Abstract
Background:
No treatment is available to reverse injury associated with traumatic brain injury (TBI). Progenitor cell therapies show promise in both pre-clinical and clinical studies. We conducted a meta-analysis of pre-clinical studies using progenitor cells to treat TBI.
Methods:
EMBASE, MEDLINE, Cochrane Review, Biosis, and Google Scholar were searched for articles using pre-specified search strategies. Studies meeting inclusion criteria underwent data extraction. Analysis was performed using Review Manager 5.3 according to a fixed-effects model, and all studies underwent quality scoring.
Results:
Of 430 abstracts identified, 38 met inclusion criteria and underwent analysis. Average quality score was 4.32 out of 8 possible points. No study achieved a perfect score. Lesion volume (LV) and Neurologic Severity Score (NSS) outcomes favored cell treatment with standard mean difference (SMD) of 0.86 (95%CI 0.64, 1.09) and 1.36 (95%CI 1.11, 1.60) respectively. Rotarod (RR) and Morris Water Maze (MWM) outcomes also favored treatment with improvements in SMD of 0.34 (95%CI 0.02, 0.65) and 0.46 (95%CI 0.17, 74) respectively. While LV and NSS were robust to publication bias assessments, RR and MWM were not. Heterogeneity (I2) ranged from 74% to 85% among the analyses, indicating a high amount of heterogeneity among studies. Precision as a function of quality score showed a statistically significant increase in the size of the confidence interval as quality improved.
Conclusions:
Our meta-analysis study reveals an overall positive effect of progenitor cell therapies on LV and NSS with a trend towards improved motor function and spatial learning in different TBI animal models.
Keywords: Meta-Analysis, Traumatic Brain Injury, Progenitor Cells, Animal TBI Model, Pre-Clinical Studies
Background
Traumatic brain injury (TBI) is major public health and socio-economic problem that affects both civilians and members of the armed forces worldwide (1, 2). Monitoring by the Centers for Disease Control and Prevention (CDC) shows that with 1.7 million incidents annually, TBI contributes to 30.5% of all injury-related deaths in the United States. TBI survivors experience long-term disabling changes in physical, cognitive, and psychosocial states and disease management costs more than $77 billion per year. The scope of TBI ranges from limited focal damage due to cerebral contusion, laceration, or hemorrhage to multifocal damage due to acceleration-deceleration injuries, or both (3). Both local and multifocal damage can lead to clinical conditions that require careful assessment and management to prevent long-term disabilities. The pathophysiology of TBI is complex and the physiologic response of the brain to injury initiates a cascade of cellular pathways, which left unchecked become pathologic (4). TBI causes stretching of axons, which causes dysregulation of axonal Na+/K+ pumps and an increase in intracellular Ca2+ concentrations (5, 6). Increased intracellular Ca2+ levels lead to excitotoxicity and neuronal cell death (7). In addition to disturbances of ionic homeostasis, initiation of inflammatory and immune responses also occur in the central nervous system after TBI (8, 9). These events contribute to blood–brain barrier (BBB) disruption and the development of cerebral edema (10).
Considering the complexity of the disease pathomechanism, the development of a therapy that can maintain or restore neuronal function would provide the most comprehensive approach to treating TBI. Progenitor cell therapies hold great promise in TBI because of their inherent biological properties of plasticity, self-renewal, and migration. Transplanted progenitor cells could either regenerate dead neurons or repair damaged neuronal cells by producing neurotrophic factors, scavenging toxic molecules, or by exerting immunomodulatory effects. In the past decade, several preclinical studies have shown promising outcomes using progenitor stem cells in TBI animal models (11, 12). In this study, we performed systemic review of the literature and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate the efficacy of different progenitor cell therapies in animal models of TBI.
Methods
A protocol for article search, data extraction, and analysis was developed by the lead author, and agreed upon by all authors (see supplementary materials). To meet inclusion criteria studies needed to have an animal model of TBI, administer a progenitor cell therapy that had not been genetically manipulated, and analyze at least one of our four pre-specified outcome measures, which were: (i) lesion volume (LV), (ii) rotarod test (RR), (iii) neurologic severity score (NSS) test, and (iv) Morris Water Maze (MWM) test. These outcomes were selected for their wide use, allowing us to compare as many studies as possible. Those studies that were not available translated into the English language, used concomitant therapies, such as but not limited to gene modification, protein administration, or scaffolding, were excluded as having a confounding therapy.
Lesion volume (LV) was defined as the volume of the brain injury caused by the TBI injury model (for example, the volume of injury cavity caused by a controlled cortical injury). The RR test is a locomotor function test where a mouse or rat must balance on a rotating rod which is gradually accelerated. Longer times on the rotarod denote improved locomotor performance. The NSS was defined as a composite score including motor function, alertness, and seeking behavior. By design, the NSS is scored so that a higher score denotes more severe injury. Therefore an improvement will be a decrease in the score. The Morris Water Maze (MWM) is a behavioral test for spatial learning and memory. For the purpose of our study we extracted latency to the platform, which is the time it takes for a pre-trained subject to find a hidden platform in the water maze.
EMBASE and MEDLINE, accessed through Ovid and PubMed respectively were selected for the main article search. Biosis, a database of meeting proceedings and abstracts, was also searched, as well as Google Scholar and the Cochrane Review. These were each searched with a pre-specified search entry, available in the protocol in the supplemental materials. Our search was designed to be exceedingly sensitive, so that we might capture all possible studies, then exclude those that were not pertinent. Periodically throughout the project the searches were updated with the final search date being November 28, 2016.
Search was performed by two independent investigators, and data were extracted by the two investigators (MLJ and AKS). Disagreements were put to a third investigator (CSC) for mediation. Those studies that did not present numerical data and only presented graphical data of their findings underwent graphical data extraction using Adobe Illustrator CS6 software. Those studies that presented only a p-value and effect direction were excluded. All included studies underwent quality assessment as follows: one point each was assigned for evidence in the manuscript of (i) sample size calculation, (ii) randomization, (iii) body temperature control during surgery, (iv) avoidance of ketamine anesthesia, (v) IACUC approval for animal use and IRB approval for human subject cell extraction, if applicable, (vi) a statement of disclosures/conflict of interest. Two points were assigned for evidence of blinded outcome assessment. These criteria are a combination of recommendations for quality assessment in the stroke pre-clinical literature (13) and A Call for Transparent Reporting to Optimize the Predictive Value of Preclinical Research by Landis et al. (14).
Data extracted for each outcome included the mean, number of subjects, and standard deviation (SD) for each study arm, as is necessary for meta-analysis of continuous variables. When standard error of the mean (SEM) was presented it was converted to SD. Once extracted, the data was entered into RevMan 5.3 [The Cochrane Collaboration, Oxford, UK] for analysis using a fixed-effects model and calculating standardized mean differences (SMD) between control and treatment groups. RevMan 5 uses Hedges’ adjusted g, which is very similar to Cohen’s d, but includes an adjustment for small sample bias, as is appropriate with the animal studies included in our analysis. Equations used by the software can be found in the statistical algorithms supplement to RevMan 5 (15). Quality score metrics were entered into Microsoft Excel 2010, and simple linear and multiple regression were run in Stata 13 [StataCorp, College Station, Texas]. Regression analyses were designed to test the two primary hypotheses that (i) increased quality score correlated with a larger treatment effect, and (ii) increased quality score correlated with a smaller confidence interval. As a secondary outcome, multiple regression model tested the hypotheses that year, cell line, timing of treatment, dose, and type of injury affect the treatment effect size.
Results
Study Selection
Four hundred and thirty records were identified through classical database searching (Figure 1). Three additional records were found on Biosis, but were found to be duplicates of studies already found in the general search. These 430 abstracts were screened, and 366 records were excluded. Nineteen studies were excluded because they used concomitant (and therefore confounding) therapies including genetic modifications to the progenitor cells, 190 studies did not use a model of TBI, 77 were excluded because they were reviews and not original research, 13 were not available in English, 16 were excluded because they did not report an outcome we were assessing, 28 were excluded because no component of the study included animals, and 23 were excluded because they used a stem cell derivative therapy such as exosomes or conditioned media. Sixty-four studies underwent full text evaluation, at which point another 26 were excluded. Two studies did not model traumatic brain injury, 22 did not examine an outcome that we were assessing in our analysis, 1 was not an animal model, and 1 used a confounding concomitant therapy. Thirty-eight studies met inclusion criteria and underwent data extraction and quality assessment. Of these studies, some reported more than one pertinent outcome measure so that a total of 10 studies presented data for RR outcomes (16–25), 14 presented data on LV outcomes (23, 26–38), 21 assessed NSS outcomes (17–21, 23–26, 28, 29, 31, 36, 38–53), and 13 presented data on MWM testing (17, 18, 24, 26, 28, 29, 36, 38, 49–53). Specific study characteristics are listed in Table 1.
Figure 1:

Flow diagram of the studies evaluated.
Table 1:
Summary characteristics of 38 studies included in meta-analysis. Data are reported as number of studies (N) followed by the percentage of all studies included in parentheses. There was even divide among years of publication and syngenic or xenogenic administration. A preponderance of studies (58%) used the controlled cortical impact TBI model, and administration in the 1-4 day time window made up a large proportion of the included studies.
| Characteristics | |
|---|---|
| Year of publication | N (%) |
| 2001-2006 | 8 (21.1) |
| 2007-2012 | 12 (31.5) |
| 2013-2015 | 18 (47.4) |
| Cell source | N (%) |
| Syngeneic | 20 (52.6) |
| Xenogeneic | 18 (47.4) |
| Cell dose | N (%) |
| <1 million cells | 12 (31.6) |
| 1-<2 million cells | 6 (15.8) |
| 2-<3 million cells | 6 (15.8) |
| >3 million cells | 5 (13.2) |
| Other* | 9 (23.7) |
| Timing | N (%) |
| <1 day | 7 (18.4) |
| 1-4 days | 18 (47.4) |
| 5-7 days | 9 (23.7) |
| >7 days | 1 (2.6) |
| Other$ | 3 (7.9) |
| Injury types | N (%) |
| Controlled cortical impact | 22 (57.9) |
| Fluid percussion injury | 5 (13.2) |
| Weight drop injury | 10 (26.3) |
| Freezing injury | 1 (2.6) |
Either multiple doses or dose not reported
Multiple time points
Pooled Effect Sizes
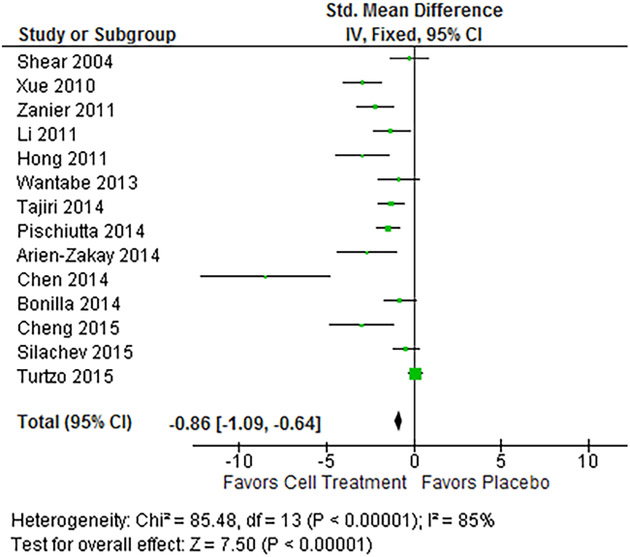
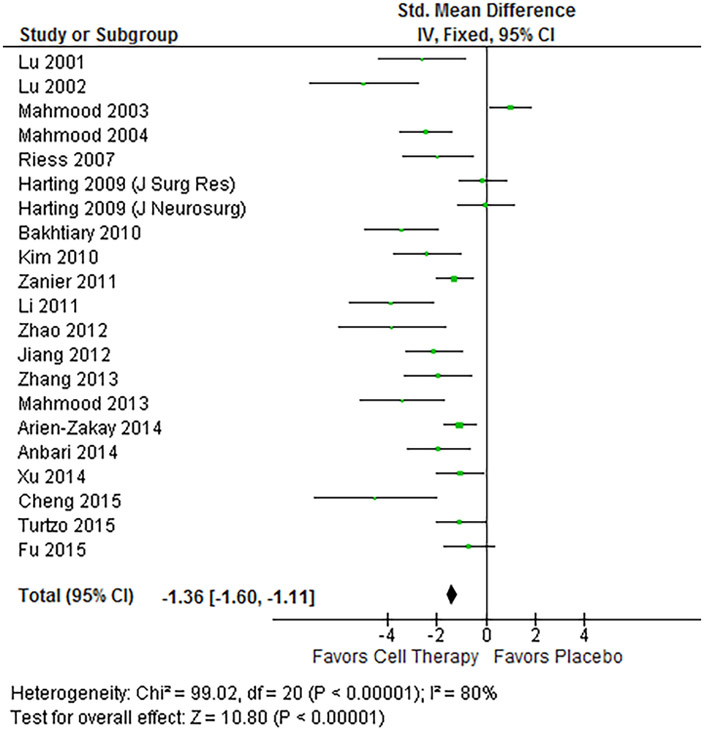
Using a fixed-effects model assessing the standardized mean difference (SMD), all outcomes showed statistically significant pooled estimates favoring treatment (p-value < 0.05). The LV analysis (Figure 2) pooled a total of 209 treated subjects and 185 control subjects, and showed a standard mean difference of −0.86 (95% CI −1.09, −0.64), showing an overall statistically significant reduction in LV in treated animals. NSS analysis (Figure 3) pooled 225 treated and 187 control subjects, and showed a SMD of −1.36 (95% CI −1.60, −1.11), showing a statistically significant reduction in overall severity of injury as assessed by the test. Heterogeneity in these two outcome assessments was quite high with an I2 of 85% and 80% respectively.
Figure 2:
Forest plot of LV results. The fixed-effects pooled SMD for LV outcomes was −0.86 (95%CI −1.09, −0.64), which signifies a large treatment effect. A negative effect in this instance indicates a reduction in the size of the lesion volume and improvement after treatment. Heterogeneity was 85%, and p-value for the model was < 0.001.
Figure 3:
Forest plot of NSS results. The fixed-effects pooled SMD for NSS outcomes was −1.36 (95%CI −1.60, −1.11), which signifies a large treatment effect. A negative result indicates a lower degree of injury in those subjects treated with progenitor cell therapy. Heterogeneity was 80%, and p-value for the model was < 0.001.
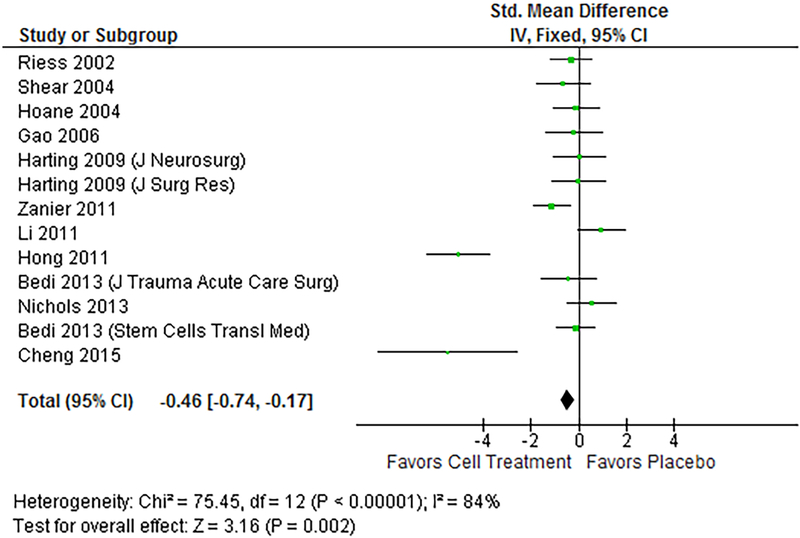
Fewer subjects were assessed in the RR (Figure 4) and MWM (Figure 5) assessments. Ten studies assessed RR as an outcome measure, with a total of 104 treated subjects and 83 control subjects. SMD in the RR test was 0.34 (95% CI 0.02, 0.62), showing an overall improvement in the ability of the animals to perform the test. The MWM pooled estimates included 135 treated subjects and 113 control subjects, and found a standard mean difference of −0.46 (95% CI −0.74, −0.17), showing decreased latency to platform time (i.e., an improvement in the animal’s ability to perform the test). Heterogeneity in these two outcomes was 74% and 84% respectively on I2 test.
Figure 4:
Forest plot of RR results. The fixed-effects pooled SMD for RR outcomes was 0.34 (95%CI 0.02, 0.65) which signifies a small to moderate treatment effect. A positive result indicates a better score on the RR test and overall improved outcome after treatment. Heterogeneity was 74%, and p-value for the model was 0.04.
Figure 5:
Forest plot of MWM results. The fixed-effects pooled SMD for MWM outcomes was −0.46 (95%CI −0.74, −0.17), which signifies a moderate treatment effect. A negative result indicates that the treated animals had a lower latency, or time to reach the platform in the MWM, and overall better outcomes. Heterogeneity was 84%, and p-value for the model was 0.002.
Regression models were analyzed with year, cell line, timing of treatment, dose, and type of injury. Within the 38 studies included in our analysis these were not factors that predicted effect size in any of the four subgroups.
Study Quality
The mean study quality of all 38 studies included for analysis was 4.32, with a standard deviation of 1.58, and a median of 5. Range was 1–7 out of 8 possible points. No study included a power analysis/sample size calculation. The most commonly included parameters in the individual studies were the avoidance of ketamine and IRB/IACUC approval.
Linear regression analysis showed that effect size was not a function of the quality score in any of the four outcome analyses. In addition, for the RR, LV, and MWM, precision (width of the 95%CI) was not a function of the quality score. In those studies that assessed the NSS, however, studies with a higher quality have a 0.363 increase in the width of their confidence interval (95%CI 0.0432, 0.683; p-value < 0.05), making higher quality a negative predictor of precision. Pearson correlation coefficient was −0.47 (p-value 0.03), indicating a moderate correlation.
Risk of Bias
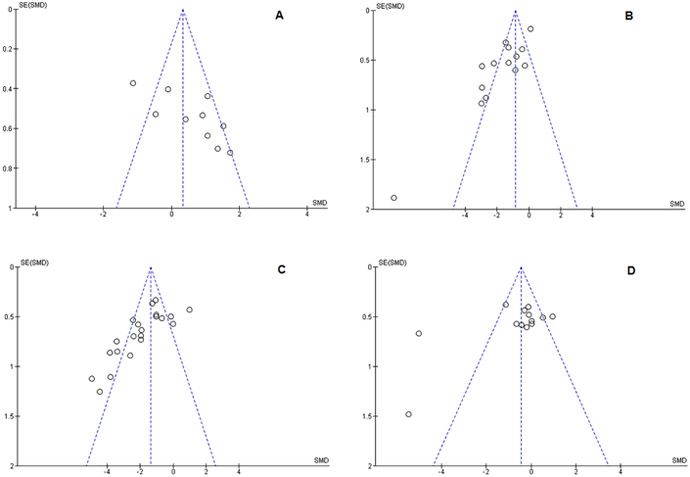
Risk of publication bias was assessed with funnel plots using the Eggar method (54), and are shown in Figure 6. Data points outside the funnel lines on the plot indicate studies outside the 95%CI of where studies would be expected to lie if there is no publication bias. None of our assessments were free of these outliers. When the trim-and-fill method was applied to the LV and NSS outcomes, the models were robust and retained their statistical significance with a minimally adjusted SMD. However the RR and MWM were not robust to the trim-and-fill method, losing statistical significance. This denotes significant publication bias in these two outcome measures.
Figure 6:
Funnel plots depicting publication bias. Funnel plots show the reverse standard error on the Y-axis and the SMD on the X-axis. The vertical dotted line shows the pooled treatment effect, and diagonal lines show the 95% confidence interval if there is no heterogeneity or publication bias present. Publication bias can be seen by an absence of studies to the LEFT of the treatment effect in (A) the RR plot, and to the RIGHT of the treatment effect in (B) LV, (C) NSS, and (D) MWM. All outcomes evaluated were susceptible to varying degrees of publication bias. Trim-and-fill test indicated that (A) RR and (D) MWM lost their statistically significant treatment effect when adjusted, though (B) LV and (C) NSS retained their statistical significance.
Discussion
Our results show that when diverse pre-clinical progenitor cell therapies in TBI are analyzed together there is an overall treatment effect as shown by histologic, motor, and neurocognitive function improvements. As are all meta-analyses, this study is limited by the quality of the available data, its heterogeneity, and publication bias. However, to our knowledge this is the first meta-analysis to examine a histologic outcome in an animal model of TBI. There are two other meta-analyses that evaluate progenitor cell treatment for traumatic brain injury. In the analysis by Peng et al. (55) only behavioral data were extracted from studies in animals that received only human mesenchymal stromal cells (hMSC), limiting the number of studies that could be included. In the meta-analysis by Chang et al.(56) studies were included that examined modified and naïve progenitor cells for the treatment of traumatic brain injury. This was not the primary endpoint of our authors, and so this study has a different population of studies included for analysis. In addition, Chang et al.examined only MWM and NSS. While both of these papers are important in the field, our meta-analysis includes a broader range of studies, and looks at more specific outcomes across four endpoints.
Figures 2, 3, 4, and 5 show the forest plots of our results. For detailed information about study weighting and individual SMD please see our Supplemental Materials. The absolute values of the effect sizes found by our analysis range from 0.34 in the RR to 1.36 in the NSS, showing a small to medium effect in the RR (0.34) and MWM (0.46) to large effects in the LV (0.86) and NSS (1.36). (The reader should note that for all outcomes except the RR a negative effect denotes improvement after treatment. However, here we are discussing absolute values.) Effect sizes are generally accepted to be small at 0.2, medium at 0.5, and high at 0.8. It is interesting that the two outcomes measures which showed the greatest effect sizes had the largest number of subjects and individual papers included: the NSS assessment included a total of 412 animal subjects from 21 papers, and the LV assessment included 394 subjects from 14 papers. The MWM and RR analyses had roughly half that number of subjects with 248 and 187 total participants respectively. Thirteen papers were included in the MWM analysis, while 10 were included in the RR. Lesion volume is also a histologic outcome and less subjective to variances between animal handling practices and animal stress. This range of effect sizes probably reflects the diversity in applicability of outcomes: small changes in lesion volume may or may not translate to the MWM assessment, which evaluates higher cortical functioning of the rat and spatial memory and learning. The NSS on the other hand, incorporates motor function, alertness, and seeking behavior, which gives a broader picture of neurologic impairment and may therefore be more likely to show an improvement as compared to the MWM or RR.
Our regression analysis did not show any signal to direct future therapies in the realm of timing, dose, or cell line of treatment. However, included studies were by design so heterogeneous as to make multivariate regression unlikely to show a signal. This lack of signal is evidence in itself that progenitor cells as a drug class are effective, and there is no “secret ingredient” such as cell line, dose, or timing that makes them so.
As in the previously published meta-analyses, quality analysis revealed that the pre-clinical literature has only mediocre quality. This is a known fault of basic-science research (57–60), with increasing movements to improve transparency of reporting (14, 58, 60). However, scientific research can only improve slowly, and with buy-in from investigators, funding agencies, and journal editors. In our regression analyses, quality was not correlated with effect size in either direction in any of the four outcomes assessed. However, increased quality did correlate with a wider confidence interval in the NSS group, echoing what has been seen in previous pre-clinical studies (57). This is interesting as general belief holds that increased methodologic rigor leads to increased precision; indeed in the statistical model RevMan gives higher weight to those studies that have greater precision (a smaller standard deviation), using greater precision to denote greater power (15, 61).
In the meta-analysis by Chang et al., higher quality index scores were associated with smaller MWM treatment effect sizes, but greater precision (56). Peng et al. ran a stratified meta-analysis, and found that the quality score of 5 (possible scores from 0–10) correlated with the largest effect sizes (62). However, neither of these meta-analyses used the same quality metrics. While a number of criteria are widely accepted as markers of higher quality basic science research (blinded outcome assessments, randomization, sample size calculation, conflict of interest statement) there are others which are harder to tease out in individual papers. The meta-analysis by Peng et al. used a 10-point scale which, while including the above parameters, also gave points for “peer review publication,” and “assessment of dose-response relationship” (62), criteria that may be out of the control of the investigators who design and conduct the experiments. Indeed, according to the PRISM guidelines, a meta-analysis must attempt to include negative studies, which may not have been accepted to peer-reviewed journals. The meta-analysis by Chang et al. used a 14-point quality score which, while including the pertinent factors above, we found to be too exhaustive to be applicable. As basic science improves its overall rigor, standardized metrics for quality will be established. We chose our scale because it hit the highlights of what investigators must have to be high quality, without affecting autonomy of study design.
As in all meta-analyses, there were methodological barriers to overcome in order to pursue the analysis. Preliminary research before starting the meta-analysis showed vast heterogeneity between studies; however, the authors decided that a meta-analysis of pre-clinical literature could not be performed without significant heterogeneity, thus decided to accept a large level of heterogeneity without rejecting the analysis (as discussed in the Supplemental Material). In order to compensate for heterogeneity among studies we measured standardized mean differences (SMD) which measure effect size as a function of the group’s standard deviation as opposed to measuring raw differences in means. In addition, meticulous care was taken in comparing studies that looked at the same specific outcome variable (e.g., RR, LV, NSS, and MWM) in order to decrease heterogeneity where possible in our samples.
Some degree of publication bias is unavoidable in pre-clinical literature, despite mounting calls for increased publication of negative studies (57, 58, 60). Some believe that smaller studies tend to be analyzed with less methodological rigor than larger studies. We would propose that all animal studies are de facto small studies because of financial, housing, and ethical constraints. Sterene (63) points out that funnel plot asymmetry is a means of displaying “small-study effects” and not representative of publication bias, but since in the pre-clinical animal model literature there are no large studies, we believe this does not apply in this subset, and that the funnel plot is sufficient evidence of the presence or absence of publication bias, which is shown in our plots especially in the RR analysis (Figure 4) where the studies included from the first decade of progenitor cell for TBI investigation were all positive, and only in the last few years have negative findings been reported. Indeed this is one of the outcomes that does not pass the trim-and-fill test for publication bias, nor does the MWM assessment. This could be due to smaller sample size of animals in these assessments as well as heterogeneity among the studies. However, the results of our study have the benefit of already being supported by two Phase I/II clinical trials, overcoming to some extent the hurdle faced by so many successful animal models which fail to show the same benefit in clinical trials (59, 64–67).
The main limitation of this meta-analysis is in the outcomes measures that we chose. Because there is such great heterogeneity in the pre-clinical literature, any attempts to refine our searches to papers that could be compared to each other by definition excluded other papers. We chose RR, LV, NSS, and MWM because they were frequently reported across studies and could be reliably compared to each other. However, this excluded a large body of literature that did not report on those specific outcomes measures. Within our included studies, however, we had large variations in cell source, dose, timing, and TBI model. Because we were limited to the 38 included studies, we did not have a large n for multiple regression analysis to model the effects of these variations. Indeed, future studies may uncover variations between these methods that we could not see with our limited methods. However, data compiled in this meta-analysis are striking in their generally similar treatment effects despite these diverse cell types, routes of delivery, and dosing. Specifically, a range of bone marrow derived cells such as mesenchymal stromal cells or MSC, multipoint adult progenitor cells or MAPC, and bone marrow mononuclear cells (from which MSC and MAPC are derived) produced under varying culture conditions all produce similar effects. This meta-analysis also underestimates the other positive treatment effects noted in numerous studies (68–74), as they often focus on specific biochemical outcomes that are not generalized across multiple papers. Examples include neurogenesis measured in various ways: neuroinflammation measured with either cytokine profiles, microglial activation with various cell surface markers or morphology; or blood-brain barrier permeability as either edema, tight junctional protein configuration/expression, or macromolecule extravasation. These important variables can be concordant but not merged for a meta-analysis. For example Evans Blue dye extravasation can show a 40% reduction in blood-brain barrier permeability with bone marrow derived mononuclear cells (52), and similar results can be obtained using an Alexa-Fluor fluorescent dye with permeability mapping (75). However, accuracy is lost in trying to harmonize the effect magnitude using different measurement techniques. This meta-analysis of progenitor cell treatments for TBI in pre-clinical data shows a positive treatment signal in both lesion volume and combined neurologic severity score which is robust to heterogeneity and publication bias assessments, paving the way for future clinical studies.
Future Directions
Though many papers have been able to show a benefit in behavioral outcomes with progenitor cell treatment, and indeed, this is the third meta-analysis to conclude that there is benefit, the mechanism of action has not been completely delineated. To that end some investigators are working with modified progenitor cells that are being reprogrammed to over-express certain factors secreted by progenitor cells, and some groups are directly injecting the factors themselves (such as TIMP3 or Wnt3a) (74, 76). As this research progresses the mechanism or mechanisms of action will be more fully understood, and cellular therapies can be better harnessed and used to the best benefit.
Though the physical impact may be short in TBI, the consequences are long lasting. There are few, if any, chronic diseases in medicine that are treated with a single dose of medication. Though four included papers in our analysis looked at multiple doses (16, 23, 31, 52), this is not currently standard in the pre-clinical or the clinical literature. Future directions may include multiple doses for a greater and longer-lasting effect.
As always, the next step after animal models is clinical trials in humans. To date, two clinical trials have been published here in the Unites States showing promising results for improved outcomes with progenitor cell therapy for TBI (65, 77), and five more clinical trials are in the process of recruiting on ClinicalTrials.gov. The results published in this meta-analysis should help prove that these trials are worthy of further pursuit, and further funding.
Conclusion
This study shows that though there is great heterogeneity in the data, and not all studies are published with the most rigorous quality standards, there is an overall improvement in the overall neurologic severity score of animals treated with progenitor cell therapies, and a decrease in the size of their lesion volumes after injury with treatment.
Supplementary Material
Table 2:
Quality score results showing the frequencies of possible scores 0-8. No study received a score of 0 or 8. The most frequent score was 5, followed by a 3 or 4. Just over 80% of studies included had a score of 5 or below, and only 7 studies (18.42%) had a score of 6 or 7.
| Quality Score | Frequency | Percent (%) | Cumulative Percent (%) |
|---|---|---|---|
| 0 | 0 | 0.00 | 0.00 |
| 1 | 1 | 2.63 | 2.63 |
| 2 | 5 | 13.16 | 15.79 |
| 3 | 6 | 15.79 | 31.58 |
| 4 | 6 | 15.79 | 47.37 |
| 5 | 13 | 34.21 | 81.58 |
| 6 | 3 | 7.89 | 89.47 |
| 7 | 4 | 10.53 | 100.00 |
| 8 | 0 | 0.00 | |
| Total | 38 | 100.00 |
Acknowledgements
This work was supported by the Department of Health and Human Services, grant numbers 5T32GM008792–13 and 4T3200GM8792–14.
Footnotes
Disclosures/Conflict of Interest:
CSC: equity interest in Cellvation, Inc., a company which seeks to develop cellular therapies for neurological injuries.
MLJ and AKS: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Maas AI, Stocchetti N, Bullock R Moderate and severe traumatic brain injury in adults. Lancet Neurol 2008:7:728–741. [DOI] [PubMed] [Google Scholar]
- 2.Cole TB Global road safety crisis remedy sought: 1.2 million killed, 50 million injured annually. JAMA 2004:291:2531–2532. [DOI] [PubMed] [Google Scholar]
- 3.Werner C, Engelhard K Pathophysiology of traumatic brain injury. Br J Anaesth 2007:99:4–9. [DOI] [PubMed] [Google Scholar]
- 4.Prins M, Greco T, Alexander D, Giza CC The pathophysiology of traumatic brain injury at a glance. Dis Model Mech 2013:6:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson VE, Stewart W, Smith DH Axonal pathology in traumatic brain injury. Exp Neurol 2013:246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 2001:21:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkhoudarian G, Hovda DA, Giza CC The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011:30:33–48, vii-iii. [DOI] [PubMed] [Google Scholar]
- 8.Goodman JC, Van M, Gopinath SP, Robertson CS Pro-inflammatory and pro-apoptotic elements of the neuroinflammatory response are activated in traumatic brain injury. Acta Neurochir Suppl 2008:102:437–439. [DOI] [PubMed] [Google Scholar]
- 9.Morganti-Kossmann MC, Satgunaseelan L, Bye N, Kossmann T Modulation of immune response by head injury. Injury 2007:38:1392–1400. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, et al. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest 1996:74:315–342. [PubMed] [Google Scholar]
- 11.Aertker BM, Bedi S, Cox CS Jr. Strategies for CNS repair following TBI. Exp Neurol 2016:275 Pt 3:411–426. [DOI] [PubMed] [Google Scholar]
- 12.Walker PA, Shah SK, Harting MT, Cox CS Jr. Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis Model Mech 2009:2:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009:40:2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012:490:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JJDaJP Statistical algorithms in Review Manager 5. In: Collaboration SMGoTC ed 2010. [Google Scholar]
- 16.Han EY, Chun MH, Kim ST, Lim DP Injection time-dependent effect of adult human bone marrow stromal cell transplantation in a rat model of severe traumatic brain injury. Curr Stem Cell Res Ther 2013:8:172–181. [DOI] [PubMed] [Google Scholar]
- 17.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg 2009:110:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harting MT, Sloan LE, Jimenez F, Baumgartner J, Cox CS Jr. Subacute neural stem cell therapy for traumatic brain injury. J Surg Res 2009:153:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HJ, Lee JH, Kim SH Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma 2010:27:131–138. [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Mahmood A, Wang L, Li Y, Lu M, et al. Adult bone marrow stromal cells administered intravenously to rats after traumatic brain injury migrate into brain and improve neurological outcome. Neuroreport 2001:12:559–563. [DOI] [PubMed] [Google Scholar]
- 21.Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant 2002:11:275–281. [PubMed] [Google Scholar]
- 22.Osanai T, Kuroda S, Sugiyama T, Kawabori M, Ito M, et al. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats--in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery 2012:70:435–444; discussion 444. [DOI] [PubMed] [Google Scholar]
- 23.Turtzo LC, Budde MD, Dean DD, Gold EM, Lewis BK, et al. Failure of intravenous or intracardiac delivery of mesenchymal stromal cells to improve outcomes after focal traumatic brain injury in the female rat. PLoS One 2015:10:e0126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedi SS, Hetz R, Thomas C, Smith P, Olsen AB, et al. Intravenous multipotent adult progenitor cell therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. Stem cells translational medicine 2013:2:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmood A, Lu D, Lu M, Chopp M Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 2003:53:697–702; discussion 702–693. [DOI] [PubMed] [Google Scholar]
- 26.Shear DA, Tate MC, Archer DR, Hoffman SW, Hulce VD, et al. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res 2004:1026:11–22. [DOI] [PubMed] [Google Scholar]
- 27.Xue S, Zhang HT, Zhang P, Luo J, Chen ZZ, et al. Functional endothelial progenitor cells derived from adipose tissue show beneficial effect on cell therapy of traumatic brain injury. Neurosci Lett 2010:473:186–191. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Jiang Q, Qu CS, Ding GL, Li QJ, et al. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: in vivo MRI study. J Neurotrauma 2011:28:535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med 2011:39:2501–2510. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe J, Shetty AK, Hattiangady B, Kim DK, Foraker JE, et al. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol. Dis 2013:59:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arien-Zakay H, Gincberg G, Nagler A, Cohen G, Liraz-Zaltsman S, et al. Neurotherapeutic effect of cord blood derived CD45+ hematopoietic cells in mice after traumatic brain injury. J Neurotrauma 2014:31:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonilla C, Zurita M, Aguayo C, Rodríguez A, Vaquero J Is the subarachnoid administration of mesenchymal stromal cells a useful strategy to treat chronic brain damage? Cytotherapy 2014:16:1501–1510. [DOI] [PubMed] [Google Scholar]
- 33.Chen SH, Wang JJ, Chen CH, Chang HK, Lin MT, et al. Umbilical cord blood-derived CD34+ cells improve outcomes of traumatic brain injury in rats by stimulating angiogenesis and neurogenesis. Cell Transplant. 2014:23:959–979. [DOI] [PubMed] [Google Scholar]
- 34.Pischiutta F, D’Amico G, Dander E, Biondi A, Biagi E, et al. Immunosuppression does not affect human bone marrow mesenchymal stromal cell efficacy after transplantation in traumatized mice brain. Neuropharmacology 2014:79:119–126. [DOI] [PubMed] [Google Scholar]
- 35.Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci 2014:34:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng T, Yang B, Li D, Ma S, Tian Y, et al. Wharton’s Jelly Transplantation Improves Neurologic Function in a Rat Model of Traumatic Brain Injury. Cell Mol Neurobiol 2015:35:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silachev DN, Plotnikov EY, Babenko VA, Danilina TI, Zorov LD, et al. Intra-Arterial Administration of Multipotent Mesenchymal Stromal Cells Promotes Functional Recovery of the Brain After Traumatic Brain Injury. Bull Exp Biol Med 2015:159:528–533. [DOI] [PubMed] [Google Scholar]
- 38.Hong S-Q, Zhang H-T, You J, Zhang M-Y, Cai Y-Q, et al. Comparison of Transdifferentiated and Untransdifferentiated Human Umbilical Mesenchymal Stem Cells in Rats after Traumatic Brain Injury. Neurochemical Research 2011:36:2391. [DOI] [PubMed] [Google Scholar]
- 39.Mahmood A, Lu D, Chopp M Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery 2004:55:1185–1193. [DOI] [PubMed] [Google Scholar]
- 40.Riess P, Molcanyi M, Bentz K, Maegele M, Simanski C, et al. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J Neurotrauma 2007:24:216–225. [DOI] [PubMed] [Google Scholar]
- 41.Bakhtiary M, Marzban M, Mehdizadeh M, Joghataei MT, Khoei S, et al. Comparison of transplantation of bone marrow stromal cells (BMSC) and stem cell mobilization by granulocyte colony stimulating factor after traumatic brain injury in rat. Iran Biomed J 2010:14:142–149. [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang J, Bu X, Liu M, Cheng P Transplantation of autologous bone marrow-derived mesenchymal stem cells for traumatic brain injury. Neural Regen Res 2012:7:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Chen N, Shen N, Zhao H, Wang D, et al. Transplantation of human umbilical cord blood mesenchymal stem cells to treat a rat model of traumatic brain injury. Neural Regen Res 2012:7:741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmood A, Wu H, Qu C, Xiong Y, Chopp M Effects of treating traumatic brain injury with collagen scaffolds and human bone marrow stromal cells on sprouting of corticospinal tract axons into the denervated side of the spinal cord. Journal of neurosurgery 2013:118:381–389. [DOI] [PubMed] [Google Scholar]
- 45.Zhang R, Liu Y, Yan K, Chen L, Chen XR, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation 2013:10:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anbari F, Khalili MA, Bahrami AR, Khoradmehr A, Sadeghian F, et al. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res 2014:9:919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu HS, Ma C, Cao L, Wang JJ, Fan XX Study of co-transplantation of SPIO labeled bone marrow stromal stem cells and Schwann cells for treating traumatic brain injury in rats and in vivo tracing of magnetically labeled cells by MRI. European review for medical and pharmacological sciences 2014:18:520–525. [PubMed] [Google Scholar]
- 48.Fu XM, Liu SJ, Dan QQ, Wang YP, Lin N, et al. Combined Bone Mesenchymal Stem Cell and Olfactory Ensheathing Cell Transplantation Promotes Neural Repair Associated With CNTF Expression in Traumatic Brain-Injured Rats. Cell Transplant 2015:24:1533–1544. [DOI] [PubMed] [Google Scholar]
- 49.Riess P, Zhang C, Saatman KE, Laurer HL, Longhi LG, et al. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery 2002:51:1043–1052; discussion 1052–1044. [DOI] [PubMed] [Google Scholar]
- 50.Hoane MR, Becerra GD, Shank JE, Tatko L, Pak ES, et al. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J Neurotrauma 2004:21:163–174. [DOI] [PubMed] [Google Scholar]
- 51.Gao J, Prough DS, McAdoo DJ, Grady JJ, Parsley MO, et al. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp Neurol 2006:201:281–292. [DOI] [PubMed] [Google Scholar]
- 52.Bedi SS, Walker PA, Shah SK, Jimenez F, Thomas CP, et al. Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. The journal of trauma and acute care surgery 2013:75:410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichols JE, Niles JA, DeWitt D, Prough D, Parsley M, et al. Neurogenic and neuro-protective potential of a novel subpopulation of peripheral blood-derived CD133+ ABCG2+CXCR4+ mesenchymal stem cells: development of autologous cell-based therapeutics for traumatic brain injury. Stem cell research & therapy 2013:4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egger M, Smith GD, Schneider M, Minder C Bias in meta-analysis detected by a simple, graphical test. BR. MED. J. 1997:315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng W, Sun J, Sheng C, Wang Z, Wang Y, et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res. Ther 2015:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang J, Phelan M, Cummings BJ A meta-analysis of efficacy in pre-clinical human stem cell therapies for traumatic brain injury. Exp. Neurol 2015:273:225–233. [DOI] [PubMed] [Google Scholar]
- 57.Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, et al. Survey of the Quality of Experimental Design, Statistical Analysis and Reporting of Research Using Animals. PLoS ONE 2009:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moher D, Simera I, Schulz KF, Hoey J, Altman DG Helping editors, peer reviewers and authors improve the clarity, completeness and transparency of reporting health research. BMC Med 2008:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prinz F, Schlange T, Asadullah K Believe it or not: How much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov 2011:10:712–713. [DOI] [PubMed] [Google Scholar]
- 60.Van der Worp HB, Macleod MR Preclinical studies of human disease: Time to take methodological quality seriously. J. Mol. Cell. Cardiol 2011:51:449–450. [DOI] [PubMed] [Google Scholar]
- 61.Cochrane Handbook for Systemic Reviews of Interventions In: Higgins J, Green S eds.The Cochrane Collaboration, 2011. [Google Scholar]
- 62.Peng W, Sun J, Sheng C, Wang Z, Wang Y, et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem cell research & therapy 2015:6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Online) 2011:343. [DOI] [PubMed] [Google Scholar]
- 64.Cox CS Jr., Baumgartner JE, Harting MT, Worth LL, Walker PA, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 2011:68:588–600. [DOI] [PubMed] [Google Scholar]
- 65.Cox CS Jr., Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, et al. Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells. Stem cells (Dayton, Ohio) 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao GP, Harting MT, Hetz RA, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cells reduce therapeutic intensity for severe traumatic brain injury in children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2015:16:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arrowsmith J, Miller P Trial Watch: Phase II and Phase III attrition rates 2011–2012. Nat. Rev. Drug Discov 2013:12:569. [DOI] [PubMed] [Google Scholar]
- 68.Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, et al. Traumatic brain injury: Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci. Transl. Med 2012:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muthuraju S, Pati S, Rafiqul M, Abdullah JM, Jaafar H IntelliCage provides voluntary exercise and an enriched environment, improving locomotive activity in mice following fluid percussion injury. Basal Ganglia 2012:2:143–151. [Google Scholar]
- 70.Muthuraju S, Pati S, Rafiqul M, Abdullah JM, Jaafar H Effect of normabaric hyperoxia treatment on neuronal damage following fluid percussion injury in the striatum of mice: A morphological approach. J. Biosci 2013:38:93–103. [DOI] [PubMed] [Google Scholar]
- 71.Walker PA, Harting MT, Jimenez F, Shah SK, Pati S, et al. Direct intrathecal implantation of mesenchymal stromal cells leads to enhanced neuroprotection via an NFκB-mediated increase in interleukin-6 production. Stem Cells Dev 2010:19:867–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker PA, Shah SK, Jimenez F, Gerber MH, Xue H, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: Preserving the blood brain barrier via an interaction with splenocytes. Exp. Neurol 2010:225:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao J, Pati S, Redell JB, Zhang M, Moore AN, et al. Caffeic acid phenethyl ester protects blood-brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J. Neurotrauma 2012:29:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Gibb SL, Zhao J, Moore AN, Hylin MJ, et al. Wnt3a, a Protein Secreted by Mesenchymal Stem Cells Is Neuroprotective and Promotes Neurocognitive Recovery Following Traumatic Brain Injury. Stem Cells 2016:34:1263–1272. [DOI] [PubMed] [Google Scholar]
- 75.Liao GP, Olson SD, Kota DJ, Hetz RA, Smith P, et al. Far-red tracer analysis of traumatic cerebrovascular permeability. J. Surg. Res 2014:190:628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibb SL, Zhao Y, Potter D, Hylin MJ, Bruhn R, et al. TIMP3 Attenuates the Loss of Neural Stem Cells, Mature Neurons and Neurocognitive Dysfunction in Traumatic Brain Injury. Stem Cells 2015:33:3530–3544. [DOI] [PubMed] [Google Scholar]
- 77.Cox CS Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery 2011:68:588–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.