Abstract
Age-related thymic involution is characterized by a decrease in thymic epithelial cell (TEC) number and function parallel to a disruption in their spatial organization, resulting in defective thymocyte development and proliferation as well as peripheral T cell dysfunction. Deficiency of Klotho, an anti-aging gene and modifier of fibroblast growth factor signaling, causes premature aging. To investigate the role of Klotho in accelerated age-dependent thymic involution, we conducted a comprehensive analysis of thymopoiesis and peripheral T cell homeostasis using Klotho-deficient mice. At 8 weeks of age, Klotho-deficient mice displayed a severe reduction in the number of thymocytes (10–100 fold reduction), especially CD4 and CD8 double positive cells, and a reduction of both cortical and medullary TEC. To address a cell-autonomous role for Klotho in TEC biology, we implanted neonatal thymi from Klotho-deficient and -sufficient mice into athymic hosts. Klotho-deficient thymus grafts supported thymopoiesis equivalently to Klotho-sufficient thymus transplants, indicating that Klotho is not intrinsically essential for TEC support of thymopoiesis. Moreover, lethally-irradiated hosts given Klotho-deficient or WT bone marrow had normal thymocyte development and comparably reconstituted T cells, indicating Klotho is not inherently essential for peripheral T cell reconstitution. Because Klotho-deficient mice have higher levels of serum phosphorus, calcium, and vitamin D, we evaluated thymus function in Klotho-deficient mice fed with vitamin D-deprived diet. We observed that vitamin D-deprived diet abrogated thymic involution and T cell lymphopenia in 8-week-old Klotho-deficient mice. Taken together, our data suggests that Klotho-deficiency causes thymic involution via systemic effects that include high active vitamin D levels.
Introduction
Thymic aging is a major contributing factor to immunological senescence as the thymus begins to involute with the onset of puberty, leading to a progressive in the ability to generate naïve T cells (1, 2). The primary elements of the thymic microenvironment affected by age-related involution are structural thymic epithelial cells (TECs). TECs are replaced by adipocytes and peripheral lymphocytes, resulting in decreased thymopoietic activity and T cell selection (3). Consequently, both quantity and quality of the T cell repertoire are affected, with decreased de novo T cell generation that is compensated by a homeostatic oligoclonal expansion of T cells in the periphery (4, 5). These dynamic changes result in an increased susceptibility to infections (6, 7), suboptimal responses to vaccines (8–12), and an increased risk to develop cancer and autoimmune diseases (13–15). The age-related decline in thymopoietic activity (1) is especially apparent in patients who have undergone chemotherapy (16) or allogeneic hematopoietic stem cell transplantation (17). The necessary preparative regimen with cytotoxic chemotherapy and/or radiation severely damages the thymus, the recovery of which is extremely limited in aged individuals (18, 19).
To study the process of aging in mice, Klotho-deficient (Kl/Kl) animals have been used as they grow normally up to 3 weeks of age and then begin to show premature aging phenotypes (20). Klotho encodes a beta-glucuronidase-related molecule in two separate isoforms, transmembrane and secreted; the transmembrane molecule serves as a co-receptor for fibroblast growth factor 23 (FGF23) by transporting this cytokine to its receptor, FGFR1c, and thereby regulating mineral metabolism (21–23). Klotho is expressed in the kidney and parathyroid gland and the secreted form also be found in the blood, CSF and urine (24). FGF23 suppresses phosphate reabsorption and Vitamin D synthesis in the kidney, causing negative phosphate balance due both to its phosphaturic hormone function and as a counter-regulatory hormone for Vitamin D(24). The secreted form of Klotho inhibits insulin growth factor 1 signaling and confers increased resistance to oxidative stress (25–27). Mice transgenic for Klotho live 20–30% longer than wild-type (WT) controls (28), while the protein’s absence results in an advanced aging syndrome resembling progeria. Multiple organs are affected in Kl/Kl mice resulting in growth retardation, pituitary abnormalities, arteriosclerosis, ectopic calcification of various organs, osteoporosis, skin atrophy, emphysema, and atrophy of both the genital organs and the thymus (20). Interestingly, mice that are FGF23 deficient or Klotho deficient have phenotypes similar to one another. These deficits can be ameliorated by reversing the effects of hyperphosphatemia either genetically or by diet, suggesting a link between aging and phosphate(24).
The Kl/Kl mouse model has provided insight into the process of aging in humans. Indeed, human KLOTHO shares 86% amino acid identity with its mouse ortholog (29). Individuals homozygous for Klotho variants that disrupt the molecule’s trafficking and catalytic functions experience a decreased life expectancy (29), have increased cardiovascular risk factors, such as elevated high-density lipoprotein cholesterol levels and high systolic blood pressure (30), and demonstrate an increased risk for stroke and coronary artery disease (31). Polymorphisms in KLOTHO (loss of function) have been associated with an increased risk for osteoporosis and spondylosis (32) and reduced KLOTHO protein expression has been noted in patients with chronic renal failure (33).
While the effects of Klotho-deficiency on kidney development and function, mineral metabolism, and bone maintenance are well studied in Kl/Kl mice, the direct effect of Klotho-deficiency on the TECs is unknown. We therefore sought to determine whether the effects of Klotho on thymic aging are cell intrinsic or reflect a systemic metabolic consequence of a lack of the Klotho protein.
Methods
Mice
B6.Cg-Foxn1nu/J mice were purchased from Jackson Labs and were used at 8–12 weeks of age. Kl/+ mice (B6-CD45.2+) were generously provided by the University of California Davis mouse mutant resource center and were intercrossed (Kl/+ by Kl/+) in our animal colony under the guidance of in-house veterinary staff. B6-Ly5.2/Cr (B6-CD45.1+) were purchased from the National Cancer Institute and were used at 7 weeks of age. Mice were housed in a specific pathogen-free facility and used with the approval of the University of Minnesota Institutional Animal Care and Use Committee (IACUC). For vitamin D experiments, breeders were fed and pups were maintained on vitamin D-deprived diet purchased from Harlan, product TD. 89123.
Flow cytometry and Klotho expression
Mouse thymus, spleen, and lymph node (LN) were processed into single cell suspensions, and analyzed by flow cytometry. Thymic epithelial cells (TECs) were isolated as previously described (34). Dead cells were stained by a fixable viability dye conjugated to eFluor780 (eBioscience). Fixation and intracellular/intranuclear staining were performed using the eBioscience Foxp3 staining kit or BD Fixation/Permeabilization Solution Kit. The following antibodies were purchased from BD biosciences: TCRβ (H57–597), TCRγδ (GL-3) and EpCAM (G8.8). The following antibodies were purchased from eBioscience: CD3 (145–2C11), CD4 (GK1.5), CD11c (N418), CD25 (PC61.5), CD45R (B220, RA3–6B2), MHCII (M5/114) and Ki67 (SolA15). The following antibodies were purchased from BioLegend: CD8 (53–6.7), CD11b (M1/70), CD44 (IM7), CD45 (30-F11), CD62L (MEL-14), CD122 (TM-β1), Gr-1 (RB6–8C5), Ly-51 (6C3), NK-1.1 (PK136) and TER-119 (TER-119). UEA-1 was purchased from Vector Laboratories. Data were collected using a BD LSR II flow cytometer and were analyzed using FlowJo V10 (Treestar Inc.). The TaqMan Gene Expression Assay (ID: Mm00502000_m1, Thermo Fisher Scientific) for quantification of Klotho gene expression.
Neonatal Thymus Transplantation
Mice heterozygous for Klotho were mated overnight and then separated. At the time of harvest, neonate pups were screened for Klotho via PCR. WT or Kl/Kl thymi were placed under the kidney capsule of B6.Cg-Foxn1nu/J mice in the previously described manner (35, 36).
Bone Marrow Transplantation
B6-CD45.1+ recipients were lethally irradiated using 1100 cGy total body irradiation by x-ray one day before infusion. On the second day, bone marrow cells (BM) were harvested from Kl/Kl mice and littermates. Mature T-cells were removed from donor BM using anti-CD4, anti-CD8 antibodies and low-toxicity rabbit complement and given intravenously at a cell dose of 1 × 107.
Immunofluorescence staining
Thymi were harvested and snap frozen in O.C.T. compound. Frozen sections (8 μm) were cut using a CM1900 cryostat (Leica). Slides were dried for 30 min and then were immerged in acetone for 5 min at room temperature. The sections were blocked in PBS with 3% BSA (PBSB) for 1 h at room temperature and stained with the rabbit anti-mouse K5 polyclonal antibody (MBL International) and rat anti-mouse K8 monoclonal antibody (TROMA-I, Development Studies Hybridoma Bank) followed by Dylight 550 donkey anti-rabbit IgG antibody and Dylight 650 donkey anti-rat IgG (Invitrogen). ProLong Gold antifade reagent (Invitrogen) was used to prevent photobleaching. Images were obtained using a microscope (DM5500B; Leica) with a camera (DFC 340FX; Leica) operating with the Leica Application Suite Advanced Fluorescence (LAS AF; Leica) software and analyzed using ImageJ (NIH) software.
Statistical Analyses
Prism software (Graphpad) was used for statistical analysis. Data sets were compared using an unpaired Mann-Whitney test. Data are shown as mean values +/− SD. Significance was defined as p<0.05.
Results
Klotho-deficient mice showed profound involution of thymus at young adult age
The thymus is the primary lymphoid organ for the development of T cells by providing a unique microenvironment able to attract T cell precursors from the blood and control their T cell lineage commitment, differentiation and selection (37). The earliest stages of intrathymic T cell development are marked by the absence of CD4 and CD8 expression as well as other lineage markers (designated double negative, DN, cells). These immature DN cells are located in the cortex, where they significantly expand in number before they acquire the concomitant expression of both CD4 and CD8 (double positive, DP) and express an αβ T cell antigen receptor (TCR). DP thymocytes constitute the most abundant subpopulation of thymocytes and are subjected first to a process of positive selection which assures that thymocytes express a TCR with sufficient affinity for a peptide-MHC complex expressed on cortical thymic epithelial cells (cTECs) (38). Positively selected thymocytes differentiate into TCRβ-positive CD4 and CD8 single positive (CD4SP and CD8SP) cells, respectively, and migrate to the medulla continuing their selection and maturation (39, 40). Self-reactive thymocytes are either eliminated by negative selection (clonal deletion) or differentiate into regulatory T cells (41). Subsequently, thymocytes are selected for tolerance to tissue-restricted antigens but responsiveness to foreign antigens presented by the individual’s MHC haplotype (39).
We investigated the thymic microenvironment of Kl/Kl mice from 4 weeks of age, shortly after which these mice begin to display signs of advanced aging (20). At 4 weeks of age, the thymi of Kl/Kl mice were comparable to that of their wild type (WT) littermates in size, cellularity and subpopulation distribution (Fig. 1A, 1B, 1C). Kl/Kl mice at 8 weeks of age (young adult) displayed a significantly reduced thymic size when compared to that of WT littermate controls (Fig. 1A) and a profound decrease of thymocytes in total cellularity (Fig. 1B). Flow cytometry revealed that the frequency of DP thymocytes was significantly decreased but that of DN, CD4SP and CD8SP thymocytes yet remained unchanged in 8-week-old Kl/Kl mice when compared to age-matched controls (Fig. 1C, Supplemental Fig. 1A-1D). All thymocyte subpopulations had, however, a significantly reduced cellularity in 8-week-old Kl/Kl mice (Fig. 1D) Semi-mature SP thymocytes were barely detected in 8-week-old Kl/Kl mice (Fig. 1E, 1F, Supplemental Fig. 1E), suggesting that de novo generation of SP thymocytes was severely blocked (42). Thus, the thymus of 8-week-old Kl/Kl mice was prematurely involuted to an extent only observed in older, physiologically aged mice.
Figure 1.
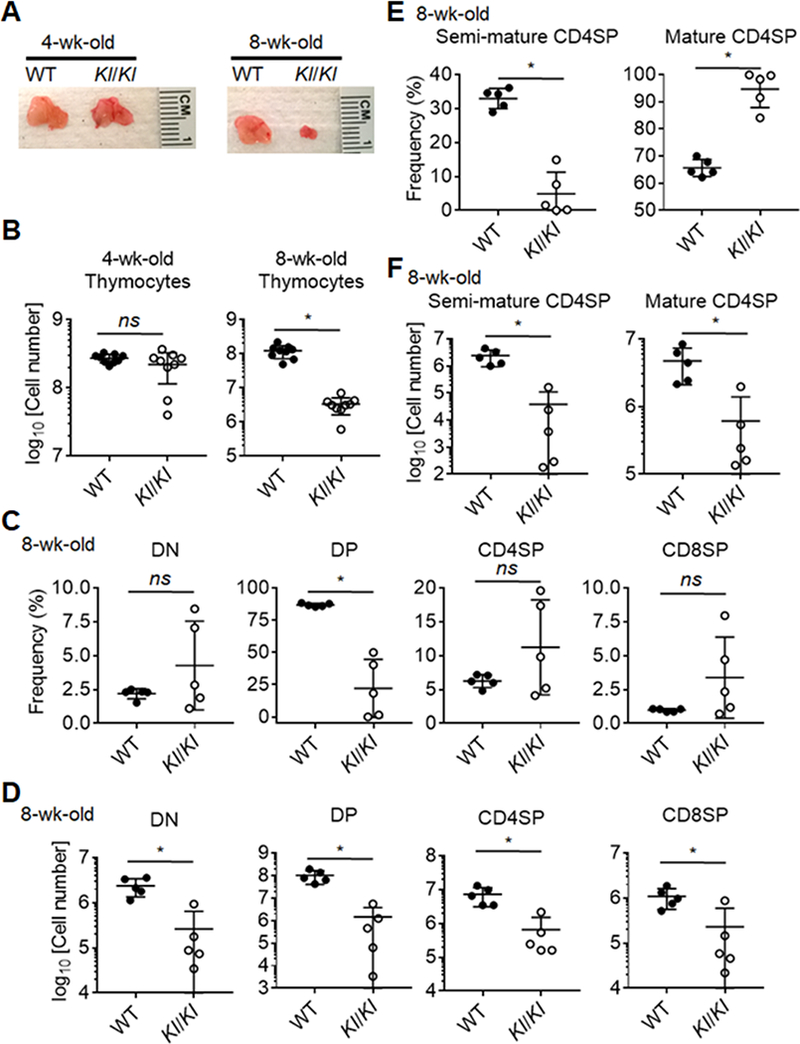
Profound thymus involution in Klotho-deficient (Kl/Kl) mice at 8 weeks of age.
(A) A representative image of the thymus from Kl/Kl and littermate control mice at 4 and 8 weeks of age. (B) Quantification of total thymocytes in Kl/Kl mice and littermate controls. (C) A summary of percentages of thymocyte subpopulations, including DN (Lineage-CD4-CD8-), DP (CD4+CD8+), CD4SP (TCRβ+CD4+CD8-) and CD8SP (TCRβ+CD4-CD8+) cells. (D) A graph shows quantification data of absolute numbers of thymocyte subpopulations. (E) Frequencies of semi-mature (CD69+MHC-I-) and mature (CD69+/−MHC-I+) subpopulations in CD4SP cells. (F) Quantification of absolute numbers of semi-mature and mature CD4SP in the thymi. Each symbol in the graphs represents an individual mouse (n ≥ 5); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *, P < 0.05.
Thymus involution in 8-week-old Klotho-deficient mice is associated with a severe reduction in thymic epithelial cells.
In order to assess the effect of Klotho on TECs, both cortical TECs (cTECs) and medullary TECs (mTECs) were evaluated. They are defined by Ly51 expression and binding capacity to the lectin, UEA-1. cTECs are critical for T cell progenitor expansion and positive selection of thymocytes whereas both cTEC and mTEC effect the negative selection of self-reactive thymocytes (43). To address whether TEC development and maintenance are impaired in the absence of Klotho, we assessed TEC cellularity and frequencies in 4 and 8 week old Kl/Kl mice. Total TEC cellularity and the frequencies of their individual subsets were comparable at 4 weeks of age with that of wildtype or heterozygote littermate controls (Fig. 2A, 2B). In contrast, 8-week-old Kl/Kl mice showed a significant reduction in the number of total TECs (Fig. 2A), affecting especially mTECs (Fig. 2B, 2C). These data suggest that a Klotho deficiency intrinsically or extrinsically reduced TEC cellularity in 8 week old animals.
Figure 2.
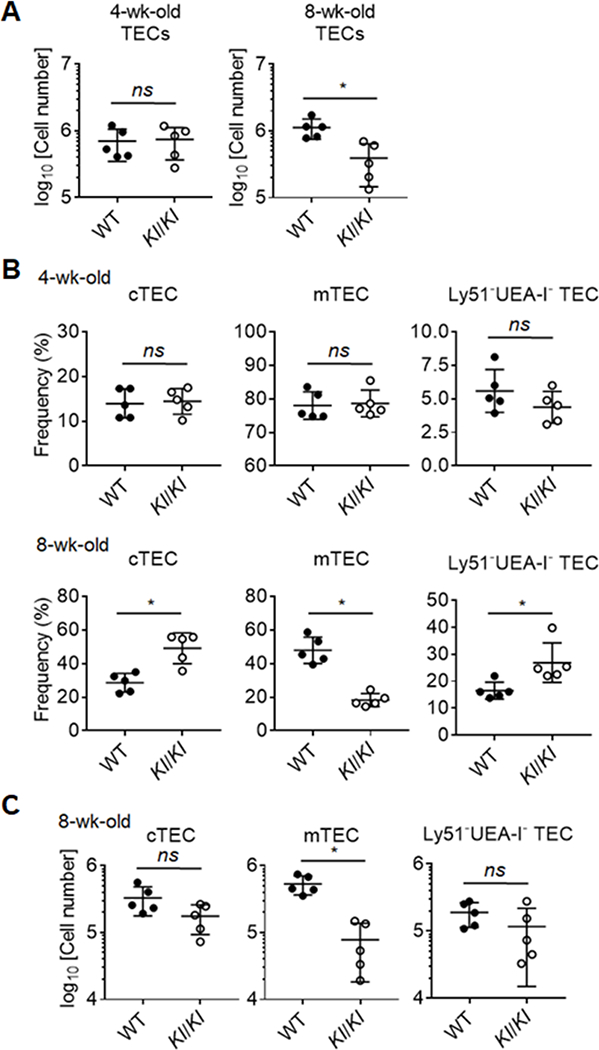
Reduction of thymic epithelial cells (TECs) in 8-wk-old Klotho-deficient mice.
(A) A graph shows the absolute number of TECs in the thymus of Kl/Kl and control mice at 4 and 8 weeks of age. (B) Frequencies of TEC Subpopulations, cTEC (UEA-I-Ly51+) and mTEC (UEA-I+Ly51-). (C) Quantification of cTECs and mTECs in Kl/Kl mice and littermate control at 8-week of age. Each symbol in the graphs represents an individual mouse (n ≥ 5); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *P < 0.05.
Thymic epithelial cells do not require Klotho expression to support thymopoiesis.
In order to determine the expression of Klotho in TECs, we performed RT-PCR on sorted cTEC, mTEClo and mTEChi cells in both wildtype and Kl/Kl animals. Klotho expression was found in both mTEC compartments, but was minimal in the cTEC compartment in WT animals. As expected, there was no expression in the TECs of Kl/Kl animals. Thymic involution is the result of cell autonomous changes in cell maintenance as a function of age but can also occur as a result of systemic abnormalities via various paracrine effector mechanisms. To distinguish between these two explanations for the observed pre-senescent changes in thymic cellularity, we grafted thymic lobes from either neonatal Kl/Kl or WT mice under the kidney capsule of haploidentical athymic (i.e. nude, Foxn1nu/nu (44)) recipients and compared their growth and differentiation. Foxn1nu/nu mice are homozygously deficient for the expression of functional Foxn1, a master regulator of TEC growth, differentiation and function of TEC, and therefore athymic and T cell deficient but have otherwise intact hematopoietic stem cells. To have a chronotypic comparison with 8-week old Kl/Kl mice, thymus grafts and peripheral lymphoid tissues of grafted recipients were analyzed 8 weeks after transplantation. The size and cellularity of Kl/Kl and WT thymus grafts were comparable (Fig. 3A and 3B) and displayed identical proportions and cellularity of the distinct thymocyte subpopulations (Fig. 3C). The ostensibly normal thymopoietic activity of Klotho-deficient grafts resulted in a comparable peripheral T cell reconstitution in both groups of transplanted mice (Fig. 3E, 3F). In aggregate, these results demonstrated that non-hematopoietic thymic stromal cells including TEC do not rely on Klotho expression for the organs’ thymopoietic activity.
Figure 3.
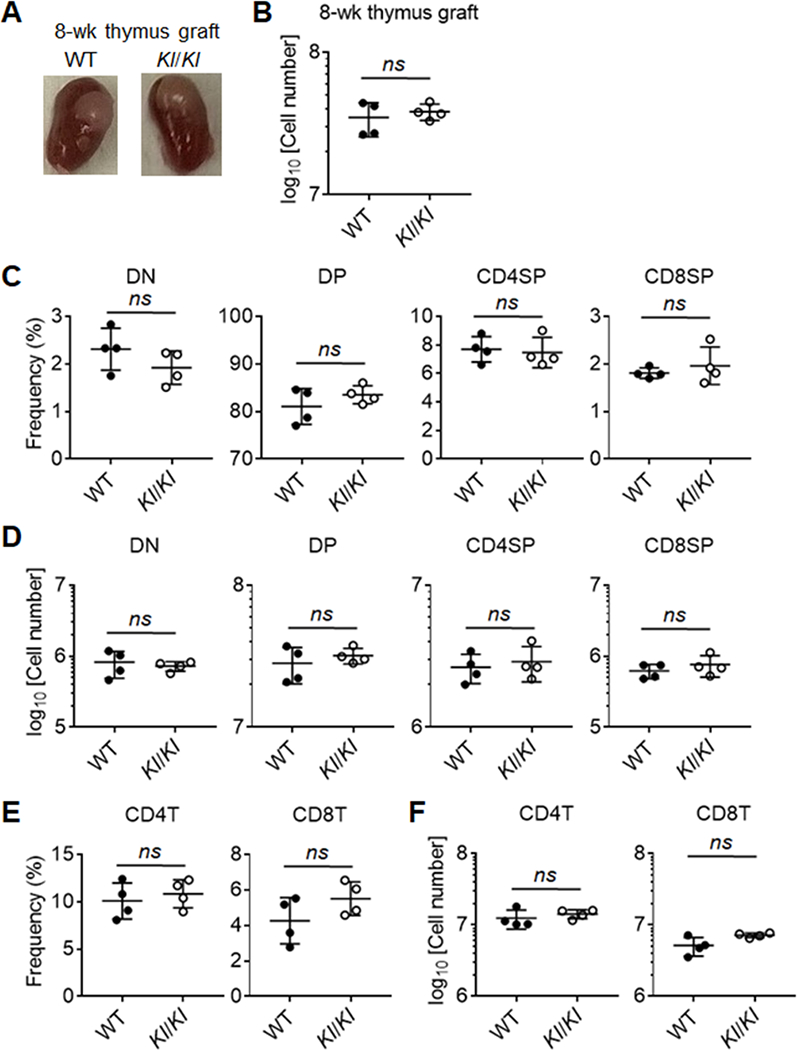
Stromal cells from Klotho-deficient and -sufficient thymus showed comparable ability to support T cell development after thymus transplantation.
(A) Representative images of thymus grafts under kidney capsule in athymic host mice after 8 weeks of transplantation. (B) Quantification of total thymocytes of Klotho-deficient and -sufficient thymus grafts in the recipients after 8 weeks of transplantation. (C) Frequencies and cellularity (D) of thymocyte subpopulations in Klotho-deficient and -sufficient thymus grafts after 8 weeks of transplantation. (E) Frequencies and cellularity (F) of T cell subsets in the spleen from athymic recipients with Klotho-deficient and -sufficient thymus grafts after 8 weeks of transplantation. Each symbol in the graphs represents an individual mouse (n ≥ 4); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *, P < 0.05.
Selective Klotho deficiency in bone marrow cells does not impair thymocyte differentiation
Although mature blood cells, including T cells, do not express Klotho, findings from the transplant experiments did not formally exclude the requirement of Klotho for progenitor cells to commit to a T cell fate and differentiate into mature thymocytes able to promote TEC maturation via thymus cross-talk. To examine this possibility, lethally irradiated H-2-matched WT mice were grafted with either Klotho-deficient or –proficient hematopoietic stem cells (HSCs) and analyzed 8 weeks later (Fig. 4A). Recipients rescued with Klotho-deficient HSCs displayed thymocyte cellularity and differentiation comparable to mice grafted with WT HSCs (Fig. 4B-4D). These results demonstrate that thymic Klotho expression in thymocytes is dispensable to repopulate the T cell lineage in irradiated hosts
Figure 4.
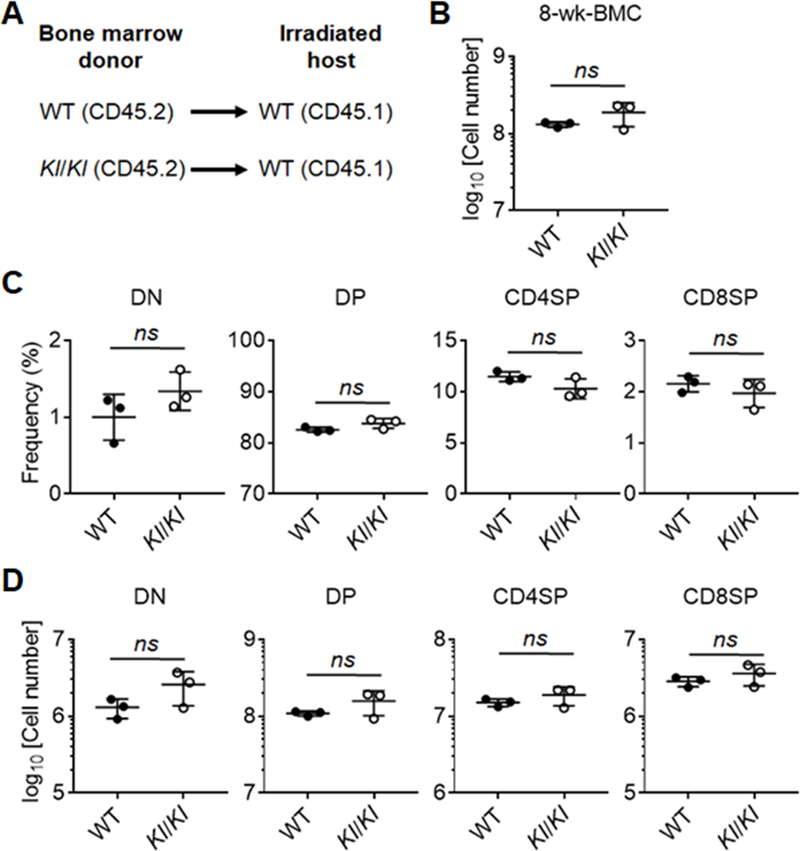
Klotho-deficient and -sufficient bone marrow cells showed equivalent capability to generate T cells in the bone marrow chimeras.
(A) An experimental schema of making bone marrow chimeras. (B) Quantification of absolute cell numbers of thymocytes in recipients 8 weeks after bone marrow transplantation. (C) A summary of percentages of thymocyte subpopulations in the thymi from the hosts. (D) Quantification of absolute numbers of thymocyte subpopulations in the hosts. Each symbol in the graphs represents an individual mouse (n = 3); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *, P < 0.05.
Thymus involution and peripheral T-lymphopenia in 8-week-old Klotho-deficient mice is averted by vitamin D-deprivation.
Previous studies have shown that elimination of vitamin D in Kl/Kl mice diet corrected the hypervitaminosis D typically observed in these animals, and consequently improved some of their disease-related phenotype (25, 45, 46). As our transplantation data indicated that premature thymic involution in Klotho-deficient mice was not caused by Klotho deficiency in either hematopoietic cells or thymic stromal cells, we next investigated whether a reduction in vitamin D levels improved the thymic changes and, as a result, the peripheral T cell compartment of Kl/Kl mice. In this experiment, both breeder mice and offspring were maintained on a vitamin D-deprived diet throughout th e experiment. Eight week old Kl/Kl mice exposed since conception to low Vitamin D levels displayed a total thymus cellularity and thymocyte differentiation comparable to that of age-matched WT animals whereas KI/KI mice fed with a vitamin D-replete diet displayed as expected the typical hallmarks of Klotho deficiency (Fig. 5A, 5B). The thymus architecture of 8-week-old, conventionally fed Kl/Kl mice showed a structural disorganization, especially of the medulla (Fig. 5C), consistent with our previous observations (47). In contrast, the histological structure of the thymus in both KI/KI and WT mice fed with a Vitamin D-deprived diet displayed separate and well demarcated cortical and medullary compartments (Fig. 5C). These data specify that low Vitamin D levels correct the thymus phenotype of Kl/Kl mice and suggest that the premature thymus involution observed in Klotho-deficient mice is the result of high systemic levels of Vitamin D.
Figure 5.
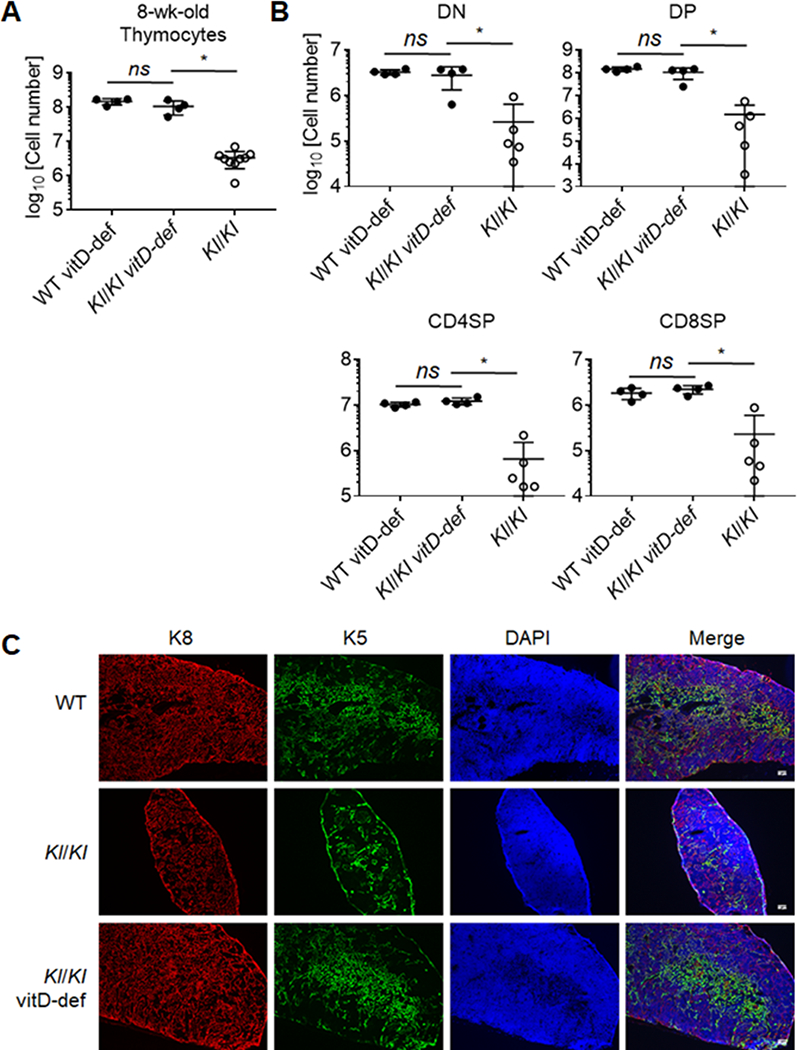
Thymus involution in 8-wk-old Klotho-deficient mice was abrogated through feeding vitamin D-deprived diet.
(A) Cellularity of total thymocytes in the thymi from 8-wk-old Kl/Kl mice and littermate controls fed with vitamin D-deprived diet, and Kl/Kl mice fed with vitamin D-replete diet at. (B) Quantification of frequencies of thymocyte subpopulations in the indicated mice. Each symbol in the graphs represents an individual mouse (n ≥ 3); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *P < 0.05. (C) Immunofluorescence staining of thymus sections using anti-K5, anti-K8 antibodies and DAPI (4’,6-diamidino-2-phenylindole).
We considered the possibility that hypervitaminosis D in Kl/Kl mice may inhibit the emigration of mature thymocytes to the periphery possibly contributing to T cell proliferation and/or survival, contributing to T cell lymphopenia. We therefore probed in Kl/Kl and control mice the cellularity and frequency of CD4 and CD8 single positive T cells (SP4 and SP8) in spleen and lymph nodes. At 8 weeks of age, Kl/Kl fed a vitamin D-depleted diet had comparable percentages (Fig. 6A) but decreased numbers (Fig. 6B) of both CD4T and CD8T cells compared to controls.
Figure 6.
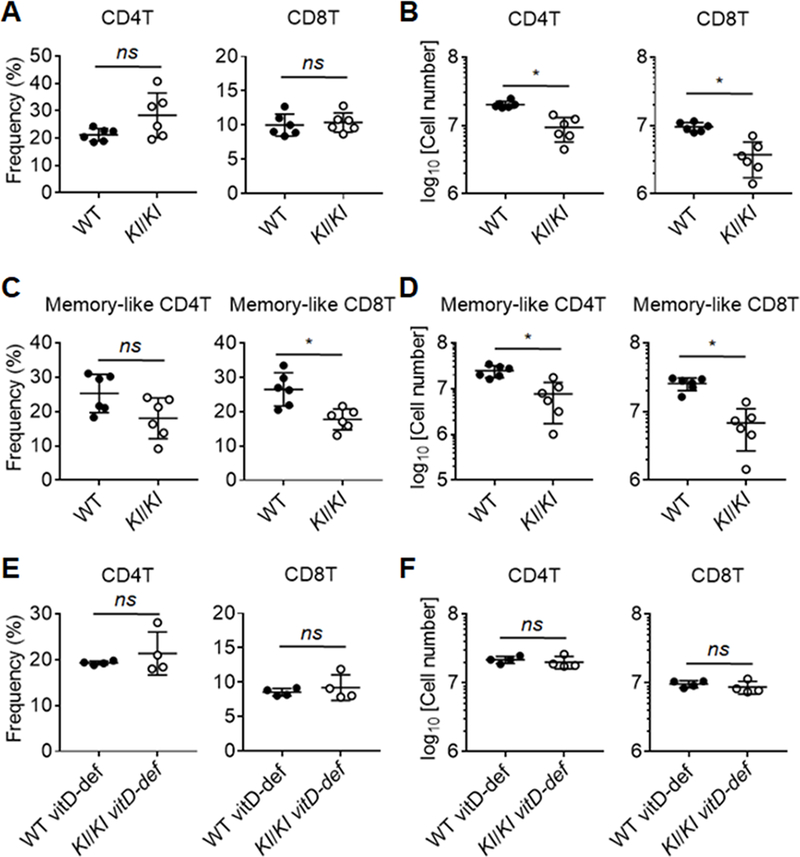
Reduction of T cells was found in the periphery in 8-week-old Klotho-deficient mice that can be prevented by deprivation of vitamin D.
(A) Frequency and (B) cellularity of CD4T and CD8T cells in the spleen from Kl/Kl mice and littermate controls at 8 weeks of age. (C) Frequency and (D) cellularity of CD44hi memory-like T cells subpopulations in the spleen from Kl/Kl mice and littermate controls at 8 weeks of age. (E) Frequency and (F) cellularity of CD4T and CD8T cells in the spleen from 8-wk-old Kl/Kl mice and littermate controls fed with vitamin D-deprived diet. Each symbol in the graphs represents an individual mouse (n ≥ 4); small horizontal lines indicate the group mean (± s.d.). ns, not significant (P ≥ 0.05); *, P < 0.05.
An increased frequency of CD44hi memory-like cells is a phenotypic hallmark of T cell lymphopenia. Interestingly, Kl/Kl mice also may have impaired IL-7 dependent lymphopenia-driven differentiation, expansion or survival of memory-like T cells due to decreased IL-7 production in stromal cells (e.g. BM) in Kl/Kl mice (48). The frequency of SP4 CD44hi but not SP8 CD44hi memory-like T cells was unaffected (Fig. 6C, Supplemental Fig. 2), although their cellularity were both reduced in Kl/Kl mice on a vitamin D depleted diet (Fig. 6D), suggesting a defect in memory-like T cell support. Moreover, the absolute cellularity of splenic SP4 and SP8 T cell lymphopenia was comparable between Kl/Kl mice fed a vitamin D-deprived diet and control animals (Fig. 6E, 6F). Overall, the data show that Klotho animals at 8 weeks of age have significant losses of both thymocytes and TECs and that these deficits can be overcome by decreasing Vitamin D levels in vivo.
Discussion
Our results demonstrate that thymus function in young Kl/Kl mice is unaffected but severe atrophy with reduced thymopoiesis is observed in these mice by 8 weeks of age. This change in thymus function was neither the consequence of a cell-intrinsic loss of Klotho expression in hematopoietic cells nor the result of a cell-autonomous deficiency in expression of this protein by thymic stromal cells. Rather, thymic hypocellularity and the loss of a regular thymic stromal architecture in Kl/Kl mice are the consequence of high Vitamin D levels, inferring that Klotho deficiency disrupts thymus function in an indirect, systemic fashion.
The molecular interactions of FGF23, klotho and vitamin D coordinate to regulate phosphate metabolism (24) (46, 49–51). FGF23 decreases renal tubular and phosphate reabsorption and stimulates Vitamin D3, which results in increased renal klotho synthesis (46). Klotho binds to FGFR1(IIIc) that reduces Vitamin D3 synthesis. In Kl/Kl mice, FGF23 is unable to bind the klotho transmembrane molecule precluding its transport to FGFR1c resulting in a failure to negatively regulate Vitamin D3 synthesis via FGF23 receptor mediated inhibition of 1α-hydroxylase (52).Thus, high levels of active vitamin D accumulate in Kl/Kl mice causing a state of calcium and phosphate imbalance.
To counteract the abnormal calcium and phosphorus state, vitamin D deficient diets have been tested and have been shown to ameliorate other consequences related to accelerated aging including decreased ectopic calcification of tissues, lack of skin atrophy, and increased life span (45). Feeding kl/kl mice a vitamin D deficient diet indeed improved thymic architecture, TEC differentiation, thymocyte development and maturation and peripheral T cell reconstitution. Therefore, it may be tempting to speculate that the vitamin D deficient diet had direct effects on TECs, despite the fact that the genetic deficiency of klotho in TECs would be unaffected. Similarly, the data from kidney capsule implants indicated that the TECs from kl/kl mice are able to develop and support thymopoiesis despite the klotho deficiency. Taken together, we favor the hypothesis that correction of calcium and phosphorus imbalance and high vitamin D levels are responsible for improvement in thymopoiesis and TEC development.
Both indirect and direct TECs effects of klotho deficiency can be envisioned. Metabolic imbalance of calcium, phosphorus and vitamin D3 may indirectly impair TEC function and development due to apoptosis and stress in the animals. High vitamin D3 levels may indirectly impair TEC development and maturation by interfering with the essential cross-talk signaling mechanisms between TECs and thymocytes needed for their mutual development (43, 54). In support of this hypothesis, thymocytes express the Vitamin D receptor according to gene expression data ((Accession Number GSE81163, http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE81163). High Vitamin D levels directly impair TEC development since cells within the thymus that express high levels of vitamin D receptor are sensitive to vitamin D-induced signaling. In the absence of Klotho, levels of FGF-23 are elevated (59), which may be toxic to developing thymocytes as it is to the kidney (60) or exert yet unknown effects independent of vitamin D levels. Klotho suppresses NF-κB translocation and therefore suppresses inflammatory cytokine production (57) and conversely, Klotho deficiency increases proinflammatory cytokines such as IL-6 or TNFα in monocytes (58), which could be driving thymocyte death leading to insufficient support of TECs.
Medullary thymocytes also express the vitamin D receptor and vitamin D signaling inhibits mitogenic stimulation of these cells (53, 55) which may negatively impact upon peripheral T-lineage reconstitution. We observed a reduction of regulatory T cells (Tregs) in the thymus and in the periphery of 8-week-old Kl/Kl mice (data not shown). Thymic Tregs express vitamin D receptor, suggesting that their development could be affected by vitamin D levels in a manner similar to conventional thymocytes (56). Additional potential mechanisms that might lead to thymic involution and T cell lymphopenia seen in Kl/Kl mice include decreased expression of positive cell cycle regulators, such as Cyclin D1 and c-Myc that may be lacking in developing thymocytes, resulting in low proliferative rates. Previously, we reported that keratinocyte growth factor (fibroblast growth factor-7) administration, known to stimulate the proliferation of TECs and other epithelial cells, can improve IL-7 production and thymopoiesis in 2-week but not 6-week old Kl/Kl mice that have substantially defective IL-7 production and thymopoiesis (47). Although it is not yet clear whether reduction of IL-7 production directly associates with hypervitaminosis D, mice given a vitamin D-deficient diet did not have evidence of poor T cell content in the periphery nor manifestations of IL-7 deficiency in the thymus. Further studies are needed to identify the molecular mechanisms downstream of reduced Vitamin D levels that account for the preservation of regular thymopoiesis in Kl/Kl mice.
In conclusion, our data suggest that Klotho is not cell-autonomously needed for differentiation, maintenance or function of the thymic microenvironment. However, under conditions that stress the thymus and developing T cells then Klotho is required to maintain normal levels of thymopoiesis. In this way, Klotho may be important to opposing the process of thymic involution, which naturally occurs with age.
Supplementary Material
Acknowledgments
This work was supported by NIH P01 CA065493; R01 AI081918
This work was supported in part by R01 AI081918, 2P01 CA065493, and the Children’s’ Cancer Research Fund.
References:
- 1.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, and Koup RA 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396: 690–695. [DOI] [PubMed] [Google Scholar]
- 2.Palmer DB 2013. The effect of age on thymic function. Front Immunol 4: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezzani R, Nardo L, Favero G, Peroni M, and Rodella LF 2014. Thymus and aging: morphological, radiological, and functional overview. Age (Dordr) 36: 313–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, and Nikolich-Zugich J 2000. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol 165: 2367–2373. [DOI] [PubMed] [Google Scholar]
- 5.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, and Nikolich-Zugich J 2004. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. The Journal of experimental medicine 200: 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, and Blackman MA 2008. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. The Journal of experimental medicine 205: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikolich-Zugich J 2008. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol 8: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes L, and Swain SL 2006. Why aging T cells fail: implications for vaccination. Immunity 24: 663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davitt CJ, McNeela EA, Longet S, Tobias J, Aversa V, McEntee CP, Rosa M, Coulter IS, Holmgren J, and Lavelle EC 2016. A novel adjuvanted capsule based strategy for oral vaccination against infectious diarrhoeal pathogens. J Control Release 233: 162–173. [DOI] [PubMed] [Google Scholar]
- 10.Aspinall R, Del Giudice G, Effros RB, Grubeck-Loebenstein B, and Sambhara S 2007. Challenges for vaccination in the elderly. Immun Ageing 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicin-Sain L, Smyk-Pearson S, Smyk-Paerson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugich D, Park B, Hobbs T, Doane CJ, Mori M, Axthelm MK, Axthelm MT, Lewinsohn DA, and Nikolich-Zugich J 2010. Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol 184: 6739–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davitt CJ, and Lavelle EC 2015. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev 91: 52–69. [DOI] [PubMed] [Google Scholar]
- 13.Prelog M 2006. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev 5: 136–139. [DOI] [PubMed] [Google Scholar]
- 14.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, and Larbi A 2010. Potential role of immunosenescence in cancer development. Annals of the New York Academy of Sciences 1197: 158–165. [DOI] [PubMed] [Google Scholar]
- 15.Foster AD, Sivarapatna A, and Gress RE 2011. The aging immune system and its relationship with cancer. Aging health 7: 707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Horowitz ME, Magrath IT, Shad AT, and Steinberg SM 1995. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 332: 143–149. [DOI] [PubMed] [Google Scholar]
- 17.Hakim FT, Memon SA, Cepeda R, Jones EC, Chow CK, Kasten-Sportes C, Odom J, Vance BA, Christensen BL, Mackall CL, and Gress RE 2005. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest 115: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wils EJ, and Cornelissen JJ 2005. Thymopoiesis following allogeneic stem cell transplantation: new possibilities for improvement. Blood Rev 19: 89–98. [DOI] [PubMed] [Google Scholar]
- 19.Krenger W, Blazar BR, and Hollander GA 2011. Thymic T-cell development in allogeneic stem cell transplantation. Blood 117: 6768–6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, and Nabeshima YI 1997. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51. [DOI] [PubMed] [Google Scholar]
- 21.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, and Kuro-o M 2006. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, and Yamashita T 2006. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774.17086194 [Google Scholar]
- 23.Chen G, Liu Y, Goetz R, Fu L, Jayaraman S, Hu MC, Moe OW, Liang G, Li X, and Mohammadi M 2018. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature 553: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John GB, Cheng CY, and Kuro-o M 2011. Role of Klotho in aging, phosphate metabolism, and CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation 58: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuro-o M 2009. Klotho and aging. Biochim Biophys Acta 1790: 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, and Sun Z 2009. Current understanding of klotho. Ageing Res Rev 8: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Château MT, Araiz C, Descamps S, and Galas S 2010. Klotho interferes with a novel FGF-signalling pathway and insulin/Igf-like signalling to improve longevity and stress resistance in Caenorhabditis elegans. Aging (Albany NY) 2: 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, and Kuro-o M 2005. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arking DE, Krebsova A, Macek M, Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, and Dietz HC 2002. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci U S A 99: 856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arking DE, Atzmon G, Arking A, Barzilai N, and Dietz HC 2005. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res 96: 412–418. [DOI] [PubMed] [Google Scholar]
- 31.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC, and Dietz HC 2003. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet 72: 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogata N, Matsumura Y, Shiraki M, Kawano K, Koshizuka Y, Hosoi T, Nakamura K, Kuro-O M, and Kawaguchi H 2002. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone 31: 37–42. [DOI] [PubMed] [Google Scholar]
- 33.Koh N, Fujimori T, Nishiguchi S, Tamori A, Shiomi S, Nakatani T, Sugimura K, Kishimoto T, Kinoshita S, Kuroki T, and Nabeshima Y 2001. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun 280: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 34.Xing Y, and Hogquist KA 2014. Isolation, identification, and purification of murine thymic epithelial cells. J Vis Exp: e51780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing Y, Jameson SC, and Hogquist KA 2013. Thymoproteasome subunit-β5T generates peptide-MHC complexes specialized for positive selection. Proc Natl Acad Sci U S A 110: 6979–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morillon YM, Manzoor F, Wang B, and Tisch R 2015. Isolation and transplantation of different aged murine thymic grafts. Journal of visualized experiments : JoVE: e52709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodewald HR 2008. Thymus organogenesis. Annu Rev Immunol 26: 355–388. [DOI] [PubMed] [Google Scholar]
- 38.Takada K, Kondo K, and Takahama Y 2017. Generation of Peptides That Promote Positive Selection in the Thymus. J Immunol 198: 2215–2222. [DOI] [PubMed] [Google Scholar]
- 39.Starr TK, Jameson SC, and Hogquist KA 2003. Positive and negative selection of T cells. Annu Rev Immunol 21: 139–176. [DOI] [PubMed] [Google Scholar]
- 40.Xing Y, Wang X, Jameson SC, and Hogquist KA 2016. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat Immunol 17: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing Y, and Hogquist KA 2012. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogquist KA, Xing Y, Hsu FC, and Shapiro VS 2015. T Cell Adolescence: Maturation Events Beyond Positive Selection. J. Immunol. 195: 1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahama Y, Ohigashi I, Baik S, and Anderson G 2017. Generation of diversity in thymic epithelial cells. Nat Rev Immunol 17: 295–305. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya HJ, Briones Leon A, and Blackburn CC 2016. FOXN1 in thymus organogenesis and development. Eur J Immunol 46: 1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, and Nabeshima Y 2003. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17: 2393–2403. [DOI] [PubMed] [Google Scholar]
- 46.Razzaque MS 2012. FGF23, klotho and vitamin D interactions: What have we learned from in vivo mouse genetics studies? Adv Exp Med Biol 728: 84–91. [DOI] [PubMed] [Google Scholar]
- 47.Min D, Panoskaltsis-Mortari A, Kuro-O M, Holländer GA, Blazar BR, and Weinberg KI 2007. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood 109: 2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okada S, Yoshida T, Hong Z, Ishii G, Hatano M, Kuro-O M, Nabeshima Y, and Tokuhisa T 2000. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol 12: 861–871. [DOI] [PubMed] [Google Scholar]
- 49.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, Haussler MR, and Kerr Whitfield G 2011. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun 414: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goetz R, Ohnishi M, Ding X, Kurosu H, Wang L, Akiyoshi J, Ma J, Gai W, Sidis Y, Pitteloud N, Kuro OM, Razzaque MS, and Mohammadi M 2012. Klotho coreceptors inhibit signaling by paracrine fibroblast growth factor 8 subfamily ligands. Mol Cell Biol 32: 1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torres PU, Prie D, Molina-Bletry V, Beck L, Silve C, and Friedlander G 2007. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int 71: 730–737. [DOI] [PubMed] [Google Scholar]
- 52.Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, Goetz R, Mohammadi M, Kuro-O M, Olsen BR, and Lanske B 2008. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol 182: 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravid A, Koren R, Novogrodsky A, and Liberman UA 1984. 1,25-Dihydroxyvitamin D3 inhibits selectively the mitogenic stimulation of mouse medullary thymocytes. Biochem Biophys Res Commun 123: 163–169. [DOI] [PubMed] [Google Scholar]
- 54.Holländer GA, Wang B, Nichogiannopoulou A, Platenburg PP, van Ewijk W, Burakoff SJ, Gutierrez-Ramos JC, and Terhorst C 1995. Developmental control point in induction of thymic cortex regulated by a subpopulation of prothymocytes. Nature 373: 350–353. [DOI] [PubMed] [Google Scholar]
- 55.Provvedini DM, Sakagami Y, and Manolagas SC 1989. Distinct target cells and effects of 1 alpha,25-dihydroxyvitamin D3 and glucocorticoids in the rat thymus gland. Endocrinology 124: 1532–1538. [DOI] [PubMed] [Google Scholar]
- 56.Porto G, Giordano RJ, Marti LC, Stolf B, Pasqualini R, Arap W, Kalil J, and Coelho V 2011. Identification of novel immunoregulatory molecules in human thymic regulatory CD4+CD25+ T cells by phage display. PLoS One 6: e21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buendía P, Ramírez R, Aljama P, and Carracedo J 2016. Klotho Prevents Translocation of NFκB. Vitam Horm 101: 119–150. [DOI] [PubMed] [Google Scholar]
- 58.Wöbke TK, Sorg BL, and Steinhilber D 2014. Vitamin D in inflammatory diseases. Front Physiol 5: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohnishi M, Nakatani T, Lanske B, and Razzaque MS 2009. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int 75: 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Razzaque MS 2009. Does FGF23 toxicity influence the outcome of chronic kidney disease? Nephrol Dial Transplant 24: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


