Abstract
Background:
Exposure to trace elements may affect health, including breast cancer risk. Trace element levels in toenails are potentially useful biomarkers of exposure, but their reliability is not established. We assessed the reproducibility of toenail element concentrations over time and whether concentrations change following a breast cancer diagnosis.
Methods:
We assessed trace element levels in toenails collected at two time points from 221 women (111 with and 110 without an intervening breast cancer diagnosis). We measured levels of arsenic, cadmium, chromium, cobalt, copper, iron, mercury, manganese, molybdenum, nickel, lead, antimony, selenium, tin, vanadium, and zinc using inductively coupled plasma mass spectrometry in samples collected at baseline and 4–10 years later. We compared trace element concentrations over time using Spearman’s rank correlation coefficient (R). We used linear models to examine the magnitude and direction of changes and the influence of a breast cancer diagnosis.
Results:
Overall, we observed positive correlations (R=0.18–0.71) between paired samples for all trace elements. However, nickel (R=−0.02) and antimony (R=0.12) were not correlated among cases. We observed decreases in cadmium, chromium, mercury, manganese, molybdenum, nickel, and lead between baseline and follow-up, but case status was unrelated to these changes. The declines are consistent with decreases over calendar-time rather than age-time.
Conclusions:
Toenail trace element concentrations were correlated over time, but many elements showed systematic decreases by calendar year. Aside from nickel and antimony, post-diagnostic toenail levels correlated with pre-diagnostic levels, providing support for using post-diagnostic toenail samples in retrospective studies.
Keywords: trace elements, toenails, breast cancer, reliability
Introduction
Metals and other trace elements are widespread environmental exposures. Trace elements have been associated with adverse health outcomes including hypertension, impaired cognition, cardiovascular disease, kidney disease, and breast cancer.1–8 Certain trace elements are essential for human health,9 but even these may become toxic at high concentrations.10 Although trace elements arise from naturally-occurring sources, industrial and agricultural operations contribute to population exposure levels.1,11 The general population is exposed to trace elements in their diet and from tobacco use, as well as from contaminated air and water.12–16
Because of this diversity of sources, it is of interest to use a biomarker that integrates exposures from multiple pathways. Epidemiologic studies have often assessed trace elements in urine17–22 or blood samples.22–24 Toenails are another potential medium for measuring trace element exposure,4,5,25–30 but the validity and reliability of trace element concentrations in toenails are not well established.4,31 Toenails are a promising biologic matrix for trace element assessment in large studies, as collection is non-invasive and nail clippings are easy to obtain, ship, and store.32 If all five digits (left, right, or both) are assessed together, toenails are estimated to reflect individual’s trace element exposure during a 4–6 month window occurring approximately 6–12 months before collection.29,33 While urine is another possible non-invasive matrix for assessing trace elements, such assessments may be problematic because of the need to adjust for urine dilution34,35 and the potential impact of kidney disease on urine and urinary dilution measures.36,37 This is particularly complicated if the elements themselves influence kidney function.38 Moreover, for some elements, urine concentrations may reflect only very recent exposure.39,40
Although studies have considered the relationship between trace elements in toenails and disease outcomes,4,5,25,30,41 very few studies have evaluated the reliability of nail-based trace element measurement over time or the potential influence of disease diagnosis. Prior studies have suggested that toenail element levels are moderately correlated within individuals sampled as controls when considering samples taken years apart.42–44 We conducted this reliability study with the intent to investigate whether one could validly assess the association between young-onset breast cancer and trace elements using retrospectively collected (i.e. post-diagnosis in cases) toenail trace element concentrations. Such an approach relies on the assumption that the disease does not cause systematic changes in the levels, so we specifically sought to assess the correlations of trace elements in toenail samples collected before and after a breast cancer diagnosis, and compare those to correlations among women without an intervening diagnosis across similar timeframes.
Methods
We conducted this reliability study using data from the Sister Study, a US-based, observational cohort enrolled between 2003–2009. To be eligible, women had to be aged 35–74 and have a sister with a history of breast cancer, but never have had breast cancer themselves. All 50,884 study participants completed computer-assisted telephone interviews, which included questions on a large range of topics.45 Toenail clippings were self-collected. Women were asked to first remove any nail polish, and then take a clipping from each toe. All participants provided written informed consent. Study approval and oversight was provided by the Institutional Review Boards of the National Institute of Environmental Health Sciences and the Copernicus Group.
Sister Study participants are re-contacted at least once a year to obtain updated health information, including any cancer diagnoses. Self-reported breast cancer cases are confirmed via medical records, when possible. As of September 2016 (data release 6.0), 3,075 Sister Study participants had developed invasive breast cancer or ductal carcinoma in situ.
In 2013–14, a subset of 3,762 participants was asked to provide a second set of biospecimens, including toenails. This included 1,918 women who had developed breast cancer between enrollment and 2013 and 1,844 women who had not. We collected second biospecimens for 1,227 cases (64% response rate) and 1,203 non-cases (65% response rate). For this reliability study, we focused on young-onset breast cancer diagnoses (age<50), identifying 111 women who were diagnosed between baseline and 2013 (“cases”), and 111 women frequency matched to cases based on age group at enrollment (35–39, 40–44, 45–49) with no intervening breast cancer diagnosis (“controls”).
We assessed levels of 16 different elements (arsenic, cadmium, chromium, cobalt, copper, iron, mercury, manganese, molybdenum, nickel, lead, antimony, selenium, tin, vanadium, and zinc). Briefly, toenails were washed to remove exogenous contaminants, then air-dried on a clean bench and acid digested in 9:1 HNO3/HCl. They were then diluted with de-ionized water and analysed by inductively-coupled plasma mass spectrometry (Agilent 8800 ICP-MS triple quad; Santa Clara, CA). Vanadium (51), chromium (52), manganese (55), cobalt (59), nickel (60), copper (63), zinc (66), molybdenum (95), cadmium (111), tin (118), antimony (121), mercury (201), and lead (206, 207, 208) were measured in collision (He) mode, iron (56) was measured in reaction (H2) mode, and arsenic and selenium were measured as AsO and SeO in oxygen mode.
Each individual’s pair of samples were analyzed in the same batch and laboratory staff were masked to case–control status. Quality control included continuing calibration verification, analysis of duplicates and spikes, digestion and analysis of standard reference material (Japan NIES #13 Hair), and within- and between-batch analysis of a laboratory-prepared toenail matrix digest.
Samples with values ≤0 after quality control adjustments (7 of 7,072 measures) were assigned a value of 0.001 μg/g, which was lower than the smallest observed value for those elements (vanadium [n=1], manganese [n=4], molybdenum [n=1], or mercury [n=1]). No other corrections were made for low concentrations, as samples with levels below quantification limits were still assigned measured values. We log-transformed measured concentrations to make the exposure distribution more normally distributed and then corrected these log-transformed values for batch using a random effects model. One control participant’s toenail samples were not useable, bringing our final count to 111 cases, 110 controls.
We compared within-individual trace element concentrations from the two time points using Spearman’s rank correlation coefficients (R), as well as intraclass correlation coefficients. We then used paired t-tests to assess changes over time between individuals’ samples. We also applied linear regression models to evaluate the influence of young-onset breast cancer diagnoses on those changes. Specifically, we assessed whether case status was associated with the difference between the log-transformed element levels at time 2 versus time 1: (log10 trace element level time 2 – log10 trace element level time 1) = α + β*case + ε, with or without adjustment for time between sample collection.
Finally, to better assess whether changes over time were attributable to age or calendar time, we examined age trends in predicted mean trace element concentrations standardized to the observed year distribution. This was done using generalized estimating equations to account for repeated measures (two per individual), and with restricted cubic spline terms for age and year (knots at the 5th, 35th, 65th, and 95th percentiles). These modeled age trends were compared to modeled year trends, which were based on predicted mean element concentrations standardized to the observed age distribution.
Results
Mean age at first toenail collection was 44.1 years for controls (standard deviation [std] =3.8) and 43.6 years for cases (std=3.5; Table 1). Second toenails were collected an average of 7.6 years later for controls and 8.0 years for cases. Cases were diagnosed at age 46.4 (std=3.0), on average (eTable 1). Most of the participants in both groups were Non-Hispanic white (87% and 88% for controls and cases, respectively). Geometric means and interquartile ranges for all 16 trace elements are also shown in Table 1.
Table 1.
Description of reliability sample
| Controls (n=110) | Cases (n=111) | |
|---|---|---|
| Non-Hispanic White Race; n (%) | 96 (87) | 98 (88) |
| Current Smoker; n (%) | 9 (8) | 5 (5) |
| Age at first toenail sample collection, y; Mean (std) | 44.1 (3.8) | 43.6 (3.5) |
| Age at second toenail sample collection, y; Mean (std) | 51.7 (3.8) | 51.6 (3.5) |
| Years between sample collections; Mean (range) | 7.6 (4.4–10.6) | 8.0 (4.6–10.6) |
| Baseline metal levels (μg/g); Geometric Mean (IQR)a | ||
| Arsenic | 0.054 (0.038–0.075) | 0.055 (0.036–0.080) |
| Cadmium | 0.006 (0.003–0.011) | 0.007 (0.003–0.012) |
| Cobalt | 0.008 (0.004–0.013) | 0.007 (0.004–0.012) |
| Chromium | 0.247 (0.119–0.548) | 0.263 (0.148–0.578) |
| Copper | 3.64 (2.99–4.05) | 3.99 (3.16–4.55) |
| Iron | 14.9 (8.12–23.0) | 13.0 (7.89–18.5) |
| Mercury | 0.084 (0.045–0.222) | 0.087 (0.040–0.200) |
| Manganese | 0.228 (0.113–0.359) | 0.189 (0.098–0.342) |
| Molybdenum | 0.009 (0.005–0.013) | 0.008 (0.005–0.012) |
| Nickel | 0.589 (0.142–1.18) | 0.457 (0.144–1.13) |
| Lead | 0.134 (0.064–0.229) | 0.144 (0.062–0.262) |
| Antimony | 0.020 (0.010–0.036) | 0.021 (0.012–0.029) |
| Selenium | 0.941 (0.860–1.04) | 0.973 (0.869–1.06) |
| Tin | 0.091 (0.055–0.143) | 0.067 (0.038–0.114) |
| Vanadium | 0.010 (0.005–0.019) | 0.010 (0.005–0.018) |
| Zinc | 105 (91.8–116) | 104 (92.5–114) |
Includes those below the limit of quantification: Arsenic (4% visit 1, 8% visit 2); Cadmium (13% visit 1, 19% visit 2); Cobalt (2% visit 1, 5% visit 2); Chromium (33% visit 1, 46% visit 2); Copper (0% visit 1, 0% visit 2); Iron (3% visit 1, 3% visit 2); Mercury (7% visit 1, 9% visit 2); Manganese (5% visit 1, 9% visit 2); Molybdenum (52% visit 1, 63% visit 2); Nickel (9% visit 1, 14% visit 2); Lead (4% visit 1, 7% visit 2); Antimony (33% visit 1, 37% visit 2); Selenium (0% visit 1, 0% visit 2); Tin (1% visit 1, 5% visit 2); Vanadium (30% visit 1, 38% visit 2); Zinc (0% visit 1, 0% visit 2). IQR indicates interquartile range.
Overall, we observed modest correlations (Spearman R=0.18–0.71) between paired samples for all trace elements (Table 2; eFigures 1a-p). The highest correlations were seen for mercury (R=0.71) and zinc (R=0.64). These patterns held among controls, but nickel and antimony were not correlated among cases (R=−0.02, 95% confidence interval [CI]: −0.21, 0.16; and R=0.12, 95% CI: −0.07, 0.30, respectively). Intraclass correlation coefficient results mirrored these findings, with a range of 0.13–0.72 overall, and low values seen for nickel and antimony among cases (−0.08 for nickel, 0.10 for antimony; eTable 2). Correlations remained consistent even after excluding values below the limit of quantification (eTable 3) or excluding outliers from the nickel analysis.
Table 2.
Comparison of metal levels in toenails measured at baseline versus 4–10 years later; Sister Study (2003–2009). All cases were breast cancer free at baseline.
| Metal | Overall (n=221) |
Controls (n=110) |
Cases (n=111) |
Mean differencea | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| Spearman R | 95% CI | Spearman R | 95% CI | Spearman R | 95% CI | |||
| Arsenic | 0.40 | (0.28, 0.50) | 0.37 | (0.20, 0.52) | 0.42 | (0.25, 0.56) | −0.04 | (−0.10, 0.01) |
| Cadmium | 0.38 | (0.26, 0.49) | 0.36 | (0.19, 0.51) | 0.40 | (0.23, 0.54) | −0.19 | (−0.24, −0.13) |
| Cobalt | 0.34 | (0.22, 0.45) | 0.46 | (0.30, 0.59) | 0.24 | (0.05, 0.41) | −0.05 | (−0.11, 0.01) |
| Chromium | 0.23 | (0.10, 0.35) | 0.29 | (0.11, 0.45) | 0.19 | (0.01, 0.37) | −0.18 | (−0.25, −0.10) |
| Copper | 0.54 | (0.44, 0.62) | 0.59 | (0.45, 0.70) | 0.49 | (0.34, 0.62) | −0.003 | (−0.02, 0.02) |
| Iron | 0.40 | (0.28, 0.51) | 0.45 | (0.29, 0.59) | 0.36 | (0.18, 0.51) | −0.03 | (−0.08, 0.03) |
| Mercury | 0.71 | (0.63, 0.77) | 0.74 | (0.64, 0.82) | 0.67 | (0.55, 0.76) | −0.07 | (−0.12, −0.01) |
| Manganese | 0.41 | (0.29, 0.51) | 0.46 | (0.30, 0.60) | 0.37 | (0.19, 0.52) | −0.10 | (−0.18, −0.01) |
| Molybdenum | 0.35 | (0.23, 0.46) | 0.25 | (0.06, 0.42) | 0.45 | (0.29, 0.59) | −0.07 | (−0.12, −0.01) |
| Nickel | 0.20 | (0.07, 0.32) | 0.40 | (0.23, 0.54) | −0.02 | (−0.21, 0.16) | −0.16 | (−0.28, −0.03) |
| Lead | 0.47 | (0.36, 0.57) | 0.42 | (0.25, 0.56) | 0.52 | (0.37, 0.64) | −0.28 | (−0.34, −0.22) |
| Antimony | 0.18 | (0.05, 0.31) | 0.23 | (0.04, 0.40) | 0.12 | (−0.07, 0.30) | −0.01 | (−0.08, 0.05) |
| Selenium | 0.58 | (0.48, 0.66) | 0.65 | (0.53, 0.75) | 0.51 | (0.35, 0.63) | −0.002 | (−0.02, 0.01) |
| Tin | 0.26 | (0.14, 0.38) | 0.21 | (0.02, 0.38) | 0.24 | (0.06, 0.41) | −0.04 | (−0.10, 0.02) |
| Vanadium | 0.45 | (0.34, 0.55) | 0.41 | (0.24, 0.56) | 0.49 | (0.33, 0.62) | −0.05 | (−0.12, 0.01) |
| Zinc | 0.64 | (0.55, 0.71) | 0.66 | (0.54, 0.76) | 0.62 | (0.49, 0.72) | 0.01 | (−0.00, 0.02) |
CI: Confidence Interval
Comparing log10 metal levels from toenails collected at the first versus second time points
log10 metal level time 2 – log10 metal level time 1
Concentrations decreased over time for all trace elements except zinc (Table 2). Case status was unrelated to these differences (Figure 1), with or without adjustment for time between sample collections (eTable 4). The largest reductions were seen for lead, cadmium, and chromium, all three of which decreased with calendar time but increased slightly with age (Figure 2).
Figure 1.
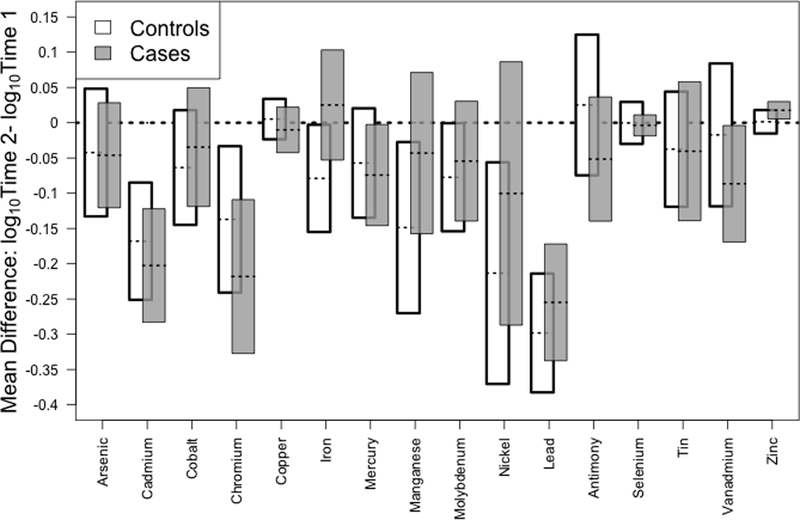
Mean difference and 95% confidence intervals for log10 metal levels: Time 2- Time 1. There were no differences in metal concentrations by case status.
Figure 2.
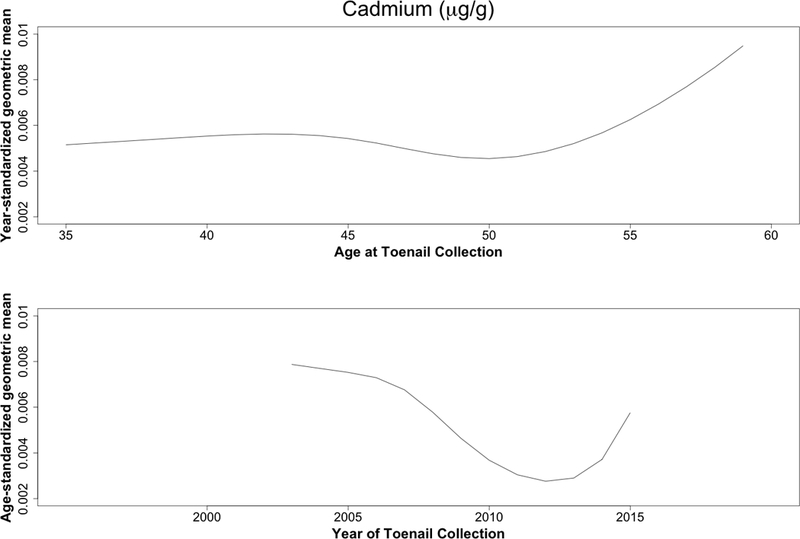
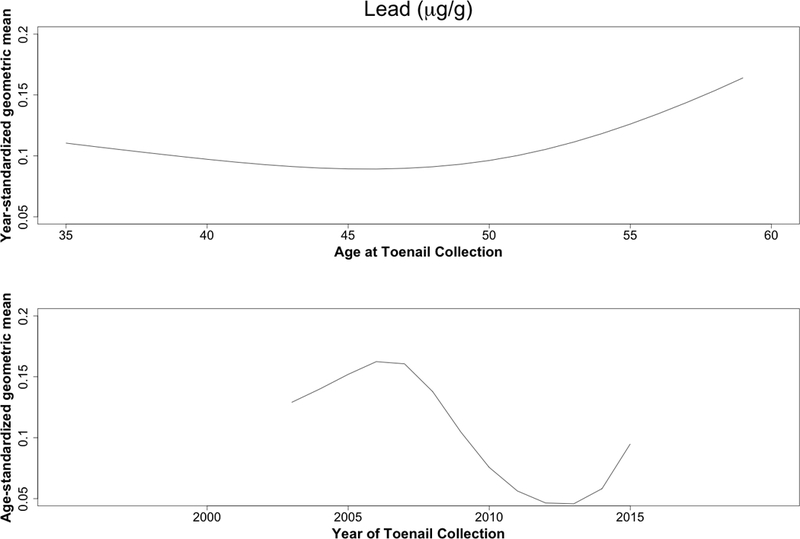
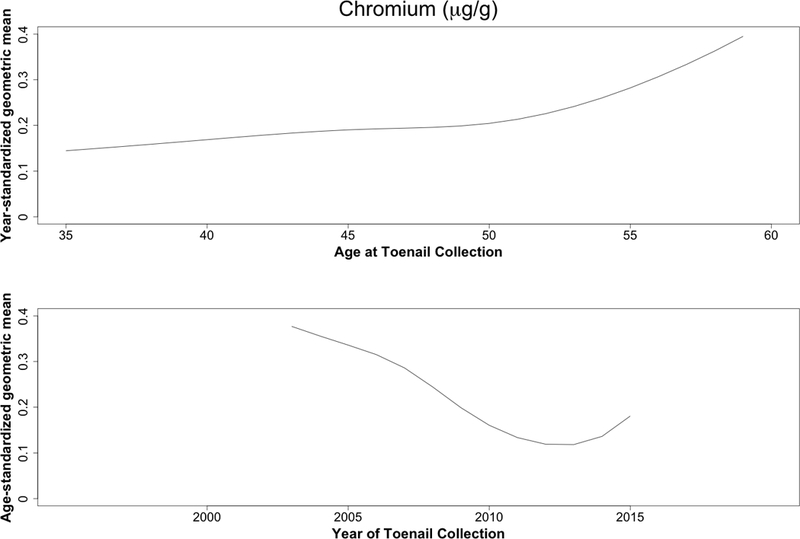
Predicted geometric mean cadmium (A), lead (B), and chromium (C) levels (μg/g) based on year (top) and age-standardized (bottom) models. Age and year effects were modeled using restricted cubic splines with four knots (5th, 35th, 65th, and 95th percentiles).
As nickel and antimony were not correlated among cases, we ran a stepwise model to assess which factors were related to changes in levels over time among cases. The model allowed for inclusion of terms for average age at toenail collection, time between diagnosis and second toenail collection, education, physical activity, alcohol consumption, estimated air pollution exposure (NO2 and PM2.5), birth control use, hormone therapy use, income, menopausal status, urbanicity, proximity to a factory, and time between toenail sample collections, as well as disease or treatment-related variables, including stage, estrogen receptor status, progesterone receptor status, human epidermal growth factor receptor-2 status, surgery type, and whether or not the participants received chemotherapy, Herceptin/lapatinib, hormonal treatment, or radiation. None of these factors was associated with changes in nickel over time. Only stage of disease was associated with changes in antimony, with in situ disease associated with a 0.25 μg/g decrease in log10 antimony levels relative to stage I disease.
Discussion
In this reliability study, we observed that toenail trace element concentrations were modestly correlated over 4–10 years. While the toenail levels decreased over time, particularly for regulated toxic metals like cadmium, chromium, and lead, the extent of decline did not vary by breast cancer status. With the exception of nickel and antimony, post-diagnostic toenail levels may serve as reasonable proxies for pre-diagnostic levels. Future investigations should consider the temporal stability of each element on a case-by-case basis, but we believe that these results support the use of post-diagnostic toenail samples to assess levels of most of the trace elements in retrospective breast cancer studies.
Toenail trace element concentrations were of similar magnitude to those reported in prior studies conducted during the same general time period (e.g. geometric mean cadmium levels of 0.006 μg/g in our 2003–2009 sample, versus 0.005 in the New Hampshire Birth Cohort Study, 2009–2016,46 versus a median of 0.002 in the Normative Aging Study, 1999–2009).4 Our findings of moderate correlation in toenail trace element concentrations over time are consistent with previous studies conducted among individuals sampled as controls. In the Nurses’ Health Study (n=127), Garland and colleagues43 compared toenail concentrations of 16 trace elements over a 6-year period, finding correlations across the trace elements ranging from 0.26–0.58, and decreases in concentrations over time for most elements. As would be expected given the time trends we observed, the magnitudes of correlations were similar for our two samples, but the average concentrations in our study tended to be lower than those measured in the Nurses’ Health Study, which collected its toenail samples in the 1980s and used different laboratory techniques.43 In other study samples, toenail selenium levels measured a year apart were well-correlated (r=0.57),42 as were arsenic concentrations in toenail samples collected 3–5 years apart (ICC=0.6).44
As previously noted, we observed the strongest declines over time for lead, cadmium, and chromium, which is consistent with known nationwide trends.47,48 Average ambient lead concentrations in the United States decreased by an estimated 92% between 1980 and 2013.49 Although the majority of the decrease occurred in the 1980s and early 1990s, levels continued to drop during our study period, with estimated mean ambient lead concentrations of 0.22 μg/m3 in 2003 and 0.03 μg/m3 in 2014.50 Levels of cadmium and chromium, both constituents of tobacco smoke,36 may have declined in part because of the decreased prevalence of tobacco use in the United States.51 However, as only a small proportion (6%, n=14) of our participants were smokers at baseline, and only six quit between samplings, changes in ambient air, water, and soil pollution over this time period are more likely to be responsible for the decrease observed here.
Nickel and antimony were not correlated in women who had an intervening breast cancer diagnosis, suggesting that toenail levels may not be useful as proxies for pre-diagnosis nickel and antimony levels. Nickel assessment in toenails in particular may suffer from misclassification due to contamination from toenail clippers. Cobalt, iron, and chromium may also be present in toenail clippers, though we observed stronger within-individual correlations for those elements.
A major strength of this study is our assessment of a large panel of trace elements in toenails. Toenails are an understudied matrix and it is important to assess their reliability for capturing trace element exposure in epidemiologic studies. Our findings suggest that results may be reliable over a period of years and do not change following diagnosis. However, it is often difficult to know exactly what exposure window is most pertinent to disease development, especially for diseases with a long latency periods, such as cancers. Nonetheless, toenails provide researchers with an opportunity to examine a different time window than would studies assessing trace element levels in blood or urine, which may reflect either cumulative or very recent exposure, depending in the element of interest.39,40,46,52
We further addressed concerns about how an intervening breast cancer diagnosis and its treatment might affect this reliability, either through effects of the disease, its treatment, or changes in behavior post-diagnosis. However, we acknowledge that our study sample was predominately non-Hispanic white with relatively high educational attainment, and thus these findings may not be generalizable to all women, especially if individuals of certain socioeconomic statuses or racial/ethnic groups had higher or more variable levels of trace element exposure. Our results may also not be generalizable to studies of other diagnoses, though we hypothesize that other types of cancer would act similarly and not strongly influence trace element levels, unless the elements are part of a particular therapeutic formulation.
Although toenail levels of some elements markedly decrease over time, we observed that levels remained moderately correlated over the 4–10 year study period. We saw no evidence to support that the occurrence of breast cancer systematically influenced toenail trace element concentrations. The findings from this study support the use of retrospective case–control studies to assess how trace elements as measured in post-diagnosis toenails are related to breast cancer risk. More generally, these findings support using a single measurement of toenail trace elements as proxies for long-term exposure, which is relevant for other health outcomes including other cancers and chronic diseases.
Supplementary Material
Acknowledgements:
The authors would like to thank Drs. Helen Chin and Kristen Upson for their feedback on an early draft of this paper.
Funding: This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005 to DPS and Z01-ES102245 to CRW) and Superfund (P42ES007373 to MRK, BPJ) and National Cancer Center Support (5P30CA023108 to MRK, BPJ) grants.
Footnotes
Data requests: Requests for de-identified Sister Study data, including the data used in this manuscript can be requested through the study website (https://sisterstudy.niehs.nih.gov/English/data-requests.htm). The Sister Study is an ongoing prospective study and the data sharing policy was developed to protect the privacy of study participants. It is consistent with study informed consent documents as approved by the NIEHS Institutional Review Board.
Conflict of Interest: The authors declare they have no conflicts of interests.
References
- 1.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72. doi:10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci. 2015;16(12):29592–29630. doi:10.3390/ijms161226183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease - A systematic review. Environ Health Perspect. 2007;115(3):472–482. doi:10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mordukhovich I, Wright RO, Hu H, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the Normative Aging Study. Environ Health Perspect. 2012;120(1):98–104. doi:org/10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garland M, Morris JS, Colditz G a, et al. Toenail trace element levels and breast cancer: a prospective study. Am J Epidemiol. 1996;144(7):653–660. doi:10.1093/oxfordjournals.aje.a008977. [DOI] [PubMed] [Google Scholar]
- 6.Khanjani N, Jafarnejad A- B, Tavakkoli L. Arsenic and breast cancer: a systematic review of epidemiologic studies. Rev Environ Health. 2017;0(0):267–277. doi:10.1515/reveh-2016-0068. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger D, Leviton A, Waterneaux C, Needleman H, Rabinowitz M. Longitudinal Analysis of Prenatal and Postnatal Lead Exposure and Early Cognitive Development. N Engl J Med. 1987;316(17):1037–1043. [DOI] [PubMed] [Google Scholar]
- 8.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dL in US children and adolescents. Public Health Rep. 2000;115(6):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. [DOI] [PubMed] [Google Scholar]
- 10.Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. 2005;26(4–5 SPEC. ISS.):235–244. doi:10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Järup L Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi:10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 12.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Metals and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(1):63–73. doi:10.1007/s10911-013-9273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Volume 3: Environmental Toxicology. Vol 101; 2012. doi:10.1007/978-3-7643-8340-4. [Google Scholar]
- 14.Castro-González MI, Méndez-Armenta M. Heavy metals: Implications associated to fish consumption. Environ Toxicol Pharmacol. 2008;26(3):263–271. doi:10.1016/j.etap.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Bernhard D, Rossmann A, Wick G. Metals in cigarette smoke. IUBMB Life. 2005;57(12):805–809. doi:10.1080/15216540500459667. [DOI] [PubMed] [Google Scholar]
- 16.Sanders AP, Messier KP, Shehee M, Rudo K, Serre ML, Fry RC. Arsenic in North Carolina: Public Health Implications. Environ Int. 2012;38(1):10–16. doi:10.1016/j.envint.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams S V, Shafer MM, Bonner MR, et al. Urinary Cadmium and Risk of Invasive Breast Cancer in the Women’s Health Initiative. Am J Epidemiol. 2016;183(9):815–823. doi:10.1093/aje/kwv285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksen KT, McElroy JA, Harrington JM, et al. Urinary Cadmium and Breast Cancer: A Prospective Danish Cohort Study. J Natl Cancer Inst. 2017;109(2):djw204. doi:10.1093/jnci/djw204. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Orsini N, Wolk A. Urinary Cadmium Concentration and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis. Am J Epidemiol. 2015;182(5):375–380. doi:10.1093/aje/kwv085. [DOI] [PubMed] [Google Scholar]
- 20.McElroy JA, Shafer MM, Gangnon RE, Crouch LA, Newcomb PA. Urinary Lead Exposure and Breast Cancer Risk in a Population-Based Case–control Study. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2311–2317. doi:10.1158/1055-9965.EPI-08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-Carrillo L, Hernández-Ram\’\irez RU, Gandolfi AJ, Ornelas-Aguirre JM, Torres-Sánchez L, Cebrian ME. Arsenic methylation capacity is associated with breast cancer in northern Mexico. Toxicol Appl Pharmacol. 2014;280(1):53–59. doi:10.1016/j.taap.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Pineda-Belmontes CP, Hernández-Ramírez RU, Hernández-Alcaraz C, Cebrián ME, López-Carrillo L. Genetic polymorphisms of PPAR gamma, arsenic methylation capacity and breast cancer risk in Mexican women. Salud Publica Mex. 2016;58(2):220–227. [DOI] [PubMed] [Google Scholar]
- 23.Muszyńska M, Jaworska-Bieniek K, Durda K, et al. Arsenic (As) and breast cancer risk. Hered Cancer Clin Pract. 2012;10(Suppl 4):A8. doi:10.1186/1897-4287-10-S4-A8. [Google Scholar]
- 24.Sanders AP, Flood K, Chiang S, Herring AH, Wolf L, Fry RC. Towards prenatal biomonitoring in North Carolina: Assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS One. 2012;7(3):1–7. doi:10.1371/journal.pone.0031354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders AP, Miller SK, Nguyen V, Kotch JB, Fry RC. Toxic metal levels in children residing in a smelting craft village in Vietnam: a pilot biomonitoring study. BMC Public Health. 2014;14(1):114. doi:10.1186/1471-2458-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders AP, Burris HH, Just AC, et al. Altered miRNA expression in the cervix during pregnancy associated with lead and mercury exposure. Epigenomics. 2015;7(6):885–896. doi:10.2217/epi.15.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR. Placental Metal Concentrations in Relation to Maternal and Infant Toenails in a U.S. Cohort. Environ Sci Technol. 2016;50(3):1587–1594. doi:10.1021/acs.est.5b05316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grashow R, Zhang J, Fang SC, et al. Inverse association between toenail arsenic and body mass index in a population of welders. Environ Res. 2014;131:131–133. doi:10.1016/j.envres.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Cavallari JM. Toenail metal concentration as a biomarker of occupational welding fume exposure. J Occup Environ Hyg. 2014;11(6):397–405. doi:10.1080/15459624.2013.875182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Rorke MA, Cantwell MM, Abnet CC, Brockman AJD, Murray LJ. Toenail trace element status and risk of Barrett’s oesophagus and oesophageal adenocarcinoma: Results from the FINBAR study. Int J Cancer. 2012;131(8):1882–1891. doi:10.1002/ijc.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuiper N, Rowell C, Nriagu J, Shomar B. What do the trace metal contents of urine and toenail samples from Qatar׳s farm workers bioindicate? Environ Res. 2014;131:86–94. doi:10.1016/j.envres.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: A review. Environ Res. 2006;102(1):125–139. doi:10.1016/j.envres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatology Venereol. 2010;24:420–423. doi:10.1111/j.1468-3083.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien KM, Upson K, Cook NR, et al. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ Health Perspect. 2016;124(2):220–227. doi:10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Env Heal Perspect. 2005;113(2):191–200. doi:10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernard A Confusion about Cadmium Risks: The Unrecognized Limitations of an Extrapolated Paradigm. Env Heal Perspect. 2015;124(1). doi:10.1289/ehp.1509691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien KMKM, Upson K, Buckley JPJP, O’Brien KM, Upson K, Buckley JPJP. Lipid and Creatinine Adjustment to Evaluate Health Effects of Environmental Exposures. Curr Environ Heal reports. 2017;4(1). doi:10.1007/s40572-017-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madden EF, Fowler BA. Mechanisms of Nephrotoxicity From Metal Combinations: a Review. Drug Chem Toxicol. 2000;23(1):1–12. doi:10.1081/DCT-100100098. [DOI] [PubMed] [Google Scholar]
- 39.Laohaudomchok W, Lin X, Herrick RF, et al. Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med. 2011;53(5):506–510. doi:10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson LR, Farmer JG. Use of human metabolic studies and urinary arsenic speciation in assessing arsenic exposure. Bull Environ Contam Toxicol. 1991;46(1):53–61. [DOI] [PubMed] [Google Scholar]
- 41.Platz EA, Helzlsouer KJ, Hoffman SC, Morris JS, Baskett CK, Comstock GW. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate. 2002;52(4):288–296. doi:10.1002/pros.10115. [DOI] [PubMed] [Google Scholar]
- 42.Krogh V, Pala V, Vinceti M, et al. Toenail selenium as biomarker: reproducibility over a one-year period and factors influencing reproducibility. J Trace Elem Med Biol. 2003;17(Suppl 1):1–36. [PubMed] [Google Scholar]
- 43.Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2(5):493–497. [PubMed] [Google Scholar]
- 44.Karagas MR, Le CX, Morris S, et al. Markers of low level arsenic exposure for evaluating human cancer risks in a US population. Int J Occup Med Environ Health. 2001;14(2):171–175. [PubMed] [Google Scholar]
- 45.Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study: Baseline methods and participant characteristics. Env Heal Perspect. 2017;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White AJ, O’Brien KM, Jackson BP, Karagas MR. Urine and toenail cadmium levels in pregnant women: a reliability study. Rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsoi MF, Cheung CL, Cheung TT, Cheung BMY. Continual Decrease in Blood Lead Level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am J Med. 2016;129(11):1213–1218. doi:10.1016/j.amjmed.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Hernandez A, Navas-Acien A, Pastor-Barriuso R, et al. Declining exposures to lead and cadmium contribute to explaining the reduction of cardiovascular mortality in the US population, 1988–2004. Int J Epidemiol. 2017;46(6):1903–1912. doi:10.1093/ije/dyx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Environmental Protection Agency. Lead Concentrations. Rep Environ. 2014. [Google Scholar]
- 50.Environmental Protection Agency. Air trends: Lead. https://www.epa.gov/air-trends/lead-trends. Accessed April 28, 2018.
- 51.Jamal A, Homa DM, O’Connor E, et al. Current Cigarette Smoking Among Adults — United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. doi:10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- 52.Järup L, Åkesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. doi:10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


