Abstract
Knockdown of orexin/hypocretin 2 receptor (Orx2) in the basolateral amygdala (BLA) affects anxious and depressive behavior. We use a new behavioral paradigm, the Stress-Alternatives Model (SAM), designed to improve translational impact. The SAM induces social stress in adult male mice by aggression from larger mice, allowing for adaptive decision-making regarding escape. In this model mice remain (Stay) in the oval SAM arena or escape from social aggression (Escape) via routes only large enough for the smaller mouse. We hypothesized intracerebroventricular (icv) stimulation of Orx2 receptors would be anxiolytic and antidepressive in SAM-related social behavior and the social interaction/preference (SIP) test. Conversely, we predicted that icv antagonism of Orx2 receptors would promote anxious and depressive behavior in these same tests. Anxious behaviors such as freezing (both cued and conflict) and startle are exhibited more often in Stay compared with Escape phenotype mice. Time spent attentive to the escape route is more frequent in Escape mice. In Stay mice, stimulation of Orx2 receptors reduces fear conditioning, conflict freezing and startle, and promotes greater attention to the escape hole. This anxiolysis was accompanied by activation of a cluster of inhibitory neurons in the amygdala. A small percentage of those Stay mice also begin escaping; whereas Escape is reversed by the Orx2 antagonist. Escape mice were also Resilient, and Stay mice Susceptible to stress (SIP), with both conditions reversed by Orx2 antagonism or stimulation respectively. Together these results suggest that the Orx2 receptor may be a useful potential target for anxiolytic or antidepressive therapeutics.
Keywords: anxiety, defeat, depression, fear conditioning, freezing, hypocretin, resilience, susceptibility, startle, Stress-Alternatives Model
1. Introduction
Anxious Behavior is widely expressed across vertebrate species (Kandel, 1983), and while anxiety is an evolutionarily conserved and adaptive suite of emotions (Bergstrom and Meacham, 2016; Smith et al., 2016; Trimmer et al., 2015), in their pathological forms also constitute the most common human psychological disorders (Kessler et al., 2010). It is highly comorbid with numerous other conditions such as depression (Spinhoven et al., 2011) and Post-Traumatic Stress Disorder (PTSD) (Zlotnick et al., 1999). Evidence is mounting that a relatively newly identified pair of neuropeptides, called the orexins or hypocretins (Broberger et al., 1998; Peyron et al., 1998; Sakurai et al., 1998), are intimately involved in stress, motivation, and affective disorders (Allard et al., 2004; Giardino and de Lecea, 2014; James et al., 2017a; James et al., 2017b; Johnson et al., 2010; Nollet et al., 2011; Ozsoy et al., 2017).
The orexin/hypocretin system consists of two peptides (OrxA = Hcrt1, OrxB = Hcrt2) cleaved in equal quantities from one pro-orexin molecule synthesized in the perifornical area of the lateral, dorsomedial hypothalamus (LH-DMH/PeF) (Broberger et al., 1998; Nambu et al., 1999). Orexin-related effects classically include promotion of foraging and feeding, maintaining homeostasis, arousal, modulation of sleep-wake circadian cycles (whereas lack of Orx or it receptors may result in sleep dysregulation or narcolepsy), and motivation (Berridge and Espana, 2005; Chemelli et al., 1999; Rodgers et al., 2002; Saper et al., 2005; Scammell and Saper, 2005). These functions and behaviors are mediated via two orexin receptors, Orx1 and Orx2, which have equally high binding affinity for OrxA. The Orx2 receptor binds OrxA and OrxB with similar high affinities, but Orx1 binds OrxB with significantly reduced affinity (Ammoun et al., 2003). Orexinergic neurons in the LHDMH/PeF have extensive anatomical associations and functional interactions via the two orexin receptors in most brain regions (Chen et al., 1999; Marcus et al., 2001; Nambu et al., 1999; Sakurai, 2005; Trivedi et al., 1998; Yoshida et al., 2006; Zhang et al., 2005) and these projections are responsive to stressors (Arendt et al., 2014; Arendt et al., 2013; Berridge et al., 2010; Giardino et al., 2018).
There are existing pharmacological and behavioral treatment options for anxiety disorders, but the quality of and satisfaction with these treatments is poor to moderate (Young et al., 2001), which indicates a need for more effective treatment options. Recent examinations of classic tests for anxiety and depression using animal models suggest that the results have little translatable relevance for trials in clinical populations, with the caveat that tests including social measures have promise (Blanchard et al., 2013; Haller and Alicki, 2012; Haller et al., 2013; Holsboer and Ising, 2010; Keifer and Summers, 2016; Nestler and Hyman, 2010). Therefore, new models are necessary to specifically address the complexity of social interactions in tests of anxiety and depression (Blanchard and Blanchard, 1989a, b; Keifer and Summers, 2016; Robertson et al., 2015).
The Stress-Alternatives Model (SAM) is designed to test simple decision-making during socially stressful situations that produce anxious and depressive behaviors (Smith et al., 2014). The SAM consists of an oval open field (OF) arena that provides two escape routes which lead to enclosed areas (Fig. 1B). In the absence of social interaction, the SAM apparatus produces OF anxiety, but when small test mice are paired with a novel larger aggressor over four days, interactions produce robust social anxiety as well (Robertson et al., 2015). The escape holes are just large enough for the smaller non-aggressive animals, but of those only a proportion of the population (usually half) ever makes use of them.
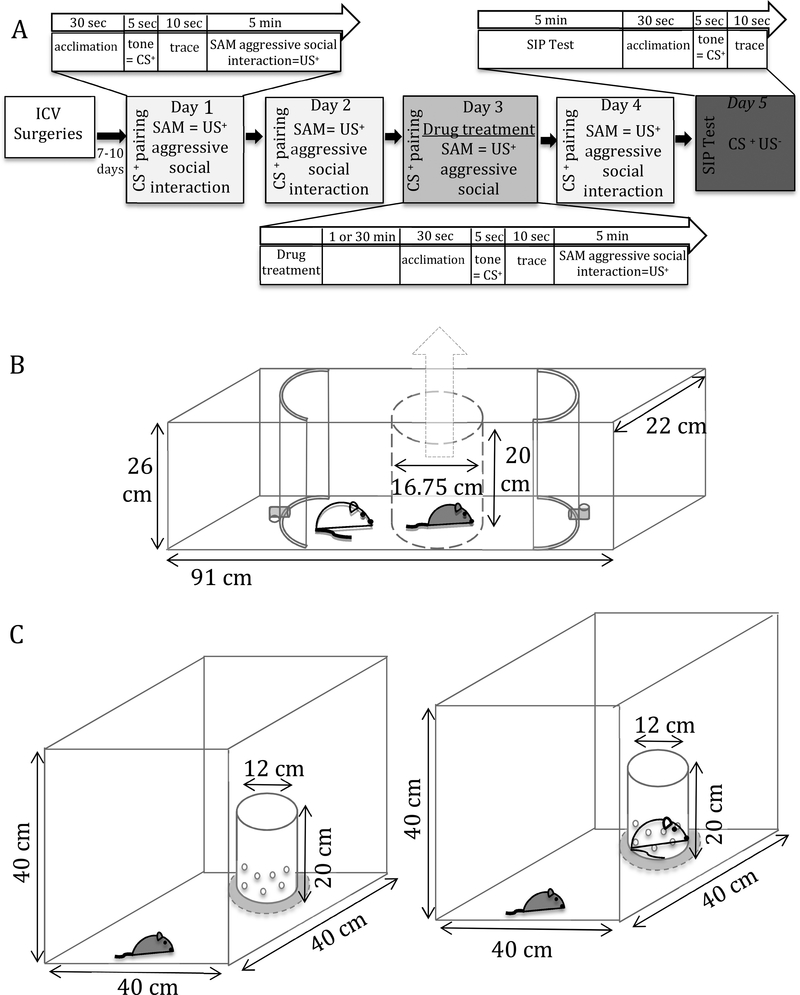
Fig. 1. Experimental design, Stress-Alternatives Model (SAM), and Social Interaction/Preference test (SIP).
A) Experimental timeline: 5-day acclimation, icv surgeries , recovery for 7–10 days, followed by aggressive social interaction (SAM). Days 1–4 included the pairing of the CS+ (tone) with US+ (aggressive social interaction) prior to social interaction. Drug treatments were given prior to social interaction on day 3. On day 5, test mice underwent an SIP test followed by a final SAM exposure that included the CS+ but not the US+, and sampled thereafter. B) Design of the SAM apparatus: a clear rectangular (91 × 22 × 26 cm) apparatus, containing an oval escapable open field (OF) arena, divided into three sections by curved opaque barriers with L-shaped tunnel escape routes at the apex (only large enough for the test mouse to pass through) that lead to safe areas. The test mouse is placed in an opaque cylinder (16.75 × 20 cm) in the middle of the OF and the aggressor is placed outside this area. The cylinder is removed just prior to social interaction. C) Design of SI apparatus; an opaque square arena (40 × 40 × 40 cm) with a clear perforated container set at the middle of one wall. Test mice are first placed in the apparatus at the opposite end from an empty container (left) for 2.5 min before being removed. The empty container is then replaced with a container that has a novel CD1 mouse strain/size matched to mice used as aggressors in SAM (right). The test mouse is placed back into the apparatus in the same manner as previously described for another 2.5 min before being removed.
Therefore, decisions made by the test mice result in two behavioral phenotypes: 1. Escape or 2. Stay. It is important to note that both phenotypes readily discover and examine the escape routes, then learn how to escape, but only some (50% is average) choose to use this option (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014). Escaping during aggressive interactions behaviorally ameliorates the stress involved, and reverses rising stress hormone and gene expression levels (Carpenter and Summers, 2009; Robertson et al., 2017; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017). Animals that choose to remain with the larger aggressor (Stay) exhibit much higher elevated plasma corticosterone, behavioral Pavlovian fear conditioning, elevated anxiolytic neuropeptide S (NPS) gene expression in the central amygdala (CeA), and enhanced gene expression of cannabinoid 2 (Cb2) receptors in the hippocampus (Robertson et al., 2017; Smith et al., 2014; Smith et al., 2016). While Escape and Stay behavioral phenotypes are relatively stable, both reserve the requisite behavioral plasticity to reverse phenotype under the influence of experience or pharmacological treatments. For example, the anxiolytic corticotropin releasing factor type 1 receptor (CRF1) antagonist antalarmin allows escape in mice that were previously only submissive (Stay). Alternatively, the Escape phenotype can be reversed through the anxiogenic α2receptor antagonist, yohimbine (Smith et al., 2016). This model (Fig. 1B) produces a gradient of anxiety, where familiar escape is least stressful, such that animals which have escaped more than once exhibit reduced apparatus and escape tunnel-related novelty stress that was expressed during the initial escape. As such, the latency to escape is dramatically reduced, which is another reliable measure of diminished anxious behavior in the SAM (Robertson et al., 2015; Smith et al., 2014). Other anxiolytic factors, such as wheel-running exercise and icv NPS, reduce the initial latency to escape (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018). Alternatively, remaining submissively (Stay) leads to social defeat, which is the most stressful condition (Koolhaas et al., 1997) in our model. The pharmacological results of anxiogenic and anxiolytic agents clearly demonstrate that the stress of socially aggressive interaction is the mechanism that inhibits escape for the Stay phenotype. Recent work from our lab and others suggests that the orexin (Orx; hypocretin, Hcrt) system interacts with the circuitry producing this social stress-induced anxiety gradient (Giardino et al., 2018; Winsky-Sommerer et al., 2005; Winsky-Sommerer et al., 2004), with type 1 receptors (Orx1) creating anxiogenic and depressive responses, and type 2 receptors (Orx2) producing contrasting anxiolytic or antidepressive results (Arendt et al., 2014; Arendt et al., 2013).
Not surprisingly, stress reactive orexins play important roles in anxiety, fear, and depression through activation of both Orx receptor subtypes (Abbas et al., 2015; Arendt et al., 2014; Arendt et al., 2013; Eacret et al., 2018; Giardino et al., 2018; Johnson et al., 2015; Johnson et al., 2010; Khalil and Fendt, 2017; Lungwitz et al., 2012; Wang et al., 2017). Intracranial or icv injection of OrxA promotes anxious behavior (Lungwitz et al., 2012; Suzuki et al., 2005), and similar types of anxiogenic (including panic) physiological and behavioral responses can be reversed by blocking Orx1 receptors (Johnson et al., 2015; Johnson et al., 2010; Staples and Cornish, 2014; Vanderhaven et al., 2015). Designer receptor activation of Orx neurons plus antagonism of Orx2 receptors (Grafe et al., 2017) potentially increases activity only at Orx1 receptors, or at receptors for Orx-colocalized factors such as glutamate, dynorphin, galanin or nitric oxide (Cheng et al., 2003; Chou et al., 2001; Hakansson et al., 1999; Torrealba et al., 2003), and results in increased anxiety, as well as decreased social interaction and latency to defeat, similar to Orx neuron stimulation alone (Eacret et al., 2018). We have previously suggested that Orx1 function is diametrically opposed to that of Orx2 (Arendt et al., 2013). We also demonstrated that knockdown of Orx2 receptors in the basolateral amygdala (BLA) produces anxiogenic responses, suggesting that the natural function of Orx2 is anxiolytic (Arendt et al., 2014). Amygdalar inhibition of anxious behavior is thought to be regulated through GABAergic interneurons in the BLA or intercalated region of the amygdala (ItC) (Busti et al., 2011; Lee et al., 2013; Tovote et al., 2016; Tovote et al., 2015). Recent experimentation with orexin-deficient mice resulted in increased anxiety (Abbas et al., 2015; Khalil and Fendt, 2017), which we speculate may be due to lack of Orx2 activation.
Therefore, we posited that modulation of Orx2 function would result in modification of adaptive emotional and defensive behavior in mice. As Orx has been shown to be functionally effective following intra-nasal delivery (Baier et al., 2008; Dhuria et al., 2009; Modi et al., 2017; Van de Bittner et al., 2018), a putatively systemic brain Orx2-induced anxiolysis could be of therapeutic value. We hypothesized that icv delivery of an Orx2 agonist would promote resilience, result in a reversal of submissive behavior, and decrease anxious and depressive behaviors. In relation to this agonist effect, we hypothesized that specific parvalbumin positive GABAergic neurons in the BLA or ItC would be activated by Orx2 stimulation in submissive Stay mice. We also hypothesized that intracerebroventricular (icv) delivery of an Orx2 antagonist would inhibit escape behavior, promote stress susceptibility, and increase anxious and depressive behaviors, such as startle responses and freezing time.
2. Methods
2.1. Subjects and housing
Adult (6–8 weeks) male C57BL/6N mice weighing ~22–26 g (Envigo, Indianapolis, IN; N = 56) were group housed (4–5 per cage) for 5 days of acclimation (Fig. 1A). They were singly housed (including cage controls) following icv cannulation for the remainder of the experiment (12 days), on a 12:12 light-dark cycle (lights off at 6 PM) at 22°C, with ad libitum food and water. A separate cohort of male Hsd:ICR (CD1) retired breeder mice weighing ~50 g (Envigo, Indianapolis, IN; N = 37) were similarly housed, and used to provide aggression during social interaction in the Stress-Alternatives Model (SAM; Fig. 1B) and a target for the Social Interaction/Preference (SIP) tests. During the dark active phase mice (C57BL/6N) were exposed to daily handling starting 2 days after icv cannulation, then behavioral aggression and testing. Handling included loosening and retightening the cannula insert for ~1 min. All surgical and behavioral procedures were performed in a manner that minimized suffering and the number of animals used was in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and approved by the Institutional Animal Care and Use Committee of the University of South Dakota.
2.2. Experimental Design
Experimental groups included: home cage control (N = 10; used to derive baseline hormone and protein [immunohistochemistry, IHC] expression levels), vehicle control (N = 20), Orx2 receptor antagonist (N = 17), and Orx2 receptor agonist (N = 9). All treatment groups, except cage control, underwent 4 days of aggressive social interaction in the Stress-Alternatives Model apparatus (SAM; Figs. 1A, B), which was preceded by fear conditioning on each day (described below; Fig. 1A). On day 5, treated animals underwent a Social Interaction/Preference (SIP; Fig. 1C) test, followed by measurement of freezing response in the SAM to the conditioned stimulus in the absence of social interaction (CR = conditioned response to tone; Fig. 1A). Drug treatments were administered on day 3. After testing on day 5, mice were briefly anesthetized with isoflurane (5% at 1.0 min/L for ~1.5 min) and rapidly decapitated. Whole brains and trunk blood plasma were collected and stored at - 80°C for further analysis.
2.2.1. Stress Alternatives Model (SAM)
The SAM apparatus is constructed of a clear rectangular box (91 cm long, 22 cm wide, and 26 cm high) with curved opaque dividers (r = 10.25 cm) that partition it into 3 sections: two enclosed areas (10 × 22 × 26 cm) at each end of the oval-shaped open field (OF = 71 × 22 × 26 cm) arena for social interaction (Fig. 1B). All behavioral interactions were conducted during scotophase (dark; active period). The two enclosed areas are accessible from the OF arena through escape routes (one each at the distal ends of the oval) only large enough for a smaller C57BL/6N test mouse to pass through (Fig. 1B).
A short time before social interaction began, a novel large retired breeder CD1mouse (aggressor) was placed into the SAM OF arena outside of a centered opaque cylindrical divider (15 cm diameter and 20 cm tall). A smaller C57BL/6N mouse (test mouse) was then placed inside the opaque cylinder (Fig. 1B). Our lab has previously shown that fear conditioning influences gene expression of Orx receptors, endocannabinoid receptors (Cb), and brain-derived neurotropic factor (BDNF) in the amygdala and hippocampus (Robertson et al., 2017; Smith et al., 2014); therefore, to incorporate a SAM-inclusive fear conditioning paradigm, after a 30 sec acclimation period inside the cylindrical divider, a 5 sec tone (2500 Hz at 75 dB, CS+) was presented. Following the tone there was a 10 sec trace period, after which the divider was lifted allowing test and aggressor (US+) mice to freely interact within the SAM arena for 5 min.
Retired breeder CD1 mice are naturally aggressive toward other male mice, including smaller C57BL/6N, and interactions led to high intensity social aggression and defeat (Golden et al., 2011). Different from traditional social defeat models (Golden et al., 2011), test mice in the SAM are provided the option to either exit the arena (Escape) through one of the two escape routes or remain in the arena (Stay) with the larger aggressive mouse. Mice that escape the arena (Escape) have been shown to produce lower physiological and behavioral measurements of stress when compared to their submissive non-escaping (Stay) counterparts (Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). It is important to note, that both Escape and Stay mice experience high levels of aggression from the novel CD1 mouse, and have elevated hormonal, behavioral, and gene expression stress responses, though these measures are significantly higher in the Stay mice. Results from this and previous experiments have demonstrated that test mice generally choose a behavioral phenotype (Escape or Stay) by the second day of SAM interaction.
During interactions in which an aggressor mouse threatened the life of a test mouse, a clear, perforated divider (15 cm wide and 20 cm high) was used to briefly interrupt (5–10 seconds) these intensely aggressive bouts. Life threatening attacks included repeated bites to the head or neck of the test mouse. After 5 min of interaction, both mice were removed from the arena and placed back into their home cages. If test mice escaped, they were left in the enclosed area of the SAM until the end of the 5 min interaction period.
Test mice were exposed to 4 consecutive days of the SAM social aggressive interaction, following fear conditioning. Drug infusions were given prior to SAM interaction on day 3 (described below). A novel aggressor mouse was used on each day. On day 5 the Social Interaction/Preference Test (SIP) was performed (described below), followed by a final SAM exposure: placement in SAM cylinder, 5 sec CS+(tone), and 10 sec trace period, testing for a conditioned response (CR). However, on this day the cylinder was not lifted and there was no large mouse present, and therefore, no social aggression. All interactions were recorded manually and digitally using GoPro Hero3+ cameras under red light. Digitally recorded interactions were scored by individuals naive to the treatment of each test mouse.
2.2.1.1. Fear Conditioning Behavioral Analysis
Freezing behavior, commonly used to assess fear conditioning (Blanchard and Blanchard, 1988), was measured for 4 days prior to aggressive social interaction (US), and on the 5th day in the absence of the US. Freezing is the amount of time (> 1 s) that mice remained motionless, except for movements related to respiration. Freezing was measured during the time that a test mouse spent inside the opaque dividing cylinder; which included time prior to the tone (CS), during the tone, and after the tone (ending when the cylinder was removed). Percent fear freezing ratio was calculated as: [(freezing time after tone/15 seconds) / (freezing time before tone/30 seconds)] x 100; this ratio has the advantage of revealing fear conditioned freezing, as opposed to spontaneous freezing, as a number greater than 100. Significant fear conditioning should always be represented by a mean > 100.
2.2.1.2. SAM Behavioral Analysis
Behavioral analysis for SAM socially aggressive interaction began following the removal of the cylinder and ended when the test mouse escaped, or at the end of the 5 min interaction time. Mice were designated as escapers (Escape) if they left the arena through an available escape tunnel on one or more of the two test days prior to drug treatment. Mice that did not escape, remained submissively (Stay) with the aggressor CD1. Interaction time was measured as the time from the removal of the cylinder until the end of the interaction (when the test mouse was removed from the arena or escaped). Freezing, startle, and time attentive to the escape route measurements were normalized by the total time spent interacting with an aggressor. Latency to escape was measured as the time starting from the removal of the cylinder and ending when the torso of the test mouse was through the escape hole. Locomotor activity was measured following SAM interactions on the day of drug treatment, in the home cage by counting line crossings of a grid with 10 cm between lines.
Freezing behavior during SAM interactions (conflict freezing) was defined in the same way as freezing during assessment of fear conditioning (before the interaction begins). Conflict freezing always occurs during social interaction, and includes freezing in anticipation of aggression, in response to aggression, and contextual freezing in response to the arena in which aggression is experienced (especially on days 2–4 of SAM interactions). Freezing time was converted into percent freezing time ([time (s) frozen/total interaction time] x 100%).
Startle responses (whole-body flinching) measure an animal’s reaction to sudden environmental stimuli, often acoustic (Heesink et al., 2017; Sallinen et al., 1998). We surmised, that as the intensity and amplitude of the acoustic-startle response are affected by stress-related events (Belzung and Griebel, 2001; Risbrough et al., 2004), the frequency of socially-induced startle might also be enhanced by increasing stress during aggressive interactions. With this in mind, we pirated the concept of startle for use in the SAM, because we noticed that active approach of an aggressor mouse also elicits a similar startle response. Therefore, we made use of a stimulus that exists as a part of aggressive social interaction, the threat of attack, and measured the number of flinches or recoils that were induced by the approach of the larger CD1 mouse during each entire social interaction.
The amount of time the test mouse spent in contact with the escape tunnel is an indicator of stress-sensitive novelty exploration (Eagle et al., 2013; Jacinto et al., 2013). Although the entire SAM arena is novel initially, the escape route is a unique attribute, distinctly different from the rest of the apparatus, as it clearly presents a physically different path for movement. The chamber at the other end of the escape route is also unknown, until the first passage. Thus, this indicator of novelty assessment is also specifically associated with the stress-sensitive act by which the Escape and Stay Phenotypes are demarcated. We defined the amount of time spent attentive to the hole as only including time when the test mouse was actively interested in and investigating the escape hole and tunnel directly (sniffing and placing head in the hole). This measure is a novel indicator of anxious behavior and decision-making unique to this model, which has been effectively used in SAM experiments on rainbow trout (Summers et al., 2017). We consider Escape and Stay behavioral outcomes to be the result of decision-making because early responses are variable, become stable with experience, and are modifiable by learning as well as anxiogenic or anxiolytic drugs (Carpenter and Summers, 2009; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018).
2.2.2. Social Interaction/Preference Test (SIP)
The Social Interaction/Preference (SIP) test was performed on day 5 as a reliable indicator of whether mice are resilient or susceptible to social stress following the SAM interactions and drug treatments (Arendt et al., 2014; Krishnan et al., 2007). The SIP test was conducted in a square box (40 cm3) with a perforated clear container (12 cm diameter and 20 cm high) placed alongside the middle of one wall (Fig. 1C). To begin, test mice were placed into the arena at the opposite end from the empty container and allowed to explore for 2.5 min, before being removed. The empty vessel was then replaced with an identical container holding a novel CD1 mouse. Test mice were then placed back into the arena for another 2.5 min.
2.2.2.1. SIP Behavioral Analysis
The amount of time test mice spent in close proximity to (within 3 cm) the empty vessel, and the one containing a novel CD1 mouse was recorded. To determine whether animals were resilient or susceptible to social stress, the amount of time near the jar containing the aggressor mouse was divided by the time spent near the empty jar ([Time 3 cm or less from aggressor’s jar/Time 3 cm near empty jar] x 100). Animals with values of 100% or greater were interpreted as being resilient, and those less than 100% were construed to be susceptible to social stress (Arendt et al., 2014; Krishnan et al., 2007).
2.3. icv Stereotaxic Surgeries
Following a 5 day acclimation period, but prior to exposure to behavioral paradigms, stereotaxic surgery for intracerebroventricular (icv) cannula implantation was performed on C57BL/6N mice that were to undergo Vehicle, Orx2 receptor agonist, or antagonist treatment (N = 46). Mice were anesthetized with isoflourane (2% at 1.0 L/min flow rate) and a guide cannula (26 ga cut to 1.5 mm) was inserted into the right lateral ventricle (Bregma; AP: −0.50 and ML: −1.0). Animals were allowed 7–10 days to recover before aggressive social interaction began.
2.4. Drugs & Infusion
Drug treatments were given icv, to mice on day 3, either 1 (antagonist or vehicle) or 30 min (agonist; to allow for longer peptide diffusion) prior to being placed into the SAM arena. Drug plus placement in the SAM was followed by the CS and then social aggression interaction, similar to all SAM interaction days (Fig. 1A, B). The SAM was designed to include both within subject and across treatment group comparisons. Injection on day 3 allows comparison of each animal to itself on days 2 and 3, before and after injection, as well as comparisons with the vehicle control. The design was intended to measure acute drug effects on day 3, just after injection. However, three kinds of longer-term effects are possible: 1) carryover effects for drugs that degrade slowly, 2) effects associated with persistent modification of stress responsiveness that yields longer-term changes in anxious or depressive behaviors, and 3) drug effects that promote neural plasticity and yield gene expression or learning.
The Orx2 receptor antagonist, MK-1064 (0.3 nmol, MCE, Monmouth Junction, NJ), and Orx2 receptor agonist, which is a modified OrxB peptide, [Ala11, D-Leu15]-OrxB (0.3 nmol, TOCRIS, Minneapolis, MN), were dissolved in 0.9% saline plus 5% DMSO (= vehicle). The doses for both drugs were chosen to avoid enhanced locomotor activity or induction of sleep. Of the two drugs used, only [Ala11, D-Leu15]-OrxB has been administered icv previously, at a dose that increased locomotion (Samson et al., 2010), and we chose a dose that was ten-fold smaller (Fig. 3D). For MK-1064, we chose a dose that was 10,000 fold smaller than that given orally, avoiding induction of sleep or acute HPA axis activity (Gotter et al., 2016; Grafe et al., 2017), but equivalent to the icv dose chosen for [Ala11, D-Leu15]-OrxB, because the IC50 (half-maximal inhibitory) and EC50 (half-maximal effective) concentrations are similar for these two drugs. All treatments were directed into the lateral ventricle via micro-syringe (extended 0.2 mm below the guide cannulae) and delivered at a rate of 0.5 μl/min, with a total injection volume of 1 μl. All icv injections (1 or 30 min prior) were followed by placement into the SAM arena, plus an additional 45 s (for fear conditioning) before initiation of the aggressive interaction. As MK-1064 crosses the blood-brain barrier, and substances delivered to the cerebrospinal fluid via icv injections are transferred to the cerebral microvasculature via the choroid plexus (Pardridge, 2016), we assume this drug is delivered throughout the brain. In contrast, similar sized peptides that include [Ala11, D-Leu15]-OrxB, OrxA, and OrxB have been administered icv previously (Flores et al., 2014; Gotter et al., 2016), producing behavioral results such as enhanced locomotion and influencing consolidation of fear memories, the latter function dependent on the BLA (Flores et al., 2017). We followed this precedent, suggesting that the Orx peptides are functionally available following icv injections to brain regions associated with fear, anxiety and depression.
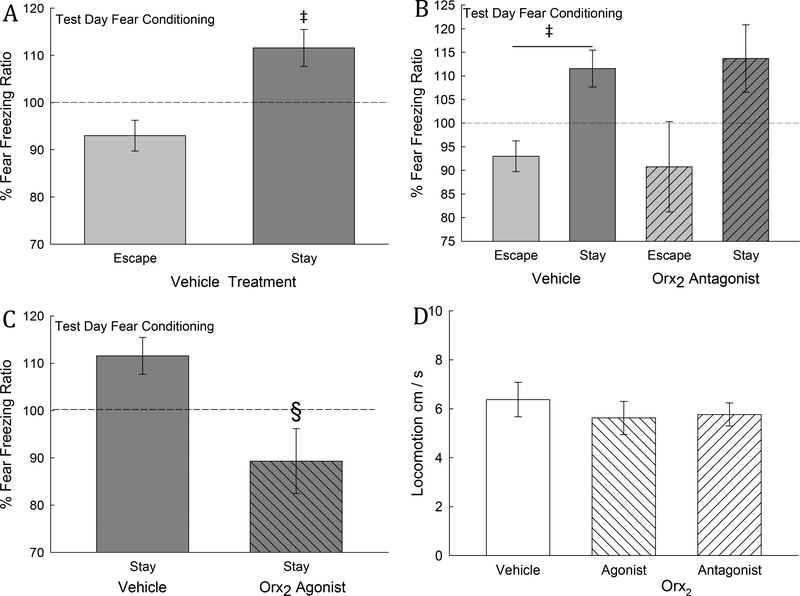
Fig. 3. Fear-conditioning is represented by freezing produced by pairing a conditioned stimulus (CS = tone) with an unconditioned stimulus (US = aggression). It is only increased in Stay mice, and is reversed by Orx2 agonism.
A) Mean (± SEM) fear freezing ratio (FFR = [(freezing time before tone/30 seconds)/(freezing time after tone/15 seconds)] x 100) of Stay phenotype mice (dark gray bar) on test day (two days after vehicle injection) reflects a conditioned response (CR) which is significantly (‡) greater than the FFR for Escape phenotype animals. B) Treatment with an Orx2 antagonist (hatched bars) did not significantly alter the fear freezing ratio 2 days after injection, compared with vehicle treated mice. C) In Stay mice, which exhibit a CR (significantly elevated FFR) in vehicle treated animals, icv injection of an Orx2 agonist (dark gray hatched bars) blocks the fear freezing conditioned response, producing a FFR which is significantly (§) reduced compared with vehicle controls (dark gray bars). D) Home cage activity is not altered by icv injection of Orx2 agonist or antagonist.
2.6. Hormone Analysis
Following testing on day 5, trunk blood was taken and centrifuged for ~5 min to separate plasma. Plasma was frozen at −80° C for further analysis. Plasma corticosterone concentrations [B] were quantified in duplicate, in a single run, using a corticosterone enzyme-linked immunosorbent assay kit (Enzo Life Sciences, Farmingdale, NY).
2.7. Immunohistochemistry
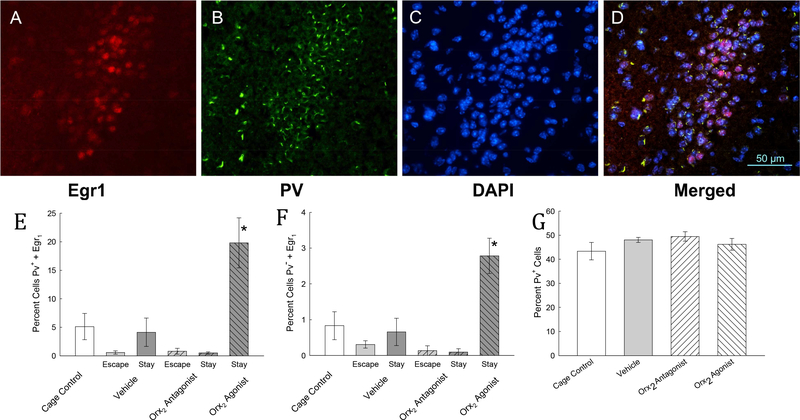
Frozen coronal sections (16 μm) from AP −0.94 to −1.70 relative to bregma were incubated with EGR1 (1:500; rabbit monoclonal from Cell Signaling 4154S) and parvalbumin (Pv, 1:100; goat polyclonal from ABcam ab32895) primary antibody combination in solution, 5% bovine serum albumin (BSA) in phosphate buffered saline (PBS), at 4°C overnight (Sathyanesan et al., 2017). Antibodies were used as per manufacturer’s instructions and specificity was tested using incubation in antibody solutions lacking primary antibody. Following primary antibody incubation, slides were washed in 1xPBS three times for 5 minutes each at room temperature. Slides were then incubated in fluorescent secondary antibody (1:500, Alexa-488 and 594: AF488 donkey anti-goat - A11055 for Pv and AF594 chicken anti-rabbit - AF21442 for EGR1; Life Technologies) in 2.5% BSA in PBS for 2 hours at room temperature. The slides were then rinsed in 1x PBS three times for five minutes each and coverslipped with VECTASHIELD HardSet mounting medium with 4′,6-diamidino-2-phenylindole (DAPI). Three sections from each mouse were analyzed, from which an area (210 × 240 μm) was defined at AP 1.06, centered on ML + or - 2.65 and DV 4.50 relative to bregma, in which all DAPI labeled neurons were counted and scored for EGR1 and Pv labeling. Sections were viewed by an unbiased observer to evaluate differences and images were captured using a Nikon Eclipse Ni microscope equipped with a DS-Qi1 monochrome, cooled digital camera and NIS-AR 4.20 Elements imaging software, using 20x and 40x objective magnifications. Sections from stressed and control mice were captured using identical exposure settings.
2.8. Statistical Analysis
All experimental designs and statistical analyses were based on a priori hypotheses. For conditions that changed over 4 days of SAM social interaction, we compared outcomes using a two-way repeated measures ANOVA (Orx2 drug x behavioral phenotype x day of SAM interaction design), where phenotype was either Stay or Escape. In addition, two-way ANOVA (Orx2 drug x Phenotype design) was utilized to determine the influence of activating Orx2 receptors (Orx2 Effects) relative to the expression of behavioral phenotypes (Stay vs Escape; Phenotype Effects), Phenotype by Conditioning (Interaction Effects), and analysis of BLA EGR1, Pv, and DAPI triple-labeling. To compare changes occurring within a treatment group across SAM interaction days, a one-way repeated measures ANOVA (Orx2 drug x day of SAM interaction design) was performed. While the statistics for behavior did not include cage controls; these controls were necessary for interpretation of hormonal corticosterone levels, because samples from SAM treatments were compared to baseline levels determined by the mean [B] value of home-cage control animals. Therefore home-cage controls were added for specific one-way ANOVA comparisons. Comparison of locomotion in the home cage after drug treatment was also accomplished by one-way ANOVA. Comparisons between two treatments (Vehicle, Orx2 receptor antagonist, or Orx2 receptor agonist) within a given phenotype (Escape or Stay) were analyzed by Student’s t-tests. To determine differences in percentage of escape, a chi-square statistical analysis was performed, where results from previous days were utilized as expected values.
Each animal provided only a singular datum for all analyses. Five assumptions of parametric statistics were applied to the data, which were transformed when necessary, but graphed in their raw form. Analyses with both non-parametric and parametric statistics (previously mentioned) were performed along with examination for multiple comparisons using the Holm-Sidak method, and when the statistical analyses match, as they do for the data herein, we report the parametric results without α adjustment (Feise, 2002; Jennions and Moller, 2003; Moran, 2003; Nakagawa, 2004; Perneger, 1998; Rothman, 1990). Significant effects between groups for one-way analyses were examined with Student–Newman–Keuls post hoc analyses (to minimize Type I error) and Duncan’s Multiple Range Test (to minimize Type II error).
3. Results
3.1. Stress-Alternatives Model
In previous experiments using the SAM arena for mice, rats, and trout, the proportion of animals exhibiting Escape and Stay phenotypes have been approximately 50:50 (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). Recent work in rainbow trout suggested that these phenotypes were fungible, even without drug treatment, and that the proportions of the two phenotypes may be altered by the degree of stress effects from the captive environment (Summers et al., 2017). As occasionally happens there was not an even distribution of phenotypes in these experiments, such that prior to any treatment the proportion of escaping mice was only 28.26%, leaving 71.74% submissive (Stay) mice. This ratio of phenotype distribution required non-random division of phenotypes into groups. Since the Orx2 receptor antagonist (MK-1064) was expected to reduce escape behavior, a relatively higher proportion of Escape mice (40%) were used for this treatment. In contrast, since application of the Orx2 receptor agonist ([Ala11, D-Leu15]OrxB) was hypothesized to promote escape behavior, and the availability of this phenotype was low, we allotted 0% of the individuals displaying Escape behavior to this treatment group (100% Stay).
3.1.1. Escape vs. stay behavior; escape latency
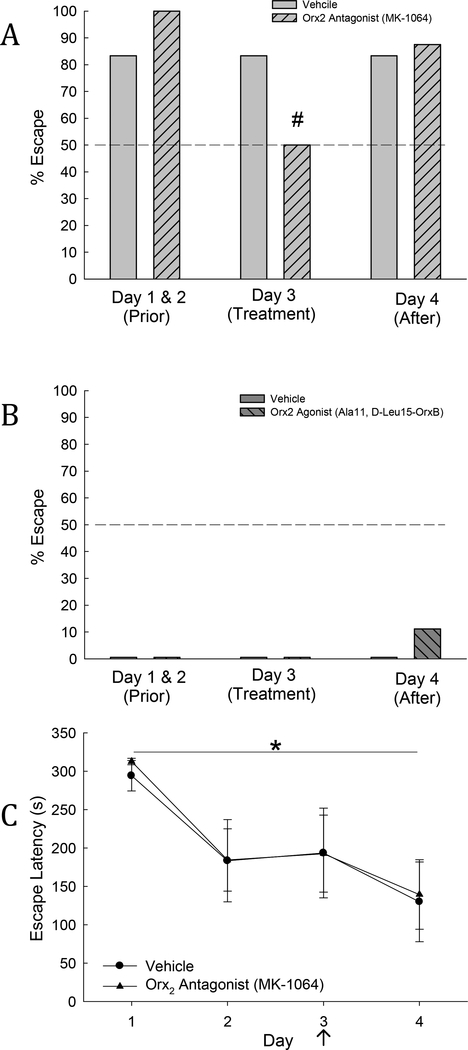
As predicted, in Escape mice, escaping behavior did not change following injection of vehicle (Fig. 2A), suggesting that the additional stress of icv injection was not enough to modify behavioral phenotype (Smith et al., 2014). Escape behavior was significantly reduced (within subject comparison, χ2: F1=4.5, P ≤ 0.034) by injection of the Orx2 receptor antagonist (MK-1064) on day 3 (50% Escape), in comparison with the first two days of interaction (100% Escape; Fig. 2A). In contrast, day 3 injection of Orx2 receptor agonist in Stay animals slightly increased escape behavior one day after icv administration (11% Escape on day 4), in comparison with the first three days of interaction (0% Escape; Fig 2B).
Fig. 2. Escape behavior is promoted by Orx2 agonism, and limited by Orx2 antagonism, while latency to escape is unchanged.
A) Escape behavior was significantly reduced, following icv injection of Orx2 receptor antagonist (MK-1064) on day 3 (light gray hatched bar) in mice that were previously Escape phenotype (light gray hatched bar; # indicates significance in antagonist treated animals from Days 1&2), but remained unchanged in vehicle treated animals (light gray bar). B) Escape behavior was promoted following day 3 icv injection of Orx2 receptor agonist ([Ala11,DLeu15]-Orexin B), on day 4 (dark gray hatched bar; not significant) in mice that were previously Stay phenotype. (C) Escape latency was significantly reduced (*) on day 4 of SAM interaction compared to day 1. There was no change seen between Escape animals treated with vehicle and animals treated with Orx2 antagonist.
Escape latency was progressively reduced (Repeated measures one-way ANOVA: F3,39 = 6.18, p ≤ 0.002; Fig. 2C) by experience in all icv injected mice taken together. As is typical of results previously reported for untreated animals (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014) the latency for days 2–4 was significantly reduced compared to day 1. However, comparison to previous studies also reveals that all latency times in icv injected animals were greater than those of untreated mice, even though the stressful icv injection occurred only on day 3. Since the availability of escaping mice was limiting, the Orx2 agonist was not given to Escape mice. The Orx2 receptor antagonist MK1064 blocked escape in some animals, but did not increase the latency to escape for those that continued to do so (Fig. 2C), suggesting that future experiments should also include Orx2 agonist treatment in Escape mice to determine if latency would be reduced.
3.1.2. Fear conditioning response prior to the SAM interaction
As in previous studies (Smith et al., 2014), only Stay mice reacted to fear conditioning stimuli (CS + US pairing = tone + aggression from a larger CD1 mouse) during isolation in the opaque cylinder. The Stay phenotype froze significantly (t13= 2.7, P ≤ 0.018) more than Escape mice on test day (5) in the absence of the US (Fig. 3A). The data are presented as a ratio of (freezing during and after the tone/ time during and after tone)/(freezing prior to the tone/time prior to tone) x 100, such that significant conditioned freezing [= CR] is greater than 100%. Importantly, the distinction between the significant CR in Stay mice and not in Escape mice was extant despite the additional stress of icv vehicle injection on day 3. Administration of the Orx2 antagonist (MK-1064) did not change the fear freezing ratio (Two-Way ANOVA, treatment effect: F1,19 = 0.06, P ≤ 0.817; phenotypic effect: F1,19 = 3.28, P ≤ 0.086; interaction effect: F1,19 = 0.86, P ≤ 0.37) when compared to vehicle treated Escape or Stay mice (Fig. 3B). However, freezing (and the fear freezing ratio) was significantly reduced in Stay mice (t10= 2.92 , P ≤ 0.015) that received icv injection of the Orx2 agonist ([Ala11, D-Leu15]-OrxB) two days before testing (Fig. 3C). For this or any behavior reported, activity changed by Orx2 agonist or antagonist treatment is unlikely to be the cause, as home cage locomotion is unchanged (One-way ANOVA: F2,35 = 0.391, P ≥ 0.679) immediately after icv injection and SAM social interaction on day 3 (Fig. 3D).
3.1.3. Freezing during SAM interaction
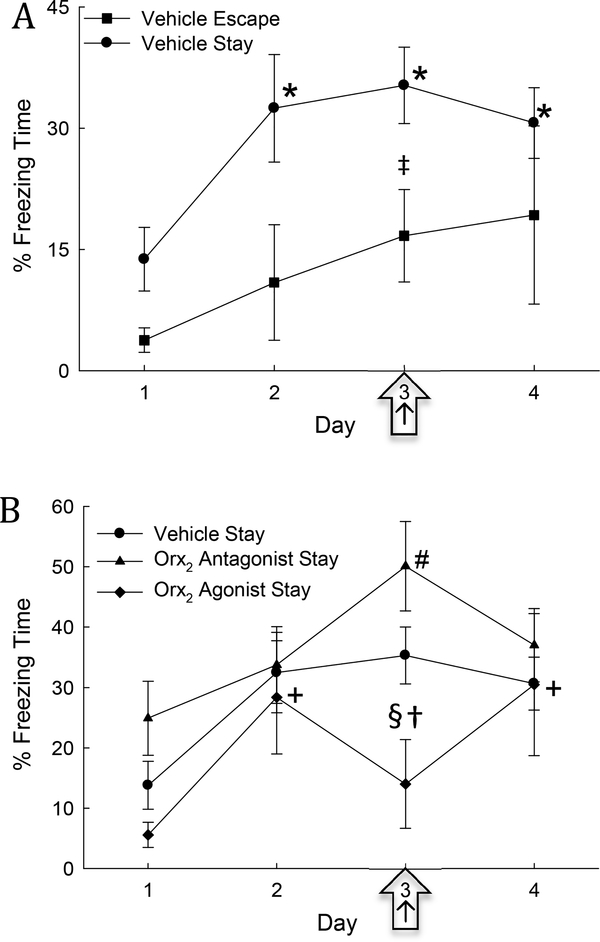
Conflict freezing behavior in C57BL/6N test mice during the SAM social interaction with larger CD1 aggressors, increases significantly (One-Way Repeated Measures ANOVA: F3,30 = 5.08, P ≤ 0.006) in mice adopting the Stay phenotype after 1 day of interaction (Figs. 4A,B). This increase includes continued longer freezing time on the day of vehicle treatment (day 3, Figs. 4A,B), suggesting that the additional stress of icv injection did not increase the total duration of freezing. In addition, greater freezing time was exhibited by vehicle-treated Stay compared to Escape mice on all days (Two-Way Repeated Measures ANOVA, phenotype effect: F1,42 = 5.17, P ≤ 0.039; time effect: F3,42 = 4.43, P ≤ 0.009; Fig 4A), including injection day (day 3; t16=2.38, P ≤ 0.03; Fig. 4A), but not day 4. Importantly, there were no significant differences between Stay mice in different icv injection groups on days 1 & 2 prior to icv injection or day 4 after treatment (Fig. 4B).
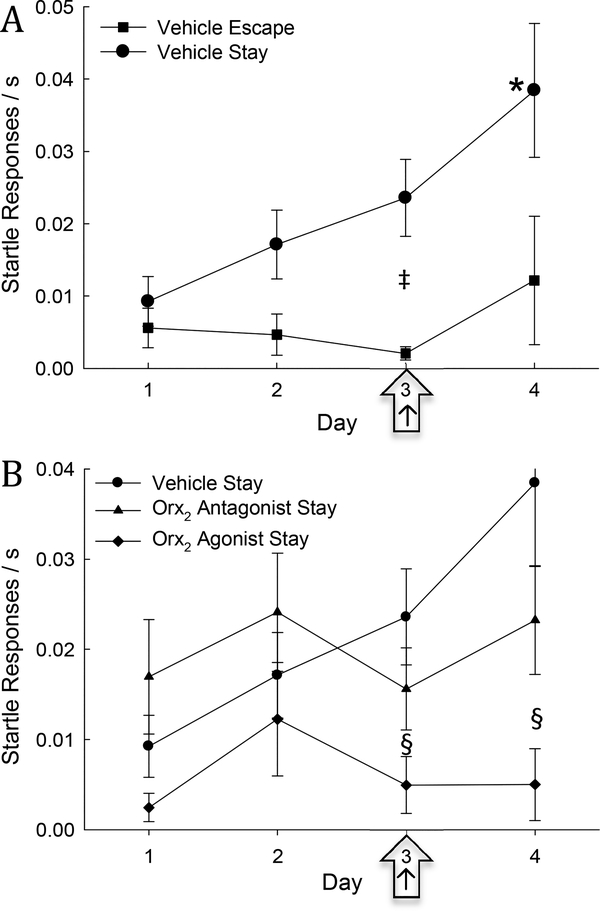
Fig. 4. Freezing time is elevated in Stay mice during SAM interaction and can be increased by Orx2 receptor antagonism and decreased by Orx2 receptor agonism.
A) Mean (± SEM) contextual freezing time in vehicle treated Stay animals (solid dots) is significantly increased (*) compared to day one on days 2–4 of SAM interaction; and compared to vehicle treated Escape mice (square ‡) on injection day (3; arrow ‡). B) Mean (± SEM) % freezing time increases in all groups on day 2, compared to day 1, prior to treatment (+ significance in agonist treated animals, # significance in antagonist treated animals). Injection of Orx2 antagonist (icv; triangles) increased freezing on day 3 compared to day 1, day 2, and day 4, and icv Orx2 agonist (diamonds, arrow) significantly decreased freezing relative to vehicle (§) and antagonist treatments (†) on day 3 (arrow).
On day 3, injection of icv Orx2 receptor antagonist MK-1064 in mice that Stay produces significantly greater time freezing compared to days without treatment (1, 2, and 4; One-Way Repeated Measures ANOVA: F3,27 = 3.3, P ≤ 0.035). Escaping animals also exhibit a nearly significant (One-Way Repeated Measures ANOVA: F3,15 = 2.94, P ≤ 0.067) increase in percent freezing time on the day of the Orx2 antagonist treatment (data not shown). Conversely, Stay animals treated with the Orx2 agonist [Ala11, D-Leu15]-OrxB exhibit reduced freezing on day 3 compared to vehicle-treated mice (One-Way Repeated Measures ANOVA: F3,9 = 4.68, P ≤ 0.031), despite being elevated similar to vehicle-controls on days without treatment (2 and 4; Fig. 4B). Additionally, when comparing injection treatment groups in Stay mice based on a priori hypotheses regarding each Orx receptor treatment (Two-way repeated measures ANOVAs 1. Vehicle vs antagonist: treatment effect: F1,60 = 6.92, P ≤ 0.016; time effect: F3,60 = 6.23, P < 0.001; 2. Vehicle vs agonist: time effect: F3,39 = 6.06, P ≤ 0.002; 3. Agonist vs antagonist: treatment effect: F1,39 = 6.28, P ≤ 0.026), freezing time in Orx2 agonist-injected Stay mice is significantly less than that for animals given vehicle (t14=2.30, P ≤ 0.037) and Orx2 antagonist (t14=2.64, P ≤ 0.02) on injection day (Fig. 4B).
3.1.4. Startle Responses during SAM interactions
Startle responses during SAM social interactions steadily increased over time in vehicle-injected Stay mice, significantly so by day four (One-Way Repeated Measures ANOVA: F3,24 = 4.12, P ≤ 0.017: Fig. 5A, B). Notably, vehicle-injected Stay and Escape mice did not exhibit a significant difference in startle responses on injection day (3) compared to the first two days of interaction (1 & 2), suggesting that the additional stress from icv injection had minimal effects on startle responses (Fig. 5A). Additionally, within the vehicle-injected cohort, Stay animals exhibit a significant increase in startle responses compared to Escape animals (Two-way repeated measures ANOVA 1. phenotype effect: F1,35 = 11.46, P ≤ 0.004; 2. time effect: F3,35 = 3.41, P ≤ 0.028; Fig. 5A), including on the day of treatment (day 3; t14=2.66, P ≤ 0.019).
Fig. 5. Startle responses in Stay mice are increased, but can be diminished by stimulating Orx2 receptors.
A) Mean (± SEM) number of startle responses/time in SAM for vehicle treated Stay animals (solid dots) is significantly elevated on day 4 compared to days 1, 2 and 3 (*) and to Escape vehicle treated animals (‡, solid squares) on the day of vehicle injection (arrow, ‡). B) Compared across days, Stay mice treated with vehicle (solid dots) have an increased number of startle responses on day 4 compared to all other days (* indicates significance in vehicle treated animals). Stay mice treated with Orx2 agonist (diamonds) are significantly (§) different from vehicle on day 3 (arrow) and day 4; but not compared to Orx2 antagonist treatment (triangles).
Mice (Stay) to be injected icv with Orx2 receptor antagonist MK-1064 and agonist [Ala11, D-Leu15]-OrxB exhibited similar changes in startle response on days 1 and 2 prior to injection on day 3 (Fig. 5B), and were not significantly different over time. However, when comparing injection treatment groups in Stay mice based on a priori hypotheses regarding each Orx receptor treatment (Two-way repeated measures ANOVAs 1. Vehicle vs antagonist: interaction effect: F3,59 = 2.67, P ≤ 0.05; 2. Vehicle vs agonist: treatment effect: F1,38 = 12.14, P ≤ 0.004; 3. Agonist vs antagonist: treatment effect: F1,39 = 5.67, P ≤ 0.033), startle responses after treatment in Orx2 agonist-injected Stay mice are reduced compared to vehicle (t13=2.02, P ≤ 0.064; Fig. 5B). This reduction was not observed after Orx2 antagonist (t14=1.29, P ≤ 0.217) treatment in mice on injection day (Fig. 5C, D), nor on day 4 (Fig 5B). Furthermore, there was not a significant difference between Stay animals given vehicle and those given Orx2 antagonist (Fig. 5B).
3.1.5. Time Attentive to Hole
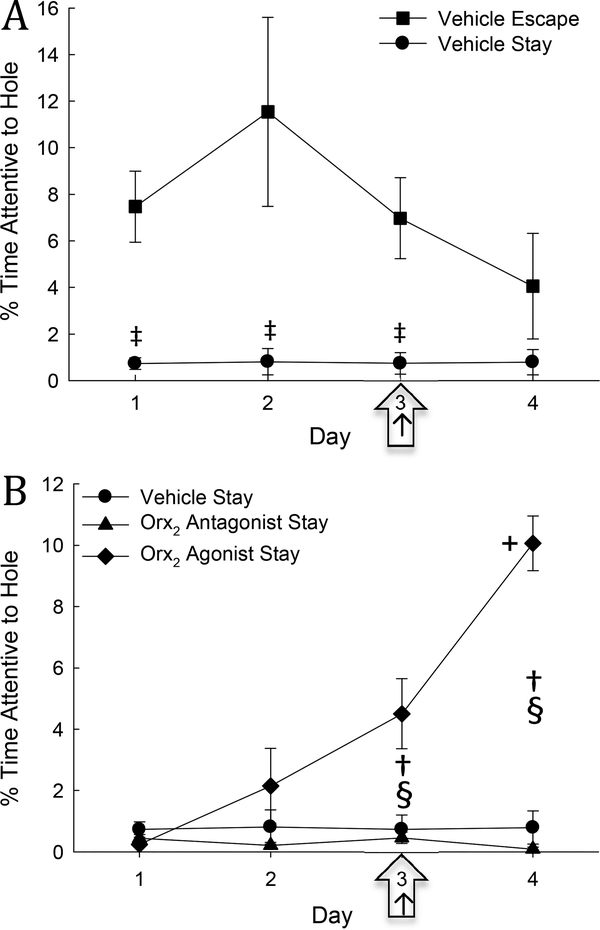
Time spent attentive to the escape hole did not change across days in Stay mice injected with vehicle (Fig. 6A, B). However, prior to (days 1 & 2) and on the day (day 3) of treatment, vehicle-treated Escape mice spent significantly more time attentive to the escape hole compared to Stay vehicle-treated mice (Two-Way Repeated Measures ANOVA, treatment effect: F1,42 = 31.99, P ≤ 0.001, time effect: F3,42 = 4.37, P ≤ 0.009, interaction effect: F3,42= 4.2 , P ≤ 0.011; Fig 6A), including treatment day (3; t16=4.59, P ≤ 0.001; Fig. 6A). Stay mice injected with Orx2 receptor antagonist MK-1064 on day 3 of SAM social interactions exhibited no differences in time spent attentive to the escape hole on any day (Fig. 6B).
Fig. 6. Attentiveness to the escape route is low in Stay mice but can be improved with icv administration of Orx2 agonist.
A) Vehicle treated mice exhibiting the Stay phenotype (solid dots) spend significantly (‡) less time attentive to the escape hole compared to Escape phenotype animals (squares), across days, and on the day of vehicle injection (arrow). B) Vehicle and Orx2 antagonist (triangles) treated Stay mice are equally disinterested in the escape route. However, mice treated with Orx2 agonist (diamonds) display a significant increase in % time attentive to the escape hole on days 3 and 4 when compared to day 1 (+). Specifically on day 3, Orx2 agonist treated mice (arrow) spend a significant more percentage of time attentive to escape hole compared to both vehicle (§) and Orx2 antagonist (†).
On the other hand, Stay mice injected with Orx2 receptor agonist [Ala11, D-Leu15]-OrxB display a significant and robust increase in time spent attentive to the escape hole (days 3 & 4) compared to the first and second day of SAM interaction prior to treatment (day 1 & 2; One-Way Repeated Measures ANOVA: F3,9 = 4.23, P ≤ 0.04; Fig. 6B). When, based on a priori hypotheses, Stay mice treated icv with Orx2 receptor agonist were compared to vehicle (Two-Way Repeated Measures ANOVA 1. Vehicle treated: treatment effect: F1,34 = 14.29, P ≤ 0.003, time effect: F3,34 = 36.74, P ≤ 0.001, interaction effect :F3,34= 36.01, P ≤ 0.001) and Orx2 antagonist-treated (Two-Way Repeated Measures ANOVA 1. Vehicle treated:treatment effect: F1,34 = 14.29, P ≤ 0.003, time effect: F3,34 = 36.74, P ≤ 0.001, interaction effect : F3,34= 36.01, P ≤ 0.001) mice, a significant increase in time spent attentive to hole on injection day (3; vehicle: t14=2.64, P ≤ 0.02, Orx2 antagonist: t14=2.64, P ≤ 0.02) and after (day 4; vehicle: t14=2.64, P ≤ 0.02, Orx2 antagonist: t14=2.64, P ≤ 0.02; Fig. 6B) was evident.
3.1.6. Social interaction/preference test
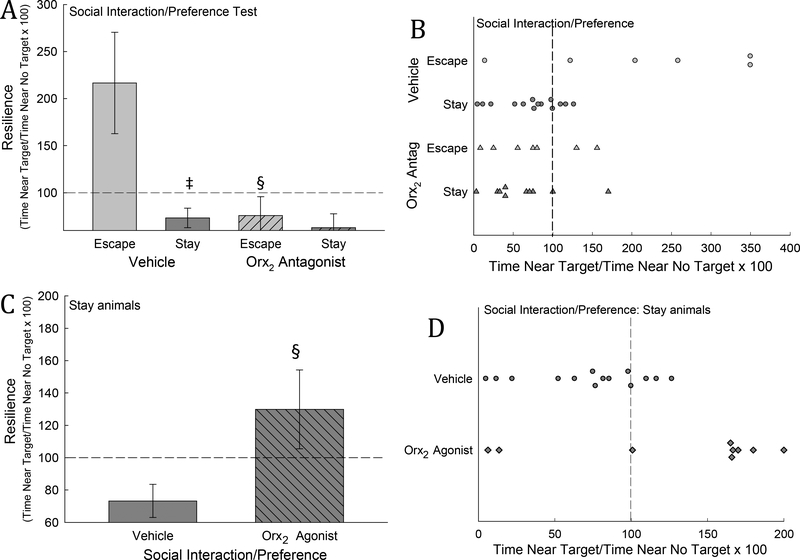
During the SIP test on day 5, after SAM social interactions were completed, significant differences in time spent near the novel container with a social target / time near the container alone x 100 [often used as a measure of social stress resilience/susceptibility (Arendt et al., 2014; Krishnan et al., 2007) ] emerged between phenotypes. Among vehicle injected mice, the Stay phenotype were significantly less resilient than mice that escape (t18=3.82, P ≤ 0.002; Figs. 7A, B). Importantly, this indicates susceptibility to stress in Stay mice that is not seen in Escape mice (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). However, in Escaping mice, time spent near the target was significantly decreased by treatment with Orx2 receptor antagonist MK-1064, when compared with vehicle-injected Escape mice (t11 = 2.6, P ≤ 0.025; Figs. 7A, B). Conversely, icv injection of Orx2 receptor agonist [Ala11, D-Leu15]-OrxB to Stay mice resulted in a significant increase in social novelty seeking when compared to vehicle-treated Stay animals (t16 = 3.71, P ≤ 0.002; Fig. 7C, D).
Fig. 7. Time near container plus social target / time near a novel container lacking a social target (stress resilience) is significantly greater in Escape compared to Stay mice, which is diminished by Orx2 antagonism (Escape mice) and reversed by Orx2 agonist treatment.
A, B) Vehicle treated Stay mice (dark gray bar and dots) are significantly (‡) less stress-resilient compared to Escape mice (light gray bar and dots), but when Orx2 antagonist is administered to a cohort of Escape mice (hatched light gray bar and triangles) there is a significant (§) decrease compared to vehicle treated Escape mice. C, D) When Orx2 agonist is administered icv to Stay mice (hatched dark gray bar and diamonds) there is a significant (§) increase in stress-resilience compared to vehicle-treated Stay mice (dark gray bar and dots).
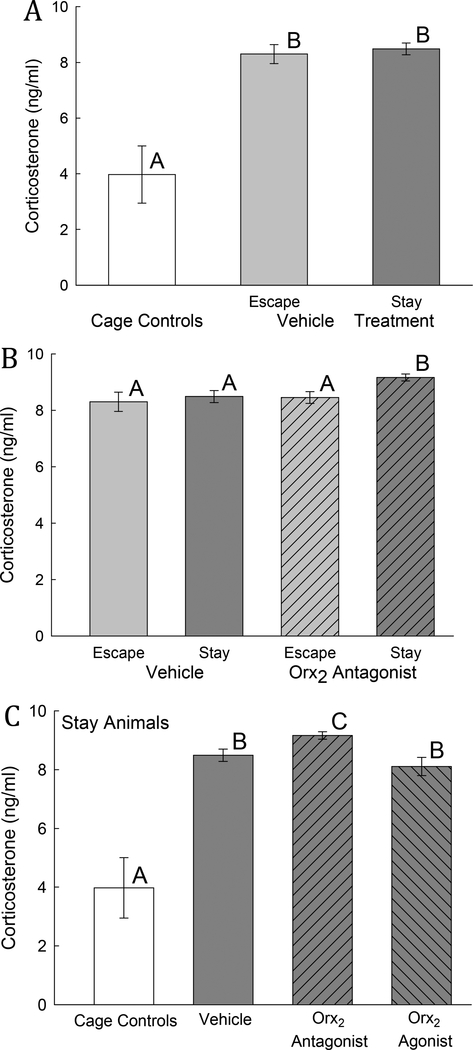
3.1.7. Corticosterone Concentrations
Plasma corticosterone levels taken on test day in both Escape and Stay animals treated with vehicle have significantly increased plasma corticosterone levels (One-way ANOVA: F2,14 = 23.27, P < 0.001) when compared to home cage controls (Fig. 8A). There was no significant change observed between phenotypes (Escape vs Stay vehicle treated mice; Fig 8A). However, Stay mice that were treated with Orx2 antagonist had significantly higher corticosterone levels (Two-way ANOVA: 1. Treatment effect: F1,22 = 4.27, P ≤ 0.05; 2. Phenotype effect: F1,22 = 5.02, P ≤ 0.035) compared to Stay mice treated with vehicle (t13 = 2.85, P ≤ 0.014) and to Escape mice also treated with MK-1064 (t13 = 3.03, P ≤ 0.01; Fig 8B). Comparing only Stay mice, plasma corticosterone concentrations in mice treated with Orx2 agonist [Ala11, D-Leu15]-OrxB were significantly lower (One-way ANOVA: F3,24 = 27.9, P < 0.001) than those treated with Orx2 antagonist MK-1064 (Fig. 8C).
Fig. 8. Social stress and Orx2 antagonist treatment increase plasma corticosterone concentrations.
A) Mean (± SEM) plasma corticosterone concentrations are significantly (bars with differing letters above, such as A vs B) elevated by aggressive social interactions, in both Escape (light gray bar) and Stay (dark gray bar) phenotype mice compared to home-cage controls (white bar). B) Among Escape mice, icv injection with an Orx2 antagonist 2 days prior (on day 3, light gray left hatched bar) does not affect plasma corticosterone on test (5) day. In contrast, among Stay phenotypes, icv injection on day 3 with an Orx2 antagonist (dark gray, left hatched bar) stimulates increased plasma corticosterone measured on day 5 compared to vehicle treated Stay mice (§), antagonist treated Escape mice (‡), and C) Orx2 agonist (dark gray, right hatched bar) Stay mice.
3.1.8. EGR1/Pv immunohistochemistry
After immunolabeling four sets of brains from each group (Vehicle-Escape, Vehicle-Stay, Orx2 agonist-Escape, Orx2 agonist-Stay, Orx2 antagonist-Escape, Orx2 antagonist-Stay), taken on day 5 after CR testing, for the immediate–early gene product early growth response protein 1 (EGR1, Zif268, NGF1A, Krox24, or TIS8), the nuclear marker 4′,6-diamidino2-phenylindole (DAPI), and the GABA neuron calcium-binding protein Parvalbumin (Pv), the images (Fig. 9) clearly indicated that a distinct cluster of cells in the BLA, or intercalated region of the amygdala (ItC) (Busti et al., 2011; Lee et al., 2013), are activated by Orx2 agonist binding. A small tight cluster of cells located in the region AP −1.06, centered on ML + or - 2.65 and DV 4.50 relative to bregma were triple labeled, but only in the Orx2 agonisttreated Stay animals (Fig. 9). This group of cells had clear boundaries, when viewed with DAPI and/or Pv labeling, even though significant DAPI and Pv labeling was evident throughout the BLA, which were not significantly different in number based on treatment group (One-way ANOVA: F3,21 = 1.38, P ≥ 0.28). For all vehicle and antagonist (MK-1064) treated groups, there was no significant effect on triple-labeled neuron count (Two-way ANOVA: 1. Treatment effect: F1,12 = 1.75, P ≥ 0.21; 2. Phenotype effect: F1,12 = 1.59, P ≥ 0.23; 3. Interaction effect: F1,12 = 2.25, P ≥ 0.16) of Pv-positive neurons, or double-labeled count of Pv-negative cells (Two-way ANOVA: 1. Treatment effect: F1,12 = 2.96, P ≥ 0.11; 2. Phenotype effect: F1,12 = 0.52, P ≥ 0.48; 3. Interaction effect: F1,12 = 0.89, P ≥ 0.38). However, one group, Stay mice treated with the Orx2 agonist [Ala11, D-Leu15]-OrxB, displayed significantly increased EGR1 labeling in Pv-postive cells (One-way ANOVA: F3,13 = 8.4, P ≤ 0.002, power = 0.944) and in Pv-negative neurons (One-way ANOVA: F3,13 = 3.9, P ≤ 0.034, power = 0.551) although in a much smaller percentage of cells, with no other treatment or phenotype having more than nominal EGR1, Pv, and DAPI labeling. Importantly, while evidence of EGR1 labeling in the BLA is suggestive of the Orx2 agonist stimulation of these cells; we cannot be sure that icv delivery reaches these neurons or that the activation is direct. There were significantly more neurons that exhibited triple labeling in Stay mice treated with the Orx2 agonist (T-test: t7 = 3.88, P < 0.006), than in Stay mice treated with either Orx2 antagonist. Additionally, the Pv labeling was unique in this area, with a smaller region labeled in each cell, than in the surrounding BLA neurons. At this time point after icv injection, this tight cluster of cells was uniquely triple labeled only in Stay mice receiving Orx2 agonist treatment (Fig. 9).
Fig. 9. Anxiolysis in Stay mice is accompanied by activation of BLA or ItC inhibitory interneurons.
Photomicrographs (magnification 40x) of the amygdala in the AP −1.06, centered on ML + or - 2.65 and DV 4.50 relative to bregma were triple labeled with A) the immediate–early gene product early growth response protein 1 (EGR1, Zif268, NGF1A, Krox24, or TIS8), B) the GABA neuron calcium-binding protein Parvalbumin (Pv), and C) the nuclear marker 4′,6-diamidino-2-phenylindole (DAPI). D) The EGR1/Pv/DAPI triple labeling is visible in the merged image; scale bar equals 50 μm. This confluence of labeling, particularly the presence of EGR1, is only evident in Stay mice that have been injected icv with the Orx2 agonist [Ala15, D-Leu11]-OrxB. In Pv-positive neurons E) only in Stay mice treated with Orx2 agonist [Ala15, D-Leu11]-OrxB was the EGR1 labeling significantly increased (dark gray right-hatched bar) compared to cage control (clear bar), vehicle (dark gray bar) and Orx2 antagonist (MK-1064)treated mice (dark gray left-hatched bar). F) In Pv-negative neurons, a much smaller, but similar increase in EGR1 labeling occurred. G) Labeling of Pv was similar in all treatment groups.
4. Discussion
The primary thrust of experiments on the orexinergic system relative to affective disorders, has been to describe anxiogenic and pro-depressive actions of OrxA (Eacret et al., 2018; Ito et al., 2009; Lungwitz et al., 2012; Nollet et al., 2011), through actions of Orx1 receptors (Johnson et al., 2010; Lu et al., 2017; Ozsoy et al., 2017; Staples and Cornish, 2014), although dual Orx receptor antagonists have been shown to be antidepressive (Nollet et al., 2011; Nollet et al., 2012). There have also been hints that Orx1 and Orx2 receptors have opposing actions (Flores et al., 2016; Lu et al., 2017), including stress-reduced thalamic and hypothalamic Orx2 expression that was reversed by the antidepressant fluoxetine (Nollet et al., 2011). Our recent work suggests that activation of Orx2 receptors may be anxiolytic and antidepressive, at least in the amygdala or hippocampus, and may have opposing actions to those of Orx1 (Arendt et al., 2014; Arendt et al., 2013). Here we describe, for the first time, that intracerebroventricularly activated Orx2 receptors are anxiolytic and antidepressive. This icv Orx2 agonist injection also produced activation, measured by labeling of the immediate-early gene EGR1, of a small cluster of GABAergic cells, co-expressing the calcium binding protein Pv, in the BLA or ItC, suggesting that these inhibitory cells are at least partially responsible for the anxiolysis (Fig. 9A-E). A small group of non-Pv containing neurons may also play a role (Fig. 9F). Activation of these inhibitory neurons coincident with reduced anxious behavior is supported by experiments using Orx2 KD in the BLA (Arendt et al., 2014). This conclusion is confirmed additionally by the contrast of anxiogenic responses plus increased plasma corticosterone triggered by Orx2 antagonist treatment. This conclusion is not based on a singular behavioral result, but includes several preclinical measures in a social stress protocol (SAM), addressing recent concerns that anxiety measures that do not include social dynamics also do not translate well to human clinical treatments (Haller and Alicki, 2012; Haller et al., 2013). Our work suggests that promoting Orx2 receptor activity (via the Orx2 agonist [Ala11, D-Leu15]-OrxB ) decreases anxious and/or depressive behaviors such as fear conditioned-, as well as conflict-freezing and startle responses (Figs. 3C, 4B, 5B). Stimulation of Orx2 receptors also increases such adaptive, anxiolytic, and antidepressive behaviors as the amount of time the test mouse spends attentive to the escape hole in the SAM arena and to a novel social target during the SIP test (resilience/susceptibility to stress). Together, these results are suggestive of an anxiolytic profile which stems from whole brain pro-Orx2 treatments.
The proportion of Escape to Stay mice in these experiments was particularly low (28:72) compared with the more typical (50:50) self-imposed division of phenotype from previous studies (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). Among researchers using various social defeat models it is anecdotally noted that cohorts of fish, mice or rats are not equivalently partitioned into stresssusceptible or resilient animals, which may explain our skewed distribution. However, we have recently noticed in fish and mice, that the proportions of the two phenotypes may be altered by stress effects from the captive environment (Summers et al., 2017; Yaeger et al., 2018). This recent work also suggests that these phenotypes are plastic, and may be affected by prior stress, exercise, or experience (Robertson et al., 2017; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018). Our evidence suggests that skewed distribution of Escape and Stay phenotypes devolve from prior non-experimental stressors in the captive environment. This allowed greater emphasis to be placed on anxiolytic and antidepressive treatment of Stay animals. It is important to note that the mutable status of these phenotypes is most common in the first two days of the SAM, but that a proportion of the mice in either phenotype is susceptible to reversal of phenotype, if the neuromodulatory elements of the stress circuitry are altered, through pharmacological treatments or environmental conditions, such as exercise or familiarity to reduce stress (Robertson et al., 2017; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018). The result is, for example in Stay mice, that a subset of that phenotype is disposed to amelioration (Yaeger et al., 2018), which produces some variability in the treatment results (Fig. 7B, D).
Freezing in response to conditioned stimuli (CS) or contexts associated with fearful environments represents adaptive behavior that reduces the exposure of the quiescent animal to predators and competitors (Demuth et al., 2017). This conditioned-fear response (Blanchard and Blanchard, 1988; Iwata and LeDoux, 1988; Iwata et al., 1986) is intensity dependent, such that anxious animals develop a higher freezing response rate to a CS as the severity of the fearful conditions increase (Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). Consistent with our previous findings (Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016), these results demonstrate that phenotype plays an important role in the development of classical conditioning, as Escape mice did not exhibit freezing in response to the CS, but Stay mice did. This test day result is suggestive of increased anxiety in Stay mice, which develops as a result of decision-making in the SAM arena (Smith et al., 2014). Therefore, escape is an adaptive behavior that results in reduced anxiety and stress reactivity (Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016). However, when Stay mice were injected with Orx2 agonist, freezing in response to the CS decreased to the level of vehicle-treated escape mice, suggesting that the treatment reduced anxiety. Fear conditioning has been shown to be regulated by activation of GABAergic neurons in the ItC and BLA (Busti et al., 2011; Lee et al., 2013; Tovote et al., 2016; Tovote et al., 2015), including by Orx stimulation (Flores et al., 2017; Flores et al., 2014), comparable to those stimulated (EGR1 labeling) in this study by Orx2 agonist treatment in Stay mice. The agonist of Orx2 receptors also limited the amount of conflict freezing during SAM interaction. Therefore, regardless of the cued or contextual nature of the fearful US (aggression), escape reduced this anxious behavior. Interestingly, after the acquisition of fear memory, in cue and context conditioning, OrxA impairs extinction of fear memories, which is reversed by an Orx1 receptor antagonist (Flores et al., 2014). These effects are manifest via the basolateral amygdala (Flores et al., 2017; Flores et al., 2014), the same brain region for which our results suggested anxiolytic actions of the Orx2 receptor (Arendt et al., 2014). Fear conditioning also has specific effects on gene expression, including opposing actions on Orx1 and Orx2 receptor mRNA expression in the hippocampus, which are coincident with cannabinoid Cb2 receptor expression changes there (Robertson et al., 2017). Anxiolytic and antinociceptive actions of phytocannabinoids are modulated via actions of Orx2 receptors (Flores et al., 2016), a result that corroborates the conclusions of the work presented here.
Fear potentiated startle is another reliable paradigm for analyzing anxious behavior (Davis, 1979, 1986; Davis et al., 2003). We measured startle, in addition to freezing, during SAM social interactions, in which startle occurs during approach from a CD1 aggressor. In the context of repeated aggressive social interactions, which produces intensely stressful responses (Robertson et al., 2015; Smith et al., 2016), startle reactions are significantly escalated in Stay phenotype mice over time as the aggressive interactions are reiterated (Fig. 5A). Escape provides an anxiolytic behavioral solution, which nearly eliminates conflict startle responses (Fig. 5A). Injection (icv) of an Orx2 agonist, significantly reduces the number of Stay animal startle responses (Fig. 5B), after which startle responding remains low on the fourth day of social interaction (Fig. 5B), potentially revealing a longerterm effect of the drug. In Stay mice, which don’t make use of the escape route, startle responses escalate over time and freezing increases by the second day and remained high for the duration of the experiment (Fig. 4A). Although, icv Orx2 antagonist (MK-1064) injection appeared to increase freezing to over 50% of the time in the SAM arena, startle was not similarly affected. It may be that more time freezing left less social interaction time for startle responses. As both startle and freezing anxious responses are ameliorated by Orx2 agonism, it suggests the possibility that proactive anxiety reducing behaviors are also influenced by Orx2 activation.
Since escape via the L-shaped tunnel at either end of the SAM arena is potently anxiolytic (Robertson et al., 2015; Smith et al., 2016), we hypothesized that time spent being attentive to the escape route would represent an anxiolytic response. Escape phenotype mice spend a significantly greater amount of time attentive to the hole (Fig. 6A). Consistent with our hypotheses, icv injection of Orx2 agonist significantly increased attentiveness to the escape tunnel on injection day (Fig. 6B). Furthermore, similar to startle responses, attentiveness to the hole was present and significantly greater the day after injection (day 4). These results suggest longer-term effects of the drug, or alternatively, Orx2-induced neuroplastic changes. These results, in combination with Orx2 agonism promoting escape, and Orx2 antagonism reducing escape behavior suggests that Orx2 activation encourages active, anxiolytic, stress-resilient behavior.
In order to validate these SAM-specific measurements of Orx2–induced anxiolysis we replicated the classic paradigm for indicating social stress susceptibility and resilience, the Social Interaction / Preference test (SIP) (Krishnan et al., 2007). First, we compared SIP results in vehicle-treated animals based on behavioral phenotype. The results indicated that Escape mice spent more time near the novel container occupied with a social target (CD1 mouse) than near the novel container alone (Fig. 7A, B). This result is typically interpreted as resilience to stress, anxiety and depression (Arendt et al., 2014; Berton et al., 2007; Golden et al., 2011; Krishnan et al., 2007). Escaping mice had a resilience / susceptibility ratio which is significantly greater than that of Stay phenotype mice. In mice with the Stay phenotype, more time was spent near the empty container than near the social target (Fig. 7A), suggesting that Stay mice are susceptible to stress, anxious and depressive behavior. Approach and avoidance behaviors are regulated by parallel reciprocal orexinergic circuits to limbic regions such as the bed nucleus of the stria terminalis (BNST), paraventricular nucleus (PVN) and amygdala (Giardino et al., 2018). Elevated plasma corticosterone from samples taken on the day of SIP and CR testing in Stay mice supports this interpretation. These results indicate that anxiety- and depression-related behaviors in the SAM paradigm are specifically associated with Escape and Stay phenotypes. That is, Escape is a stress-resilient phenotype, and Stay is associated with stress vulnerability. The effect of icv Orx2 antagonist MK-1064 is to reverse stress-resilience in Escape animals (Fig. 7A), consistent with our previous work showing that Orx2 gene knockdown in the BLA promoted the same anxiogenic response (Arendt et al., 2014). Here we also demonstrate the opposite effect for icv injection of the Orx2 agonist [Ala11, DLeu15]-OrxB, that is, enhanced resilience as indicated by more time spent with the container holding a social target by Stay phenotype mice (Fig. 7C).
5. Conclusions
Consistent with our previous work, the results reported here indicate that Orx2 receptor activity, in contrast with Orx1 receptor actions, are anxiolytic and anti-depressive (Arendt et al., 2014; Arendt et al., 2013). Results that devolve specifically from social interactions in the SAM arena, including Orx2 activation that reduced cued and conflict fear conditioned freezing, reduced startle responses, increased attentiveness to the escape route, and promoted stress resilience, as well as recruitment of a discrete cluster of ItC or BLA GABAergic Pv-containing inhibitory interneurons, well placed to promote anxiolysis. Corroborating evidence comes from Orx2 antagonism which reversed the Escape phenotype and increased plasma corticosterone in Stay mice, give evidence in multiple formats for this assertion. What is more, the SAM-specific results are supported and validated by the classic social defeat model measure of susceptibility and resilience (SIP test). As such, we suggest that the use of the SAM can be applied to evaluate social susceptibility and resilience in a shorter period of time with more behavioral valuations to verify the outcome. Finally, given that measurements from both SAM and SIP suggest that Orx2 receptors mediate anxiolytic and antidepressive actions, targeting these receptors may lead to new potential pharmacotherapies.
Highlights.
Stress-Alternatives Model (SAM) produces resilient (Escape) and susceptible (Stay) phenotypes
icv Orx2 agonist reduces anxious and depressive behaviors in Stay mice
icv Orx2 antagonist increases anxious and depressive behavior plus plasma corticosterone
Stay mice exhibit fear conditioning, reversed by an Orx2 agonist
icv Orx2 agonist increases immediate-early gene EGR1 in BLA parvalbumin GABA interneurons
Acknowledgements
We would like to thank Robert Garner for the construction of the SAM arena. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Numbers R15MH104485 (to CHS), and R01MH106640 (to SSN), a CBBRe Research Enhancement Pilot grant (to CHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Veterans Affairs or the United States Government.
Abbreviations:
- [Ala11, D-Leu15]–OrxB
a modified OrxB peptide used as an Orx2 receptor agonist
- AP
anterior-posterior
- [B]
corticosterone concentration
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- BSA
bovine serum albumen
- C57BL/6N
a strain of black mice used for stress testing
- Cb2
cannabinoid 2 receptors
- CD1
Hsd:ICR retired breeder mice used as aggressors
- CeA
central amygdala
- cm
centimeter
- cm3
centimeter cubed
- CR
conditioned response
- CRF1
corticotropin releasing factor 1 receptors
- CS
conditioned stimulus
- DAPI
4′,6-diamidino-2-phenylindole
- DMSO
dimethylsulfoxide
- DV
dorsal-ventral
- EC50
half-maximal effective concentration
- EGR1
early growth response protein 1
- Escape
mice that respond to social stress by leaving
- FFR
fear freezing ratio
- GABA or GABAergic
γ-aminobutyric acid
- Hcrt
orexin/hypocretin
- Hcrt1
orexin A/hypocretin 1
- Hcrt2
orexin B/hypocretin 2
- IC50
half-maximal inhibitory concentration
- icv
intracerebroventricular
- IHC
immunohistochemistry
- ItC
intercalated region of the amygdala
- LH-DMH/PeF
the perifornical area of the lateral, dorsomedial hypothalamus
- min
minutes
- MK-1064
5“-chloro-N-[(5,6-dimethoxy-2-pyridinyl)methyl][2,2’:5’,3“-terpyridine]-3’-carboxamide - an Orx2 antagonist
- ML
medial-lateral
- NIH
National Institutes of Health
- NIMH
National Institute of Mental Health
- NPS
neuropeptide S
- OF
open field test
- Orx
orexin/hypocretin
- OrxA
orexin A/hypocretin 1
- OrxB
orexin B/hypocretin 2
- Orx1
orexin 1 receptors
- Orx2
orexin 2 receptors
- PBS
phosphate buffered saline
- Pv
parvalbumin
- PVN
paraventricular nucleus of the hypothalamus
- PTSD
posttraumatic stress disorder
- s
seconds
- SAM
Stress-Alternatives Model
- SIP
social interaction / preference test
- Stay
socially defeated submissive mice
- US
unconditioned stimulus
- Ala11, D-Leu15]–OrxB
a modified OrxB peptide used as an Orx2 receptor agonist
- MK-1064
5“-chloro-N-[(5,6-dimethoxy-2pyridinyl)methyl]-[2,2’:5’,3“-terpyridine]-3’-carboxamide - an Orx2 antagonist
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T, 2015. Comprehensive Behavioral Analysis of Male Ox1r (−/−) Mice Showed Implication of Orexin Receptor1 in Mood, Anxiety, and Social Behavior. Frontiers in Behavioral Neuroscience 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K, 2004. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides 38, 311–315. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP, 2003. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther 305, 507–514. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, DiLeone RJ, Ronan PJ, Summers CH, 2014. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH, 2013. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci 127, 86–94. [DOI] [PubMed] [Google Scholar]
- Baier PC, Weinhold SL, Huth V, Gottwald B, Ferstl R, Hinze-Selch D, 2008. Olfactory dysfunction in patients with narcolepsy with cataplexy is restored by intranasal Orexin A (Hypocretin-1). Brain 131, 2734–2741. [DOI] [PubMed] [Google Scholar]
- Belzung C, Griebel G, 2001. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural brain research 125, 141–149. [DOI] [PubMed] [Google Scholar]
- Bergstrom CT, Meacham F, 2016. Depression and anxiety: maladaptive byproducts of adaptive mechanisms. Evol Med Public Health 2016, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, 2005. Hypocretins: waking, arousal, or action? Neuron 46, 696–698. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, Vittoz NM, 2010. Hypocretin/orexin in arousal and stress. Brain Res 1314, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Covington HE 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ, 2007. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron 55, 289–300. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, 1988. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol 39, 43–68. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ, 2013. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci Biobehav Rev 37, 1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1989a. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol 103, 70–82. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1989b. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry 13 Suppl, S3–14. [DOI] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T, 1998. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol 402, 460–474. [PubMed] [Google Scholar]
- Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F, 2011. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci 31, 5131–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, Summers CH, 2009. Learning strategies during fear conditioning. Neurobiol Learn Mem 91, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M, 1999. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK, 1999. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett 260, 161–164. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Kuchiiwa S, Gao HZ, Kuchiiwa T, Nakagawa S, 2003. Morphological study of orexin neurons in the hypothalamus of the Long-Evans rat, with special reference to co-expression of orexin and NADPH-diaphorase or nitric oxide synthase activities. Neurosci Res 46, 53–62. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE, 2001. Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21, RC168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, 1979. Diazepam and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology (Berl) 62, 1–7. [DOI] [PubMed] [Google Scholar]
- Davis M, 1986. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci 100, 814–824. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM, 2003. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci 985, 218–232. [DOI] [PubMed] [Google Scholar]
- Demuth BS, Ferrari MCO, Weber LP, Janz DM, Chivers DP, 2017. Exposure to a contextually neutral stressor potentiates fear conditioning in juvenile rainbow trout, Oncorhynchus mykiss. Horm Behav 94, 124–134. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH 2nd, , 2009. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci 98, 2501–2515. [DOI] [PubMed] [Google Scholar]
- Eacret D, Grafe LA, Dobkin J, Gotter AL, Rengerb JJ, Winrow CJ, Bhatnagar S, 2018. Orexin signaling during social defeat stress influences subsequent social interaction behaviour and recognition memory. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Eagle AL, Fitzpatrick CJ, Perrine SA, 2013. Single prolonged stress impairs social and object novelty recognition in rats. Behav Brain Res 256, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feise RJ, 2002. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Herry C, Maldonado R, Berrendero F, 2017. Facilitation of contextual fear extinction by Orexin-1 Receptor Antagonism Is associated with the Activation of Specific Amygdala Cell Subpopulations. Int J Neuropsychopharmacol 20, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Julia-Hernandez M, Maldonado R, Berrendero F, 2016. Involvement of the orexin/hypocretin system in the pharmacological effects induced by Delta9tetrahydrocannabinol. Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F, 2014. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology 39, 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L, 2014. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol 29, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, de Lecea L, 2018. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington III HE, Berton O, Russo SJ, 2011. A standardized protocol for repeated social defeat stress in mice. Nature protocols 6, 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Forman MS, Harrell CM, Stevens J, Svetnik V, Yee KL, Li X, Roecker AJ, Fox SV, Tannenbaum PL, Garson SL, Lepeleire ID, Calder N, Rosen L Struyk A, Coleman PJ, Herring WJ, Renger JJ, Winrow CJ, 2016. Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man. Sci Rep 6, 27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, 2017. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience 348, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson M, de Lecea L, Sutcliffe JG, Yanagisawa M, Meister B, 1999. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 11, 653–663. [DOI] [PubMed] [Google Scholar]
- Haller J, Alicki M, 2012. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr Opin Psychiatry 25, 59–64. [DOI] [PubMed] [Google Scholar]
- Haller J, Aliczki M, Gyimesine Pelczer K, 2013. Classical and novel approaches to the preclinical testing of anxiolytics: A critical evaluation. Neurosci Biobehav Rev 37, 2318–2330. [DOI] [PubMed] [Google Scholar]
- Heesink L, Kleber R, Hafner M, van Bedaf L, Eekhout I, Geuze E, 2017. Anger and aggression problems in veterans are associated with an increased acoustic startle reflex. Biol Psychol 123, 119–125. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M, 2010. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol 61, 81–109, C101–111. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Nagai T, Oikawa T, Yamada H, Hanawa T, 2009. A possible mechanism underlying an antidepressive-like effect of Kososan, a Kampo medicine, via the hypothalamic orexinergic system in the stress-induced depression-like model mice. Biol Pharm Bull 32, 1716–1722. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, 1988. Dissociation of associative and nonassociative concomitants of classical fear conditioning in the freely behaving rat. Behav Neurosci 102, 66–76. [DOI] [PubMed] [Google Scholar]
- Iwata J, LeDoux JE, Reis DJ, 1986. Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res 368, 161–166. [DOI] [PubMed] [Google Scholar]
- Jacinto LR, Reis JS, Dias NS, Cerqueira JJ, Correia JH, Sousa N, 2013. Stress affects theta activity in limbic networks and impairs novelty-induced exploration and familiarization. Frontiers in Behavioral Neuroscience 7, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Dayas CV, 2017. a. Role of the Orexin/Hypocretin System in Stress-Related Psychiatric Disorders. Curr Top Behav Neurosci 33, 197–219. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017b. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci 33, 247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennions MD, Moller AP, 2003. A survey of the statistical power of research in behavioral ecology and animal behavior. Behavioral Ecology 14, 438–445. [Google Scholar]
- Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, Bonaventure P, Shekhar A, 2015. Orexin 1 and 2 Receptor Involvement in Co2 Induced Panic-Associated Behavior and Autonomic Responses. Depress Anxiety 32, 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat Med 16, 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, 1983. From metapsychology to molecular biology: Explorations into the nature of anxiety. American Journal of Psychiatry 140, 1277–1293. [DOI] [PubMed] [Google Scholar]
- Keifer J, Summers CH, 2016. Putting the “Biology” Back into “Neurobiology”: The Strength of Diversity in Animal Model Systems for Neuroscience Research. Front Syst Neurosci 10, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Ruscio AM, Shear K, Wittchen HU, 2010. Epidemiology of anxiety disorders. Curr Top Behav Neurosci 2, 21–35. [PubMed] [Google Scholar]
- Khalil R, Fendt M, 2017. Increased anxiety but normal fear and safety learning in orexindeficient mice. Behav Brain Res 320, 210–218. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A, 1997. Social stress in rats and mice. Acta Physiol Scand Suppl 640, 69–72. [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH, 2013. Inhibitory networks of the amygdala for emotional memory. Front Neural Circuits 7, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao J, Balesar R, Fronczek R, Zhu QB, Wu XY, Hu SH, Bao AM, Swaab DF, 2017. Sexually Dimorphic Changes of Hypocretin (Orexin) in Depression. EBioMedicine 18, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA, 2012. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav 107, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Modi HR, Wang Q, Gd S, Sherman D, Greenwald E, Savonenko AV, Geocadin RG, Thakor NV, 2017. Intranasal post-cardiac arrest treatment with orexin-A facilitates arousal from coma and ameliorates neuroinflammation. PLoS One 12, e0182707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MD, 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100, 403–405. [Google Scholar]
- Nakagawa S, 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behavioral Ecology 15, 1044–1045. [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K, 1999. Distribution of orexin neurons in the adult rat brain. Brain Res 827, 243–260. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, 2010. Animal models of neuropsychiatric disorders. Nat Neurosci 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S, 2011. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S, 2012. Neurogenesisindependent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology 37, 2210–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy S, Olguner Eker O, Abdulrezzak U, Esel E, 2017. Relationship between orexin A and childhood maltreatment in female patients with depression and anxiety. Soc Neurosci 12, 330–336. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, 2016. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv 13, 963–975. [DOI] [PubMed] [Google Scholar]
- Perneger TV, 1998. What’s wrong with Bonferroni adjustments. BMJ 316, 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA, 2004. Corticotropinreleasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci 24, 6545–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Achua JK, Smith JP, Prince MA, Staton CD, Ronan PJ, Summers TR, Summers CH, 2017. Anxious behavior induces elevated hippocampal Cb2 receptor gene expression. Neuroscience 352, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Blanchard DC, Summers CH, 2015. Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety. Physiol Behav 146, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Ishii Y, Halford JC, Blundell JE, 2002. Orexins and appetite regulation. Neuropeptides 36, 303–325. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
- Sakurai T, 2005. Reverse pharmacology of orexin: from an orphan GPCR to integrative physiology. Regul Pept 126, 3–10. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, 1998. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M, 1998. Adrenergic alpha2C-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci 18, 3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM, 2010. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiol (Oxf) 198, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J, 2005. Hypothalamic regulation of sleep and circadian rhythms. Nature 437, 1257–1263. [DOI] [PubMed] [Google Scholar]
- Sathyanesan M, Haiar JM, Watt MJ, Newton SS, 2017. Restraint stress differentially regulates inflammation and glutamate receptor gene expression in the hippocampus of C57BL/6 and BALB/c mice. Stress 20, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell TE, Saper CB, 2005. Orexin, drugs and motivated behaviors. Nat Neurosci 8, 1286–1288. [DOI] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH, 2014. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Frontiers in Behavioral Neuroscience 8: 121, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH, 2016. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology 63, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinhoven P, van Balkom AJ, Nolen WA, 2011. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry 72, 341–348. [DOI] [PubMed] [Google Scholar]
- Staples LG, Cornish JL, 2014. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of Trial 1 and Trial 2 effects. Horm Behav 65, 294–300. [DOI] [PubMed] [Google Scholar]
- Summers TR, Summers TL, Carpenter RE, Smith JP, Young SL, Meyerink B, Orr TZ, Arendt DH, Summers CH, 2017. Learning and CRF-Induced Indecision during Escape and Submission in Rainbow Trout during Socially Aggressive Interactions in the Stress-Alternatives Model. Front Neurosci 11, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T, 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res 1044, 116–121. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Yanagisawa M, Saper CB, 2003. Colocalization of orexin a and glutamate immunoreactivity in axon terminals in the tuberomammillary nucleus in rats. Neuroscience 119, 1033–1044. [DOI] [PubMed] [Google Scholar]
- Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Luthi A, 2016. Midbrain circuits for defensive behaviour. Nature 534, 206–212. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A, 2015. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience 16, 317–331. [DOI] [PubMed] [Google Scholar]
- Trimmer PC, Higginson AD, Fawcett TW, McNamara JM, Houston AI, 2015. Adaptive learning can result in a failure to profit from good conditions: implications for understanding depression. Evol Med Public Health 2015, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438, 71–75. [DOI] [PubMed] [Google Scholar]
- Van de Bittner GC, Van de Bittner KC, Wey HY, Rowe W, Dharanipragada R, Ying X, Hurst W, Giovanni A, Alving K, Gupta A, Hoekman J, Hooker JM, 2018. Positron Emission Tomography Assessment of the Intranasal Delivery Route for Orexin A. ACS Chem Neurosci 9, 358–368. [DOI] [PubMed] [Google Scholar]
- Vanderhaven MW, Cornish JL, Staples LG, 2015. The orexin-1 receptor antagonist SB334867 decreases anxiety-like behavior and c-Fos expression in the hypothalamus of rats exposed to cat odor. Behav Brain Res 278, 563–568. [DOI] [PubMed] [Google Scholar]
- Wang H, Li S, Kirouac GJ, 2017. Role of the orexin (hypocretin) system in contextual fear conditioning in rats. Behav Brain Res 316, 47–53. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L, 2005. Stress and arousal: the corticotrophinreleasing factor/hypocretin circuitry. Mol Neurobiol 32, 285–294. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L, 2004. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci 24, 11439–11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeger JDW, Staton CD, Krupp KT, Summers TR, Summers CH, 2018. Anxious phenotypes: prior experience and orexin 2 (Orx2) receptor activation lead to social stress resilience Society for Neuroscience Abstracts 44. [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE, 2006. Afferents to the orexin neurons of the rat brain. J Comp Neurol 494, 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB, 2001. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry 58, 55–61. [DOI] [PubMed] [Google Scholar]
- Zhang S, Blache D, Vercoe PE, Adam CL, Blackberry MA, Findlay PA, Eidne KA, Martin GB, 2005. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept 124, 81–87. [DOI] [PubMed] [Google Scholar]
- Zlotnick C, Warshaw M, Shea MT, Allsworth J, Pearlstein T, Keller MB, 1999. Chronicity in posttraumatic stress disorder (PTSD) and predictors of course of comorbid PTSD in patients with anxiety disorders. Journal of traumatic stress 12, 89–100. [DOI] [PubMed] [Google Scholar]