Abstract
Resveratrol, a natural polyphenol compound, has been shown to possess anticancer activity. However, how resveratrol inhibits cancer cell adhesion has not been fully elucidated. Here, we show that resveratrol suppressed the basal or type I insulin-like growth factor (IGF-1)-stimulated adhesion of cancer cells (Rh1, Rh30, HT29 and HeLa cells) by inhibiting Erk1/2 pathway. Inhibition of Erk1/2 with U0126, knockdown of Erk1/2, or over-expression of dominant negative MKK1 strengthened resveratrol’s inhibition of the basal or IGF-1-stimulated of Erk1/2 phosphorylation and cell adhesion, whereas ectopic expression of constitutively active MKK1 attenuated the inhibitory effects of resveratrol. Further research revealed that both PP2A and PTEN/Akt were implicated in resveratrol-inactivated Erk1/2-dependent cell adhesion. Inhibition of PP2A with okadaic acid or over-expression of dominant negative PP2A rendered resistance to resveratrol’s suppression of the basal or IGF-1-stimulated phospho-Erk1/2 and cell adhesion, whereas expression of wild-type PP2A enhanced resveratrol’s inhibitory effects. Over-expression of wild-type PTEN or dominant negative Akt, or inhibition of Akt with Akt inhibitor X strengthened resveratrol’s inhibition of the basal or IGF-1-stimulated Erk1/2 phosphorylation and cell adhesion. Furthermore, inhibition of mTOR with rapamycin or silencing mTOR enhanced resveratrol’s inhibitory effects on the basal and IGF-1-induced inhibition of PP2A/PTEN, activation of Akt/Erk1/2, and cell adhesion. The results indicate that resveratrol inhibits Erk1/2-mediated adhesion of cancer cells via activating PP2A/PTEN signaling network. Our data highlight that resveratrol has a great potential in the prevention of cancer cell adhesion.
Keywords: Resveratrol, Cell adhesion, Erk1/2, PP2A, PTEN
1. INTRODUCTION
Resveratrol (3,4′,5-trihydroxystilbene; Res), a polyphenolic phytoalexin produced by grape and a few other plant species to protect them against fungal infections, possesses pharmacological activities such as cardioprotection, anti-aging, anti-inflammation and anti-carcinogenesis (Hasan and Bae, 2017; Park and Pezzuto, 2015). The anti-carcinogenic effect of resveratrol is attributed to its capability to inhibit proliferation, induce apoptosis and interfere with autophagy in cancer cells (Cucciolla et al., 2007; Shakibaei et al., 2009). Recently it has been reported that resveratrol exhibits anti-metastatic property in a spectrum of cancer cells (Bhattacharya et al., 2011; Salado et al., 2011; Yu et al., 2013). Adhesion of cancer cells to distant organs is a critical step during cancer metastatic process (Gupta and Massague, 2006; Steeg and Theodorescu, 2008). To date, several studies have shown that resveratrol reduced cell adherent ability in numerous malignancies (Bai et al., 2017; Mikula-Pietrasik et al., 2014; Park et al., 2009). However, how resveratrol inhibits cancer cell adhesion is not well understood.
Extracellular matrix (ECM), the non-cellular component present within all tissues and organs, is composed of water, proteins and polysaccharides (Frantz et al., 2010). ECM proteins, including collagen, elastin, fibronectin, vitronectin and laminin, mediate a wide variety of cellular interactions with ECM and play important roles in cell adhesion and migration (Frantz et al., 2010; Cescon et al., 2015; Pankov and Yamada, 2002; Yao, 2017). Cell adhesion to the ECM is mediated by ECM receptors (Humphries et al., 2006; Harburger and Calderwood, 2009; Leitinger and Hohenester, 2007). Integrins are the major receptors on the cell surface for adhesion to ECM (Hynes, 2002).
Cell adhesion is a multistep cellular process that is regulated by complex extracellular and intracellular signals (Homrich et al., 2015; Ridley et al., 2003). Increasing evidences have shown that extracellular signal-regulated kinase 1/2 (Erk1/2), an important member of mitogen-activated protein kinase (MAPK) family, is involved in cell adhesion regulation (Wang et al., 2013a; Zennadi et al., 2012). For example, shikonin inhibits proliferation, adhesion, migration and invasion by suppressing integrin β1 expression and the Erk1/2 pathway in A549 lung cancer cells (Wang et al., 2013a). Additionally, it has been reported that resveratrol regulates Erk1/2 activity in cancer cells (Kato et al., 2015; Kulkarni and Canto, 2015). For instance, resveratrol suppresses the Akt-GSK3β and Erk1/2 signaling pathways, resulting in reduction of nuclear cyclin D1 expression and cell cycle arrest in pancreatic cancer cells (Kato et al., 2015). Hence, we hypothesized that resveratrol may inhibit cancer cell adhesion by regulating Erk1/2 pathway.
Protein phosphatase 2A (PP2A) and phosphatase and tensin homologue on chromosome 10 (PTEN) are two tumor suppressor phosphatases, whose loss of function has been observed in many cancers (Kalev and Sablina, 2011; Perrotti and Neviani, 2013; Xu et al., 2014). Interestingly, recent studies have shown that both PP2A and PTEN negatively regulate Erk1/2 pathway in several malignancies (Chetram and Hinton, 2012; Xie et al., 2015). Besides, PTEN negatively regulates Akt by its lipid phosphatase activity and Akt activates Erk1/2 through protein kinase C (PKC)/Raf signaling axis (Chetram and Hinton, 2012; Polak and Hall, 2009). Importantly, PP2A, PTEN and Akt function by regulating cell adhesion and migration (Haier and Nicolson, 2002; Kraus et al., 2002; Sontag and Sontag, 2006). In addition, studies have shown that resveratrol regulates PP2A, PTEN and Akt (Liu et al., 2015; Liu et al., 2014). Collectively, these findings suggest that resveratrol may affect Erk1/2 and cancer cell adhesion through mediating PP2A and PTEN/Akt signaling pathway.
Mechanistic/mammalian target of rapamycin (mTOR), a serine/threonine protein kinase, is a central controller of cell growth and metabolism (Laplante and Sabatini, 2012; Yang et al., 2013). It is dysregulated in many human cancers and contributes to cancer pathogenesis and therapy resistance (Guertin and Sabatini, 2007). mTOR lies downstream of type I insulin-like growth factor (IGF-1) receptor and functions at least as two complexes (mTORC1 and mTORC2) in mammalian cells (Laplante and Sabatini, 2012; Yang et al., 2013). mTORC1 phosphorylates p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), while mTORC2 regulates phosphorylation or activity of Akt, glucocorticoid-inducible kinase 1 (SGK1), PKCα, focal adhesion proteins and small GTPases (Laplante and Sabatini, 2012; Yang et al., 2013). Recent studies have demonstrated that resveratrol inhibits mTOR signaling (Widlund et al., 2013). Interestingly, we recently found that both mTORC1 and mTORC2 are involved in the regulation of cell adhesion (Chen et al., 2015b). Taken together, we postulated that resveratrol might inhibit cancer cell adhesion via mediating PP2A/PTEN-Erk1/2 signaling network in an mTOR kinase activity-dependent manner.
Here we show that resveratrol attenuates IGF-1-induced adhesion of cancer cells in part by suppressing Erk1/2 pathway. Mechanistically, resveratrol blocks Erk1/2 pathway, not only by activating PP2A, but also via activating PTEN and inactivating Akt, thereby preventing IGF-1-induced adhesion in cancer cells. Our findings underline a potential beneficial role of resveratrol in the treatment of cancer, especially in the prevention of cancer cell adhesion.
2. MATERIALS AND METHODS
2.1. Materials
Resveratrol, collagen type IV (CN IV), fibronectin, laminin, U0126 and okadaic acid were purchased from Sigma (Saint Louis, MO, USA). Resveratrol was dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution (100 mM), aliquoted and stored at −80˚C. IGF-1 was from PeproTech (Rocky Hill, NJ, USA), rehydrated in 0.1 M acetic acid to prepare a 10 μg/ml stock solution and stored at −80°C. RPMI 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM) and 0.05% trypsin-EDTA were obtained from Invitrogen (Grand Island, NY, USA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT, USA). Akt inhibitor X was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rapamycin was purchased from LC Laboratories (Woburn, MA, USA). CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit was from Promega (Madison, WI, USA). Enhanced chemiluminescence solution was from Millipore (Billerica, MA, USA). Other chemicals were purchased from local commercial sources and were of analytical grade.
2.2. Cell line and culture
Human Ewing sarcoma (Rh1) and rhabdomyosarcoma (Rh30) cells were described previously (Chen et al., 2015b) and cultured in antibiotic-free RPMI 1640 medium supplemented with 10% FBS. Colon carcinoma (HT29) and cervical adenocarcinoma (HeLa) cells, obtained from American Type Culture Collection (Manassas, VA, USA), were grown in antibiotic-free DMEM supplemented with 10% FBS. The cells were maintained in a humid incubator (37°C, 5% CO2).
2.3. Recombinant adenoviral constructs and infection of cells
The recombinant adenoviruses encoding FLAG-tagged constitutively active MKK1 (MKK1-R4F), dominant-negative MKK1 (MKK1-K97M), hemagglutinin (HA)-tagged dominant-negative PP2A catalytic subunit (dn-PP2A, L199P), FLAG-tagged wild-type rat PP2Acα (Ad-PP2A), wild-type human PTEN (Ad-PTEN), hemagglutinin (HA)-tagged dominant-negative Akt (dn-Akt, T308A/S473A) and the control vector expressing green fluorescent protein (GFP) alone (Ad-GFP) were described previously (Chen et al., 2009; Liu et al., 2010a). To construct recombinant adenoviruses expressing FLAG-tagged MKK1-R4F and MKK1-K97M, DNA fragments encoding the corresponding mutants were excised from pMCL-MKK1-R4F and pMCL-MKK1-K97M (Mansour et al., 1994) (gifts from Dr. Natalie Ahn, University of Colorado, Boulder, CO, USA), and then sub-cloned to FLAG-tagged pENTR11 shuttle vector. The recombinant adenovirus was generated using ViraPower™ Adenoviral Gateway™ Expression Kit (Invitrogen, Carlsbad, CA, USA) following the manufacture’s instruction. For experiments, Rh30 and HeLa cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 1 of multiplicity of infection (MOI = 1). Subsequently, cells were used for experiments. Ad-GFP served as a control. Expression of FLAG-tagged MKK1-R4F, MKK1-K97M and PP2A, as well as HA-tagged dn-PP2A and dn-Akt was determined by Western blot analysis with antibodies to FLAG and HA, respectively.
2.4. Lentiviral shRNA cloning, production, and infection
Lentiviral shRNAs to Erk1/2, mTOR and GFP (for control) were generated and used as described (Chen et al., 2008; Chen et al., 2015b). Rh30 and HeLa cells, when grown to about 70% confluence, were infected with above lentivirus-containing supernatant in the presence of 8 μg/ml polybrene for 24 h and then exposed to 2 μg/ml puromycin for 48 h. In 5 days, cells were used for experiments.
2.5. Cell adhesion assay
Cell adhesion was evaluated by using CN IV-, fibronectin- or laminin-coated assay, as described previously (Sawhney et al., 2004). Briefly, 48-well tissue culture plates were coated for 2 h at 37°C with CN IV (0.2 μg/ml), fibronectin (0.5 μg/ml) or laminin (0.5 μg/ml) respectively, followed by blocking with 3% bovine serum albumin for 3 h, and then rinsed once with PBS. Cells for determination of adhesion functions were changed to serum-free medium and grown for 24 h. After trypsinization, serum-starved cells were plated at 5 × 104 cells/well on CN IV, fibronectin or laminin-coated plates and incubated for 4 h in the absence or presence of resveratrol (0-100 μM) and/or IGF-1 (10 ng/ml), with 6 replicates of each treatment. Non-adherent cells were removed by washing three times with serum-free medium. Afterwards, each well was added 20 μl of MTS reagent (one solution reagent) and incubated for 1 h. The relative number of attached cells was determined by measuring the optical density (OD) at 490 nm using a Victor X3 Light Plate Reader (PerkinElmer, Waltham, MA, USA).
2.6. Wound-healing assay
Cell motility was assessed by the wound-healing assay as described (Liu et al., 2006). Briefly, a monolayer of cells were grown in 6-well plates to 80% confluence and serum starved for 24 h, with 6 replicates of each treatment. Migration was initiated by removing a portion of the cell layer by scratching with a single-edge razor blade cut to ~27 mm in length. The scratch began at the diameter of the dish and extended over an area ~10 mm wide. The medium was changed to remove floating or damaged cells. Cells were pretreated with or without resveratrol (0–100 μM) for 4 h, followed by stimulation with or without IGF-1 (10 ng/ml) for 20 h. Cells migrated over the denuded area were observed and taken under a Leica inverted phase-contrast microscope equipped with Quick Imaging system (Leica DMi8, Wetzlar, Germany). The number of cells migrating per millimeter of scratch was counted.
2.7. Cell viability assay
Cell viability was evaluated using MTS assay, as described (Chen et al., 2014). Briefly, cells were seeded in a 96-well plate at a density of 1 × 104 cells/well in the growth medium and grown overnight at 37°C in a humidified incubator with 5% CO2. After serum-starvation for 24 h, cells were treated with 0–100 μM resveratrol for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h, with 6 replicates of each treatment. Subsequently, cell viability, after incubation with MTS reagent (one solution reagent) (20 μl/well) for 3 h, was evaluated by measuring the optical density (OD) at 490 nm using a Victor X3 Light Plate Reader (PerkinElmer, Waltham, MA, USA).
2.8. Assay for live cell number
Live cell number was counted by using Trypan blue exclusion as described previously (Chen et al., 2014). In short, serum-starved cells, seeded in 6-well plates at a density of 2 × 105 cells/well, were treated with 0–100 μM resveratrol for 4 h and stimulated with/without IGF-1 (10 ng/ml) for 1 h, with 6 replicates of each treatment. Then, the floating and attached cells were collected and incubated with 0.4% trypan blue solution (Sigma, Saint Louis, MO, USA) for 3 min at room temperature. Afterwards, the viable (unstained) and nonviable (stained) cells in the trypan blue/cell mixture were counted separately under a microscope using a hemacytometer. Finally, the relative number of live cells was presented by calculating the ratio of the unstained cells / the total cells.
2.9. In vitro PP2A phosphatase assay
In vitro PP2A phosphatase activity was determined as described (Liu et al., 2010a), with some modifications. Briefly, after serum-starvation for 24 h, cells were incubated for 4 h in the absence or presence of resveratrol (0–100 μM) ± IGF-1 (10 ng/ml), with 6 replicates of each treatment. Subsequently, the cells were lysed in 50 mM Tris-HCl buffer, pH 7.0, containing 1% Nonidet P-40, 2 mM EDTA, and protease inhibitor cocktail (Sigma, Saint Louis, MO, USA. 1:1000). PP2Ac in cell lysates was immunoprecipitated with antibodies to PP2Ac (Millipore, Temecula, CA, USA), and protein A/G agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Next, the beads were washed three times with the above lysis buffer, followed by washing twice with the phosphatase assay buffer (50 mM Tris-HCl, pH 7.0, 0.1 mM CaCl2). The phosphatase activity of immunoprecipitated PP2A was assayed with a Ser/Thr Phosphatase Assay kit 1 using p-nitrophenyl phosphate (pNPP) as the substrate (Millipore, Bedford, MA, USA) according to the manufacturer’s instructions. Finally, all data pooled from three different batches of experiments were statistically analyzed.
2.10. Western blot analysis
Western blotting was performed in three independent experiments, as described previously (Chen et al., 2014). Briefly, the indicated cells, after treatments, were washed with cold PBS, and then on ice, lysed in the radioimmunoprecipitation assay buffer. After that, lysates containing equivalent amounts of protein were separated on 6–12% sodium dodecyl sulfate -polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were incubated with PBS containing 0.05% Tween 20 and 5% nonfat dry milk to block nonspecific binding, and then with primary antibodies against phospho-Akt (p-Akt) (Thr308), p-Akt (Ser473), p-S6K1 (Thr389) and mTOR (Cell Signaling Technology, Danvers, MA, USA), PP5, p-Erk1/2 (Thr202/Tyr204), Erk2, demethylated-PP2A, Akt and S6K1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), PP2Acα (BD Biosciences, San Jose, CA, USA), p-PP2A, p-PTEN (Thr366) and PTEN (Epitomics, Burlingame, CA, USA), PP2A-A subunit, PP2A-B subunit (Millipore, Bedford, MA, USA), MKK1, FLAG, HA and β-tubulin (Sigma, Santa Cruz, CA, USA) overnight at 4°C, respectively, followed by incubation with appropriate secondary antibodies including horseradish peroxidase-conjugated goat anti-rabbit IgG, goat anti-mouse IgG, or rabbit anti-goat IgG (Pierce, Rockford, IL, USA) overnight at 4°C. Immunoreactive bands were visualized by using enhanced chemiluminescence solution (Millipore, Bedford, MA, USA). The blots for detected proteins were semi-quantified using NIH Image J software (National Institutes of Health, Bethesda, MD, USA).
2.11. Statistical analysis
Results were expressed as mean ± standard error of the mean (SEM). Statistically significant differences between treatment means were identified by using the Student’s t-test for non-paired replicates. One-way or two-way ANOVA followed by Bonferroni’s post-tests to compare replicate means was conducted to compare group variability and interaction. p-value of <0.05 was considered significant.
3. RESULTS
3.1. Resveratrol attenuates IGF-1-stimulated adhesion of cancer cells
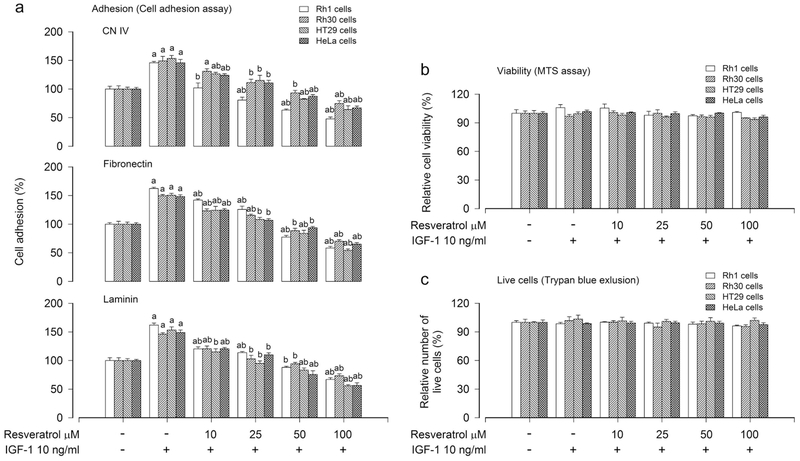
Extracellular matrix (ECM) is tissue-specific due to unique composition and topology (Frantz et al., 2011). To determine the general cell adhesion function, representative ECM substrates (CN IV, fibronectin and laminin) were selected as markers of cell adhesion assay according to our previous study (Chen et al., 2015b). To investigate the effect of resveratrol on cancer cell adhesion, human Ewing sarcoma (Rh1), rhabdomyosarcoma (Rh30), colon carcinoma (HT29) and cervical adenocarcinoma (HeLa) cells, after serum-starved for 24 h, were treated with 0–100 μM resveratrol for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. Adherent cells were determined using CN IV-, fibronectin- or laminin-coated cell adhesion assay. As shown in Figure 1a, exposure to resveratrol inhibited IGF-1-stimulated cancer cell adhesion in a concentration-dependent manner. In addition, we also determined the effect of resveratrol on cancer cell motility using the wound-healing assay, showing that treatment with resveratrol also inhibited IGF-1-stimulated cancer cell motility in a concentration-dependent manner (Figure S1). To exclude the possibility that resveratrol inhibits IGF-1-stimulated cancer cell adhesion by reducing cell viability or inducing cell death, we also examined the effect of resveratrol on cell viability and live cell number using MTS assay and trypan blue exclusion, respectively. As shown in Figure 1b, c, treatment with resveratrol (0–100 μM) for 4 h and then stimulation with/without IGF-1 (10 ng/ml) did not significantly influence cell viability or live cell number in all cell lines tested (Rh1, Rh30, HT29 and HeLa). The results indicate that resveratrol attenuates IGF-1-stimulated adhesion of cancer cells, which is not through reducing cell viability or live cell number.
FIGURE 1.
Inhibitory effect of administered resveratrol in Rh1, Rh30, HT29 and HeLa cells on IGF-1-stimulated cell adhesion. Serum-starved Rh1, Rh30, HT29 and HeLa cells were treated with resveratrol (0–100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a) Adherent cells were evaluated using CN IV-, fibronectin- or laminin-coated cell adhesion assay. (b) Cell viability was determined by the MTS assay. (c) Live cells were detected by counting viable cells using trypan blue exclusion. Results are presented as mean ± SEM (n = 6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group.
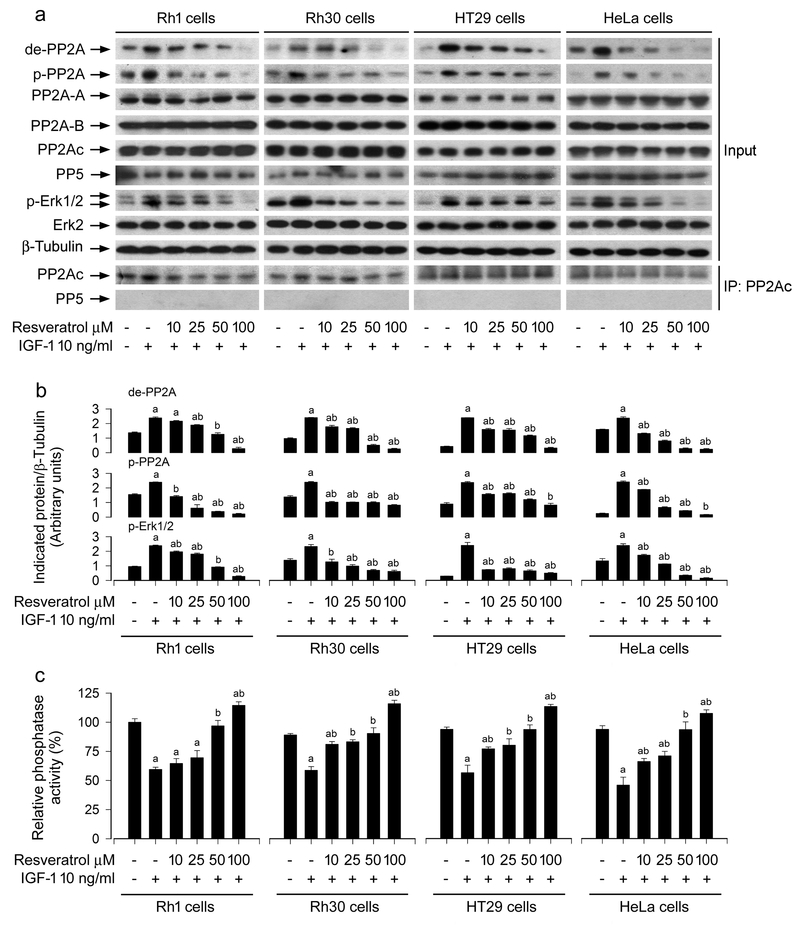
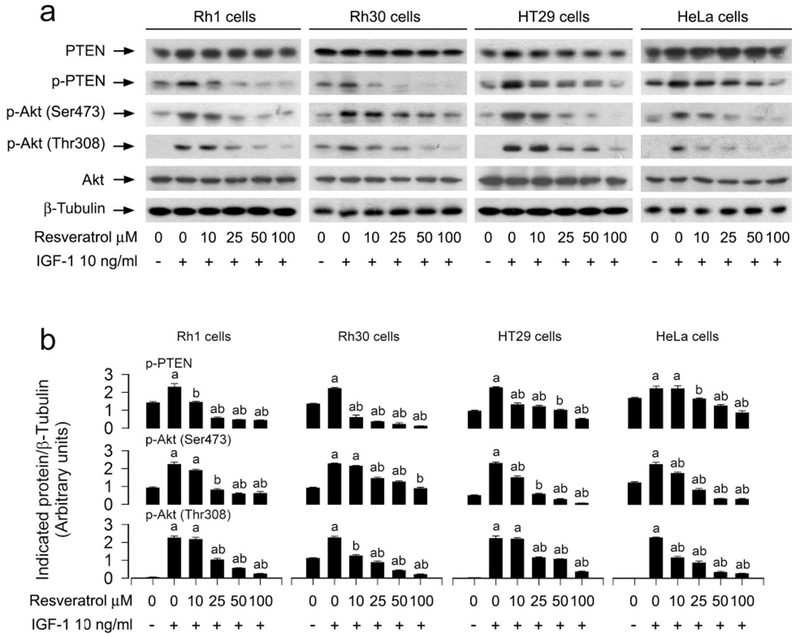
3.2. Resveratrol intervenes in IGF-1-stimulated inhibition of PP2A and activation of Erk1/2 in cancer cells
Resveratrol may activate or inhibit Erk1/2, depending on the concentration of resveratrol used (Kato et al., 2015; Miloso et al., 1999). Besides, resveratrol can regulate PP2A activity (Liu et al., 2015). Importantly, both Erk1/2 and PP2A are involved in the control of cell adhesion (Sontag and Sontag, 2006; Wang et al., 2013a). To explore the molecular mechanism of resveratrol-inhibited cancer cell adhesion, we checked the effect of resveratrol on Erk1/2 and PP2A activity in cancer cells. As shown in Figure 2a, b, resveratrol inhibited IGF-1-stimulated phosphorylation of Erk1/2 in a concentration-dependent manner, suggesting a reduction of Erk1/2 activity in cancer cells. In addition, resveratrol did not alter cellular protein levels of PP2Ac, PP2A-A or PP2A-B, but reduced IGF-1-induced demethylated-PP2Ac (de-PP2A) and phospho-PP2Ac (p-PP2A) in a concentration-dependent manner, indicating that resveratrol activates PP2A. This is further supported by the finding that resveratrol attenuated IGF-1-induced decrease of PP2A activity, as determined by the in vitro Ser/Thr phosphatase assay (Figure 2c). To make sure that PP2A is actually the only protein specifically immunoprescipitated, we also conducted Western blot analysis for PP5. As expected, only PP2Ac, but not PP5, was seen in the immunoprecipitates with anti-PP2Ac antibody (Figure 2a), indicating that in vitro PP2A phosphatase assay is specific for PP2Ac.
FIGURE 2.
Block effects of administered resveratrol in Rh1, Rh30, HT29 and HeLa cells on IGF-1-induced inhibition of PP2A and activation of Erk1/2. Serum-starved Rh1, Rh30, HT29 and HeLa cells were treated with resveratrol (0–100 μM) for 4 h and then stimulated with/without IGF-1 (10 ng/ml) for 1 h. (a) Total cell lysates were subjected to Western blotting using indicated antibodies. Immunoprecipitation (IP) was performed by incubation of cell lysates (500 μg) with antibodies to PP2Ac, followed by immunoblotting with antibodies to PP2Ac and PP5, respectively. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b) The blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (c) PP2A in cell lysates was immunoprecipitated with antibodies to PP2Ac plus protein A/G agarose beads, followed by in vitro phosphatase assay using Ser/Thr Phosphatase Assay Kit. Results are presented as mean ± SEM (n = 3–6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group.
3.3. Resveratrol suppresses IGF-1-stimulated cell adhesion through blocking Erk1/2 pathway in cancer cells
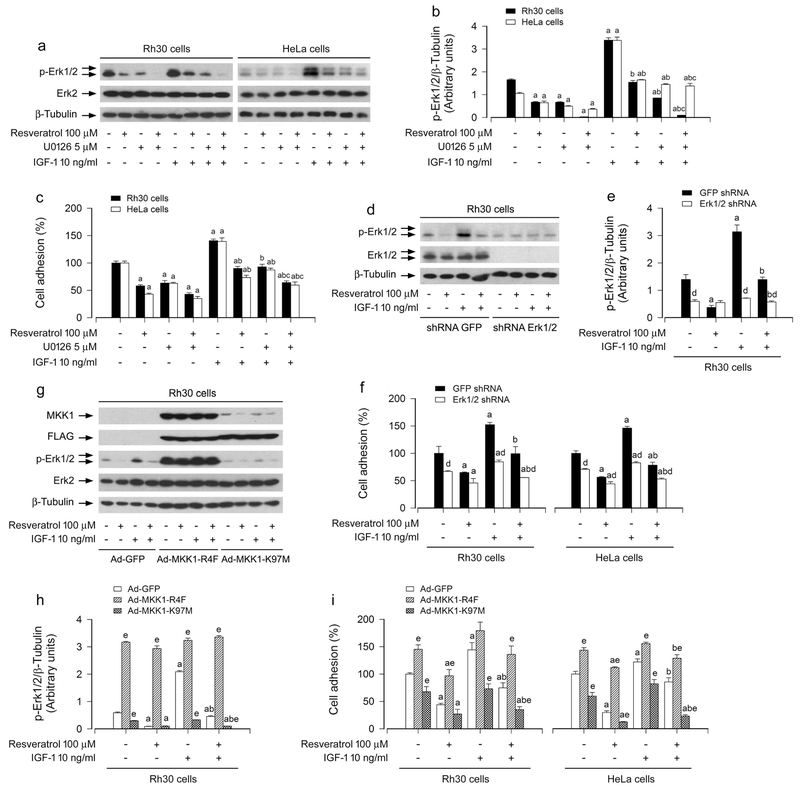
To unveil whether resveratrol inhibition of IGF-1-stimulated cancer cell adhesion is related to resveratrol blockage of Erk1/2 activation, Rh30 and HeLa cells was pre-incubated with/without U0126 (a selective inhibitor of MKK1/2, upstream of Erk1/2) alone, or in combination with resveratrol. We found that U0126 (5 μM) or resveratrol (100 μM) alone obviously suppressed the basal and IGF-1-stimulated phosphorylation of Erk1/2 (Figure 3a, b). Furthermore, co-treatment with resveratrol/U0126 exhibited a stronger inhibitory effect on the basal and IGF-1-induced phospho-Erk1/2 (p-Erk1/2) in the cells (Figure 3a, b). Interestingly, the combination of resveratrol with U0126 also exhibited more potent inhibition of IGF-1-induced cell adhesion than resveratrol or U0126 alone (Figure 3c).
FIGURE 3.
Effects of inhibition of Erk1/2 by U0126, down-regulation of Erk1/2, or ectopic expression of MKK1-R4F or MKK1-K97M in Rh30 and HeLa cells on resveratrol’s inhibition of IGF-1-induced Erk1/2 activation and cell adhesion. Serum-starved Rh30 and HeLa cells, pre-incubated with/without U0126 (5 μM) for 1 h, or serum-starved Rh30 and/or HeLa cells, infected with lentiviral shRNA to Erk1/2 or GFP (as control), or infected with Ad-MKK1-R4F, Ad-MKK1-K97M or Ad-GFP (as control), respectively, were treated with/without resveratrol (100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a, d, g) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b, e, h) The blots for p-Erk1/2 were semi-quantified using NIH image J. (c, f, i) Adherent cells were determined using CN IV-coated cell adhesion assay. Results are presented as mean ± SEM (n = 3–6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group; c p < 0.05, difference with IGF-1/resveratrol group or IGF-1/U0126 group; d p < 0.05, Erk1/2 shRNA group vs. GFP shRNA group; e p < 0.05, Ad-MKK1-R4F group or Ad-MKK1-K97M group vs. Ad-GFP group.
We also confirmed the above findings using RNA interference. As detected by Western blotting, lentiviral shRNA to Erk1/2, but not GFP, silenced the expression of Erk1/2 protein by ~90%, and silencing Erk1/2 blocked the basal and IGF-1-induced phosphorylation of Erk1/2 in Rh30 cells (Figure 3d, e) and HeLa cells (data not shown). Consistently, down-regulation of Erk1/2 reduced the basal and IGF-1-stimulated cell adhesion in Rh30 and HeLa cells (Figure 3f). Importantly, addition of resveratrol exhibited more inhibitory effects on IGF-1-induced cell adhesion in the cells infected with lentiviral shRNA to Erk1/2 than in the control cells infected with lentiviral shRNA to GFP (Figure 3f). In addition, we also employed recombinant adenoviruses Ad-MKK1-R4F and Ad-MKK1-K97M, encoding FLAG-tagged constitutively active and dominant negative MKK1, respectively. Infection with Ad-MKK1-R4F and Ad-MKK1-K97M, but not Ad-GFP (control virus), resulted in expression of high levels of FLAG-tagged MKK1 mutants in Rh30 cells (Figure 3g). Expression of MKK1-R4F led to robust phosphorylation of Erk1/2, whereas expression of MKK1-K97M downregulated phosphorylation of Erk1/2 in the cells (Figure 3g, h), indicating that the MKK1 mutants function in the cells as expected. Similar results were seen in HeLa cells infected by Ad-MKK1-R4F and Ad-MKK1-K97M (data not shown). Of note, expression of MKK1-R4F in Rh30 and HeLa cells significantly elevated the basal or IGF-1-stimulated cell adhesion, and conferred profound resistance to resveratrol’s inhibitory effects (Figure 3i). In contrast, expression of MKK1-K97M in the cells dramatically reduced the basal or IGF-1-stimulated cell adhesion, and especially addition of resveratrol reinforced the events (Figure 3i). The results clearly indicate that resveratrol suppresses IGF-1-stimulated cell adhesion in part by blocking Erk1/2 pathway in cancer cells.
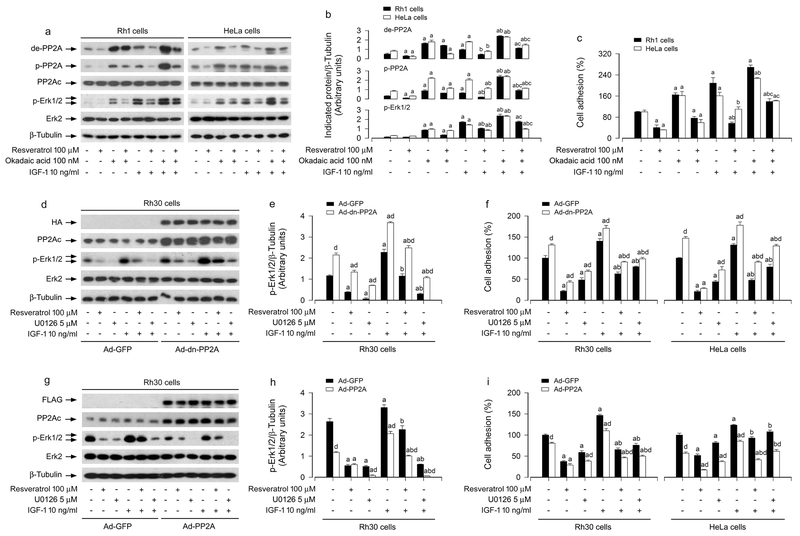
3.4. Resveratrol inhibits IGF-1-stimulated Erk1/2 activation and cell adhesion via PP2A-dependent manner in cancer cells
Since PP2A plays a crucial role in the regulation of Erk1/2 pathway (Xie et al., 2015), we next asked whether resveratrol’s blockage of IGF-1-induced activation of Erk1/2 pathway and consequential cell adhesion was due to activation of PP2A. Rh1 and HeLa cells were pretreated with 100 nM okadaic acid (a relatively specific PP2A inhibitor) alone, or in combination with 100 μM resveratrol, followed by incubation with/without 10 ng/ml IGF-1. As shown in Figure 4a, b, IGF-1 markedly stimulated the expression of de-PP2Ac, p-PP2Ac and p-Erk1/2, which was inhibited by resveratrol. Okadaic acid profoundly increased the basal or IGF-1-stimulated de-PP2Ac, p-PP2Ac and p-Erk1/2, and reversed the inhibitory effect of resveratrol on IGF-1-stimulated events (Figure 4a, b). In line with this, resveratrol inhibited the basal or IGF-1 stimulated cell adhesion in Rh1 and HeLa cells, which was significantly attenuated by okadaic acid (Figure 4c). These results imply that resveratrol inhibits the basal or IGF-1-induced Erk1/2 phosphorylation and cell adhesion through upregulation of PP2A activity in cancer cells.
FIGURE 4.
Effects of inhibition of PP2A by okadaic acid or ectopic expression of dominant negative or wild-type PP2A in Rh1, Rh30 and/or HeLa cells on resveratrol’s inhibition of IGF-1-induced Erk1/2 activation and cell adhesion. Resveratrol inhibits IGF-1-stimulated Erk1/2 activation and cell adhesion via activating PP2A. Serum-starved Rh1 and HeLa cells, pre-treated with/without okadaic acid (100 nM) for 1 h, or serum-starved Rh30 and/or HeLa cells, infected with Ad-GFP (as control), Ad-dn-PP2A and Ad-PP2A, respectively, and then pre-incubated with/without U0126 (5 μM) for 1 h, were treated with/without resveratrol (100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a, d, g) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b, e, h) The blots for de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (c, f, i) Adherent cells were determined using CN IV-coated cell adhesion assay. Results are presented as mean ± SEM (n = 3–6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group; c p < 0.05, difference with IGF-1/resveratrol group or IGF-1/okadaic acid group; d p < 0.05, Ad-dn-PP2A group or Ad-PP2A group vs. Ad-GFP group.
To gain more insights into the event that the upregulated PP2A activity is responsible for the inhibitory effects of resveratrol on the basal or IGF-1-induced Erk1/2 activation and cell adhesion, Rh30 and HeLa cells, infected with Ad-dominant negative (dn)-PP2A, Ad-PP2A and Ad-GFP (as control), respectively, were pretreated with/without U0126 (5 μM) for 1 h, and then treated with/without resveratrol (100 μM) for 4 h, followed by treatment with/without IGF-1 (10 ng/ml) for 1 h. A low basal level of p-Erk1/2 was observed in Ad-GFP-infected cells (control), whereas a higher basal level of p-Erk1/2 could be detected in Ad-dn-PP2A-infected Rh30 cells (Figure 4d, e) and HeLa cells (data not shown), suggesting the dn-PP2A was functioning in the cells. IGF-1 was able to stimulate the phosphorylation of Erk1/2 in Ad-GFP- and Ad-dn-PP2A-infected Rh30 (Figure 4d, e) and HeLa cells (data not shown). Of interest, expression of dn-PP2A markedly increased the basal cell adhesion, but conferred high resistance to resveratrol inhibition of the basal or IGF-1-stimulated cell adhesion (Figure 4f). However, in contrast, overexpression of wild-type PP2A powerfully suppressed the basal or IGF-1-induced p-Erk1/2 in the presence or absence of resveratrol in the cells (Figure 4g, h). Consistently, overexpression of wild-type PP2A notably inhibited the basal or IGF-1-induced cell adhesion in the presence or absence of resveratrol (Figure 4i). Taken together, our data strongly support the idea that resveratrol blocks the basal or IGF-1-induced Erk1/2 activation and cell adhesion via PP2A-dependent mechanism in cancer cells.
3.5. Resveratrol attenuates IGF-1-induced inactivation of PTEN and activation of Akt, leading to decreased Erk1/2 phosphorylation and cell adhesion in cancer cells
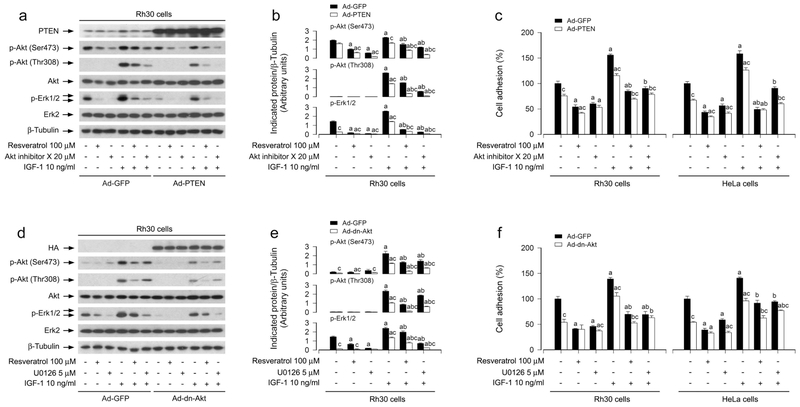
It is well-known that PTEN negatively regulates Akt pathway (Polak and Hall, 2009). In addition, Akt may activate Erk1/2 through PKC (Chetram and Hinton, 2012). Emerging studies have suggested that PTEN may also negatively regulate Erk1/2 pathway in several malignancies (Chetram and Hinton, 2012). Here, we hypothesized that resveratrol might block IGF-1-induced Erk1/2 activation and cell adhesion also partly by modulating PTEN/Akt signaling in cancer cells. As shown in Figure 5a, b, treatment with resveratrol attenuated IGF-1-induced phosphorylation of PTEN and Akt in Rh1, Rh30, HT29 and HeLa cells in a concentration-dependent manner. To identify the role and significance of PTEN/Akt pathway in resveratrol-inhibited IGF-1 stimulation of Erk1/2 activation and cell adhesion, Rh30 and HeLa cells, infected with recombinant adenovirus expressing wild-type human PTEN (Ad-PTEN) or Ad-GFP (as control), were pre-incubated with/without Akt inhibitor X (20 μM) for 1 h and then treated with/without resveratrol (100 μM) for 4 h, followed by incubation with/without IGF-1 (10 ng/ml) for 1 h. We observed that the infection with Ad-PTEN increased the expression of PTEN, compared to the infection with Ad-GFP in Rh30 cells (Figure 6a) and HeLa cells (data not shown). As expected, treatment with IGF-1 correspondingly increased phosphorylation of Akt and Erk1/2 in the cells infected with Ad-GFP (Figure 6a, b). Over-expression of PTEN blocked IGF-1-induced events in the cells (Figure 6a, b). Both resveratrol and Akt inhibitor X diminished IGF-1-induced p-Akt and p-Erk1/2 in the cells (Figure 6a, b). Furthermore, over-expression of PTEN was able to potentiate the inhibitory effects of resveratrol or Akt inhibitor X on IGF-1-induced phosphorylation of Akt and Erk1/2 in Rh30 cells (Figure 6a, b) and HeLa cells (data not shown). Moreover, we observed that over-expression of PTEN alone partially prevented IGF-1-induced cell adhesion in Rh30 and HeLa cells (Figure 6c). Addition of resveratrol or Akt inhibitor X elicited more significant suppression of IGF-1-induced cell adhesion (Figure 6c). Collectively, the findings support the notion that resveratrol inhibits IGF-1-induced activation of Erk1/2 and consequential cell adhesion in cancer cells, by attenuating IGF-1-induced inactivation of PTEN and activation of Akt.
FIGURE 5.
Preventive effects of administered resveratrol in Rh1, Rh30, HT29 and HeLa cells on IGF-1-induced inhibition of PTEN and activation of Akt. Serum-starved Rh1, Rh30, HT29 and HeLa cells were treated with resveratrol (0–100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b) The blots for p-PTEN, p-Akt (Ser473), and p-Akt (Thr308) were semi-quantified using NIH image J. Results are presented as mean ± SEM (n = 3). a p< 0.05, difference with control group; b p < 0.05, difference with IGF-1 group.
FIGURE 6.
Effects of ectopic expression of wild-type PTEN or dominant negative Akt in Rh30 and HeLa cells on resveratrol’s suppression of IGF-1-stimulated Akt/Erk1/2 activation and cell adhesion. Serum-starved Rh30 and/or HeLa cells, infected with Ad-GFP (as control), Ad-PTEN or Ad-dn-Akt, respectively, and pretreated with/without Akt inhibitor X (20 μM) or U0126 (5 μM) for 1 h, were treated with/without resveratrol (100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a, d) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b, e) The blots for p-Akt (Ser473), p-Akt (Thr308), and p-Erk1/2 were semi-quantified using NIH image J. (c, f) Adherent cells were determined using CN IV-coated cell adhesion assay. Results are presented as mean ± SEM (n = 3–6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group; c p < 0.05, Ad-PTEN group or Ad-dn-Akt group vs. Ad-GFP group.
To further verify the role of Akt in resveratrol’s blockage of IGF-1-induced Erk1/2 activation and cell adhesion in cancer cells, recombinant adenovirus expressing HA-tagged dominant negative Akt (Ad-dn-Akt) was utilized. As shown in Figure 6d, a high level of HA-tagged Akt mutant was seen in Rh30 cells infected with Ad-dn-Akt, but not in the cells infected with Ad-GFP (control virus). Similar results were observed in Ad-dn-Akt-infected HeLa cells (data not shown). Over-expression of dn-Akt remarkably suppressed IGF-1-triggered phosphorylation of Akt and Erk1/2 in the cells (Figure 6d, e). Resveratrol, but not U0126, powerfully attenuated IGF-1-increased Akt phosphorylation (Figure 6d, e). However, both resveratrol and U0126 obviously inhibited IGF-1-induced Erk1/2 phosphorylation in Rh30 cells (Figure 6d, e) and HeLa cells (data not shown). Interestingly, over-expression of dn-Akt was able to strengthen the inhibitory effects of resveratrol on IGF-1-induced phosphorylation of Akt or Erk1/2 in the cells (Figure 6d, e). Consistently, over-expression of dn-Akt also potently reinforced the inhibitory effect of resveratrol on IGF-1-induced cell adhesion in Rh30 and HeLa cells (Figure 6f). These data indicate that resveratrol blocks IGF-1-induced Erk1/2 activation and cell adhesion by suppressing IGF-1-induced activation of Akt in cancer cells.
3.6. Inhibition of mTOR potentiates resveratrol’s activation of PP2A/PTEN, leading to more inhibition of Akt/Erk1/2 and cell adhesion in IGF-1-stimulated cancer cells
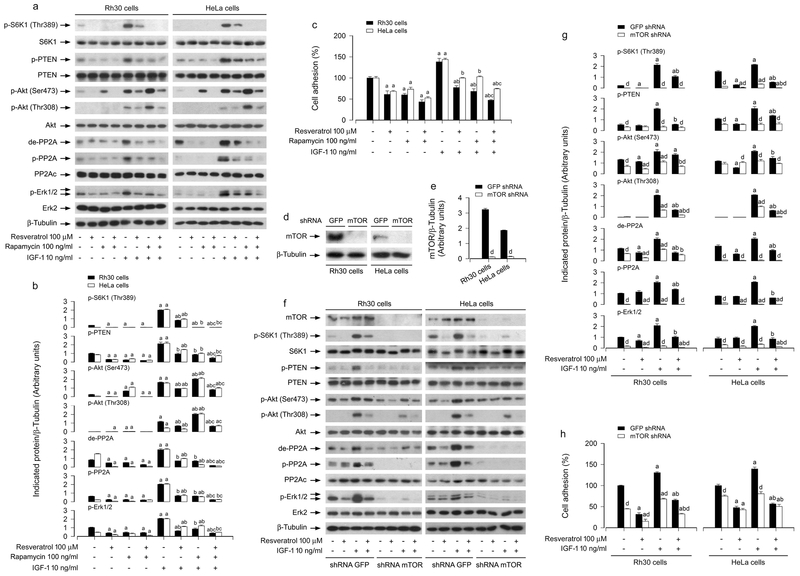
Increasing findings have shown that resveratrol can inhibit mTOR (Widlund et al., 2013). Importantly, we have demonstrated that mTOR is involved in the regulation of cell adhesion (Chen et al., 2015b). To determine whether inhibition of mTOR co-operates with resveratrol in activating PP2A and PTEN, leading to more inhibition of Erk1/2 and cancer cell adhesion, Rh30 and HeLa cells were pretreated with/without the mTORC1 inhibitor rapamycin (100 ng/ml) for 1 h, and then treated with/without resveratrol (100 μM) for 4 h, followed by incubation with/without IGF-1 (10 ng/ml) for 1 h. As shown in Figure 7a, b, rapamycin or resveratrol alone obviously suppressed the basal and IGF-1-stimulated de-PP2Ac, p-PP2Ac, p-PTEN and p-Erk1/2 in the cells. In line with previous observations (Chen et al., 2015b), rapamycin inhibited p-S6K1 and induced p-Akt. However, rapamycin potentiated the inhibitory effects of resveratrol on IGF-1-stimulated de-PP2Ac, p-PP2Ac, p-PTEN and p-Erk1/2. Consistently, the combination of resveratrol with rapamycin also exhibited more potent inhibition of IGF-1-triggered cell adhesion than resveratrol or rapamycin alone (Figure 7c). These results imply that inhibition of mTOR enhances the activating effect of resveratrol on PP2A and PTEN, resulting in more inhibition of Erk1/2 and cell adhesion, in response to IGF-1.
FIGURE 7.
Effects of inhibition of mTOR by rapamycin or down-regulation of mTOR in Rh30 and HeLa cells on resveratrol’s prevention from IGF-1-induced PP2A/PTEN inhibition, Akt/Erk1/2 activation and cell adhesion. Serum-starved Rh30 and HeLa cells, pre-incubated with/without rapamycin (100 ng/ml) for 1 h, or infected with lentiviral shRNA to mTOR or GFP (as control), respectively, were treated with/without resveratrol (100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 1 h. (a, d, f) Total cell lysates were subjected to Western blotting using indicated antibodies. The blots were probed for β-tubulin as a loading control. Similar results were observed in at least three independent experiments. (b, e, g) The blots for p-S6K1 p-PTEN, p-Akt (Ser473), p-Akt (Thr308), de-PP2A, p-PP2A, and p-Erk1/2 were semi-quantified using NIH image J. (c, h) Adherent cells were determined using CN IV-coated cell adhesion assay. Results are presented as mean ± SEM (n = 3–6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group; c p < 0.05, difference with IGF-1/resveratrol group or IGF-1/rapamycin group; d p < 0.05, mTOR shRNA group vs. GFP shRNA group.
To confirm the above findings, RNA interference was used. As demonstrated in Figure 7d, e, lentiviral shRNA to mTOR, but not GFP, silenced the expression of mTOR protein by ~90% in Rh30 and HeLa cells, as detected by Western blotting. Down-regulation of mTOR significantly decreased the mTOR kinase activity, since the basal or IGF-1-induced phosphorylation of S6K1 (Thr389), routinely used as an indicator of mTOR kinase activity, was almost not detectable by Western blotting (Figure 7f, g). Of note, down-regulation of mTOR blocked IGF-1-induced de-PP2Ac, p-PP2Ac, p-PTEN, p-Akt, and p-Erk1/2 in the cells even without treatment with resveratrol (Figure 7f, g). Furthermore, as expected, down-regulation of mTOR obviously suppressed IGF-1-induced cell adhesion, and potentiated the inhibitory effect of resveratrol (Figure 7h). Taken together, our data underscore the concept that inhibition of mTOR is co-operative with resveratrol in activating PP2A/PTEN, leading to more inhibition of Akt/Erk1/2 and cancer cell adhesion.
4. DISCUSSION
Resveratrol executes its anticancer activity in part through its anti-metastatic action in cancer cells (Chen et al., 2012; Kim et al., 2016; Lee et al., 2012). Cancer cell migration and attachment to specific distant organs are key events during cancer metastasis (Leber and Efferth, 2009; Woodhouse et al., 1997). Recently it has been shown that resveratrol inhibits cell adhesion in numerous malignancies (Wang et al., 2013b; Wu et al., 2008). Here, we report that resveratrol inhibited IGF-1-induced cell adhesion in a panel of cancer cell lines (Rh1, Rh30, HT29 and HeLa). Mechanistically, resveratrol inhibited IGF-1-induced cancer cell adhesion by activating PP2A/PTEN network, leading to inhibition of Erk1/2.
Here, we chose the most widely expressed ECM proteins such as CN IV, fibronectin and laminin to investigate the effect of resveratrol on cancer cell adhesion. The results showed that resveratrol inhibited cell adhesion in Rh1, Rh30, HT29 and HeLa cells. Since cell adhesion is an essential step in cancer metastatic process (Gupta and Massague, 2006; Leber and Efferth, 2009; Steeg and Theodorescu, 2008), inhibition of cancer cell adhesion may contribute to prevention of cancer metastasis. To verify the link between cancer cell adhesion and cancer metastasis, we further investigated the effect of resveratrol on cancer cell motility using the wound-healing assay according to our previous research (Liu et al., 2006). The results indicated that resveratrol indeed inhibited IGF-1-stimulated cell motility in Rh1, Rh30, HT29 and HeLa cells. Taken together, these data suggest that resveratrol has a great potential in the prevention of cancer metastasis by inhibiting cancer cell adhesion.
It is well known that multiple signaling molecules mediate cancer cell adhesion (Canel et al., 2013; Mitra and Schlaepfer, 2006). Erk1/2, a member of MAPK family involved in cell behavior control, has a well-established role as a regulator of cancer cell adhesion (Fincham et al., 2000; Liao et al., 2009). Similarly, PP2A, a negative regulator of Erk1/2, also regulates cell adhesion (Sontag and Sontag, 2006). Resveratrol can inhibit Erk1/2 at high concentrations (Roy et al., 2011). Recently, we have noticed that resveratrol activates PP2A activity (Liu et al., 2015). This led us to ask whether resveratrol inhibits IGF-1-induced cancer cell adhesion by targeting PP2A-Erk1/2 pathway. In this study, we found that resveratrol suppressed IGF-1-induced phosphorylation of Erk1/2 in Rh1, Rh30, HT29 and HeLa cells in a concentration-dependent manner (Figure 2a, b). Of note, resveratrol did not alter cellular protein expression of the catalytic subunit of PP2A (PP2Ac) and two regulatory subunits PP2A-A and PP2A-B, but remarkably attenuated IGF-1 induced expression of de-PP2Ac and p-PP2Ac (Tyr307) in the cells (Figure 2a, b). The in vitro PP2A phosphatase assay further confirmed that resveratrol reversed IGF-1-induced reduction of PP2A activity (Figure 2c). The results suggest that resveratrol inhibits IGF-1-induced cancer cell adhesion, probably by activating PP2A, leading to inhibition of Erk1/2.
First, to corroborate that resveratrol inhibits the basal and IGF-1-stimulated cell adhesion through inhibiting Erk1/2 phosphorylation in cancer cells, pharmacological and genetic approaches were employed. We noticed that the combination of resveratrol with U0126 exhibited a more potent inhibitory effect on IGF-1-induced activation of Erk1/2 and cell adhesion than resveratrol or U0126 alone (Figure 3a-c). Furthermore, silencing Erk1/2 or expression of dominant negative MKK1 (MKK1-K97M) reinforced resveratrol’s inhibition of IGF-1-stimulated Erk1/2 activation and cell adhesion, whereas expression of constitutively active MKK1 (MKK1-R4F) reversed resveratrol’s inhibitory effect on IGF-1-induced Erk1/2 phosphorylation and cell adhesion (Figure 3d-i). Collectively, our findings indicate that resveratrol prevents IGF-1-induced cell adhesion, at least in part, by blocking activation of Erk1/2 pathway in cancer cells.
Next, to determine whether resveratrol inhibits the basal and IGF-1-stimulated cell adhesion through activating PP2A, resulting in inhibition of Erk1/2, pharmacological/genetic inhibition or rescue experiments for PP2A were conducted. We found that over-expression of wild-type PP2Ac potentiated resveratrol’s suppression of IGF-1-induced phosphorylation of Erk1/2 and cell adhesion, whereas inhibition of PP2A by okadaic acid, or expression of dominant negative PP2A resulted in the robust phosphorylation of Erk1/2 and conferred high resistance to resveratrol inhibition of IGF-1-induced cell adhesion (Figure 4). These results support a model in which resveratrol blocks IGF-1-induced cancer cell adhesion by suppressing IGF-1 activation of Erk1/2, which is partly via activation of PP2A.
PTEN is a well-known phosphatase negatively regulating Akt pathway (Polak and Hall, 2009); Akt can activate Erk1/2 through PKC (Chetram and Hinton, 2012). Emerging studies have suggested that PTEN also negatively regulates Erk1/2 pathway in several malignancies (Chetram and Hinton, 2012). Both PTEN and Akt are involved in the regulation of cell adhesion (Haier and Nicolson, 2002; Kraus et al., 2002). In this study, treatment with resveratrol (0–100 μM) for 4 h potently blocked IGF-1-induced p-PTEN and p-Akt in Rh1, Rh30, HT29, and HeLa cells (Figure 5a, b). Putting all data together, we thus postulated that IGF-1 inactivation of PTEN and concurrent activation of Akt may result in activation of Erk1/2, which may be blocked by resveratrol. Here, for the first time, we present evidence that resveratrol inhibited IGF-1-induced cancer cell adhesion indeed by blocking IGF-1-induced inactivation of PTEN and activation of Akt, resulting in inhibition of Erk1/2. This is strongly supported by the findings that ectopic expression of wild-type PTEN or dominant negative Akt, or inhibition of Akt with Akt inhibitor X enhanced resveratrol’s inhibitory effects on IGF-1-induced p-Erk1/2 and cell adhesion in Rh30 and/or HeLa cells (Figure 6a-f). Our data underscore that resveratrol has an ability to suppress IGF-1-induced inactivation of PTEN and activation of Akt, thereby attenuating IGF-1-induced Erk1/2 activation and adhesion in cancer cells.
In addition, PI3K is a kinase that converses phosphatidylinositol (3,4)-bis-phosphate (PIP2) lipids to phosphatidylinositol (3,4,5)-tris-phosphate (PIP3) (Hemmings et al., 2012). Many studies have documented that Akt is a major downstream effector of PI3K (Hemmings et al., 2012). The PI3K/Akt signaling pathway plays an important role in regulating various cellular processes including cell adhesion and migration (Faes and Dormond, 2015). Emerging evidence has demonstrated that PI3K and Akt can act independently of each other (Mahajan and Mahajan, 2012; Bruhn et al., 2013). Definitely, more studies are needed to uncover the role of PI3K in the regulation of resveratrol-inhibited Akt and cancer cell adhesion.
Recently, we have demonstrated that both mTORC1 and mTORC2 are involved in the regulation of cell adhesion (Chen et al., 2015b). This prompted us to study whether inhibition of mTOR can potentiate the inhibitory effect of resveratrol on IGF-1-induced inhibition of PP2A/PTEN and activation of Akt/Erk1/2, as well as cell adhesion. We found that treatment with rapamycin alone inhibited p-S6K1 but induced p-Akt (Figure 7a, b), which is line with our previous observations (Chen et al., 2015b). It has been proposed that rapamycin inhibits S6K1, which may activate Akt through a S6K1-IRS negative feedback mechanism (Cornu et al., 2013; Lamming et al., 2013). Rapamycin treatment alone inhibited the basal and IGF-1-induced cancer cell adhesion (Figure 7c), which is also consistent with our recent finding (Chen et al., 2015b). Rapamycin blocked IGF-1-induced inactivation of PP2A and PTEN, thus potentiating the inhibitory effects on IGF-1-induced p-Erk1/2 and cell adhesion (Figure 7a-c). This is further supported by the findings that silencing mTOR more potently enhanced the inhibitory effects of resveratrol on IGF-1-induced inhibition of PP2A/PTEN and activation of Akt/Erk1/2 activation as well as cell adhesion (Figure 7d-h). These results clearly indicate that inhibition of mTOR potentiates the inhibitory effect of resveratrol on IGF-1-induced cell adhesion.
Dysregulation of cell adhesion molecules (CAMs), including integrins, cadherins, immunoglobulin-like CAMs, selectins and CD44s, is a characteristic in human cancers (Schmidmaier and Baumann, 2008). Focal adhesion proteins (FAK, paxillin and p130Cas) and small GTPases (RhoA, Cdc42 and Rac1) have been recognized as key players in the regulation of cell motility and adhesion (Havel et al., 2015; Liu et al., 2008; Liu et al., 2010b). It has been reported that Erk1/2 pathway regulates integrins and cadherins (Chen et al., 2015a; Tashiro et al., 2016; Ying et al., 2017). Our group previously has found that IGF-I stimulates the phosphorylation of focal adhesion proteins and the activity of small GTPases, which is substantially attenuated by rapamycin (Liu et al., 2008; Liu et al., 2010b). Whether the suppression of Erk1/2 and Akt pathways leads to inhibition of cancer cell adhesion by altering the expression of these CAMs, focal adhesion proteins and small GTPases remains to be determined. Moreover, basement membrane (BM), a unique ECM, is not just a physical barrier, and it also contributes to cell adhesion, migration, proliferation, and survival (LeBleu et al., 2007). Laminins are major components of BM (Yao, 2017). Laminin-binding integrins are associated with both inhibitory and stimulatory roles in cancer (Ramovs et al., 2017). IGF-1 stimulated cell adhesion relies essentially on β1 integrins, which are privileged receptors for laminin molecules available to invading cancer cells in basal laminae (Takada et al., 2017). It has been shown that resveratrol affects ovarian cancer cell adhesion to human peritoneal mesothelial cells by decreasing cellular α5β1 integrin level (Mikula-Pietrasik et al., 2014). These data suggest that resveratrol inhibition of IGF-1-stimulated cancer cell adhesion seems through inhibiting laminin-β1 integrin interaction. Further research should be conducted to address this issue.
In addition, reactive oxygen species (ROS) has been found to regulate PP2A and PTEN/Akt activity (Nakahata and Morishita, 2014; Wang et al., 2016). Resveratrol, as a natural antioxidant, inhibits hypoxia- and hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells through suppression of Hedgehog and Erk/p38 MAPK signaling pathway, respectively (Cao et al., 2016; Li et al., 2016). Undoubtedly, more studies are required to address whether resveratrol suppresses ROS level, which results in activation of PP2A and PTEN, thereby leading to inhibition of Erk1/2-mediated adhesion in cancer cells.
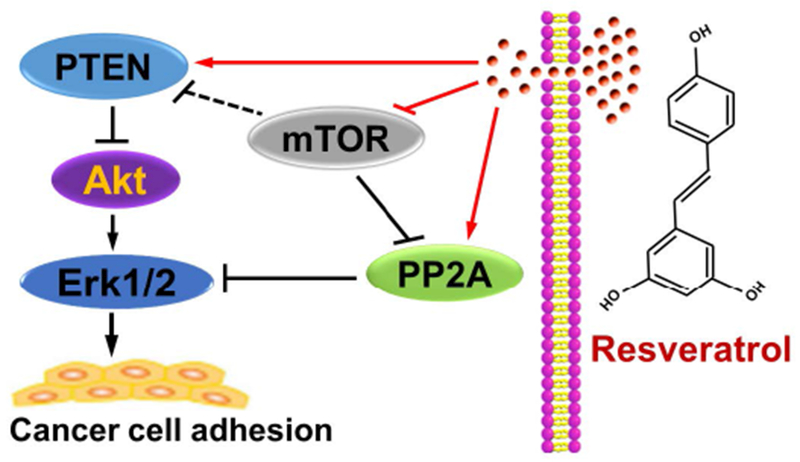
In summary, here we have identified that resveratrol attenuates IGF-1-induced cancer cell adhesion by blocking Erk1/2 pathway. Mechanistically, resveratrol blocks Erk1/2 pathway, not only by activating PP2A, but also via activating PTEN and inactivating Akt, thereby preventing adhesion in cancer cells (Figure 8). Inhibition of mTOR enhanced the inhibitory effect of resveratrol on the events (Figure 8). Our findings suggest that resveratrol has a great potential in the inhibition of cancer cell adhesion, which may contribute to its prevention of cancer metastasis.
FIGURE 8.
Schematic model of the preventive effect of resveratrol on cancer cell adhesion. Resveratrol prevents cancer cells from Erk1/2-mediated adhesion, not only by activating PP2A, but also via activating PTEN and inactivating Akt. Inhibition of mTOR potentiates the inhibitory effect of resveratrol on the events.
Supplementary Material
SUPPLEMENTARY FIGURE 1 Inhibitory effect of resveratrol on IGF-1-stimulated motility of Rh1, Rh30, HT29 and HeLa cells. Serum-starved Rh1, Rh30, HT29 and HeLa cells were treated with resveratrol (0–100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 20 h. Cell motility was determined by the wound-healing assay, as described in Materials and methods. (a) A representative experimental result was shown. Bar=50 μm. (b) The quantitative data were indicated. Results are presented as mean ± SEM (n = 6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group.
ACKNOWLEDGEMENTS
We are grateful to Dr. Natalie Ahn for generously providing the constructs of pMCL-MKK1-R4F and pMCL-MKK1-K97M. This work was supported in part by the grants from National Natural Science Fundation of China (No. 81271416; LC), National Institutes of Health (CA115414; SH), Project for the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (PAPD-14KJB180010; LC), American Cancer Society (RSG-08-135-01-CNE; SH), and Louisiana Board of Regents (NSF-2009-PFUND-144; SH).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Bai Y, Yang H, Zhang G, Hu L, Lei Y, Qin Y, … Mao Q (2017). Inhibitory effects of resveratrol on the adhesion, migration and invasion of human bladder cancer cells. Molecular Medicine Reports, 15, 885–889. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Darjatmoko SR, & Polans AS (2011). Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Research, 21, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn MA, Pearson RB, Hannan RD, & Sheppard KE (2013). AKT-independent PI3-K signaling in cancer—Emerging role for SGK3. Cancer Management and Research, 5, 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canel M, Serrels A, Frame MC, & Brunton VG. (2013). E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci, 126(Pt 2), 393–401. [DOI] [PubMed] [Google Scholar]
- Cao L, Chen X, Xiao X, Ma Q, & Li W (2016). Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. International Journal of Oncology, 49, 735–743. [DOI] [PubMed] [Google Scholar]
- Cescon M, Gattazzo F, Chen P, & Bonaldo P (2015). Collagen VI at a glance. Journal of Cell Science, 128, 3525–3531. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chen YJ, Lin CY, Fong YC, Hsu CJ, Tsai CH, …Tang CH (2015a). Amphiregulin enhances alpha6beta1 integrin expression and cell motility in human chondrosarcoma cells through Ras/Raf/MEK/ERK/AP-1 pathway. Oncotarget, 6, 11434–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu L, Luo Y, & Huang S (2008). MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. Journal of Neurochemistry, 105, 251–261. [DOI] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y, & Huang S (2009). Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. The International Journal of Biochemistry & Cell Biology, 41, 1284–1295. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Liu C, Luo Y, Chen X, … Huang S (2015b). Both mTORC1 and mTORC2 are involved in the regulation of cell adhesion. Oncotarget, 6, 7136–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Chang WW, Kuan YD, Lin ST, Hsu HC, & Lee CH (2012). Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immunity, 18, 685–693. [DOI] [PubMed] [Google Scholar]
- Chen X, Gu Y, Singh K, Shang C, Barzegar M, Jiang S, & Huang S (2014). Maduramicin inhibits proliferation and induces apoptosis in myoblast cells. PLoS One, 9, e115652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetram MA, & Hinton CV (2012). PTEN regulation of ERK1/2 signaling in cancer. Journal of Receptor and Signal Transduction Research, 32, 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Albert V, & Hall MN (2013). mTOR in aging, metabolism, and cancer. Current Opinion in Genetics & Development, 23, 53–62. [DOI] [PubMed] [Google Scholar]
- Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, & Della Ragione F (2007). Resveratrol: from basic science to the clinic. Cell Cycle, 6, 2495–2510. [DOI] [PubMed] [Google Scholar]
- Faes S, & Dormond O (2015). PI3K and AKT: Unfaithful Partners in Cancer. International Journal of Molecular Sciences, 16, 21138–21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, & Winder SJ (2000). Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. The EMBO Journal, 19, 2911–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, & Weaver VM (2010). The extracellular matrix at a glance. Journal of Cell Science, 123, 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, & Sabatini DM (2007). Defining the role of mTOR in cancer. Cancer Cell, 12, 9–22. [DOI] [PubMed] [Google Scholar]
- Gupta GP, & Massague J (2006). Cancer metastasis: building a framework. Cell, 127, 679–695. [DOI] [PubMed] [Google Scholar]
- Haier J, & Nicolson GL (2002). PTEN regulates tumor cell adhesion of colon carcinoma cells under dynamic conditions of fluid flow. Oncogene, 21, 1450–1460. [DOI] [PubMed] [Google Scholar]
- Harburger DS, & Calderwood DA (2009). Integrin signalling at a glance. Journal of Cell Science, 122, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, & Bae H (2017). An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules, 22, pii: E294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel LS, Kline ER, Salgueiro AM, & Marcus AI (2015) Vimentin regulates lung cancer cell adhesion through a VAV2-Rac1 pathway to control focal adhesion kinase activity. Oncogene, 34, 1979–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings BA, & Restuccia DF (2012). PI3K-PKB/Akt pathway. Cold Spring Harbor Perspectives in Biology, 4, a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homrich M, Gotthard I, Wobst H, & Diestel S (2015). Cell Adhesion Molecules and Ubiquitination-Functions and Significance. Biology (Basel), 5, pii: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries JD, Byron A, & Humphries MJ (2006). Integrin ligands at a glance. Journal of Cell Science, 119, 3901–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO (2002). Integrins: bidirectional, allosteric signaling machines. Cell, 110, 673–687. [DOI] [PubMed] [Google Scholar]
- Kalev P, & Sablina AA (2011). Protein phosphatase 2A as a potential target for anticancer therapy. Anti-Cancer Agents in Medicinal Chemistry, 11, 38–46. [DOI] [PubMed] [Google Scholar]
- Kato A, Naiki-Ito A, Nakazawa T, Hayashi K, Naitoh I, Miyabe K, … Takahashi S (2015). Chemopreventive effect of resveratrol and apocynin on pancreatic carcinogenesis via modulation of nuclear phosphorylated GSK3beta and ERK1/2. Oncotarget, 6, 42963–42975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CW, Hwang KA, & Choi KC (2016). Anti-metastatic potential of resveratrol and its metabolites by the inhibition of epithelial-mesenchymal transition, migration, and invasion of malignant cancer cells. Phytomedicine, 23, 1787–1796. [DOI] [PubMed] [Google Scholar]
- Kraus AC, Ferber I, Bachmann SO, Specht H, Wimmel A, Gross MW, … Schuermann M (2002). In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene, 21, 8683–8695. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, & Canto C (2015). The molecular targets of resveratrol. Biochimica et Biophysica Acta, 1852, 1114–1123. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Sabatini DM, & Baur JA (2013). Rapalogs and mTOR inhibitors as anti-aging therapeutics. The Journal of Clinical Investigation, 123, 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, & Sabatini DM (2012). mTOR signaling in growth control and disease. Cell, 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber MF, & Efferth T (2009). Molecular principles of cancer invasion and metastasis (review). International Journal of Oncology, 34, 881–895. [DOI] [PubMed] [Google Scholar]
- LeBleu VS, Macdonald B, & Kalluri R (2007). Structure and function of basement membranes. Experimental Biology and Medicine, 232, 1121–1129. [DOI] [PubMed] [Google Scholar]
- Lee HS, Ha AW, & Kim WK (2012). Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutrition Research and Practice, 6, 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitinger B, & Hohenester E (2007). Mammalian collagen receptors. Matrix Biology, 26, 146–155. [DOI] [PubMed] [Google Scholar]
- Li W, Cao L, Chen X, Lei J, & Ma Q (2016). Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncology Reports, 35, 1718–1726. [DOI] [PubMed] [Google Scholar]
- Liao YC, Shih YW, Chao CH, Lee XY, & Chiang TA (2009). Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. Journal of Agricultural and Food Chemistry, 57, 8933–8941. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhang R, Sun C, Zhang H, Xu C, Liu W, … Chen L (2015). Resveratrol prevents cadmium activation of Erk1/2 and JNK pathways from neuronal cell death via protein phosphatases 2A and 5. Journal of Neurochemistry, 135, 466–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen L, Chung J, & Huang S (2008). Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene, 27, 4998–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, … Huang S (2010a). Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS One, 5, e10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li F, Cardelli JA, Martin KA, Blenis J, & Huang S (2006). Rapamycin inhibits cell motility by suppression of mTOR-mediated S6K1 and 4E-BP1 pathways. Oncogene, 25, 7029–7040. [DOI] [PubMed] [Google Scholar]
- Liu L, Luo Y, Chen L, Shen T, Xu B, Chen W, … Huang S (2010b). Rapamycin inhibits cytoskeleton reorganization and cell motility by suppressing RhoA expression and activity. The Journal of Biological Chemistry, 285, 38362–38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng ZJ, … He BC (2014). The PTEN/PI3K/Akt and Wnt/beta-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. International Journal of Oncology, 45, 104–112. [DOI] [PubMed] [Google Scholar]
- Mahajan K, & Mahajan NP (2012). PI3K-independent AKT activation in cancers: A treasure trove for novel therapeutics. Journal of Cellular Physiology, 227, 3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour SJ, Matten WT, Hermann AS, Candia JM, Rong S, Fukasawa K, Vande Woude GF, & Ahn NG (1994). Transformation of mammalian cells by constitutively active MAP kinase kinase. Science, 265, 966–970. [DOI] [PubMed] [Google Scholar]
- Mikula-Pietrasik J, Sosinska P, & Ksiazek K (2014). Resveratrol inhibits ovarian cancer cell adhesion to peritoneal mesothelium in vitro by modulating the production of alpha5beta1 integrins and hyaluronic acid. Gynecologic Oncology, 134, 624–630. [DOI] [PubMed] [Google Scholar]
- Miloso M, Bertelli AA, Nicolini G, & Tredici G (1999). Resveratrol-induced activation of the mitogen-activated protein kinases, ERK1 and ERK2, in human neuroblastoma SH-SY5Y cells. Neuroscience Letters, 264, 141–144. [DOI] [PubMed] [Google Scholar]
- Mitra SK, & Schlaepfer DD (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Current Opinion in Cell Biology, 18, 516–523. [DOI] [PubMed] [Google Scholar]
- Nakahata S, & Morishita K (2014). PP2A inactivation by ROS accumulation. Blood, 124, 2163–2165. [DOI] [PubMed] [Google Scholar]
- Pankov R, & Yamada KM (2002). Fibronectin at a glance. Journal of Cell Science, 115, 3861–3863. [DOI] [PubMed] [Google Scholar]
- Park EJ, & Pezzuto JM (2015). The pharmacology of resveratrol in animals and humans. Biochimica et Biophysica Acta, 1852, 1071–1113. [DOI] [PubMed] [Google Scholar]
- Park JS, Kim KM, Kim MH, Chang HJ, Baek MK, Kim SM, & Jung YD (2009). Resveratrol inhibits tumor cell adhesion to endothelial cells by blocking ICAM-1 expression. Anticancer Research, 29, 355–362. [PubMed] [Google Scholar]
- Perrotti D, & Neviani P (2013). Protein phosphatase 2A: a target for anticancer therapy. The Lancet Oncology, 14, e229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P, & Hall MN (2009). mTOR and the control of whole body metabolism. Current Opinion in Cell Biology, 21, 209–218. [DOI] [PubMed] [Google Scholar]
- Ramovs V, Te Molder L, & Sonnenberg A (2017). The opposing roles of laminin-binding integrins in cancer. Matrix Biology, 57-58, 213–243. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, … Horwitz AR (2003). Cell migration: integrating signals from front to back. Science, 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- Roy SK, Chen Q, Fu J, Shankar S, & Srivastava RK (2011). Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One, 6, e25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salado C, Olaso E, Gallot N, Valcarcel M, Egilegor E, Mendoza L, & Vidal-Vanaclocha F (2011). Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. Journal of Translational Medicine, 9, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RS, Cookson MM, Sharma B, Hauser J, & Brattain MG (2004). Autocrine transforming growth factor alpha regulates cell adhesion by multiple signaling via specific phosphorylation sites of p70S6 kinase in colon cancer cells. The Journal of Biological Chemistry, 279, 47379–47390. [DOI] [PubMed] [Google Scholar]
- Schmidmaier R,, & Baumann, P. (2008). ANTI-ADHESION evolves to a promising therapeutic concept in oncology. Current Medicinal Chemistry, 15, 978–990. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Harikumar KB, & Aggarwal BB (2009). Resveratrol addiction: to die or not to die. Molecular Nutrition & Food Research, 53, 115–128. [DOI] [PubMed] [Google Scholar]
- Sontag JM, & Sontag E (2006). Regulation of cell adhesion by PP2A and SV40 small tumor antigen: an important link to cell transformation. Cellular and Molecular Life Sciences, 63, 2979–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS, & Theodorescu D (2008). Metastasis: a therapeutic target for cancer. Nature Clinical Practice Oncology, 5, 206–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y Takada YK & Fujita M (2017). Crosstalk between insulin-like growth factor (IGF) receptor and integrins through direct integrin binding to IGF1. Cytokine and Growth Factor Reviews, 34, 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro E, Henmi S, Odake H, Ino S, & Imoto M (2016). Involvement of the MEK/ERK pathway in EGF-induced E-cadherin down-regulation. Biochemical and Biophysical Research Communications, 477, 801–806. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu C, Wan S, Zhang H, Zhou S, & Liu G (2013a). Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin beta1 expression and the ERK1/2 signaling pathway. Toxicology, 308, 104–112. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang H, Tang L, Chen H, Wu C, Zhao M, … Liu G (2013b). Resveratrol inhibits TGF-beta1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology, 303, 139–146. [DOI] [PubMed] [Google Scholar]
- Wang SQ, Wang C, Chang LM, Zhou KR, Wang JW, Ke Y, … Liu HM (2016). Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget, 7, 72990–73002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widlund AL, Baur JA, & Vang O. (2013). mTOR: more targets of resveratrol? Expert Rev Mol Med, 15, e10. [DOI] [PubMed] [Google Scholar]
- Woodhouse EC, Chuaqui RF, & Liotta LA (1997). General mechanisms of metastasis. Cancer, 80, 1529–1537. [DOI] [PubMed] [Google Scholar]
- Wu H, Liang X, Fang Y, Qin X, Zhang Y, & Liu J (2008). Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomedicine & Pharmacotherapy, 62, 613–621. [DOI] [PubMed] [Google Scholar]
- Xie F, Bao X, Yu J, Chen W, Wang L, Zhang Z, & Xu Q (2015). Disruption and inactivation of the PP2A complex promotes the proliferation and angiogenesis of hemangioma endothelial cells through activating AKT and ERK. Oncotarget, 6, 25660–25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang Z, Zhou SF, & Lu N (2014). Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Design, Development and Therapy, 8, 1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, & Pavletich NP (2013). mTOR kinase structure, mechanism and regulation. Nature, 497, 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y (2017). Laminin: loss-of-function studies. Cellular and Molecular Life Sciences, 74, 1095–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying HY, Gong CJ, Feng Y, Jing DD, & Lu LG (2017). Serine protease inhibitor Kazal type 1 (SPINK1) downregulates E-cadherin and induces EMT of hepatoma cells to promote hepatocellular carcinoma metastasis via the MEK/ERK signaling pathway. Journal of Digestive Diseases, 18, 349–358. [DOI] [PubMed] [Google Scholar]
- Yu YH, Chen HA, Chen PS, Cheng YJ, Hsu WH, Chang YW, … Su JL (2013). MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene, 32, 431–443. [DOI] [PubMed] [Google Scholar]
- Zennadi R, Whalen EJ, Soderblom EJ, Alexander SC, Thompson JW, Dubois LG, … Telen MJ (2012). Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium. Blood, 119, 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY FIGURE 1 Inhibitory effect of resveratrol on IGF-1-stimulated motility of Rh1, Rh30, HT29 and HeLa cells. Serum-starved Rh1, Rh30, HT29 and HeLa cells were treated with resveratrol (0–100 μM) for 4 h, followed by stimulation with/without IGF-1 (10 ng/ml) for 20 h. Cell motility was determined by the wound-healing assay, as described in Materials and methods. (a) A representative experimental result was shown. Bar=50 μm. (b) The quantitative data were indicated. Results are presented as mean ± SEM (n = 6). a p < 0.05, difference with control group; b p < 0.05, difference with IGF-1 group.