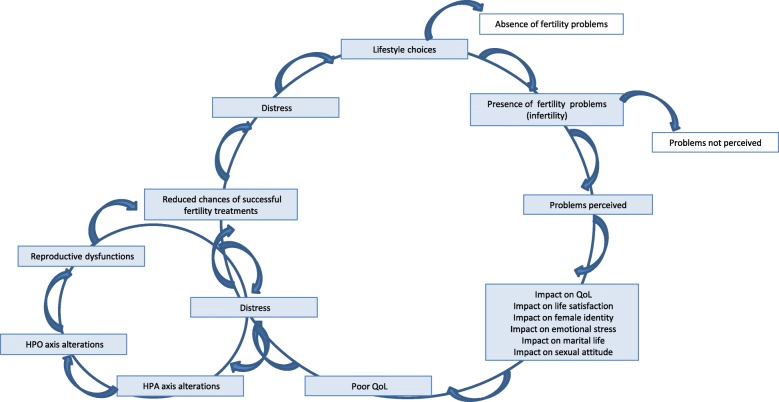
Figure 3 summarizes the theory of vicious circle between stress, QoL and altered female fertility, as suggested by Taymor’s and Bresnick’s hypothesis [125], leaving unresolved the cause-effect question point. However, we can address to further studies the following criticisms of current literature. Determining what is stressful is complex because individual responses to stressful stimuli can differ dramatically converging to the major issue of stress response rather than stress itself. Unfortunately, no optimal stress response marker is available as well as standardized measures defined independently of matching group comparisons. This hampers the possibility to conduct more studies using valid and standard tools as it is actually difficult to reproduce and generalize data from literature in this field. The identification of factors explaining stress, or that may be targets for intervention, would be important to social workers in health care (for instance, to plain programs screening aimed to decrease stress levels). Ultimately, there are quite studies that reported on health-related QoL in infertile couples.
Fig. 3.
The vicious circle between stress, QoL and altered female fertility. No cause-effect relationship can be assessed inside the intriguing relationship between stress, female infertility and QoL as it mainly depends on what stressor is considered between being infertile and an impaired QoL. Dependently on the individual perception of the problem, infertility can be a serious psychological and relationship stressor that can contribute to poor QoL levels or the clinical consequences of stress from external forms of stressful stimuli. Once infertility is manifested, difficulties arise to establish in which mechanism and because of which reason women become part of the vicious circle
In summary, at the moment the FertiQoL constitutes recommended PROs measures of female infertility related to QoL. Although gaps in evidence remain including test-retest reliability and thresholds for interpreting clinically important changes [84], further use of FertiQoL in future interventional studies is warranted to address the intriguing relationship on the physiological mechanism orchestrating stress and QoL in female fertility.
Acknowledgements
The authors are grateful to Dr. V. Davoli, “Antonio Vallisneri” Study Center, Reggio Emilia, Italy, for her assistance in collecting studies selected.
Funding
No specific funding was sought for the study. Departmental funds from the Center of Reproductive Medicine and Surgery ASMN-IRCCS of Reggio Emilia (Italy) were used to support the Authors throughout manuscript preparation.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- ACTH
Adrenocorticotrophic hormone
- ART
Assisted reproductive technology
- BMI
Body mass index
- CRH
Corticotrophin-releasing hormone
- ESHRE
European Society of Human Reproduction and Embryology
- FertiQoL
Fertility quality of life
- FPI
Fertility problem inventory
- GnIH
Gonadotropin-Inhibitory Hormone
- GnRH
Gonadotropin releasing hormone
- HPA
Hypothalamic-Pituitary-Adrenal axis
- HPO
Hypothalamic-Pituitary-Ovary axis
- KISS1
Kisspeptin 1
- LH
Luteinizing hormone
- PCOS
Polycystic ovary syndrome
- PRO
Patient self-reported outcome
- QoL
Quality of Life
- SAM
Sympathetic-Adrenal-Medullary axis
- WHO
World Health Organization
Authors’ contributions
SP performed the literature search, selected the papers, interpreted and analyzed data, revised the manuscript and approved its final version; JD performed the literature search, selected the papers, wrote and drafted the manuscript, and approved its final version; SR performed the literature search, drafted the manuscript, and approved its final version; FAB analyzed data, wrote and drafted the manuscript, and approved its final version; RM interpreted and analyzed data, revised the manuscript and approved its final version; GBLS analyzed data, revised the manuscript for intellectual content and approved its final version.
Ethics approval and consent to participate
Not applicable (a review article)
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefano Palomba, Email: stefanopalomba@tin.it.
Jessica Daolio, Email: jessica.daolio@ausl.re.it.
Sara Romeo, Email: sara.romeo_sr@libero.it.
Francesco Antonino Battaglia, Email: franbatt@alice.it.
Roberto Marci, Email: roberto.marci@unife.it.
Giovanni Battista La Sala, Email: giovannibattista.lasala@ausl.re.it.
References
- 1.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138(3479, July 4):32. doi: 10.1038/138032a0. [DOI] [PubMed] [Google Scholar]
- 2.Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wöhr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Elliott GR, Eisdorfer C, editors. Institute of Medicine National Academy of Sciences (U.S.). Stress and human health: analysis and implications of research: a study. New York: Springer Publishing; 1982.
- 4.Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization World Health Organization quality of life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403–1409. doi: 10.1016/0277-9536(95)00112-K. [DOI] [PubMed] [Google Scholar]
- 6.Sandler B. Emotional stress and infertility. Practitioner. 1960;184:355–361. [PubMed] [Google Scholar]
- 7.Selye H. The stress concept. Can Med Assoc J. 1976;115:718. [PMC free article] [PubMed] [Google Scholar]
- 8.Aarts JW, van Empel IW, Boivin J, Nelen WL, Kremer JA, Verhaak CM. Relationship between quality of life and distress in infertility: a validation study of the Dutch FertiQoL. Hum Reprod. 2011;26:1112–1118. doi: 10.1093/humrep/der051. [DOI] [PubMed] [Google Scholar]
- 9.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–E276. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- 10.Brkovich AM, Fisher WA. Psychological distress and infertility: forty years of research. J Psychosom Obstet Gynecol. 1998;4:218–228. doi: 10.3109/01674829809025700. [DOI] [PubMed] [Google Scholar]
- 11.Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13:209–223. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Fedor J, Biedenharn K, Agarwal A. Lifestyle factors and oxidative stress in female infertility: is there an evidence base to support the linkage? J Expert Rev Obstet Gynecol. 2013;8:607–624. doi: 10.1586/17474108.2013.849418. [DOI] [Google Scholar]
- 13.Domar AD. Psychological stress and infertility. In: Silver JM, Solomon D, editors. Waltham: UpToDate Inc; 2017.
- 14.Schmidt L, Holstein BE, Boivin J, Sångren H, Tjørnhøj-Thomsen T, Blaabjerg J, Hald F, Andersen AN, Rasmussen PE. Patients’ attitudes to medical psychosocial aspects of care in infertility clinics: findings from the Copenhagen multi-center psychosocial infertility (COMP1) research Programme. Hum Reprod. 2003;18:628–637. doi: 10.1093/humrep/deg149. [DOI] [PubMed] [Google Scholar]
- 15.Gift T, O’M S. Infertility and quality of life: findings from a literature review. Sexually Transmitted Diseases. 2014;41(Suppl 1):115. [Google Scholar]
- 16.Alvarez S, Devouche E. First French national survey on lifestyle and toxic factors in infertile couples. Gynecol Obstet Fertil. 2012;40:765–771. doi: 10.1016/j.gyobfe.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Wright J, Duchesne C, Sabourin S, Bissonnette F, Benoit J, Girad Y. Psychosocial distress and infertility: men and women respond differently. Fertil Steril. 1991;55:100–108. doi: 10.1016/S0015-0282(16)54067-9. [DOI] [PubMed] [Google Scholar]
- 18.Klemetti R, Raitanen J, Sihvo S, Saarni S, Koponen P. Infertility, mental disorders and well-being--a nationwide survey. Acta Obstet Gynecol Scand. 2010;89:677–682. doi: 10.3109/00016341003623746. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen TB. General psychosocial and work-related stress and reduced fertility. Scand J Work Environ Health. 1999;25(Suppl 1):38–39. [PubMed] [Google Scholar]
- 20.Merari D, Feldberg D, Elizur A, Goldman J, Modan B. Psychological and hormonal changes in the course of in vitro fertilization. J Assist Reprod Genet. 1992;9:161–169. doi: 10.1007/BF01203757. [DOI] [PubMed] [Google Scholar]
- 21.Harlow CH, Jahy UM, Talbot WM, Wardle PG, Hull MGR. Stress and stress-related hormones during in-vitro fertilization treatment. Hum Reprod. 1996;11:274–279. doi: 10.1093/HUMREP/11.2.274. [DOI] [PubMed] [Google Scholar]
- 22.Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod. 1998;13:2296–2300. doi: 10.1093/humrep/13.8.2296. [DOI] [PubMed] [Google Scholar]
- 23.Sheiner E, Sheiner EK, Potashnik G, Carel R, Shoham-Vardi I. The relationship between occupational psychological stress and female fertility. Occup Med (Lond) 2003;53:265–269. doi: 10.1093/occmed/kqg069. [DOI] [PubMed] [Google Scholar]
- 24.Anderhein L, Holter H, Bergh C, Müller A. Does psychologocal stress affect the outcome of in vitro fertilization? Hum Reprod. 2005;20:2969–2975. doi: 10.1093/humrep/dei219. [DOI] [PubMed] [Google Scholar]
- 25.Cooper BC, Gerber IR, McGettrick AL, Johnson JV. Perceived infertility-related stress correlates with in vitro fertilization outcome. Fertil Steril. 2007;88:714–717. doi: 10.1016/j.fertnstert.2006.11.158. [DOI] [PubMed] [Google Scholar]
- 26.Griel AL. Infertility and psychological distress: a critical review of literature. Soc Sci Med. 1997;45:1679–1704. doi: 10.1016/S0277-9536(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 27.Wilson JF, Kopitzke EJ. Stress and infertility. Curr Women Health Rep. 2002;2:194–199. [PubMed] [Google Scholar]
- 28.Wischmann TH. Psychogenic infertility--myths and facts. J Assist Reprod Genet. 2003;20:485–494. doi: 10.1023/B:JARG.0000013648.74404.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biringer E, Howard LM, Kessler U, Stewart R, Mykletun A. Is infertility really associated with higher levels of mental distress in the female population? Results from the north-Trøndelag health study and the medical birth registry of Norway. J Psychosom Obstet Gyneacol. 2015;36:38–45. doi: 10.3109/0167482X.2014.992411. [DOI] [PubMed] [Google Scholar]
- 30.Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- 31.Whirledge S, Cidlowski JA. A role for glucocorticoids in stress-impaired reproduction: beyond the hypothalamus and pituitary. Endocrinology. 2013;154:4450–4468. doi: 10.1210/en.2013-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28:399–415. doi: 10.1016/j.tem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulrich-Lai YM, Herman JP. Neural Regulation of Endocrine and Autonomic Stress Responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schenker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;16:1–8. doi: 10.1016/0028-2243(92)90186-3. [DOI] [PubMed] [Google Scholar]
- 35.Negro-Vilar A. Stress and other environmental factors affecting fertility in men and women: overview. Environ Health Perspect. 1993;101(Suppl 2):59–64. doi: 10.1289/ehp.93101s259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman J, Plotsky PM, Nemeroff CB, Chamey D. Effects of early adverse experiences on brain structure and function: clinical implications. Biol Psychiatry. 2000;48:778–790. doi: 10.1016/S0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- 37.McEwen BS. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- 38.Bablis P, Pollard H, Monti DA. Resolution of anovulation infertility using neuro emotional technique: a report of 3 cases. J Chiropr Med. 2006;5:13–21. doi: 10.1016/S0899-3467(07)60128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sominsky L, Fuller EA, Hodgson DM. Factors in early-life programming of reproductive fitness. Neuroendocrinology. 2015;102:216–225. doi: 10.1159/000431378. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Vale WW. The role of the hypothalamicpituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, Schisterman EF, Pyper C. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95:2184–2189. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewagalamulage SD, Clarke IJ, Rao A, Henry BA. Ewes With Divergent Cortisol Responses to ACTH Exhibit Functional Differences in the Hypothalamo-Pituitary-Adrenal (HPA) Axis. Endocrinology. 2016;157:3540–3549. doi: 10.1210/en.2016-1287. [DOI] [PubMed] [Google Scholar]
- 43.Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011;300:28–36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campagne DM. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21:1651–1658. doi: 10.1093/humrep/del078. [DOI] [PubMed] [Google Scholar]
- 45.Damti OB, Sarid O, Sheiner E, Zilberstein T, Cwikel J. Stress and distress in infertility among women. Harefuah. 2008;147:256–260. [PubMed] [Google Scholar]
- 46.Breen KM, Mellon PL. Influence of stress-induced intermediates on gonadotropin gene expression in gonadotrope cells. Mol Cell Endocrinol. 2014;385:71–77. doi: 10.1016/j.mce.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geraghty AC, Kaufer D. Glucocorticoid regulation of reproduction. Adv Exp Med Biol. 2015;872:253–278. doi: 10.1007/978-1-4939-2895-8_11. [DOI] [PubMed] [Google Scholar]
- 48.Wakodkar V. Stress related infertility and infertility related stress. Indian J Psychiatry. 2017;59(Suppl 2):159. [Google Scholar]
- 49.Pirkalani K, Talaeerad Z. Psychological stress causes relative infertility through direct change in the frequency pattern of GnRH release from the hypothalamus. Eur Psychiatry. 2014;29(Suppl 1):1.
- 50.Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531:40–45. doi: 10.1016/j.neulet.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Luo E, Stephens SB, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157:1187–1199. doi: 10.1210/en.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One. 2009;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke IJ, Bartolini D, Conductier G, Henry BA. Stress increases gonadotropin inhibitory hormone cell activity and input to GnRH cells in ewes. Endocrinology. 2016;157:4339–4350. doi: 10.1210/en.2016-1513. [DOI] [PubMed] [Google Scholar]
- 54.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci U S A. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buffet NC, Bouchard P. The neuroendocrine regulation of the human ovarian cycle. Chrobiol Int. 2001;18:893–919. [PubMed] [Google Scholar]
- 56.Monti C, Ciarrocca M, Cardella C, Capozzella A, Rosati MV, Cherubini E, Fargnoli S, Casale T, Tomei F, Tomei G. Exposure to urban stressor and effects on luteinizing hormone (LH) in female outdoor workers. J Environ Sci Helath A Tox Hazard Subst Environ Eng. 2006;41:1437–1448. doi: 10.1080/10934520600754292. [DOI] [PubMed] [Google Scholar]
- 57.Hjøllund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, Ernst E, Giwercman AJ, Skakkebaek NE, Olsen J. Distress and reduced fertility. A follow-up study of first-pregnancy planned. Reprod Endocrinol. 1999;1:47–53. doi: 10.1016/s0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 58.Smeenk JMJ, Verhaak CM, Aj V, Sweep CG, Jmm M, Sj W. Van Minnen a, Straatman H and Braat DD. Stress and outcome success in IVF: the role of self-reports and endocrine variables. Hum Reprod. 2005;29:991–996. doi: 10.1093/humrep/deh739. [DOI] [PubMed] [Google Scholar]
- 59.Boivin J, Schmidt L. Infertility-related stress in men and women predicts treatment outcome 1 year later. Fertil Steril. 2005;83:1745–1752. doi: 10.1016/j.fertnstert.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 60.Pal L, Bevilacqua K, Santoro NF. Chronic psychosocial stressors are detrimental to ovarian reserve: a study of infertile women. J Psychosom Obstet Gynaecol. 2010;31:130–139. doi: 10.3109/0167482X.2010.485258. [DOI] [PubMed] [Google Scholar]
- 61.Barut MU, Agacayak E, Bozkurt M, Aksu T, Gul T. There is a positive correlation between socioeconomic status and ovarian Reserve in Women of reproductive age. Med Sci Monit. 2016;22:4386–4392. doi: 10.12659/MSM.897620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondoh E, Okamoto T, Higuchi T, Tatsumi K, Baba T, Murphy SK, Takakura K, Konishi I, Fujii S. Stress affects uterine receptivity through an ovarian-independent pathway. Hum Reprod. 2009;24:945–953. doi: 10.1093/humrep/den461. [DOI] [PubMed] [Google Scholar]
- 63.Domar AD, Broome A, Zuttermeister PC, Seibel M, Friedman R. The prevalence and predictability of depression in infertile women. Fertil Steril. 1992;58:1158–1163. doi: 10.1016/S0015-0282(16)55562-9. [DOI] [PubMed] [Google Scholar]
- 64.Nelson CJ, Shindel AW, Naughton CK, Ohebshalom M, Mulhall JP. Prevalence and predictors of sexual problems, relationship stress, and depression in female partners of infertile couples. J Sex Med. 2008;5:1907–1914. doi: 10.1111/j.1743-6109.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 65.Lakatos E, Szabó G, Szigeti FJ, Balog P. Relationships between psychological well-being, lifestyle factors and fertility. Orv Hetil. 2015;156:483–492. doi: 10.1556/OH.2015.30104. [DOI] [PubMed] [Google Scholar]
- 66.Yusuf L. Depression, anxiety and stress among female patients of infertility; a case control study. Pak J Med Sci. 2016;32:1340–1343. doi: 10.12669/pjms.326.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rooney KL, Domar AD. The impact of stress on fertility treatment. Curr Opin Obstet Gynecol. 2016;28:198–201. doi: 10.1097/GCO.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 68.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;29(10):49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prasad S, Tiwari M, Pandey AN, Shrivastav TG, Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci. 2016;23:36. doi: 10.1186/s12929-016-0253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrmann D, Scherg H, Verres R, von Hagens C, Strowitzki T, Wischmann T. Resilience in infertile couples acts as a protective factor against infertility-specific distress and impaired quality of life. J Assist Reprod Genet. 2011;28:1111–1117. doi: 10.1007/s10815-011-9637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jisha PR, Thomas I. Quality of life and infertility: influence of gender, years of marital life, resilience, and anxiety. Psychol Stud. 2016;61:159–169. doi: 10.1007/s12646-016-0358-6. [DOI] [Google Scholar]
- 72.Domar AD. Stress and infertility in women: is there a relationship? J Clin Psychol. 1996;2:17–27. [Google Scholar]
- 73.Domar AD, Friedman R, Zuttermeister PC. Distress and conception in infertile women: a complementary approach. J Am Med Womens Assoc (1972) 1999;54:196–198. [PubMed] [Google Scholar]
- 74.Domar AD, Clapp D, Slawsby EA, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000;73:805–811. doi: 10.1016/S0015-0282(99)00493-8. [DOI] [PubMed] [Google Scholar]
- 75.Terzioglu F. Investigation into the effectiveness of counseling on assisted reproductive techniques in Turkey. J Psychosom Obstet Gynaecol. 2001;22:133–141. doi: 10.3109/01674820109049965. [DOI] [PubMed] [Google Scholar]
- 76.Kupka MS, Dorn C, Richter O, Schmutzler A, van der Ven H, Kulczycki A. Stress relief after infertility treatment - spontaneous conception, adoption and psychological counselling. Eur J Obstet Gynecol Reprod Biol. 2003;110:190–195. doi: 10.1016/S0301-2115(03)00280-X. [DOI] [PubMed] [Google Scholar]
- 77.Facchinetti F, Tarabusi M, Volpe A. Cognitive-behavioral treatment decreases cardiovascular and neuroendocrine reaction to stress in women waiting for assisted reproduction. Psychoneuroendocrinology. 2004;29:162–173. doi: 10.1016/S0306-4530(02)00170-1. [DOI] [PubMed] [Google Scholar]
- 78.Cwikel J, Gidron Y, Sheiner E. Psychological interactions with infertility among women. Eur J Ostet Gynecol Reprod Biol. 2004;117:126–131. doi: 10.1016/j.ejogrb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 79.de Liz TM, Strauss B. Differential efficacy of group and individual/couple psychotherapy with infertile patients. Hum Reprod. 2005;20:1324–1332. doi: 10.1093/humrep/deh743. [DOI] [PubMed] [Google Scholar]
- 80.Campagne DM. Stress: at what point in the medical treatment of infertility should it be treated? Papeles del Psicologo. 2008;29:197–204. [Google Scholar]
- 81.Ardakani AM, Chaharsoughi SA. Female Infertility: P-48: Iinfertility and stress. Int J Fertil Steril. 2010;4 Suppl 1.
- 82.Oron G, Allnutt E, Lackman T, Sokal-Arnon T, Holzer H, Takefman J. A prospective study using hatha yoga for stress reduction among women waiting for IVF treatment. Reprod BioMed Online. 2015;30:542–548. doi: 10.1016/j.rbmo.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 83.Frederiksen Y, Farver-Vestergaard I, Skovgård NG, Ingerslev HJ, Zachariae R. Efficacy of psychosocial interventions for psychological and pregnancy outcomes in infertile women and men: a systematic review and meta-analysis. BMJ Open. 2015;28:e006592. doi: 10.1136/bmjopen-2014-006592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matthiesen SM, Frederiksen Y, Ingerslev HJ, Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): a meta-analysis. Hum Reprod. 2011;26:2763–2776. doi: 10.1093/humrep/der246. [DOI] [PubMed] [Google Scholar]
- 85.Boivin J., Griffiths E., Venetis C. A. Emotional distress in infertile women and failure of assisted reproductive technologies: meta-analysis of prospective psychosocial studies. BMJ. 2011;342(feb23 1):d223–d223. doi: 10.1136/bmj.d223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kitchen H, Aldhouse N, Trigg A, Palencia R, Mitchell S. A review of patient-reported outcome measures to assess female infertility-related quality of life. Health Qual Life Outcomes. 2017;27(15):86. doi: 10.1186/s12955-017-0666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boivin J, Psychol C, Takefman J, Braverman A. The fertility quality of life (FertiQoL) tool: development and general psychometric properties. Fertil Steril. 2011;96:409–415. doi: 10.1016/j.fertnstert.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moura-Ramos M, Gameiro S, Canavarro MC, Soares I. Assessing infertility stress: re-examining the factor structure of the fertility problem inventory. Hum Reprod. 2012;27:496–505. doi: 10.1093/humrep/der388. [DOI] [PubMed] [Google Scholar]
- 89.Dural O, Yasa C, Keyif B, Celiksoy H, Demiral I, Yuksel Ozgor B, Gungor Ugurlucan F, Bastu E. Effect of infertility on quality of life of women: a validation study of the Turkish FertiQoL. Hum Fertil (Camb). 2016;19:186–191. doi: 10.1080/14647273.2016.1214754. [DOI] [PubMed] [Google Scholar]
- 90.Kahyaoglu Sut Hatice, Balkanli Kaplan Petek. Quality of life in women with infertility via the FertiQoL and the Hospital Anxiety and Depression Scales. Nursing & Health Sciences. 2014;17(1):84–89. doi: 10.1111/nhs.12167. [DOI] [PubMed] [Google Scholar]
- 91.Chi HJ, Park IH, Sun HG, Kim JW, Lee KH. Psychological distress and fertility quality of life (FertiQoL) in infertile Korean women: The first validation study of Korean FertiQoL. Clin Exp Reprod Med. 2016;43:174–180. doi: 10.5653/cerm.2016.43.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maroufizadeh S, Ghaheri A, Amini P, Samani RO. Psychometric Properties of The Fertility Quality of Life Instrument in Infertile Iranian Women. Int J Fertil Steril. 2017;10:371–379. doi: 10.22074/ijfs.2016.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McQuillan J, Stone RAT, Greil AL. Infertility and life satisfaction among women. J Family. 2007;28:955–981. [Google Scholar]
- 94.McQuillan J, Greil AL, White L, Jacob MC. Frustrated fertility: infertility and psychological distress among women. J Marriage Fam. 2003;65:1007–1018. doi: 10.1111/j.1741-3737.2003.01007.x. [DOI] [Google Scholar]
- 95.Donarelli Z, Lo Coco G, Gullo S, Salerno L, Marino A, Sammartano F, Allegra A. The fertility quality of life questionnaire (FertiQoL) relational subscale: psychometric properties and discriminant validity across gender. Hum Reprod. 2016;31:2061–2071. doi: 10.1093/humrep/dew168. [DOI] [PubMed] [Google Scholar]
- 96.White L, McQuillan J. No longer intending: the relationship between relinquished fertility intentions and distress. J Marriage Fam. 2006;68:478–490. doi: 10.1111/j.1741-3737.2006.00266.x. [DOI] [Google Scholar]
- 97.Jumayev I, Harun-Or-Rashid M, Rustamov O, Zakirova N, Kasuya H, Sakamoto J. Social correlates of female infertility in Uzbekistan. Nagoya J Med Sci. 2012;74:273–283. [PMC free article] [PubMed] [Google Scholar]
- 98.Andrews FM, Abbey A, Halman LJ. Is fertility-problem stress different? The dynamics of stress in fertile and infertile couples. Fertil Steril. 1992;57:1247–1253. doi: 10.1016/S0015-0282(16)55082-1. [DOI] [PubMed] [Google Scholar]
- 99.Lo SS, Kok WM. Sexual functioning and quality of life of Hong Kong Chinese women with infertility problem. Hum Fertil (Camb) 2016;19:268–274. doi: 10.1080/14647273.2016.1238516. [DOI] [PubMed] [Google Scholar]
- 100.Ebbesen SM, Zachariae R, Mehlsen MY, Thomsen D, Højgaard A, Ottosen L, Petersen T, Ingerslev HJ. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: a prospective study. Hum Reprod. 2009;24:2173–2182. doi: 10.1093/humrep/dep185. [DOI] [PubMed] [Google Scholar]
- 101.Santos C, Sobral MP, Martins MV. Effects of life events on infertility diagnosis: comparison with presumably fertile men and women. J Reprod Infant Psychol. 2017;35:1. doi: 10.1080/02646838.2016.1249834. [DOI] [PubMed] [Google Scholar]
- 102.Podfigurna-Stopa A, Luisi S, Regini C, Katulski K, Centini G, Meczekalski B, Petraglia F. Mood disorders and quality of life in polycystic ovary syndrome. Gynecol Endocrinol. 2015;31:431–434. doi: 10.3109/09513590.2015.1009437. [DOI] [PubMed] [Google Scholar]
- 103.Bahri Khomami M, Rainezani Tehrani F, Azizi F. Infertility and quality of life in women with polycystic ovary syndrome. Iran J Reprod Med. 2015;(Suppl 32–6):32.
- 104.Hadjiconstantinou M, Mani H, Patel N, Levy M, Davies M, Khunti K, Stone M. Understanding and supporting women with polycystic ovary syndrome: a qualitative study in an ethnically diverse UK sample. Endocr Connect. 2017;6:323–330. doi: 10.1530/EC-17-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aliasghari Fatemeh, Mirghafourvand Mojgan, Charandabi Sakineh Mohammad-Alizadeh, lak Tahereh Behroozi. The predictors of quality of life in women with polycystic ovarian syndrome. International Journal of Nursing Practice. 2017;23(3):e12526. doi: 10.1111/ijn.12526. [DOI] [PubMed] [Google Scholar]
- 106.Panico A, Messina G, Lupoli GA, Lupoli R, Cacciapuoti M, Moscatelli F, Esposito T, Villano I, Valenzano A, Monda V, Messina A, Precenzano F, Cibelli G, Monda M, Lupoli G. Quality of life in overweight (obese) and normal-weight women with polycystic ovary syndrome. Patient Prefer Adherence. 2017;11:423–429. doi: 10.2147/PPA.S119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sendi P, Brunotte R, Potoczna N, Branson R, Horber FF. Health-related quality of life in patients with class II and class III obesity. Obes Surg. 2005;15:1070–1076. doi: 10.1381/0960892054621323. [DOI] [PubMed] [Google Scholar]
- 108.Khayata GM, Rizk DE, Hasan MY, Ghazal-Aswad S, Asaad MA. Factors influencing the quality of life of infertile women in United Arab Emirates. Int J Gynaecol Obstet. 2003;80:183–188. doi: 10.1016/S0020-7292(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 109.Aliyeh G, Laya F. Quality of life and its correlates among a group of infertile Iranian women. Med Sci Monit. 2007;13:313–317. [PubMed] [Google Scholar]
- 110.Orji EO, Kuti O, Fasubaa OB. Impact of infertility on marital life in Nigeria. Int J Gynaecol Obstet. 2002;79:61–62. doi: 10.1016/S0020-7292(02)00180-7. [DOI] [PubMed] [Google Scholar]
- 111.Gameiro S, Boivin J, Dancet E, de Klerk C, Emery M, Lewis-Jones C, Thorn P, Van den Broeck U, Venetis C, Verhaak CM, Wischmann T, Vermeulen N. ESHRE guideline: routine psychosocial care in infertility and medically assisted reproduction-a guide for fertility staff. Hum Reprod. 2015;30:2476–2485. doi: 10.1093/humrep/dev177. [DOI] [PubMed] [Google Scholar]
- 112.Verhaak CM, Smeenk JMJ, Evers AWM, Kremer JAM, Kraaimaat FW, Braat DDM. Women’s emotional adjustment to IVF: a systematic review of 25 years of research. Hum Reprod Update. 2007;13:27–36. doi: 10.1093/humupd/dml040. [DOI] [PubMed] [Google Scholar]
- 113.Knoll N, Schwarzer R, Pfüller B, Kienle R. Transmission of depressive symptoms: a study with couples undergoing assisted-reproduction treatment. Eur Psychol. 2009;14:7–17. doi: 10.1027/1016-9040.14.1.7. [DOI] [Google Scholar]
- 114.Agostini Francesca, Monti Fiorella, Andrei Federica, Paterlini Marcella, Palomba Stefano, La Sala Giovanni Battista. Assisted reproductive technology treatments and quality of life: a longitudinal study among subfertile women and men. Journal of Assisted Reproduction and Genetics. 2017;34(10):1307–1315. doi: 10.1007/s10815-017-1000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Agostini F, Monti F, Paterlini M, Andrei F, Palomba S, La Sala GB. Effect of the previous reproductive outcomes in subfertile women after in vitro fertilization (IVF) and/or intracytoplasmic sperm injection (ICSI) treatments on perinatal anxious and depressive symptomatology. J Psychosom Obstet Gynaecol. 2017;21:1–9. doi: 10.1080/0167482X.2017.1286474. [DOI] [PubMed] [Google Scholar]
- 116.Domar AD, Gross J, Rooney K, Boivin J. Exploratory randomized trial on the effect of a brief psychological intervention on emotions, quality of life, discontinuation, and pregnancy rates in in vitro fertilization patients. Fert Steril. 2015;104:440–451. doi: 10.1016/j.fertnstert.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Ősapay G, Ősapay K. Stress and fertility. Orv Hetil. 2015;156:1430–1434. doi: 10.1556/650.2015.30250. [DOI] [PubMed] [Google Scholar]
- 118.An Y, Wang Z, Ji H, Zhang Y, Wu K. Pituitary-adrenal and sympathetic nervous system responses to psychiatric disorders in women undergoing in vitro fertilization treatment. Fertil Steril. 2011;96:404–408. doi: 10.1016/j.fertnstert.2011.05.092. [DOI] [PubMed] [Google Scholar]
- 119.Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scand. 2000;79:113–118. doi: 10.1034/j.1600-0412.2000.079002113.x. [DOI] [PubMed] [Google Scholar]
- 120.Lynch CD, Sundaram R, Maisog JM, Sweeney AM, Buck Louis GM. Preconception stress increases the risk of infertility: results from a couple-based prospective cohort study--the LIFE study. Hum Reprod. 2014;29:1067–1075. doi: 10.1093/humrep/deu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luk BHK, Loke AY. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: a systematic review. J Sex Marital Ther. 2015;41:610–625. doi: 10.1080/0092623X.2014.958789. [DOI] [PubMed] [Google Scholar]
- 122.Steuber KR, High A. Disclosure strategies, social support, and quality of life in infertile women. Hum Reprod. 2015;30:1635–1642. doi: 10.1093/humrep/dev093. [DOI] [PubMed] [Google Scholar]
- 123.Aarts JW, Huppelschoten AG, van Empel IW, Boivin J, Verhaak CM, Kremer JA, Nelen WL. How patient-centred care relates to patients’ quality of life and distress: a study in 427 women experiencing infertility. Hum Reprod. 2012;27:488–495. doi: 10.1093/humrep/der386. [DOI] [PubMed] [Google Scholar]
- 124.Ried K, Alfred A. Quality of life, coping strategies and support needs of women seeking Traditional Chinese Medicine for infertility and viable pregnancy in Australia: a mixed methods approach. BMC Womens Health. 2013. doi:10.1186/1472-6874-13-17. [DOI] [PMC free article] [PubMed]
- 125.Taymor ML, Bresnick E. Emotional stress and infertility. Infertility. 1979;2:39–47. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact author for data requests.