Abstract
Background
The Neonatal Resuscitation Program recommends initial resuscitation of preterm infants with low oxygen (O2) followed by titration to target preductal saturations (SpO2). We studied the effect of resuscitation with titrated O2 on gas exchange, pulmonary and systemic hemodynamics.
Methodology
Twenty-nine preterm lambs (127d-gestation) were randomized to resuscitation with 21%O2 (n=7), 100%O2 (n=6) or initiation at 21% and titrated to target SpO2 (n=16). Seven healthy term control lambs were ventilated with 21%O2.
Results
Preductal SpO2 achieved by titrating O2 was within the desired range similar to term lambs in 21%O2. Resuscitation of preterm lambs with 21 and 100%O2 resulted in SpO2 below and above the target respectively. Ventilation of preterm lambs with 100%O2 and term lambs with 21%O2 effectively decreased pulmonary vascular resistance (PVR). In contrast, preterm lambs with 21%O2 and titrated O2 demonstrated significantly higher PVR than term lambs on 21%O2.
Conclusion(s)
Initial resuscitation with 21%O2 followed by titration of O2 led to suboptimal pulmonary vascular transition at birth in preterm lambs. Ventilation with 100%O2 in preterm lambs caused hyperoxia but reduced PVR similar to term lambs on 21%O2. Studies evaluating initiation of resuscitation at higher O2 concentration followed by titration based on SpO2 in preterm neonates are needed.
Introduction
The optimal concentration of supplemental oxygen during initial resuscitation of premature infants remains controversial. The International Liaison Committee on Resuscitation (ILCOR) recommends initiating resuscitation of preterm newborns (less than 35 weeks postmenstrual age-PMA) with a low inspired oxygen concentration (≤30%) (strong recommendation, moderate-quality evidence) (1). ILCOR does not recommend the use of high concentration (≥ 65%) of supplemental oxygen (1, 2). Similarly, the Neonatal Resuscitation Program (NRP) recommends that resuscitation of preterm newborns < 35 weeks gestation at birth should be initiated with low concentration of inspired oxygen (≤ 30%), and the oxygen concentration should be titrated to achieve preductal SpO2 targets (3). The evidence for this recommendation comes from analysis of 7 randomized control trials (RCTs) (4–10) which have shown no advantage of using high oxygen compared to low oxygen for the critical outcome of mortality (Relative risk (RR) 1.48; 95% confidence interval (CI) 0.8 – 2.73). After these recommendations were published, a large RCT by Oei et al. targeting SpO2 between 65–95% up to 5 min of postnatal life and 85–95% from 5 min until NICU admission has shown that the mortality was high in infants <28 weeks PMA initially resuscitated with 21% O2 compared to 100% O2 (RR 3.9 (1.1–13.4); P= 0.01 – non-prespecified analysis) (11). A retrospective study by Rabi et al. comparing infants ≤27 weeks’ gestation before and after 2006, when the policy regarding initial oxygen concentration for preterm infants in the delivery room was changed from 100% to <100% oxygen and titration to achieve target SpO2 was published in 2015 (12). This Canadian Neonatal Network study has shown that the adjusted odds ratio (AOR) for the primary outcome of severe neurologic injury or death was higher in the lower oxygen group (AOR 1.36; 95% CI 1.11–1.66) and in those resuscitated with 21% oxygen (AOR 1.33; 95% CI 1.04–1.69), when compared to 100% oxygen. Despite the current evidence, the optimal concentration of supplemental oxygen for initial resuscitation of preterm neonates remains unclear.
The effect of titrating inspired oxygen during resuscitation of a newly born preterm model on gas exchange and hemodynamics is not known. Our objectives were to determine the effect of resuscitating a preterm lamb model with 21% O2, 100% O2 and titrating supplemental oxygen as per NRP recommended preductal saturations on i) oxygen saturations, ii) arterial oxygenation and iii) systemic and pulmonary hemodynamics.
Methodology
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of University at Buffalo. Time-dated pregnant ewes (May Farms, Buffalo Mills, PA) at 127–128d gestation (preterm equivalent, the term equivalent 147 – 150d) were sedated, intubated and ventilated with 21–30% oxygen and anesthetized with 2% isoflurane. Before delivery, the lambs were randomized using sequential opaque envelopes (target randomization ratio- 1:1:3 – 21% O2, 100% O2 and titrated O2 respectively – see below). Preterm lambs were partially exteriorized via a cesarean section. They were instrumented as described below while on placental circulation and maternal anesthesia. The ewes did not receive prenatal steroids. Term lambs at 142d gestation (to reduce the risk of spontaneous labor) were used as controls. The instrumentation of these lambs were as described previously {Rawat, 2016 #39}.
Monitoring – Preductal saturations were measured by Masimo radical pulse oximeter probe (Masimo, Irvine, CA) which was placed on the right forelimb. We targeted preductal saturations based on current NRP recommendations (3). A respiratory monitor (NM3 Philips, Respironics, MA) with a mainstream end-tidal carbon dioxide detector (ETCO2) was attached to the tracheal tube (13, 14). Cerebral saturation was measured by NONIN (SenSmart Model X-100, Plymouth, MN) regional oximetry. The cerebral regional saturations (CrSO2) were measured in preterm lambs but not in term lambs (controls). The carotid arterial oxygen content was measured as described previously in preterm lambs (15). The oxygen extraction was calculated in preterm lambs as a product of left carotid blood flow and the difference in arterial oxygen content (carotid artery) and the venous oxygen content (jugular bulb) (15). Venous gases were not available in term lambs.
Randomization and protocol – After instrumentation and surfactant instillation (Infasurf 3ml/kg, ONY), the lamb was delivered following early cord clamping. Based on the randomization arm, preterm lambs were resuscitated with 21% O2 (group 1) or 100% O2 (group 2) regardless of preductal SpO2. In the targeted oxygen group (group 3), the O2 was initiated at 21% and titrated to maintain oxygen in the NRP specified range as mentioned above. If preductal SpO2 was outside this range, inspired oxygen was adjusted by 5% every 15 s. Term healthy lambs were ventilated with 21% irrespective of SpO2 and served as controls (16). After the first 30 min, the variable O2 group was further subdivided into three saturation target ranges to mimic NeOPrOM studies (85–89%, 90–94% and 95–99% - data not shown in this manuscript) (17). As “positive” and “negative” controls, we ventilated two group of lambs continuously with 21% oxygen and 100% oxygen irrespective of SpO2. Our original intention was to study 6 lambs in each group. In the titrated group we intended to study 18 lambs, but 2 were lost during instrumentation. Because of this reason, the variable group has approximately 3 times the number of lambs in the 21% and 100% categories.
Ventilation was initiated with the Siemens Servo 300A (Maquet Medical, Salt Lake City, Utah) with a peak inspiratory pressure (PIP) of 30–35 cm H2O and a PEEP of 5–6 cm H2O to target a tidal volume of 8–9 ml/kg in preterm lambs. Tidal volume data were not available in the term lambs. Arterial blood gases were drawn at birth, 1, 2 and 5 min after birth followed by every 5 min for the first 30 min. Systemic and pulmonary hemodynamics were continuously recorded using AcqKnowledge Acquisition and Analysis Software (BIOPAC Systems, Goleta, CA). Sample size and power calculation - Based on previous studies, to detect a difference in pulmonary vascular resistance of 0.15 mmHg/ml/kg/min we needed 6 lambs in each group (18, 19). A power estimate was performed by one-way ANOVA, using statistical analysis software 9.4 (SAS, NC). With the current randomization, the power to detect the difference in PVR is 0.85 with an alpha of 0.05.
Statistics – Categorical variables are presented as numbers and percentages while continuous variables as means and standard deviations. Data were analyzed using chi-square, t-test and ANOVA. Statistical significance was set at less than 5%.
Results
Baseline characteristics of lambs before resuscitation are shown in Table 1. There were a total of 36 lambs in the study: six preterm lambs ventilated with 100% O2, seven preterm lambs with 21% O2, sixteen preterm lambs ventilated targeting SpO2 range and seven term lambs ventilated with 21% O2. The weight of the term lambs was significantly higher compared to preterm lambs (table 1).
Table.
Baseline characteristics and carotid arterial blood gases prior to delivery
| Parameters (in mean ± S.D) | Preterm lambs | Term lambs | ||
|---|---|---|---|---|
| 100% oxygen (N=6) | Titrated Oxygen (N=16) | 21% oxygen (N=7) | 21% oxygen (N=7) | |
| Weight (kg) | 3.1±0.4 | 2.9±0.6 | 2.8±0.4 | 4.0±0.6* |
| Female n (%) | 3 (50%) | 7 (44%) | 4 (57%) | 3 (43%) |
| Twins n (%) | 2 (33%) | 5 (31%) | 2 (29%) | 2 (29%) |
| pH | 7.15±0.20 | 7.12±0.12 | 7.13±0.19 | 7.2±0.2 |
| PaCO2 – (mm Hg) | 84.9±27.0 | 83.7±26.5 | 81.3±24.0 | 77±20.5 |
p<0.05, data presented as mean & SD unless specified
Saturation: In the first 30 minutes after birth, use of 21% and 100% O2 resulted in SpO2 below and above the NRP recommended target range respectively. Titrating oxygen in preterm lambs and use of 21% oxygen in term lambs resulted in NRP recommended target saturations (Figure 1a). Among preterm lambs, the time spent within the target SpO2 as recommended by NRP was 10% in 100% O2 group, 47% with the titrated O2 group and 30% in 21% O2 group. Term lambs ventilated with 21% O2 spent 65% of the first 30 minutes within the NRP recommended target range (significantly higher than preterm lambs, p<0.001).
Figure 1.
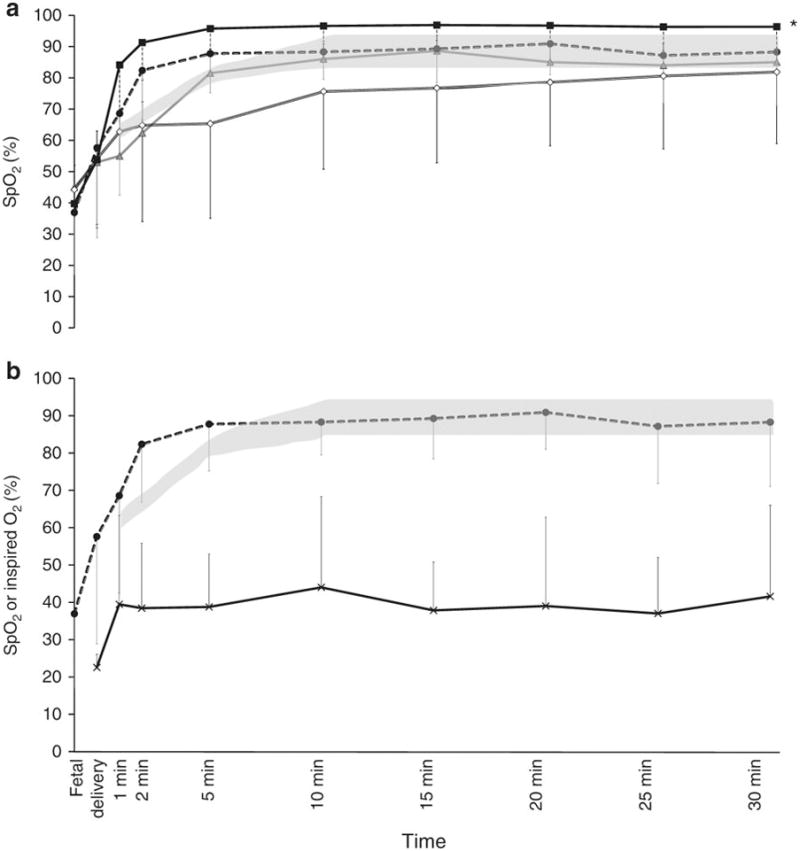
a) Changes in saturations (SpO2) with 100% inspired oxygen preterm lambs (closed square), 21% oxygen preterm lambs (open diamond), titrated oxygen (closed circle) and term 21% oxygen controls (triangle). The shaded area shows the NRP recommended saturation targets
b) Changes in titrated oxygen (cross) and saturations (closed circle) in titrated oxygen group. Hyphenated lines/shaded area shows the NRP recommended saturation targets.
FiO2: The inspired oxygen concentration required to maintain targeted saturations in the titrated FiO2 preterm group is shown in Figure 1b (Median FiO2 0.31 (interquartile range: 0.21 – 0.40)).
Tidal volume and arterial carbon dioxide (Figure S1): There was no difference in tidal volumes in preterm lambs (Figure S1a). The term lambs had lower PaCO2 compared to preterm lambs and these values were statistically different (Figure S1b) (p<0.01).
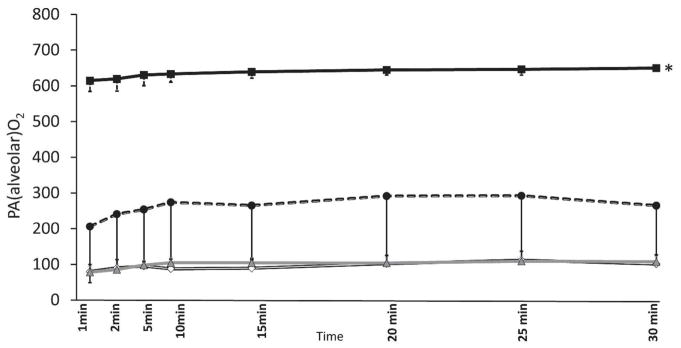
Alveolar Oxygenation (PAO2) (Figure 2): The calculated PAO2 with 100% O2, titrated O2 and 21% O2 were 643±24.4 mmHg, 244±156 mmHg and 95±24.2 mmHg and were significantly different (p<0.001) (Figure 3). The PAO2 in term lambs (99±11 mmHg) resuscitated with 21% O2 were similar to preterm 21% inspired oxygen group.
Figure 2.
PAO2 (alveolar oxygenation) are shown in figure 3. 100% inspired oxygen preterm lambs (closed square), 21% oxygen preterm lambs (open diamond), titrated oxygen (closed circle) were different. *p<0.001 by ANOVA. Use of 21% in the term (triangle) and preterm lambs showed similar PAO2.
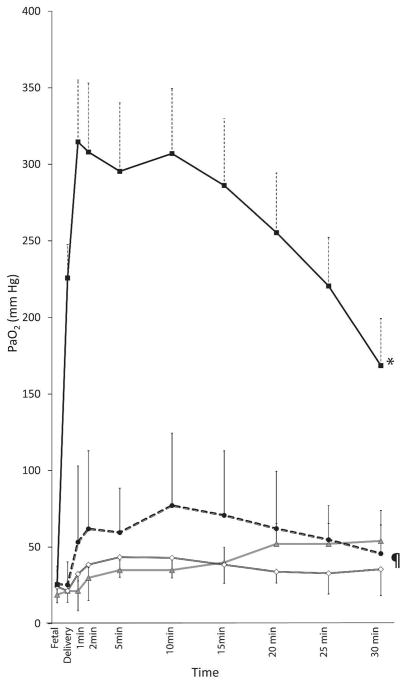
Figure 3.
Arterial Oxygen tension (PaO2) changes with 100% oxygen preterm lambs (closed square), 21% oxygen preterm lambs (open diamond), titrated oxygen (closed circle) and term 21% (triangle)oxygen controls (triangle). * 100% preterm oxygen statistically different from the rest by ANOVA (p<0.001). ¶ Titrated oxygen group different from 21% preterm by ANOVA (p<0.01).
Arterial oxygenation: Arterial oxygen tension (PaO2) was highest with 100% O2 (256±144 mmHg) and was higher than 21% O2 group of preterm lambs (39±16 mmHg, p<0.001), titrated oxygen group (60±36 mmHg, p<0.001) and in term controls (37±10 mmHg, p<0.001) in the first 30 minutes. The PaO2 was higher in preterm lambs resuscitated with titrated O2 group compared to preterm lambs resuscitated with 21% O2 (p=0.039) (Figure 3).
Heart rate: Heart rates were similar in preterm lambs resuscitated with 100% O2 (183±27/min), 21% O2 (175±27/min), titrated O2 (173±37/min) and term controls (180±25/min).
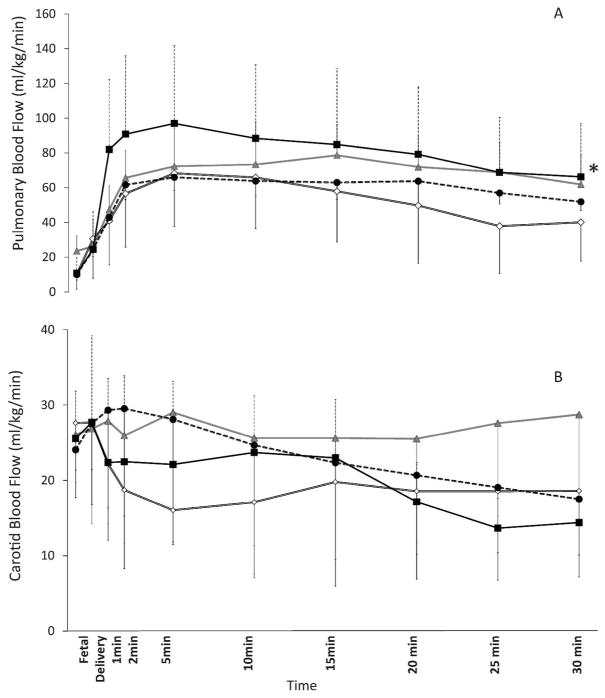
Pulmonary blood flow (PBF): The PBF was highest in preterm lambs resuscitated with 100% O2 and was different from preterm lambs resuscitated with 21% O2 (p<0.001), titrated oxygen (p<0.001) but similar to term controls (Figure 4a).
Figure 4.
a) Left pulmonary arterial blood flow (PBF) changes with 100% oxygen preterm lambs (closed square), 21% oxygen preterm lambs (open diamond), titrated oxygen (closed circle) and term 21% controls (triangle). *100% preterm lambs were different from preterm 21% and titrated O2 group. But not different from term 21% p<0.001 by ANOVA.
b) Left carotid blood flow (CBF) changes with 100% oxygen preterm lambs (closed square), 21% oxygen preterm lambs (open diamond), titrated oxygen (closed circle) and term 21% controls (triangle). The flows were not different.
Carotid blood flow (CBF): The CBF was similar in all four groups (Figure 4b).
Cerebral regional saturation (CrSO2), carotid arterial oxygen content and oxygen extraction (Figure S2): The CrSO2 (figure S2a) and carotid arterial oxygen content (figure S2b) was higher and statistically different in the lambs that received 100% O2 (p<0.01). The oxygen extraction was not different among the preterm lambs (figure S2c).
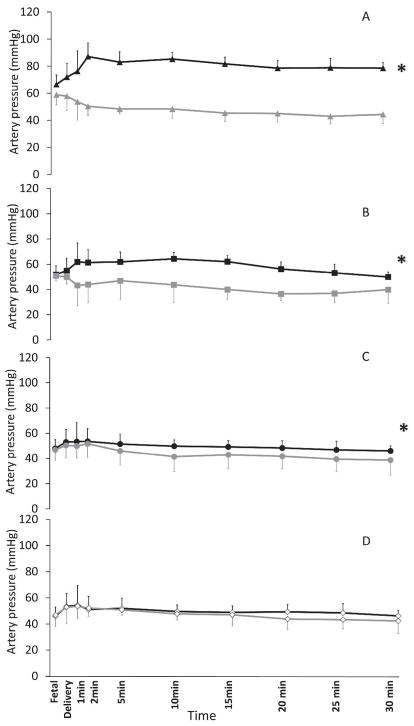
Systemic mean blood pressures (SBP) and pulmonary arterial mean blood pressures (PAP) (Figure 5): Left to right ductal flow is an essential contributor of pulmonary blood flow immediately after birth (20). The pressure gradient driving net ductal flow is the difference between systemic and pulmonary blood pressures. Mean systemic blood pressure was significantly higher than the pulmonary mean blood pressure in term lambs ventilated with 21% O2 (SBP: 80.2±14.4 mmHg, PBP: 48.4±7.2 mmHg, p<0.001), preterm lambs ventilated with 100% O2 (SBP:57.8±10.5 mmHg, PBP:38.4±8.3 mmHg, p<0.001) and in preterm lambs where the inspired oxygen was titrated to maintain saturations (SBP: 49.4±8.2mmHg, PBP: 41.5±11.1 mmHg, p<0.01). In contrast, preterm lambs ventilated with 21% O2 had similar systemic and pulmonary arterial pressures with no gradient across the ductus throughout the first 30 minutes of the postnatal period (SBP:49.1±9.0 mmHg, PBP:47.4±7.4 mmHg, p=0.25 as shown in Figure 5D). The difference in mean systemic blood pressure and pulmonary arterial pressure over the first 30 minutes after birth was significantly higher in term lambs ventilated with 21% oxygen and preterm lambs ventilated with 100% oxygen compared to preterm lambs ventilated with titrated O2 and 21% oxygen.
Figure 5.
Systemic and pulmonary arterial mean pressures in term 21% oxygen (A), preterm 100% oxygen (B), preterm titrated oxygen (C) and preterm 21% oxygen (D). A) Black and gray triangles represent the mean systemic and mean pulmonary arterial pressures in term lambs ventilated with 21% inspired oxygen (controls). B) Black and gray squares represent the mean systemic and mean pulmonary arterial pressures in preterm lambs resuscitated with 100% oxygen. C) Black and gray circles represent the mean systemic and mean pulmonary arterial pressures in preterm lambs resuscitated with oxygen titrated to achieve target SpO2. D) Open black and gray diamonds represent the mean systemic and mean pulmonary arterial pressures in preterm lambs resuscitated with 21% inspired oxygen concentration. * p<0.001 by ANOVA – the difference between systemic and pulmonary pressures.
Compared to fetal values, pulmonary arterial pressure significantly decreased over the first 30 minutes in term lambs (21% oxygen) and preterm lambs ventilated with 100% oxygen. There was no significant difference in pulmonary arterial pressure at 30 min after birth when compared to fetal values in preterm lambs ventilated with 21% oxygen. In lambs ventilated with titrated O2, the difference between fetal pulmonary arterial pressure compared to pressure at 30 min of postnatal age had a p-value of 0.05.
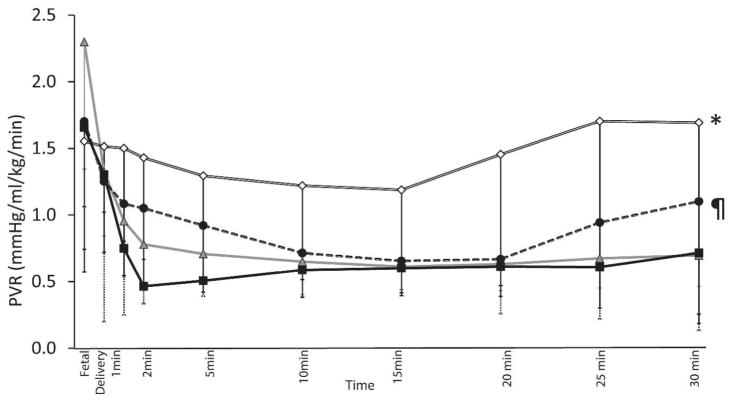
Pulmonary vascular resistance (PVR) (Figure 6): The PVR was highest in preterm lambs ventilated with 21% O2 (1.5±0.9 mmHg/ml/kg/min) and was significantly different from preterm lambs ventilated with 100% O2 (0.6±0.3 mmHg/ml/kg/min, p<0.001), titrated O2 (0.9±0.6 mmHg/ml/kg/min, p<0.001) and term lambs ventilated with 21% O2 (0.7±0.3 mmHg/ml/kg/min, p<0.001). The PVR was significantly higher in the titrated oxygen group compared to the 100% O2 group in preterm lambs (p=0.03) Both these groups were not statistically different from term lambs resuscitated with 21% O2.
Figure 6.
Pulmonary vascular resistance (PVR) changes in preterm lambs resuscitated with 100% oxygen (closed squares), 21% oxygen (open diamonds), and titrated inspired oxygen to maintain target SpO2 (closed circles). Term lambs ventilated with 21% inspired oxygen (controls - triangles) are shown by a hyphenated line. * 21% preterm oxygen statistically different from the rest by ANOVA (p<0.001).
Discussion
Effective ventilation of the lungs with optimal inspired O2 is the key factor mediating pulmonary transition at birth leading to an 8 to 10-fold increase in PBF permitting effective gas exchange (21–23). In this study, we have demonstrated that ventilation with 21% O2 in term lambs without lung pathology reduced pulmonary arterial pressure and increased PBF. Ventilation of preterm lambs with 100% O2 significantly reduced pulmonary arterial pressure, established a significant pressure gradient across the ductus and marginally increased PBF, similar to that seen in term lambs ventilated with 21% O2. In contrast, ventilation with 21% O2 in preterm lambs did not significantly reduce pulmonary arterial pressure over the first 30 minutes after birth and the increase in PBF was significantly lower than that in term lambs. Interestingly, initiation of ventilation at 21% O2 and titration of FiO2 targeting NRP recommended SpO2 range led to a modest decrease in pulmonary arterial pressure, but the increase in PBF was significantly lower than that achieved in term lambs. To our knowledge, this is the first study evaluating gas exchange, systemic and pulmonary hemodynamics, and cerebral oxygen delivery in a transitioning preterm model targeting the NRP recommended SpO2 ranges. These differences were achieved with similar tidal volumes and PaCO2 values among the three groups of preterm lambs suggesting that these differences were mainly due to variation in oxygen tension (figure S1).
The supplemental oxygen needed in a preterm neonate requiring assisted ventilation that facilitates the pulmonary vascular transition remains a subject of controversy. It is not known if the target saturation range recommended by NRP is adequate to increase pulmonary blood flow and establish lungs as the site of gas exchange in extremely preterm neonates requiring positive-pressure ventilation. We demonstrate that titrating inspired oxygen resulted in SpO2 in the desired range and 100% inspired O2 resulted in saturations above the target range. These results are different from the study in 287 preterm infants by Oei et al. (11). In their study, initiation of resuscitation with 21% oxygen and titration resulted in mean SpO2 below the target range for the first 7–8 minutes after birth. We speculate that this difference was secondary to: (a) all lambs in our study being intubated (and having received surfactant), compared to only 30% of infants in the Oei et al. study; (b) SpO2 values were available in the very first minute and O2 was adjusted immediately after birth; (c) we titrated O2 by 5% every 15 seconds as compared to 10% every minute in the clinical study; (d) our lambs were not exposed to antenatal steroids, while 97% of mothers in the Oei et al. study had received antenatal steroids and finally (e) species differences.
Dawson et al. have published changes in preductal SpO2 after birth for the term and preterm infants with no supplemental oxygen (24) (25). The supplemental oxygen required to achieve target SpO2 was higher (30% – 67%) in extremely preterm infants less than 27 6/7 weeks compared to moderate preterm infants 28 – 32 weeks (23% – 43%). Similarly, Phillipos et al. have shown that infants <28 weeks gestation requiring PPV who were initially ventilated with 30% oxygen had markedly lower saturations compared to NRP targets (25). With decreasing gestational age, higher FiO2 is necessary to achieve target oxygen saturations (25). We found similar differences between term lambs where target SpO2 could be achieved by ventilation with 21% O2 but required 44 ± 4% oxygen to achieve the same target in preterm lambs (equivalent gestational age <28 weeks in humans). Interestingly, the preductal PaO2 achieved by preterm lambs ventilated with 21% O2 was similar to that in term lambs. We do not have a physiologic explanation for the difference in SpO2 between preterm and term lambs ventilated with 21% oxygen, although for a given SpO2 value there may be a wide range of PaO2 values (26). In an individual patient analysis by Oei et al. which included 706 infants, only 11% reached NRP recommended SpO2 of 80–85% by 5 minutes (27). Infants who did not reach 80% SpO2 by 5 minutes had 2.4 times higher mortality and 4.5 times more likely to develop severe intraventricular hemorrhage (27) (28). The data from this study need to be interpreted with caution as it is unclear if the infants were sick and could not achieve > 80% SpO2 in spite of receiving high FiO2 or if less supplemental oxygen was provided resulting in SpO2 < 80%. Furthermore, a study by Follett et al. concluded that ‘there was a lag time of approximately 30 s to achieve the FiO2 at the facemask’. Slower change in oxygen could have yielded different results. Our study was different from the clinical situation where intubation and surfactant delivery was performed before delivery.
Ventilation with 100% O2 resulted in supra-physiological PaO2 (Figure 3). The PaO2 in the titrated oxygen group was significantly higher than preterm lambs resuscitated with 21%. Interestingly, delivery of more oxygen to the brain did not result in higher oxygen extraction (figure S2) but resulted in higher CrsO2. Previous studies have shown that resuscitation with higher oxygen can lead to oxidative stress injury, inflammation and chronic lung disease (9, 29). Kapadia et al. have shown that when resuscitation was initiated with low oxygen and titrated to target NRP recommended SpO2, the infants had low cumulative oxygen exposure, lower oxidative stress and decreased respiratory morbidities (5). Continuous use of 100% O2 may lead to hyperoxic injury and may cause the arrest of lung development (30–32). In an asphyxiated term lamb model, we have shown that use of 100% oxygen increases oxidative stress (33). Previously, we have also shown that oxidized glutathione activity correlated with PaO2 in a preterm lamb model (34). In a prospective clinical study by Castillo et al. maintaining saturations between 85 – 93% resulted in PaO2 levels of 40 to 80 mmHg, 87% of the time in neonate (35). In our study, titrating oxygen to maintain SpO2 in target range resulted in an average PaO2 of 54 mmHg while the continuous use of 21% resulted in an average PaO2 of 35 mmHg during the first 30 minutes after birth. A PaO2 of at least 45 mmHg is required for successful pulmonary vascular transition (16, 19, 33, 36). To achieve a delicate balance between hyperoxia and hypoxia, maintaining SpO2 in the target range suggested by NRP may be safe until more evidence is available in preterm infants.
At birth with the establishment of respiration, pulmonary vascular resistance (PVR) decreases, PBF increases and ductal shunt from right-to-left changes to left-to-right (37, 38). Heart rate is the single most important predictor of adequate ventilation (3). Since our lambs were intubated and ventilated immediately after birth, we did not find any difference in heart rate between the groups. In contrast, Oei et al. demonstrated a lower heart rate in preterm infants resuscitated initially with 21% O2 when compared to 100% O2 (11). We observed higher PBF in preterm lambs ventilated with 100% O2 similar to that seen in term lambs ventilated with 21% oxygen. Although PAO2 and PaO2 achieved by ventilation with 21% oxygen were similar in preterm and term lambs, the increase in pulmonary blood flow was significantly lower in preterm lambs. We speculate that this difference might be secondary to lung disease, ventilation-perfusion mismatch, the lower sensitivity of pulmonary vasculature to oxygen and/or due to differences in pressure gradient across the ductus.
The difference in systemic and pulmonary blood pressures were strikingly different in preterm lambs ventilated with different oxygen concentrations (Figure 5). The gradient across the ductus (difference in systemic and pulmonary pressures) in term lambs ventilated with 21% was higher than preterm lambs ventilated with 21% O2 or titrated FiO2 and similar to preterm lambs ventilated with 100% O2. The difference in pulmonary and systemic pressures reached a statistically significant difference in the titrated oxygen group but not in 21% preterm lambs.
Why is the pressure gradient across the ductus important in the golden hour of resuscitation especially in preterm infants? Once the cord is clamped, systemic blood pressure increases and with ventilation, there is a significant decrease in PVR. The increase in right ventricular output is not the sole contributor to increased blood flow to the lung after birth (38, 39). The change in shunt in the ductus which switches to left-to-right contributes to right ventricular output immediately after birth. This transition is a critical process that helps sustain and stabilize PBF, pulmonary venous return and thus maintaining left ventricular output (20, 37, 39). Our study highlights the effect of using various oxygen concentration in this critical process.
The drop in PVR was highest with the use of 100% O2 in preterm lambs and was similar to 21% oxygen use in term lambs. Use of 21% O2 in preterm lambs resulted in no change in PVR by 30 minutes of postnatal age compared to fetal values. Titrating oxygen to maintain target SpO2 lead to a drop in PVR by 57% at 30 minutes of life compared to the fetal PVR. The decrease in PVR, in this case, can be directly related to the PaO2 achieved by various oxygen concentration during resuscitation. We have previously shown in an asphyxiated model with lung disease, use of 21% O2 may not be adequate to achieve pulmonary vasodilation secondary to low PaO2 levels (16), although in a term model without lung disease use of 21% O2 did result in significant decrease in PVR (16, 19).
The suboptimal increase in PBF with the initiation of ventilation with 21% oxygen and titration to achieve target SpO2 (when compared to 100% O2 ventilation) is a concern. The recent evidence from Oei et al. study shows that extremely preterm infants (< 28 weeks’ gestation) had higher mortality when ventilation was initiated with 21% O2 as compared to 100% O2 further emphasizing the importance of this finding (11). In the same study, the most common cause of death in preterm infants resuscitated initially with 21% O2 was respiratory failure (which may potentially be secondary to RDS complicated by impaired pulmonary vascular transition). It is important to recognize that the results in < 28 weeks’ gestation preterm infants in this study were not part of a prespecified analysis and that the sample size was small. However, increased mortality with lower oxygen supplementation is a concern. These findings are similar to the retrospective Canadian study which showed higher mortality and evidence of higher neurologic injury in preterm infants resuscitated with < 100% oxygen (12). We speculate that inadequate pulmonary vascular transition in preterm infants resuscitated with lower FiO2 may potentially play a role in mediating higher respiratory morbidity.
Studies in postnatal preterm infants have shown that 100% O2 inhalation results in ~15% reduction in cerebral blood flow (40, 41). Exposure to oxygen at birth results in prolonged cerebral vasoconstriction in preterm infants (6). However, a recent study in stable preterm infants did not demonstrate cerebral vasoconstriction following hyperoxia (42). The relationship between hyperoxia and cerebral blood flow are complex and are elegantly summarized in a recent editorial (43).
Cerebral vasoconstriction following 100% oxygen may potentially protect the brain from hyperoxic injury. However, in our study, we found no reduction in CBF in spite of 100% oxygen resuscitation in preterm lambs. The cerebral saturations and the carotid arterial oxygen content were highest with 100% O2 resuscitation resulting in increased delivery of oxygen, but oxygen extraction was similar between the 3 groups of preterm lambs. Premature neonates are at risk of hyperoxic brain injury, especially when exposed to supraphysiological oxygen levels compared to their fetal environment. Gerstner et al. have shown that hyperoxia could lead to maturation-dependent cell death in the developing white matter (44). The lack of reduction in cerebral blood flow despite high PaO2 levels suggests that the risk of cerebral hyperoxia is considerably higher in preterm infants initially resuscitated with 100% oxygen and may contribute to brain injury. We acknowledge that species differences and similar PaCO2 levels between groups in our study may account for some of the variations among studies. Based on these findings, resuscitators should exercise caution and avoid initiating resuscitation with 100% oxygen in preterm infants and support the currently planned study comparing 60% and 30% oxygen for initial resuscitation of preterm infants ((PRESOX) (NCT01773746)).
There are several limitations to this study. Ventilating these lambs with an endotracheal tube and prophylactic surfactant helped achieved targeted saturations early and would have been different if bag and mask ventilation was initiated. The time spent in the prespecified saturation range in the titrated O2 group was only 47%, while 30% were above and the remainder of the 23% were below the range. We found it practically difficult to maintain saturations in 5% range in the first 5 minutes. During the 5 – 30 min period, the target range broadens to 85–95% and targets were achievable. Improved time spent within the SpO2 range may have altered the pulmonary hemodynamics in these lambs. We performed immediate cord clamping in our study and did not evaluate the effect of placental transfusion during initial ventilation (3, 45, 46). The ductal flow in this study was not measured/analyzed. None of these lambs had bradycardia and did not require chest compressions or epinephrine (47, 48). All these experiments were done in a controlled setting with personnel available to titrate oxygen every 15 seconds which may be different in a real-life scenario in a busy delivery room (49). We did not study initiation of ventilation with 65–100% oxygen followed by decreasing FiO2 based on SpO2 – this may be an important arm that requires further study. We continued ventilating these infants for 6 hours and did not obtain tissues at the end of the 30 min period. Hence, oxidative stress markers are not available at the end of 30 minutes to evaluate the injury.
Conclusion
Titrating oxygen starting from 21% O2 successfully achieved target saturations. Continuous use of 100% and 21% oxygen resulted in saturations above and below the target range. Use of 100% oxygen resulted in better pulmonary transition as evidenced by the significant difference in systemic and pulmonary hemodynamics compared to 21% use in preterm lambs but was similar to term controls ventilated with 21% O2. Ventilation with 100% O2 in preterm lambs caused hyperoxia and did not increase oxygen delivery to the brain but reduced PVR similar to term lambs on 21% O2. Initiation of resuscitation with low oxygen (21%) and titration based on NRP recommended target saturations, resulted in significantly lower increase in PBF compared to term lambs and may not be adequate. In the future, studies evaluating the effect of resuscitating preterm infants with higher initial oxygen concentration (60–100%) and titrating down targeting saturations are needed. We eagerly await additional studies such as the ‘Study of Room Air Versus 60% Oxygen for Resuscitation of Premature Infants (PRESOX) (NCT01773746)’ for more insight.
Supplementary Material
Acknowledgments
Financial support: (SL)1R01HD072929
(PC) Salary support from University at Buffalo - Dr. Henry C. and Bertha H. Buswell Grant
Footnotes
Conflict of interest: Authors have no conflicts of interest
References
- 1.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation Chapter C 2015 Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 132:S204–241. doi: 10.1161/CIR.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 2.Wyckoff MH, Aziz K, Escobedo MB, Kapadia VS, et al. Part 13: Neonatal Resuscitation: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S543–560. doi: 10.1161/CIR.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 3.Textbook of Neonatal Resuscitation (NRP) 7. 2016. p. 326. [Google Scholar]
- 4.Armanian AM, Badiee Z. Resuscitation of preterm newborns with low concentration oxygen versus high concentration oxygen. J Res Pharm Pract. 2012;1:25–29. doi: 10.4103/2279-042X.99674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapadia VS, Chalak LF, Sparks JE, Allen JR, Savani RC, Wyckoff MH. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics. 2013;132:e1488–1496. doi: 10.1542/peds.2013-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundstrom KE, Pryds O, Greisen G. Oxygen at birth and prolonged cerebral vasoconstriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;73:F81–86. doi: 10.1136/fn.73.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabi Y, Singhal N, Nettel-Aguirre A. Room-air versus oxygen administration for resuscitation of preterm infants: the ROAR study. Pediatrics. 2011;128:e374–381. doi: 10.1542/peds.2010-3130. [DOI] [PubMed] [Google Scholar]
- 8.Rook D, Schierbeek H, Vento M, et al. Resuscitation of preterm infants with different inspired oxygen fractions. J Pediatr. 2014;164:1322–1326e1323. doi: 10.1016/j.jpeds.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 10.Wang CL, Anderson C, Leone TA, Rich W, Govindaswami B, Finer NN. Resuscitation of preterm neonates by using room air or 100% oxygen. Pediatrics. 2008;121:1083–1089. doi: 10.1542/peds.2007-1460. [DOI] [PubMed] [Google Scholar]
- 11.Oei JL, Saugstad OD, Lui K, et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics. 2017:139. doi: 10.1542/peds.2016-1452. [DOI] [PubMed] [Google Scholar]
- 12.Rabi Y, Lodha A, Soraisham A, Singhal N, Barrington K, Shah PS. Outcomes of preterm infants following the introduction of room air resuscitation. Resuscitation. 2015;96:252–259. doi: 10.1016/j.resuscitation.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekharan P, Vali P, Rawat M, et al. Continuous capnography monitoring during resuscitation in a transitional large mammalian model of asphyxial cardiac arrest. Pediatr Res. 2017 doi: 10.1038/pr.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrasekharan PK, Rawat M, Nair J, et al. Continuous End-Tidal Carbon Dioxide Monitoring during Resuscitation of Asphyxiated Term Lambs. Neonatology. 2016;109:265–273. doi: 10.1159/000443303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smolich JJ, Soust M, Berger PJ, Walker AM. Indirect relation between rises in oxygen consumption and left ventricular output at birth in lambs. Circ Res. 1992;71:443–450. doi: 10.1161/01.res.71.2.443. [DOI] [PubMed] [Google Scholar]
- 16.Rawat M, Chandrasekharan PK, Swartz DD, et al. Neonatal resuscitation adhering to oxygen saturation guidelines in asphyxiated lambs with meconium aspiration. Pediatr Res. 2016;79:583–588. doi: 10.1038/pr.2015.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Askie LM, Brocklehurst P, Darlow BA, et al. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr. 2011;11:6. doi: 10.1186/1471-2431-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 19.Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62:313–318. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crossley KJ, Allison BJ, Polglase GR, Morley CJ, Davis PG, Hooper SB. Dynamic changes in the direction of blood flow through the ductus arteriosus at birth. J Physiol. 2009;587:4695–4704. doi: 10.1113/jphysiol.2009.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56:579–600. doi: 10.1016/j.pcl.2009.04.004. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshminrusimha S, Keszler M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews. 2015;16:e680–e692. doi: 10.1542/neo.16-12-e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang JA, Pearson JT, Binder-Heschl C, et al. Increase in pulmonary blood flow at birth: role of oxygen and lung aeration. J Physiol. 2016;594:1389–1398. doi: 10.1113/JP270926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawson JA, Kamlin CO, Vento M, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. 2010;125:e1340–1347. doi: 10.1542/peds.2009-1510. [DOI] [PubMed] [Google Scholar]
- 25.Phillipos E, Solevag AL, Aziz K, et al. Oxygen Saturation and Heart Rate Ranges in Very Preterm Infants Requiring Respiratory Support at Birth. J Pediatr. 2017;182:41–46e42. doi: 10.1016/j.jpeds.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol. 2015;35:8–15. doi: 10.1038/jp.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oei JL, Finer NN, Saugstad OD, et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2017 doi: 10.1136/archdischild-2016-312366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oei JL, Wright IM, Saugstad OD. Authors’ Response. Pediatrics. 2017:139. doi: 10.1542/peds.2017-0452B. [DOI] [PubMed] [Google Scholar]
- 29.Vento M, Asensi M, Sastre J, Garcia-Sala F, Pallardo FV, Vina J. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 30.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152:640–646. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 31.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 32.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 33.Lakshminrusimha S, Steinhorn RH, Wedgwood S, et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21 and 100% oxygen. J Appl Physiol (1985) 2011;111:1441–1447. doi: 10.1152/japplphysiol.00711.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel A, Lakshminrusimha S, Ryan RM, et al. Exposure to supplemental oxygen downregulates antioxidant enzymes and increases pulmonary arterial contractility in premature lambs. Neonatology. 2009;96:182–192. doi: 10.1159/000211667. [DOI] [PubMed] [Google Scholar]
- 35.Castillo A, Sola A, Baquero H, Neira F, Alvis R, Deulofeut R, Critz A. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: is 85% to 93% an acceptable range? Pediatrics. 2008;121:882–889. doi: 10.1542/peds.2007-0117. [DOI] [PubMed] [Google Scholar]
- 36.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, Swartz DD, Kumar VH. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59:137–141. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polglase GR, Miller SL, Barton SK, et al. Respiratory support for premature neonates in the delivery room: effects on cardiovascular function and the development of brain injury. Pediatr Res. 2014;75:682–688. doi: 10.1038/pr.2014.40. [DOI] [PubMed] [Google Scholar]
- 38.Rudolph AM. Fetal and neonatal pulmonary circulation. Am Rev Respir Dis. 1977;115:11–18. doi: 10.1164/arrd.1977.115.S.11. [DOI] [PubMed] [Google Scholar]
- 39.Kluckow M. Low systemic blood flow and pathophysiology of the preterm transitional circulation. Early Hum Dev. 2005;81:429–437. doi: 10.1016/j.earlhumdev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Leahy FA, Cates D, MacCallum M, Rigatto H. Effect of CO2 and 100% O2 on cerebral blood flow in preterm infants. J Appl Physiol Respir Environ Exerc Physiol. 1980;48:468–472. doi: 10.1152/jappl.1980.48.3.468. [DOI] [PubMed] [Google Scholar]
- 41.Niijima S, Shortland DB, Levene MI, Evans DH. Transient hyperoxia and cerebral blood flow velocity in infants born prematurely and at full term. Arch Dis Child. 1988;63:1126–1130. doi: 10.1136/adc.63.10_spec_no.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thing M, Sorensen LC, Pryds O. Transient hyperoxia does not affect regional cerebral tissue oxygen saturation in moderately preterm or term newborns. Acta Paediatr. 2015;104:657–662. doi: 10.1111/apa.12860. [DOI] [PubMed] [Google Scholar]
- 43.Saugstad OD. Hyperoxia and cerebral vasoconstriction in healthy newborns. Acta Paediatr. 2015;104:645–646. doi: 10.1111/apa.13004. [DOI] [PubMed] [Google Scholar]
- 44.Gerstner B, DeSilva TM, Genz K, et al. Hyperoxia causes maturation-dependent cell death in the developing white matter. J Neurosci. 2008;28:1236–1245. doi: 10.1523/JNEUROSCI.3213-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hooper SB, Binder-Heschl C, Polglase GR, et al. The timing of umbilical cord clamping at birth: physiological considerations. Matern Health Neonatol Perinatol. 2016;2:4. doi: 10.1186/s40748-016-0032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical Cord Milking Versus Delayed Cord Clamping in Preterm Infants. Pediatrics. 2015;136:61–69. doi: 10.1542/peds.2015-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vali P, Chandrasekharan P, Rawat M, et al. Evaluation of Timing and Route of Epinephrine in a Neonatal Model of Asphyxial Arrest. J Am Heart Assoc. 2017:6. doi: 10.1161/JAHA.116.004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vali P, Chandrasekharan P, Rawat M, et al. Hemodynamics and gas exchange during chest compressions in neonatal resuscitation. PLoS One. 2017;12:e0176478. doi: 10.1371/journal.pone.0176478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oei JL, Saugstad OD, Vento M. Oxygen and preterm infant resuscitation: what else do we need to know? Curr Opin Pediatr. 2018 doi: 10.1097/MOP.0000000000000610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.