Abstract
Problem:
The contribution of fibroblasts to innate immune protection of the human female reproductive tract (FRT) against viral pathogens is relatively unknown.
Method of Study:
Endometrial (EM), endocervical (Cx), and ectocervical (ECx) fibroblasts were isolated from hysterectomy patients and grown in vitro. Fibroblasts were treated with the viral mimic poly (I:C) in the presence or absence of the sex hormone estradiol (E2), with gene expression measured by real-time RT-PCR and protein secretion by ELISA.
Results:
Poly (I:C) induced the expression of the interferon-stimulated genes (ISG) MxA, OAS2 and APOBEC3G, and the cytokines MCP-1, IL-8, IL-6, CCL20, IFNβ and RANTES by fibroblasts from all three sites. ISG upregulation was dependent upon Type I IFN signaling. E2 inhibited the poly (I:C)-induced upregulation of MxA and OAS2 in EM fibroblasts, but not Cx or ECx fibroblasts. E2 upregulated SDF-1α by EM fibroblasts but had no effect on secretion of other cytokines either alone or in the presence of poly (I:C). Conditioned media (CM) from poly (I:C)-treated or E2-treated fibroblasts significantly reduced HIV infection of CD4+ T cells.
Conclusions:
Stromal fibroblasts represent a level of innate immune protection against viral pathogens in the FRT beyond that seen with epithelial cells and immune cells. Our findings indicate that fibroblasts FRT are selectively responsive to E2, capable of initiating an antiviral response against viral pathogens, and may play a role in preventing HIV infection of CD4+ T cells.
Introduction:
The immune system in the female reproductive tract (FRT) is adapted to protect against incoming pathogens, while accommodating the needs of reproduction 1. Stromal fibroblasts are the predominant cell population in the sub-epithelial layers of the FRT mucosa where they regulate tissue architecture, epithelial proliferation and barrier permeability 2–5. In premenopausal women with normal menstrual cycles, the endometrial (EM) epithelium is cyclically shed at menstruation, exposing the underlying fibroblasts to the external environment, and incoming pathogens. Furthermore, sexually transmitted viral pathogens such as HIV can cross the epithelial barrier to infect immune cells that reside in an environment dominated by fibroblasts 6. Thus, it is important to define the role of fibroblasts in host defense which is poorly understood. Currently, they are not considered to be active participants in the innate immune response to pathogens in the FRT. Defining their role in immune protection will be essential in furthering women’s reproductive health.
Pattern recognition receptors (PRR) are essential for the recognition of conserved pathogen-associated molecular patterns (PAMPs) expressed by incoming pathogens. Multiple PRRs are present in the FRT, including Toll-like receptors (TLR) and retinoic acid inducible gene (RIG)-like receptors, whose expression varies with menstrual cycle stage, hormone treatment and anatomical location in the FRT 7,8. EM fibroblasts respond to the TLR 4 ligand lipopolysaccharide (LPS), by increasing secretion of interleukin (IL) 8, a neutrophil chemoattractant, as well as regulated on activation, normal T cell expressed and secreted (RANTES), a lymphocyte and monocyte chemoattractant 9,10. Our own group has demonstrated that poly (I:C), a synthetic dsRNA viral ligand recognized by multiple PRRs (TLR3, RIG-I and MDA5) 11,12, induces the secretion of IL-27 13 and hepatocyte growth factor (HGF) 14 by EM fibroblasts.
The cyclic changes of the sex hormones estradiol (E2) and progesterone (P) during the menstrual cycle in premenopausal women regulate the multiple components of the FRT immune system 1,15. E2, acting via estrogen receptor (ER) α and β, is a key modulation of immune function in the FRT 1,15. Previous studies have shown that cytokine and chemokine expression by EM fibroblasts are under hormonal control as demonstrated by E2-mediated inhibition of monocyte chemotactic protein 1 (MCP-1) expression, and stimulation of HGF, stromal-derived factor 1α (SDF-1α), IL-13 and IL-15 expression 14,16–18. Further, our studies have shown that E2 selectively regulates the response to TLR agonists in different cell types in the FRT 19–22 including EM fibroblasts 13. Despite these observations, little is known about how E2 regulates the antiviral response of stromal fibroblasts throughout the FRT.
In this study, we demonstrate that stromal fibroblasts from different sites in the FRT mount an immune response to the viral ligand poly (I:C) characterized by the increased expression of cytokines and antiviral genes. Furthermore, E2 regulates specific aspects of this immune response specifically in the EM, but not Cx or ECx. Secretions from stromal fibroblasts inhibited infection of CD4+ T cells by both CCR5- and CXCR4-tropic strains of HIV. These findings indicate that stromal fibroblasts perform an important role in recognizing viral pathogens and determining the outcome of HIV transmission in the FRT.
Materials and Methods:
Source of Tissue:
Human endometrial (EM), endocervical (Cx) and ectocervical (ECx) tissues were obtained immediately following surgery from women (n=20) undergoing hysterectomies at Dartmouth-Hitchcock Medical Center (DHMC) (Lebanon, NH). The average age of the women was 55.2 years with a range of 32–81 years. None of the women were on supplemental hormone treatment. Reasons for surgery included prolapse, fibroids, endometriosis, pelvic pain and menorrhagia. Tissues obtained were distal to the sites of pathology and were determined to be disease-free upon inspection by DHMC Pathology. The Committee for the Protection of Human Subjects (CPHS), DHMC, provided approval for all investigations involving human subjects. All our studies were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from and with written informed consent obtained from the patients before surgery. All studies comparing the EM, Cx, and ECx used matched tissues obtained from the same donors to reduce the effects of inter-donor variability.
Isolation of Stromal Fibroblasts:
Stromal fibroblasts were isolated as previously described 23. Briefly, FRT tissues were minced into 1–2 mm fragments under sterile conditions and digested using an enzyme mixture containing final concentrations of 3.4 mg/ml pancreatin (Invitrogen Life Technologies, Carlsbad, CA), 0.1 mg/ml hyaluronidase (Worthington Biochemical, Lakewood, NJ), 1.6 mg/ml collagenase (Worthington Biochemical), and 2 mg/ml D-glucose, in 1× HBSS (Invitrogen). After enzymatic digestion for 1hr at 37°C, cells were dispersed through a 250-μm mesh screen (Small Parts, Miami Lakes, FL), washed, and resuspended in HBSS. Epithelial cell sheets were separated from stromal fibroblasts by filtration through a 20-μm nylon mesh filter (Small Parts). Epithelial sheets were retained on the 20-μm filter, while the stromal fibroblasts and immune cells passed through. The flow-through containing stromal fibroblasts and immune cells was centrifuged at 500xg for 10 min, resuspended, cell number and viability determined.
Cell Culture:
Freshly isolated stromal fibroblasts and immune cells were incubated in complete medium in a T75 cell culture flask (ThermoFisher, Logan, UT) as previously described 24. Briefly, media was replaced every 48hr and cells passaged weekly. Over 1–3 weeks the fibroblasts increase in number and out-compete immune cells. The resulting population of cells is CD45-, EpCam-, CD90+ and vimentin+. CD90 is a marker for fibroblasts arising from the stroma functionalis 25. Expression of CD45, EpCam, CD90 and vimentin were determined by flow cytometry. After reaching confluence cells were trypsinized and plated at a concentration of 1×105 cells/well in 24-well culture plates (ThermoFisher) for at least 48hr prior to treatment. Complete medium was supplemented with 20 mM HEPES (Invitrogen), 2 mM L-glutamine (Invitrogen), 50 mg/ml primocin (Invivogen) and 10% heat-inactivated defined Fetal Bovine Serum (FBS) (ThermoFisher).
Poly (I:C), IFNβ, αIFNAR2, and αTLR3 treatment:
Stromal fibroblasts were incubated with 0.25 – 25 μg/ml of poly (I:C) (Invivogen) for up to 24hr. Recombinant human IFNβ (PBL Assay Science, Piscataway, NJ) was used to stimulate fibroblasts at 1, 10 & 100 U/ml for 24hr. Interferon receptor blockade experiments were conducted using a mouse monoclonal anti-human interferon receptor 2 (IFNAR2) blocking antibody (R&D Systems, Minneapolis, MN) or an IgG2a isotype control at a final concentration of 10μg/ml for 1hr and then stimulated with poly (I:C) for 24hr. TLR3 blockade experiments were conducted with an anti-TLR3 mAb (clone TLR3.7) (Santa Cruz Biotechnology) or an IgG1 isotype control (R&D Systems) at a final concentration of 20 μg/ml for 1hr and followed by stimulation with poly(I:C) for 24hr.
Estradiol and Raloxifene Treatment:
17β-estradiol (E2) (Calbiochem, Gibbstown, NJ) or the selective estrogen receptor modulator Raloxifene hydrochloride (Rx) (Tocris Biosciences, Bristol, UK) 26 were dissolved in 100% ethanol for an initial concentration of 1×10−3 M, evaporated to dryness in a glass scintillation vial and resuspended to a concentration of 1×10−5 M in complete media containing charcoal dextran-stripped FBS as previously described 20. Further dilutions were made to achieve final working concentrations of E2 ranging from 5×10−8 M to 5×10−10 M. These values are representative of E2 concentrations detected in the human FRT at specific stages of the menstrual cycle and are distinct from those values reported in the peripheral circulation 27–32. As a control, an equivalent amount of ethanol without dissolved hormone was initially evaporated. For E2 inhibition experiments, as described previously 33, Rx was added at 100-fold excess (5×10−6M) 1hr prior to addition of E2 and maintained in cell culture till the conclusion of the experiment.
Measurement of Cytokines and Antimicrobials:
Cell culture conditioned media was analyzed by ELISA for the presence of RANTES, IL-6, TNFα, IL-8, CCL20, MCP-1, SDF-1α (all R&D Systems) and IFNβ (PBL Assay Science) according to the manufacturers recommendations.
Real-time RT-PCR:
RNA was isolated and purified using a Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s recommendations (Invitrogen) with on-column DNase digestion using the RNase-Free DNase set (Qiagen). 400ng of total RNA was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s recommendations. Gene expression was measured using the 5′ fluorogenic nuclease assay in real-time quantitative PCR using TaqMan chemistry on the ABI 7300 Prism real-time PCR instrument (Applied Biosystems, Carlsbad, CA). Gene primer/MGB probe sets were obtained from Applied Biosystems assays-on-demand. PCR was conducted using the following cycle parameters: 95°C, 12 min for 1 cycle (95°C, 20 s; 60°C, 1 min), for 40 cycles. Gene expression was normalized to β-actin which we have used in previous studies of stromal fibroblasts 13,14,24,34 and whose expression was unaffected by any of the treatments performed in this study. Analysis was conducted using the sequence detection software supplied with the ABI 7300. Relative expression levels were expressed as a fold-increase in mRNA expression and calculated using the formula 2−ΔΔCt.
Measurement of Anti-HIV activity:
HIV-1 strains IIIB (CXCR4-tropic) and BaL (CCR5-tropic) were obtained from Dr. Phalguni Gupta (Univ. of Pittsburgh, PA). CD4+ T cells were isolated by negative bead selection (Miltenyi Biotec) as per the manufacturer’s instructions from the blood of a female donor. Following isolation, CD4+ T cells were activated by PHA and IL-2 for 48hr in X-Vivo-15 media containing phenol red (Lonza) supplemented with 10% charcoal-dextran stripped human serum. Prior to HIV infection, activated CD4+ T cells were transferred to round-bottom ultra-low attachment 96 well plates (Corning, NY, USA). To measure antiviral activity in fibroblast secretions, 24hr conditioned media from EM fibroblast cultures or control media were incubated for 1hr with HIV (MOI=0.1) at 37°C in a final volume of 100μl. Following incubation, the combined virus and conditioned media were added to CD4+ T cells for a further 1hr. Subsequently, CD4+ T cells were extensively washed to remove any residual virus and conditioned media, and the cells maintained in vitro for 6 days. Media was changed every 2–3 days. Infection was determined by measuring viral p24 secretion into the cell culture media by ELISA (Advanced Bioscience Laboratories, Rockville, MD). Controls for the anti-HIV assay included HIV alone, E2 alone, CM alone, HIV plus E2, HIV plus poly (I:C), and a combination of E2/poly (I:C) plus HIV. To generate conditioned media, EM fibroblasts were first pretreated for 48hr (control or E2: 5×10−8M), followed by washout, retreated under their original conditions (control or E2) in the presence or absence of poly (I:C) (25μg/ml) for a further 12hr, followed by extensive washout and incubation with fresh media for a subsequent 24hr after which conditioned media was recovered.
Statistical Analysis:
All data analysis was performed using Graphpad Prism Version 5.0 (Graphpad Software, San Diego, CA). Statistical tests are described in the figure legends. A P-value less than 0.05 was considered significantly different.
Results:
Characterization of in vitro FRT Stromal Fibroblast Cell Cultures
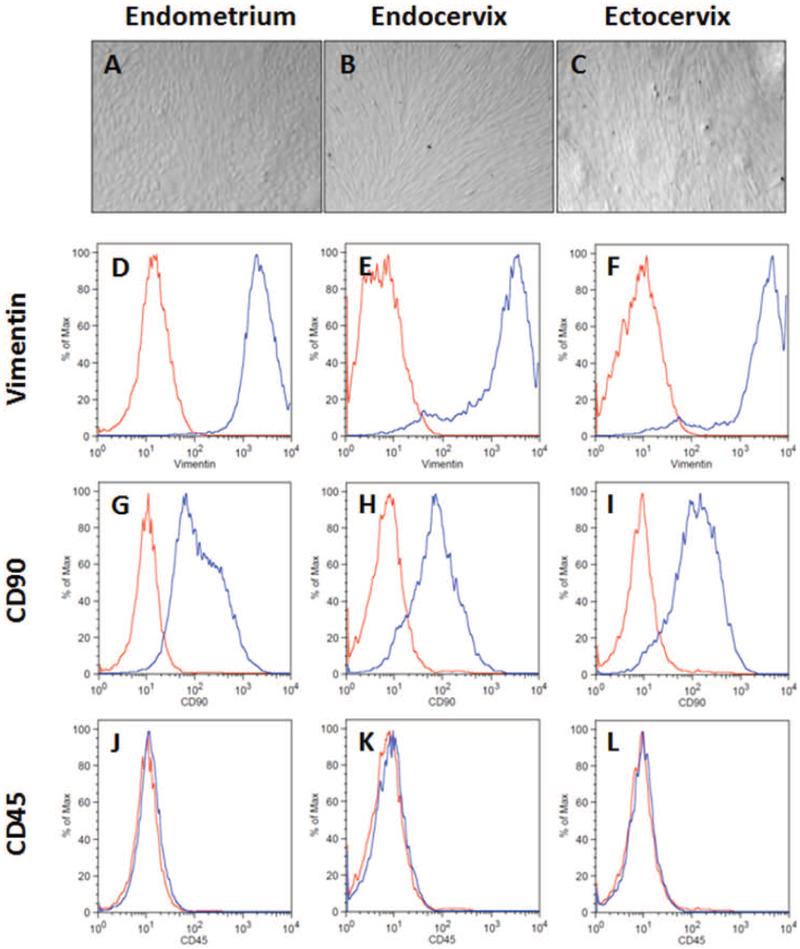
Primary human EM, Cx and ECx stromal fibroblasts were isolated and recovered from enzymatically digested tissues and grown in vitro. Confluent monolayers from the three sites were comparatively similar and composed of thin, long and spindle-shaped cells (Figure 1A-C). To assess their purity, fibroblasts in culture were analyzed by flow cytometry for the expression of the fibroblast markers vimentin (Figure 1D-F) and CD90 (Figure 1G-I), and the hematopoietic cell marker CD45 (Figure 1K-M). EM, Cx and ECx fibroblast cultures were all greater than 95% positive for vimentin. The majority of cells (>80%) at all three sites stained positive for CD90 indicating that they originate from stroma functionalis rather than the stroma basalis 25. Cell cultures were negative for the pan-leucocyte marker CD45 demonstrating a lack of immune cell contamination in our fibroblast preparations.
Figure 1: FRT stromal fibroblast morphology and marker expression.
Matched endometrial, endocervical, and ectocervical stromal fibroblasts were isolated from tissue fragments and cultured in vitro prior to analysis. (A-C) Representative images of unstained stromal fibroblasts imaged under a light microscope. Flow cytometry analysis of intracellular vimentin (D-F), surface CD90 (G-I) and surface CD45 (J-L) expression in matched endometrium, endocervix, and ectocervix from a representative individual donor. (Red – Isotype control; blue – antibody). All panels are derived from the same patient and are representative of 8 individual donors.
Human FRT Fibroblasts Express Pattern Recognition Receptors
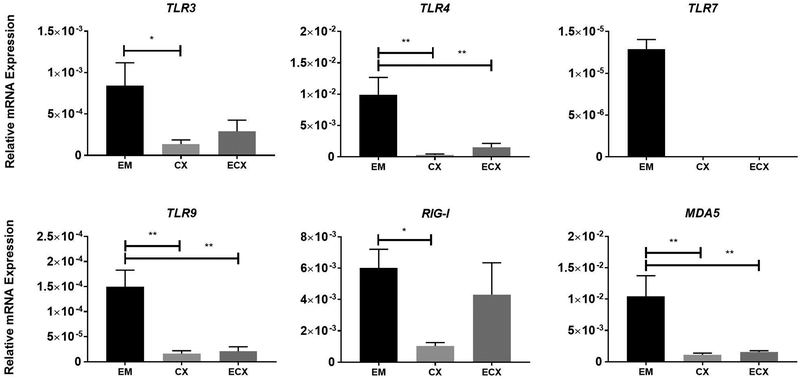
PRRs are essential for recognition of pathogens and initiation of immune responses. We therefore evaluated the relative expression by RT-PCR of a panel of PRRs in fibroblasts isolated from the EM, Cx and ECx. As seen in Figure 2, TLR3, TLR4, TLR9, RIG-I and MDA5 were detectable in fibroblasts from all three sites. In contrast, TLR7 was undetectable in Cx and ECx fibroblasts. Of the remaining TLRs, TLR4 was the highest expressed and TLR9 the lowest expressed in fibroblasts from all tissue sites. The cytoplasmic viral receptors RIG-I and MDA5 were generally expressed at higher levels than all the other TLRs with the exception of TLR4 in the EM. EM fibroblasts had the highest expression of the PRRs with significantly higher expression of TLR3, TLR4, TLR9, RIG-I and MDA5 compared to the Cx, and significantly higher expression of TLR3, TLR4, TLR9 and MDA5 compared to the ECx.
Figure 2: Constitutive pattern recognition receptors (PRRs) expression in FRT stromal fibroblasts.
mRNA from EM, Cx, and ECx stromal fibroblasts was analyzed for expression of PRRs by real-time RT-PCR (n=8). Bars and horizontal lines represent the mean relative mRNA expression +/− SEM respectively, normalized to the endogenous control β-Actin using matched EM, Cx and ECx from 8 individual donors. ND=Not detectable. * P<0.05; ** P<0.01 are significantly different when compared to the EM. Statistical analysis was performed using the non-parametric Friedmann with Dunn’s post-test correction for multiple comparison.
Poly (I:C) Induces Interferon-Stimulated Gene Expression (ISG) via Type I IFN Signaling in Stromal Fibroblasts
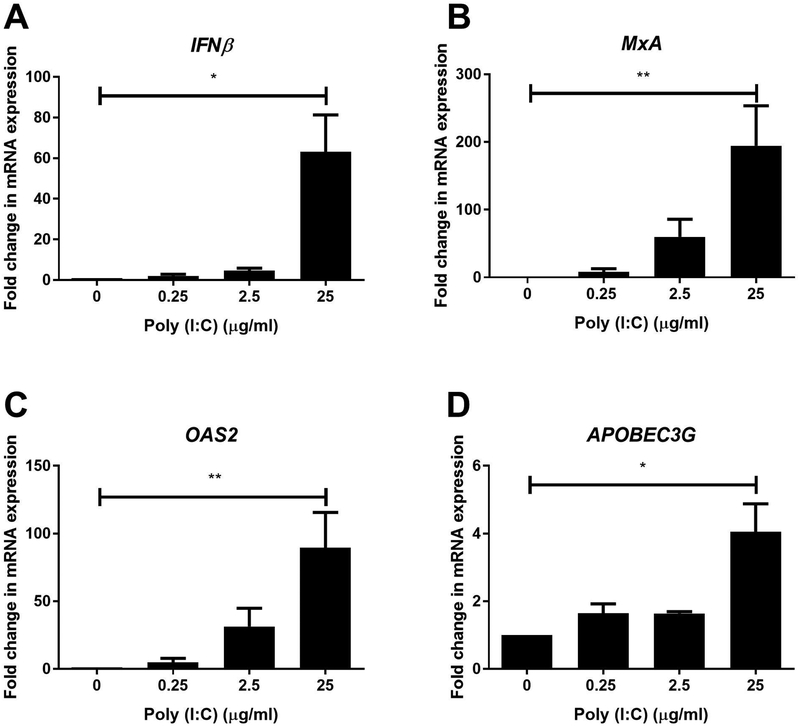
To determine whether EM fibroblasts are responsive to viral pathogens, we incubated cells with poly (I:C), a dsRNA viral analogue, and found a dose-dependent increase in mRNA expression of IFNβ, and the ISGs MxA, OAS2 and APOBEC3G with maximal upregulation in the presence of 25μg/ml of poly (I:C) (Figure 3).
Figure 3: Poly (I:C) dose-dependently induces IFNβ and antiviral gene expression in endometrial stromal fibroblasts.
mRNA from EM, Cx, and ECx stromal fibroblasts was analyzed for expression of IFNβ (A), MxA (B), OAS2 (C) and APOBEC3G (D) (n=3–4) after stimulation with 0.25, 2.5 and 25μg/ml of poly (I:C) for 24hr. Cellular mRNA was isolated and analyzed by real-time RT-PCR. Bars and horizontal lines represent the mean fold-change in mRNA expression +/− SEM respectively, from independent experiments with 3–4 individual donors with values normalized against the endogenous control β-Actin. Untreated (0) values are set to 1. * P<0.05; ** P<0.01 are significantly different compared to untreated samples. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
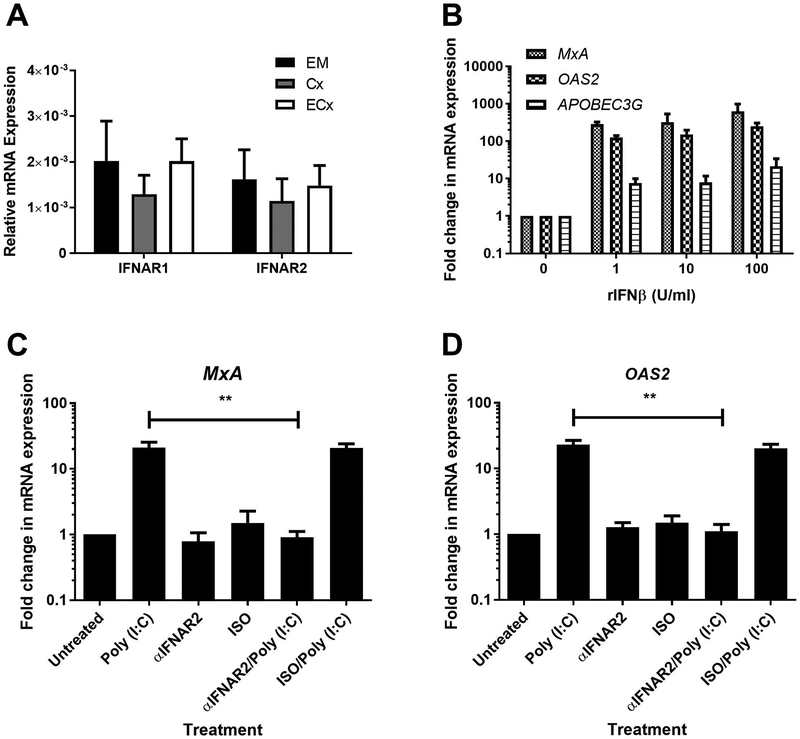
Recognizing that ISG induction was likely due to Type I IFNs secreted in response to poly (I:C), we investigated the presence of Type I IFN receptors IFNAR1 and IFNAR2 that are essential for cells to respond to IFNβ. As seen in Figure 4A, IFNAR1 and IFNAR2 mRNA expression was relatively consistent between EM, Cx and ECx fibroblasts. EM fibroblasts upregulated MxA and OAS2 mRNA expression to greater than 100-fold at concentrations ranging from 1U/ml to 100U/ml of IFNβ (Figure 4B). In contrast, APOBEC3G upregulation ranged from 10–30-fold (Figure 4B). To demonstrate that Type I IFNs were responsible for the upregulation of MxA and OAS2 after poly (I:C) exposure, we pretreated EM stromal fibroblasts with a blocking antibody against IFNAR2 prior to stimulation with poly (I:C) at 25 μg/ml for 24hr. The upregulation of both MxA and OAS2 due to poly (I:C) in EM fibroblasts was abrogated in the presence of blocking antibody (Figure 4C-D).
Figure 4: IFNβ induces antiviral gene expression.
mRNA from matched EM, Cx, and ECx stromal fibroblasts was analyzed for expression of IFNAR1 and IFNAR2 (A) by real-time RT-PCR (n=8). Bars and horizontal lines represent the mean relative mRNA expression +/− SEM respectively, normalized to the endogenous control β-Actin from 8 individual donors. Recombinant human IFNβ (rIFNβ) was used to stimulate cultured EM stromal fibroblasts for 24hr after which MxA, OAS2 and APOBEC3G expression was analyzed by real-time RT-PCR (B) (n=5). EM stromal fibroblasts were pretreated with an anti-IFNAR2 blocking antibody (αIFNAR2) (10μg/ml) or isotype control (ISO) for 1 hr followed by stimulation with poly (I:C) (25μg/ml) for 24 hours prior to mRNA expression analysis for MxA (C) and OAS2 (D) (n=5). Data shown in B-D is the mean fold-change in mRNA expression +/− SEM for independent experiments from 5 individual donors with values normalized against the endogenous control β-Actin. Bars and horizontal lines in all panels represent the mean +/− the SEM respectively. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
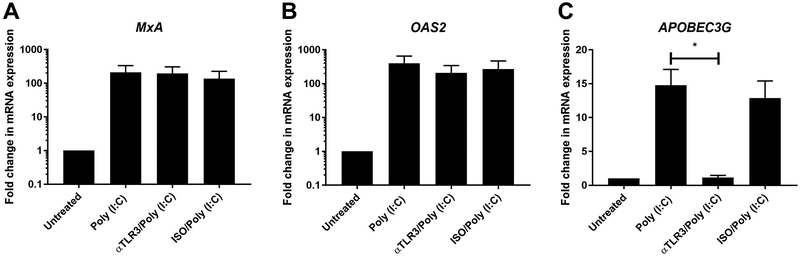
Poly (I:C) Induces APOBEC3G Expression via TLR3
Since poly (I:C) can potentially signal via TLR3, RIG-I or MDA5 12,35,36, we blocked TLR3 signaling using a blocking antibody that we have previously used 22. While upregulation of APOBEC3G was prevented in the presence of blocking antibody (Figure 5C), there was no effect on the induction of MxA and OAS2 in EM fibroblasts (Figure 5A-B). This suggests that ISG expression in response to poly (I:C) is selectively mediated by specific PRRs.
Figure 5: TLR3 blockade inhibits APOBEC3G expression.
EM stromal fibroblasts were pretreated with an anti-TLR3 blocking antibody (αTLR3) (20μg/ml) or isotype control (ISO) for 1 hr followed by stimulation with poly (I:C) (25μg/ml) for 24hr prior to mRNA expression analysis for MxA (A) and OAS2 (B) and APOBEC3G (C) (n=3). Bars and horizontal lines represent the mean fold-change in mRNA expression +/− SEM respectively, for independent experiments from 3 individual donors with values normalized against the endogenous control β-Actin. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
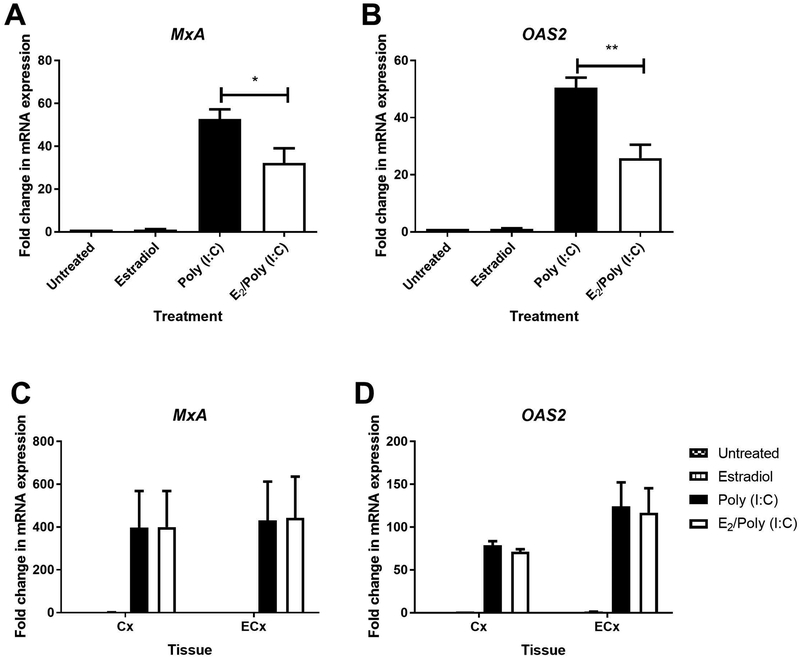
Estradiol Inhibits ISG Upregulation in EM but not Cx and ECx Fibroblasts
To assess the impact of E2 on poly (I:C)-mediated signaling, we pretreated EM, Cx and ECx fibroblasts with hormone for 48hr prior to washout, and retreatment with hormone in the presence or absence of poly (I:C) for an additional 24hr. As seen in Figures 6A-B, E2 significantly inhibited the poly (I:C)-induced upregulation of MxA and OAS2 in EM fibroblasts by approximately 40–50% but had no effect in Cx and ECx stromal fibroblasts (Figure 6C-D). To determine if this effect was via altered PRR expression, we measured TLR3, RIG-I, and MDA5 mRNA levels following 48hr of hormone treatment. However, E2 had no effect on PRR mRNA expression in EM fibroblasts (data not shown).
Figure 6: E2 inhibits poly (I:C)-induced MxA and OAS2 mRNA expression in endometrial stromal fibroblasts.
Effect of poly (I:C) (25μg/ml) and E2 (5×10−8M) on MxA and OAS2 mRNA expression in matched EM, Cx and ECx stromal fibroblasts. Cells were pretreated for 48hr with control media or media containing E2, washed out and retreated with E2 or control media for a subsequent 24hr in the presence or absence of poly (I:C) at 25μg/ml. After 24hr the total cellular mRNA was recovered and analyzed for changes in gene expression (n=4). Bars and horizontal lines represent the mean fold change in gene expression +/− SEM respectively, for independent experiments with 4 individual donors using matched EM, Cx and ECx, with β-Actin as the endogenous control. Untreated control is set to 1. * P<0.05, ** P<0.01. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
Estradiol Increases SDF-1α Secretion
As seen in Table 1, fibroblasts from all three sites constitutively secreted SDF-1α, CCL20, RANTES, MCP-1, IL-8 and IL-6 with no detectable IFNβ or TNFα. SDF-1α was present at highest levels in secretions from EM fibroblasts, while MCP-1, IL-8, IL-6 and CCL20 were highest in secretions from Cx fibroblasts. Except for SDF-1α, the concentration of all cytokines was generally lowest in secretions from EM fibroblasts. There was considerable variability in cytokine and chemokine concentrations between different individuals, possibly due to our samples being obtained from a population of pre- and post-menopausal women, and thus across a wide age range.
Table 1: Constitutive cytokine secretion by FRT stromal fibroblasts.
Conditioned media from endometrial (EM), endocervical (Cx) and ectocervical (ECx) stromal fibroblasts was collected after 48hr of cell culture and analyzed for secretion of MCP-1, IL-8, IL-6, CCL20, RANTES, and SDF-1α by ELISA. (n=14).
| MCP-1 | IL-8 | IL-6 | CCL20 | RANTES | SDF-1α | |
|---|---|---|---|---|---|---|
| EM (pg/ml) | 8377 | 748 | 22 | 40 | 113 | 2526 |
| 1727–15026 | 224–1771 | 0–37 | 12.5–50 | 12–274 | 608–3505 | |
| Cx (pg/ml) | 13252 | 864 | 384 | 499 | 121 | 276 |
| 7867–18635 | 97–1889 | 196–572 | 35–1358 | 102–148 | 0–423 | |
| ECx (pg/ml) | 11563 | 276 | 293 | 171 | 103 | 962 |
| 8590–13082 | 130–513 | 96–599 | 15–396 | 90–122 | 437–1605 |
Lower limit of detection: MCP-1 (15.6 pg/ml), IL-8 (31.2 pg/ml), IL-6 (9.38 pg/ml), CCL20 (15.6 pg/ml), RANTES (15.6 pg/ml), SDF-1α (31.2 pg/ml)
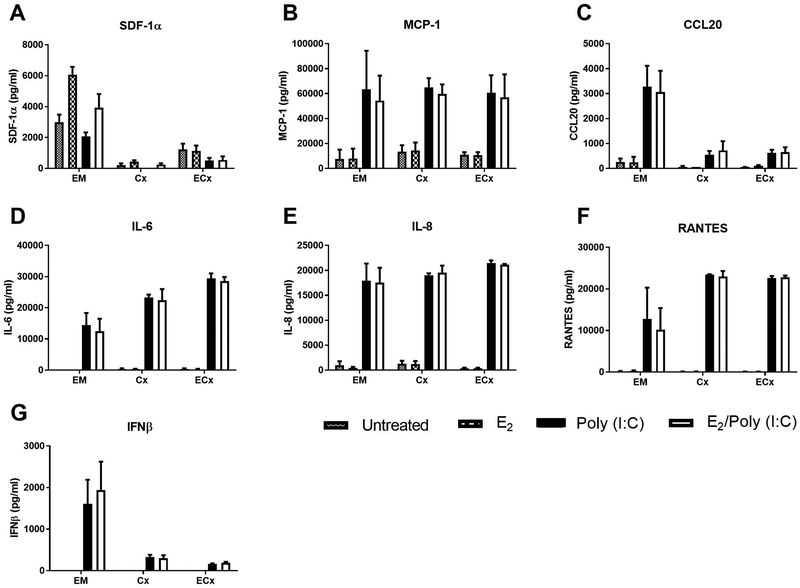
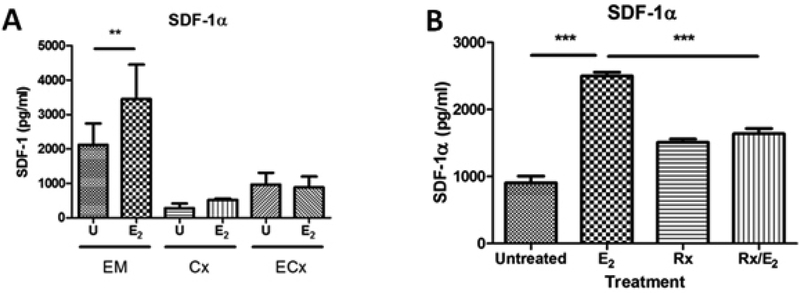
We next assessed whether E2 regulated cytokine secretion by stromal fibroblasts. E2, either alone or in the presence of poly (I:C) had no effect on the secretion of MCP-1, CCL20, IL-6, IL-8, RANTES, and IFNβ by EM, Cx, or ECx fibroblasts (Figure 7). However, treatment with E2 significantly increased the secretion of SDF-1α by EM fibroblasts (Figure 8A), but not Cx or ECx fibroblasts. To examine the mechanism behind E2 regulation of SDF-1α, we pretreated EM fibroblasts with the estrogen receptor (ER) α inhibitor Raloxifene (Rx) for 1 hr, prior to the addition of E2. As seen in Figure 8B, Rx blocked the upregulation of SDF-1α by E2, demonstrating that ERα signaling is essential for increased expression of SDF-1α.
Figure 7: Poly (I:C) upregulates cytokine secretion by FRT stromal fibroblasts.
Stromal fibroblasts from matched EM, Cx and ECx were pretreated for 48hr with E2 (5×10−8M) and subsequently retreated with poly (I:C), E2, or a combination of both for a further 24hr. Conditioned media was recovered and analyzed for SDF-1α (A), MCP-1 (B), CCL20 (C), IL-6 (D), IL-8 (E), RANTES (F) and IFNβ (G) by ELISA (n=4). Bars and horizontal lines represent the mean cytokine concentration +/− SEM respectively, for independent experiments using matched EM, Cx and ECx from 4 different donors.
Figure 8: E2 stimulates SDF-1α secretion by endometrial fibroblasts.
EM stromal fibroblasts were pretreated for 48hr with E2 (5×10−8M) and subsequently retreated with E2 for a further 24hr. Conditioned media was recovered and analyzed for SDF-1α by ELISA (A) (n=4). Cultured EM stromal fibroblasts were pretreated for 1 hr using the ERα antagonist Raloxifene (Rx) (5×10−6M), followed by addition of E2 (5×10−8M) for a further 48hr during which Rx was maintained in the culture media. Conditioned media was recovered and SDF-1α concentration determined by ELISA (B). Data shown in (A) is the mean +/− SEM of individual experiments from 4 individual donors while (B) shows the mean +/− SEM of a single donor that is representative of independent experiments from 3 individual donors. ** P<0.01, ***P<0.001. Bars and horizontal lines in both panels represent the mean +/− the SEM respectively.
Stromal Fibroblast Conditioned Media Inhibits HIV Infection
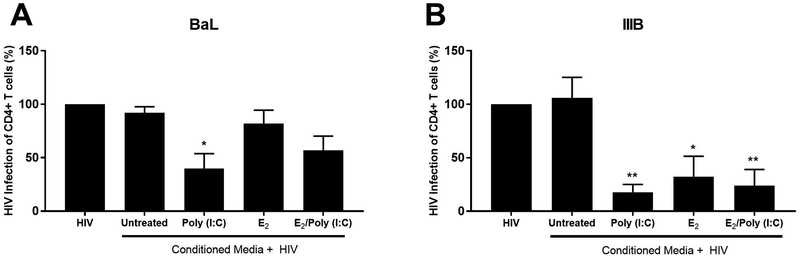
Previously we found that both EM epithelial cell and ovarian fibroblast conditioned media (CM) contains anti-HIV activity 34,37. To determine if stromal fibroblast secretions protect CD4+ T cells against viral infection, we investigated the effect of EM fibroblast CM on HIV infection of CD4+ T cells using two strains of HIV: BaL (CCR5-tropic) and IIIB (CXCR4-tropic). We incubated CM with virus (MOI=0.1) for 1hr, prior to infection of blood CD4+ T cells for 1hr. This allows for any antiviral factors in CM to interact with the virus, but minimizes the exposure time of CD4+ T cells to CM. As seen in Figure 9, CM from poly (I:C)-treated EM fibroblasts had significantly reduced BaL and IIIB infection of CD4+ T cells by 55% and 80% respectively, compared to CM from untreated cells. CM from E2-treated fibroblasts significantly inhibited IIIB infection of CD4+ T cells by approximately 70% compared to untreated cells but had no effect on BaL infection. For both BaL and IIIB, there was no difference between CM from poly (I:C)-treated cells versus E2/poly (I:C)-treated cells.
Figure 9: Endometrial stromal fibroblast conditioned media inhibits HIV infection.
Conditioned media (CM) was recovered from EM stromal fibroblasts, diluted 1:1 in X-Vivo 15 media and incubated with R5-tropic HIV-1 BaL (A) or X4-tropic HIV-1 IIIB (D) (MOI=0.1) for 1hr prior to addition to CD4+ T cells for 1hr, followed by extensive washout, and incubation with fresh media. Infection was determined by assessing levels of secreted p24 by ELISA after 7 days. * P<0.05, ** P<0.01 compared to CM from untreated cells. Bars and horizontal lines represent the percent mean HIV infection +/− SEM respectively, of independent experiments from 6 different individual donors. Statistical analysis was performed using the non-parametric Wilcoxon Signed Rank test.
Discussion:
The role and contribution of FRT fibroblasts to immune protection against sexually-transmitted infections is relatively unknown, with most research focusing on epithelial cells and immune cells. We examined the innate immune response of stromal fibroblasts from the EM, Cx and ECx to poly (I:C) and demonstrated that fibroblasts throughout the FRT are capable of initiating and directing an antiviral response and potentially have an important role in determining the outcome of infection. Studies at other mucosal surfaces have shown that fibroblasts, in addition to their role as structural cells, are essential in regulating inflammation and immune responses to pathogens and commensal organisms 38,39. Together with our studies, this demonstrates that fibroblasts are key mediators of mucosal immunity whose contributions need to be further defined in future studies.
Despite the protection conferred by an epithelial barrier throughout the FRT, pathogens can access the underlying stroma either by breaching the epithelium 6 or in the case of the EM, bypassing it entirely during menstruation when the overlying epithelial cells are shed. Thus, fibroblasts are routinely exposed to pathogens. Key to pathogen recognition are PRRs that are expressed in multiple cell types in the FRT. Responsiveness to PRR ligands is a characteristic feature of fibroblasts throughout the body. Ovarian, skin, lung, intestinal and foreskin fibroblasts respond to poly (I:C) by upregulating the expression of interferon (IFN) β, cytokines and interferon-stimulated genes (ISG) 34,40–42. Our findings indicate that following exposure to poly (I:C), a dsRNA analogue that is a common intermediate in viral replication, FRT fibroblasts upregulate the expression and secretion of ISGs, cytokines and chemokines. Together these create a hostile intracellular and extracellular environment to pathogen survival, that is likely essential in preventing successful infection.
Regular changes in sex hormone levels in pre-menopausal women have profound effects on immune function 1. For example, E2 increases secretion of HBD2, SLPI and elafin by primary uterine epithelial cells, while decreasing that by vaginal epithelial cells 19,43. In the present study the concentration of SDF-1α but not MCP-1, IL-8, IL-6, CCL20, RANTES, IFNβ and TNFα increased after E2 treatment of EM fibroblasts. Given that SDF-1α induces the proliferation of Ishikawa uterine epithelial cells 18, our finding that E2 stimulates EM fibroblasts production of SDF-1α suggests a mechanism whereby epithelial cells in the endometrium are regenerated after menstruation. SDF-1α is also chemotactic for immune cells, including plasmacytoid dendritic cells (pDC) and natural killer (NK) cells, and may be involved in the increase of NK cells between the proliferative and secretory stages of the cycle 44. SDF-1α is also upregulated by E2 in breast cancer cell lines 45 suggesting that SDF-1α is regulated by E2 elsewhere in the body. Whether E2 regulates cytokine secretion by fibroblasts at other mucosal sites remains unknown.
Intriguingly, poly (I:C) stimulation of ISG expression in the EM was decreased when cells were incubated with E2. Similarly, E2 inhibits MxA induction in human dendritic cells after infection with Newcastle Disease Virus 46. In contrast, ISG expression in EM epithelial cells is unaffected by E2 20. Our data suggests that E2 control of ISG expression in the FRT is regulated with respect to cell type (EM epithelial versus EM fibroblast) as well as anatomical location (EM versus Cx and ECx). Why E2 specifically dampens ISG induction by poly (I:C) in EM fibroblasts remains to be determined. Previous studies in the ovine and ruminant EM have demonstrated a role for hormonal regulation of specific ISGs during pregnancy 47,48. The regulation of ISG expression in EM fibroblasts may be part of the general pattern of optimization of reproductive conditions in the FRT stroma. Specifically, ISGs may not only be involved in the antiviral response, but also in generating a permissive microenvironment in the EM for successful implantation and pregnancy. Whether hormones regulate ISG expression by fibroblasts from other mucosal surfaces regularly exposed to pathogens, such as the lung or intestinal tract, is unknown, but may have profound consequences for immune protection.
Our findings that cytokine secretion by Cx and ECx fibroblasts do not change with E2 does not mean that these cells are hormonally unresponsive. We have previously shown that E2 upregulates 5’-ectonucleotidase by Cx and ECx fibroblasts 24. Similarly, in Cx fibroblasts, E2 stimulates and P inhibits hyaluronate metabolism 49 while E2 upregulates the expression of progesterone receptor 50. Together with our work, this demonstrates the specificity of E2 effects on different populations of fibroblasts. Further studies are needed to determine the extent to which E2 acts on fibroblasts in both the FRT and elsewhere in the body.
While EM fibroblasts in our system were responsive to E2, previous studies reported that E2 decreases TLR4 mRNA, and MCP-1 mRNA and protein levels by EM fibroblasts in vitro 16,51. In contrast, we observed no E2 effect on either TLR4 expression or MCP-1 secretion. Differences in experimental conditions could account for the different results of Arici et al. 16 who used serum-free cell culture medium and Hirata et al. 51 who used 2.5% FBS and a lower concentration of E2 (36.7nM) to treat their fibroblasts. In contrast, we used culture medium containing 10% charcoal-dextran stripped FBS. The presence of serum affects the expression and mRNA stability of several genes in fibroblasts 52 and could account for our different observations.
Several of the proteins secreted by fibroblasts including SDF-1α, MCP-1, CCL20 and RANTES have anti-HIV activity 53–56. We demonstrate for the first time that following PRR stimulation, stromal fibroblast secretions from the EM, similar to those from ovarian stromal fibroblasts 34, are capable of reducing HIV infection of CD4+ T cells. This is probably due to the increased secretion of antiviral cytokines such as RANTES and CCL20 in response to PRR treatment. Given the varying concentrations of proteins in the CM, antiviral activity is likely not due to a single protein, but rather the additive or synergistic effects of multiple proteins as demonstrated at other mucosal sites 57.
An unexpected finding in our study was that secretions from E2-treated EM fibroblasts reduced IIIB infection of CD4+ T cells but not BaL. One explanation for this finding is that E2 upregulates SDF-1α, which is known to competitively inhibit viral binding to the CXCR4 co-receptor 58, and thus reduce viral entry into the cell. Previous studies have shown that fibroblasts can enhance HIV infection of CD4+ T cells in a contact-dependent manner 59. Together with our study, this suggests distinct contact-independent and dependent contributions by fibroblasts to HIV susceptibility of CD4+ T cells. At present, the contributions of FRT fibroblasts to the overall balance of immune protection against HIV in the FRT remains to be determined. However, it is important to recognize that in vivo extracellular antiviral activity is not the exclusive domain of one cell type, but rather the combined effect of secretions from multiple cell types, including epithelial cells 37,43 and immune cells 60. Therefore, in vivo the contribution of fibroblasts may be a key additive or synergistic component for preventing HIV infection.
In conclusion, FRT stromal fibroblasts are active participants in antiviral immune protection of the FRT. FRT fibroblasts create a restrictive environment characterized by increased expression of ISGs and cytokines in response to viral exposure. Their sensitivity to E2 suggests that specific aspects of innate immune protection change across the menstrual cycle leading to periods of altered susceptibility to infection. Reduction of HIV infection by fibroblast secretions demonstrates that these cells may prevent the outcome of HIV transmission in women. Stromal fibroblasts in the FRT thus represent a level of immune protection against HIV beyond that seen with epithelial cells and immune cells. Further studies are needed to more fully define the complexities of the microenvironment within FRT tissues and the mechanisms through which they interact to protect against infection.
Acknowledgments
Supported by NIH AI102838, AI071761, AI117739 (Charles R. Wira)
References:
- 1.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nature Reviews Immunology. 2015;15(4):217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Human Reproduction. 2001;16(5):836–845. [DOI] [PubMed] [Google Scholar]
- 3.Malmström E, Sennström M, Holmberg A, et al. The importance of fibroblasts in remodelling of the human uterine cervix during pregnancy and parturition. Molecular Human Reproduction. 2007;13(5):333–341. [DOI] [PubMed] [Google Scholar]
- 4.Singer CF, Marbaix E, Lemoine P, Courtoy PJ, Eeckhout Y. Local cytokines induce differential expression of matrix metalloproteinases but not their tissue inhibitors in human endometrial fibroblasts. European Journal of Biochemistry. 1999;259(1–2):40–45. [DOI] [PubMed] [Google Scholar]
- 5.Grant KS, Wira CR. Effect of Mouse Uterine Stromal Cells on Epithelial Cell Transepithelial Resistance (TER) and TNFα and TGFβ Release in Culture. Biology of Reproduction. 2003;69(3):1091–1098. [DOI] [PubMed] [Google Scholar]
- 6.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation. PLoS Pathogens. 2010;6(4):e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasu K, Narahara H. Pattern Recognition via the Toll-Like Receptor System in the Human Female Genital Tract. Mediators of Inflammation. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infection and Immunity. 2004;72(10):5799–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirata T, Osuga Y, Hirota Y, et al. Evidence for the Presence of Toll-Like Receptor 4 System in the Human Endometrium. Journal of Clinical Endocrinology & Metabolism. 2005;90(1):548–556. [DOI] [PubMed] [Google Scholar]
- 10.Arima K, Nasu K, Narahara H, Fujisawa K, Matsui N, Miyakawa I. Effects of lipopolysaccharide and cytokines on production of RANTES by cultured human endometrial stromal cells. Molecular Human Reproduction. 2000;6(3):246–251. [DOI] [PubMed] [Google Scholar]
- 11.Kato H, Takeuchi O, Mikamo-Satoh E, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. The Journal of Experimental Medicine. 2008;205(7):1601–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-[kappa]B by Toll-like receptor 3. Nature. 2001;413(6857):732–738. [DOI] [PubMed] [Google Scholar]
- 13.Patel MV, Shen Z, Rossoll RM, Wira CR. IL-27 Expression and Responsiveness in Human Uterine Epithelial Cells and Fibroblasts In Vitro and the Role of Estradiol. J Interferon Cytokine Res. 2018;38(3):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman KD, Ghosh M, Crist SG, et al. Modulation of Hepatocyte Growth Factor Secretion in Human Female Reproductive Tract Stromal Fibroblasts by Poly (I:C) and Estradiol. American Journal of Reproductive Immunology. 2012;67(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. Journal of Reproductive Immunology. 2011;88(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arici A, Senturk LM, Seli E, Bahtiyar MO, Kim G. Regulation of Monocyte Chemotactic Protein-1 Expression in Human Endometrial Stromal Cells by Estrogen and Progesterone. Biology of Reproduction. 1999;61(1):85–90. [DOI] [PubMed] [Google Scholar]
- 17.Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-α and TGF-β in human endometrial epithelial and stromal cells. Molecular Human Reproduction. 2005;11(10):751–760. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi A, Okada H, Nakamoto T, Okamoto R, Yasuda K, Kanzaki H. Estrogen induces stromal cell-derived factor 1 (SDF-1/CXCL12) production in human endometrial stromal cells: a possible role of endometrial epithelial cell growth. Fertility and Sterility. 2011;95(1):444–447. [DOI] [PubMed] [Google Scholar]
- 19.Fahey JV, Wright JA, Shen L, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunology. 2008;1(4):317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel MV, Ghosh M, Fahey JV, Wira CR. Uterine Epithelial Cells Specifically Induce Interferon-Stimulated Genes in Response to Polyinosinic-Polycytidylic Acid Independently of Estradiol. PLoS ONE. 2012;7(4):e35654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahey JV, Schaefer TM, Wira CR. Sex hormone modulation of human uterine epithelial cell immune responses. Integrative and Computational Biology. 2006;46(6):1082–1087. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate Immunity in the Human Female Reproductive Tract: Antiviral Response of Uterine Epithelial Cells to the TLR3 Agonist Poly(I:C). Journal of Immunology. 2005;174(2):992–1002. [DOI] [PubMed] [Google Scholar]
- 23.Classen-Linke I, Kusche M, Knauthe R, Beier HM. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell and Tissue Research. 1996;287(1):171–185. [DOI] [PubMed] [Google Scholar]
- 24.Shen Z, Fahey JV, Bodwell JE, et al. Estradiol regulation of nucleotidases in female reproductive tract epithelial cells and fibroblasts. PLoS ONE. 2013;8(7):e69854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koumas L, King AE, Critchley HOD, Kelly RW, Phipps RP. Fibroblast Heterogeneity: Existence of Functionally Distinct Thy 1+ and Thy 1− Human Female Reproductive Tract Fibroblasts. The American Journal of Pathology. 2001;159(3):925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bryant HU, Glasebrook AL, Yang NN, Sato M. An estrogen receptor basis for raloxifene action in boneProceedings of Xth International Congress on Hormonal Steroids, Quebec, Canada, 17–21 June 1998. The Journal of Steroid Biochemistry and Molecular Biology. 1999;69(1):37–44. [DOI] [PubMed] [Google Scholar]
- 27.Huhtinen K, Desai R, Ståhle M, et al. Endometrial and Endometriotic Concentrations of Estrone and Estradiol Are Determined by Local Metabolism Rather than Circulating Levels. Journal of Clinical Endocrinology & Metabolism. 2012;97(11):4228–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baird DT, Fraser IS. Blood Production and Ovarian Secretion Rates of Estradiol-17β and Estrone in Women Throughout the Menstrual Cycle. Journal of Clinical Endocrinology & Metabolism. 1974;38(6):1009–1017. [DOI] [PubMed] [Google Scholar]
- 29.Baird DT, Fraser IS. Concentration of oestrone and oestradiol in follicular fluid and ovarian venous blood of women. Clinical Endocrinology. 1975;4(3):259–266. [DOI] [PubMed] [Google Scholar]
- 30.Fraser IS, Baird DT. Blood Production and Ovarian Secretion Rates of Estradiol-17β and Estrone in Women with Dysfunctional Uterine Bleeding. Journal of Clinical Endocrinology & Metabolism. 1974;39(3):564–570. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd CW, Lobotsky J, Baird DT, et al. Concentration of Unconjugated Estrogens, Androgens and Gestagens in Ovarian and Peripheral Venous Plasma of Women: The Normal Menstrual Cycle. Journal of Clinical Endocrinology & Metabolism. 1971;32(2):155–166. [DOI] [PubMed] [Google Scholar]
- 32.McNatty KP, Baird DT, Bolton A, Chambers P, Corker CS, McLean H. Concentration of oestrogens and androgens in human ovarian venous plasma and follicular fluid throughout the menstrual cycle. Journal of Endocrinology. 1976;71(1):77–85. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Garcia M, Biswas N, Patel MV, et al. Estradiol Reduces Susceptibility of CD4+ T Cells and Macrophages to HIV-Infection. PLoS ONE. 2013;8(4):e62069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel MV, Shen Z, Wira CR. Poly (I:C) and LPS Induce Distinct Immune Responses by Ovarian Stromal Fibroblasts. Journal of Reproductive Immunology. 2018;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunology. 2004;5(7):730–737. [DOI] [PubMed] [Google Scholar]
- 36.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. [DOI] [PubMed] [Google Scholar]
- 37.Wira CR, Ghosh M, Smith JM, et al. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunology. 2011;4(3):335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens BM. Inflammation, Innate Immunity, and the Intestinal Stromal Cell Niche: Opportunities and Challenges. Front Immunol. 2015;6:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens BM, Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6(2):224–234. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa T, Sugiura H, Koarai A, et al. TLR3 Activation Augments Matrix Metalloproteinase Production through Reactive Nitrogen Species Generation in Human Lung Fibroblasts. The Journal of Immunology. 2014;192(11):4977–4988. [DOI] [PubMed] [Google Scholar]
- 41.Farina GA, York MR, Di Marzio M, et al. Poly(I:C) drives type I IFN- and TGFbeta-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130(11):2583–2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang F, Ooka K, Sun X, et al. A synthetic TLR3 ligand mitigates profibrotic fibroblast responses by inducing autocrine IFN signaling. J Immunol. 2013;191(6):2956–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel MV, Fahey JV, Rossoll RM, Wira CR. Innate Immunity in the Vagina (Part I): Estradiol Inhibits HBD2 and Elafin Secretion by Human Vaginal Epithelial Cells. American Journal of Reproductive Immunology. 2013;69(5):463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flynn L, Byrne B, Carton J, Kelehan P, O’Herlihy C, O’Farrelly C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol. 2000;43(4):209–217. [DOI] [PubMed] [Google Scholar]
- 45.Felix AS, Stone RA, Chivukula M, et al. Survival outcomes in endometrial cancer patients are associated with CXCL12 and estrogen receptor expression. International Journal of Cancer. 2012;131(2):E114–E121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, López CB, Moran TM. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood. 2008;112(12):4574–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joyce MM, Burghardt RC, Geisert RD, et al. Pig Conceptuses Secrete Estrogen and Interferons to Differentially Regulate Uterine STAT1 in a Temporal and Cell Type-Specific Manner. Endocrinology. 2007;148(9):4420–4431. [DOI] [PubMed] [Google Scholar]
- 48.Joyce MM, White FJ, Burghardt RC, et al. Interferon Stimulated Gene 15 Conjugates to Endometrial Cytosolic Proteins and Is Expressed at the Uterine-Placental Interface throughout Pregnancy in Sheep. Endocrinology. 2005;146(2):675–684. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka K, Nakamura T, Takagaki K, Funahashi M, Saito Y, Endo M. Regulation of hyaluronate metabolism by progesterone in cultured fibroblasts from the human uterine cervix. Febs Letters. 1997;402(2–3):223–226. [DOI] [PubMed] [Google Scholar]
- 50.Ackerman WE, Summerfield TL, Mesiano S, Schatz F, Lockwood CJ, Kniss DA. Agonist-Dependent Downregulation of Progesterone Receptors in Human Cervical Stromal Fibroblasts. Reproductive Sciences. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirata T, Osuga Y, Hamasaki K, et al. Expression of toll-like receptors 2, 3, 4, and 9 genes in the human endometrium during the menstrual cycle. Journal of Reproductive Immunology. 2007;74(1–2):53–60. [DOI] [PubMed] [Google Scholar]
- 52.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Molecular and Cellular Endocrinology. 1993;94(2):195–204. [DOI] [PubMed] [Google Scholar]
- 53.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382(6594):833–835. [DOI] [PubMed] [Google Scholar]
- 54.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3α is a Novel Anti-HIV-1 Molecule of the Human Female Reproductive Tract. American Journal of Reproductive Immunology. 2009;62(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coffey MJ, Woffendin C, Phare SM, Strieter RM, Markovitz DM. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1997;272(5):L1025–L1029. [DOI] [PubMed] [Google Scholar]
- 56.Frade JM, Llorente M, Mellado M, et al. The amino-terminal domain of the CCR2 chemokine receptor acts as coreceptor for HIV-1 infection. The Journal of Clinical Investigation. 1997;100(3):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh PK, Tack BF, McCray PB, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2000;279(5):L799–L805. [DOI] [PubMed] [Google Scholar]
- 58.Bleul CC, Farzan M, Choe H, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382(6594):829–833. [DOI] [PubMed] [Google Scholar]
- 59.Neidleman JA, Chen JC, Kohgadai N, et al. Mucosal stromal fibroblasts markedly enhance HIV infection of CD4+ T cells. PLoS Pathogens. 2017;13(2):e1006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mselle TF, Howell AL, Ghosh M, Wira CR, Sentman CL. Human Uterine Natural Killer Cells but Not Blood Natural Killer Cells Inhibit Human Immunodeficiency Virus Type 1 Infection by Secretion of CXCL12. Journal of Virology. 2009;83(21):11188–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]