Abstract
Objective
Alterations in gut microbiota have been linked to host insulin resistance, diabetes, and impaired amino acid metabolism. We investigated whether changes in gut microbiota-dependent metabolite of trimethylamine N-oxide (TMAO), and its nutrient precursors (choline and L-carnitine) were associated with improvements in glucose metabolism and diabetes-related amino acids in a weight-loss diet intervention.
Design
We included 504 overweight and obese adults who were randomly assigned to 1 of 4 energy-reduced diets varying in macronutrient intake. The 6-month changes (Δ) in TMAO, choline, and L-carnitine levels after the intervention were calculated.
Results
Greater decreases in choline and L-carnitine were significantly (p <0.05) associated with greater improvements in fasting insulin concentrations and homeostasis model assessment-of-insulin resistance (HOMA-IR) at 6 months. The reduction of choline was significantly related to 2-year improvements in glucose and insulin resistance. We found significant linkages between dietary fat intake and ΔTMAO for changes in fasting glucose, insulin, and HOMA-IR (Pinteraction <0.05); a greater increase in TMAO was related to lesser improvements in the outcomes among participants who consumed a high-fat diet. In addition, ΔL-carnitine and Δcholine were significantly related to changes in amino acids (including branched-chain and aromatic amino acids). Interestingly, the associations of ΔTMAO, Δcholine, and ΔL-carnitine with diabetes-related traits were independent of the changes in amino acids.
Conclusion
Our findings underscore the importance of changes in TMAO, choline, and L-carnitine in improving insulin sensitivity during a weight-loss intervention for obese patients. Dietary fat intake may modify the associations of TMAO with insulin sensitivity and glucose metabolism.
Keywords: Trimethylamine N-oxide, Choline, L-carnitine, Amino acids, Metabolites, Glucose metabolism, Insulin sensitivity, Randomized clinical trial, Nutrition
INTRODUCTION
Alterations in gut microbiota may impact host insulin sensitivity and type 2 diabetes.1–3 Several studies have shown that a gut-microbiota derived metabolite, trimethylamine N-oxide (TMAO), is significantly associated with the risk of type 2 diabetes.4–7 The intestinal microbiota metabolizes precursors of TMAO, such as choline and L-carnitine, to produce trimethylamine,8,9 and trimethylamine is then further metabolized to TMAO by the hepatic flavin-containing enzyme monooxygenase 3 (FMO3).10,11 The TMAO-generating pathway by FMO3 has been related to insulin sensitivity and glucose metabolism.12 Also, several lines of evidence have suggested a significant role of choline in regulating insulin resistance and glucose metabolism.7,13–15
Dietary intake substantially affects intestinal microbiota and the production of TMAO.9,16–23 Although evidence has consistently demonstrated that various weight-loss diets are effective for the improvement of glucose metabolism and the prevention of diabetes,24,25 little has been clarified as to whether changes in microbial metabolites play a significant role in such protective effects. We have recently reported associations of changes in TMAO, choline, and L-carnitine with achieving successful weight-loss among overweight and obese adults who participated in a weight-loss diet intervention trial.26 However, whether diet-induced changes in the microbial metabolites are related to the improvements in glucose metabolism remains unclarified.
Recent studies have shown significant relationships between the gut microbiome and diabetes-related amino acids, such as aromatic amino acids (AAAs) and branched-chain amino acids (BCAAs), in the development of obesity and insulin resistance.2,27 Nonetheless, to the best of our knowledge, no study has comprehensively examined how alterations in diabetes-related amino acids may influence associations of changes in gut microbial metabolites with improvements in glycemia and insulin sensitivity in a weight-loss diet intervention.
Therefore in the present study, we investigated the associations of diet-induced changes in circulating levels of TMAO and its nutrient precursors (choline and L-carnitine) with changes in diabetes-related amino acids, and improvements of glucose metabolism (glycemia and insulin sensitivity) among overweight and obese adults who participated in the Preventing Overweight Using Novel Dietary Strategies (POUNDS) Lost trial.24 We hypothesized that alterations in diabetes-related amino acids might explain the association of TMAO and its precursors with the glucose metabolism. We also tested whether different macronutrient intakes (fat, protein, and carbohydrate) might modify these associations.
METHODS
Study participants
The present study included overweight and obese adults who participated in a 2-year randomized diet intervention, the POUNDS Lost trial, which was conducted from October 2004 through December 2007 at two sites: Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital in Boston, MA, and the Pennington Biomedical Research Center of Louisiana State University System, in Baton Rouge, LA. In the POUNDS Lost trial, a total of 811 participants who were overweight and obese were randomly assigned to 1 of 4 energy-reduced diets varying in the macronutrient composition to compare their effects on body weight change over two years (ClinicalTrials.gov, NCT00072995). Major exclusion criteria in this trial were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation.24 The study was powered to detect a 1.67-kg weight loss as an effect of the level of protein or fat in the diet over the 2-year period, assuming a withdrawal rate of 40%. A total of 80% of the participants completed the study.24 Macronutrient intake goals for the four diet groups were the following: 1) low-fat and average-protein: 20% fat, 15% protein, 65% carbohydrate; 2) low-fat and high-protein: 20% fat, 25% protein, 55% carbohydrate; 3) high-fat and average-protein: 40% fat, 15% protein, 45% carbohydrate; 4) high-fat and high-protein: 40% fat, 25% protein, 35% carbohydrate. The two high-fat diets were also low-carbohydrate diets. Random assignments to one of four diet groups were performed by the data manager at the coordinating center on request of a study dietitian after eligibility of a participant was confirmed. Randomization by computer occurred after the collection of baseline data and was managed by the study statistician. Diet group assignments were stratified by site with varying block sizes to ensure a balance at each site. Investigators and staff who measured outcomes were unaware of the diet assignment of the participants.24 More details of the trial have been described in detail elsewhere.24 The study was approved by the human subjects committee at each institution and by data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants gave written informed consent.
Of the 811 individuals, the present analysis included 510 participants on the basis of availability of blood samples and measurements of TMAO, choline, and L-carnitine both at the baseline examination and at 6 months during the intervention. There were no significant differences in mean values of body mass index (BMI), fasting glucose, fasting insulin, and the homeostasis model assessment-of-insulin resistance (HOMA-IR) at the baseline examination between participants who were included (n=510) or those who were not included (n=301) in the present analysis. As we described previously,26 the baseline median (25th, 75th) values of each metabolite were 2.7 (1.8, 3.8) μM for TMAO, 8.6 (7.4, 10.3) μM for choline, and 34.5 (30.0, 39.3) μM for L-carnitine among the 510 participants. Since a few participants (n=6) had missing data on fasting insulin, hemoglobin A1c (HbA1c), or 6-month changes in serum amino acid metabolites, these were excluded from the present analysis. Subsequently, a total of 504 participants were eligible, and we included those with available data on outcome measures at 6 months or 2 years in each analysis. Baseline mean (standard deviation) values were 5.1 (0.7) mmol/L for fasting glucose and 5.4% (0.4%) for HbA1c (International Federation of Clinical Chemistry and Laboratory Medicine-HbA1c, 36 (5) mmol/mol) among the 504 individuals.
Measurements of glucose and insulin concentrations
Blood samples were collected in the fasting state on one day at baseline, 6 months and 24 months of the intervention, and stored at −80 °C. The measurements were performed at the clinical laboratory at the Pennington Biomedical Research Center. Fasting glucose and insulin concentrations were measured using an immunoassay with chemiluminescent detection on the Immulite analyzer (Diagnostic Products Corporation, Los Angeles CA). HbA1c was measured on a Synchron CX5 (Beckman Coulter, Brea CA). We calculated HOMA-IR using fasting glucose and insulin levels.28
Measurements of TMAO, choline, and L-carnitine
Blood concentrations of TMAO, choline, and L-carnitine were measured at Prevention Research Laboratory and Laboratory Diagnostic Core, Cleveland Clinic (Cleveland, OH) using stable isotope dilution high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. Details of the measurements were addressed elsewhere previously.8,9,29,30 We assessed changes (Δ) in TMAO, choline, and L-carnitine from the baseline examination to 6 months of the diet intervention. In our previous study, there were no significant differences in mean or median values of ΔTMAO, Δcholine, or ΔL-carnitine across the different diets varying in fat, protein, or carbohydrate.26 The ΔTMAO was weakly correlated with Δcholine (Pearson correlation coefficient (r) =0.15, p=0.0006) or with ΔL-carnitine (r=0.14, p=0.002). There was a modest correlation between Δcholine and ΔL-carnitine (r=0.41, p <0.0001) (Supplemental Figure 1).
Measurements of serum amino acids
The profiling of amino acids was performed using electrospray tandem mass spectrometry in the Institute of Laboratory Medicine, Clinical Chemistry and Molecular Diagnostics, University Hospital Leipzig, Leipzig, Germany. We previously reported significant associations of body weight changes with changes in BCAAs (leucine, isoleucine, and valine) and AAAs (tyrosine and phenylalanine) among participants of the POUNDS Lost trial.31 Also, changes in tyrosine and alanine were significantly related to the improvement in HOMA-IR during the intervention.31 Of a total of 25 amino acids measured in the POUNDS Lost trial, we selected the following diabetes-related amino acids such as gluconeogenesis substrates (alanine, glycine, glutamine, and citrulline), BCAAs, AAAs, and some other amino acids (glutamic acid, taurine, and tryptophan) to examine in the present study, based on results of studies relating amino acids and insulin resistance, glucose concentrations, or type 2 diabetes.31–35
Other measurements
Height was measured at the baseline examination. Body weight was measured in the morning before breakfast at baseline, 6, 12, 18 and 24 months during the intervention using calibrated hospital scales. BMI was calculated as body weight in kilograms divided by the square of height in meters (kg/m2). Dietary intake was assessed in a random sample of 50% of the total participants by a review of 5-day diet records at the baseline examination.
Statistical analysis
The primary outcomes in this study were changes in fasting glucose, HbA1c, fasting insulin, and HOMA-IR at 6 months. Data on TMAO, choline, L-carnitine, and amino acids were log-transformed to improve normality before analyses. We first examined associations of ΔTMAO, Δcholine, and ΔL-carnitine with concurrent changes in the diabetes-related amino acids using the general linear model including covariates listed in Table 1. Amino acids that showed significant (p <0.05) associations for TMAO, choline, or L-carnitine were treated as covariates in subsequent analyses for the primary outcomes (changes in fasting glucose, HbA1c, fasting insulin, and HOMA-IR). The general linear models were performed to examine an effect of each 1 log-transformed decrease in ΔTMAO, Δcholine, or ΔL-carnitine for the primary outcomes. Multivariate-adjusted models including covariates of age, sex, ethnicity, parental history of diabetes, diet group, BMI at baseline, value for the respective outcome traits at baseline, and either choline or L-carnitine at baseline (model 1), and changes in the amino acids (model 2) were performed. We also performed an analysis with addition of concurrent changes in body weight in the multivariate-adjusted model. We tested potential interactions between ΔTMAO, Δcholine, or ΔL-carnitine and diet groups (high-/low-fat diet, or high-/low-protein diet) for the outcomes. Associations of the initial (6-month) changes in TMAO, choline, and L-carnitine with long-term (2-year) improvements of the primary outcomes were also examined. Statistical analyses were performed with SAS version 9.3 (SAS Institute); the p value <0.05 was considered statistically significant.
Table 1.
Associations of changes (Δ) in serum amino acids and changes in circulating levels of trimethylamine N-oxide (TMAO), choline or L-carnitine at 6 months during the dietary intervention (n=504)
| ΔTMAO
|
ΔCholine
|
ΔL-carnitine
|
||||
|---|---|---|---|---|---|---|
| Outcomes | β (SE) | P | β (SE) | P | β (SE) | P |
| Gluconeogenesis substrates | ||||||
| ΔAlanine | 0.01 (0.02) | 0.52 | –0.11 (0.05) | 0.02 | –0.2 (0.06) | 0.0007 |
| ΔGlycine | –0.02 (0.02) | 0.45 | 0.02 (0.06) | 0.68 | –0.07 (0.07) | 0.36 |
| ΔGlutamine | –0.05 (0.04) | 0.18 | 0.02 (0.1) | 0.84 | 0.1 (0.13) | 0.45 |
| ΔCitrulline | –0.01 (0.01) | 0.5 | –0.04 (0.04) | 0.31 | –0.11 (0.05) | 0.02 |
| Branched-chain amino acids (BCAAs) | ||||||
| ΔValine | 0 (0.01) | 0.77 | –0.02 (0.03) | 0.63 | –0.11 (0.04) | 0.006 |
| ΔLeucine/Isoleucine | –0.01 (0.01) | 0.53 | –0.05 (0.03) | 0.12 | –0.13 (0.04) | 0.004 |
| ΔSum of BCAAs | 0 (0.01) | 0.9 | –0.03 (0.03) | 0.3 | –0.12 (0.04) | 0.002 |
| Aromatic amino acids (AAAs) | ||||||
| ΔPhenylalanine | 0 (0.01) | 0.98 | –0.09 (0.03) | 0.01 | –0.12 (0.04) | 0.005 |
| ΔTyrosine | 0.01 (0.02) | 0.42 | –0.17 (0.05) | 0.0002 | –0.22 (0.06) | <0.0001 |
| ΔSum of AAAs | 0.01 (0.01) | 0.66 | –0.13 (0.04) | 0.0006 | –0.17 (0.05) | 0.0002 |
| Other amino acids | ||||||
| ΔGlutamic acid | 0 (0.02) | 0.85 | –0.1 (0.05) | 0.03 | –0.12 (0.06) | 0.04 |
| ΔTaurine | –0.01 (0.02) | 0.59 | –0.02 (0.07) | 0.8 | –0.03 (0.08) | 0.71 |
| ΔTryptophan | 0.03 (0.02) | 0.23 | –0.12 (0.07) | 0.07 | –0.15 (0.08) | 0.07 |
Data on changes in serum amino acids, TMAO, choline and L-carnitine were log-transformed before analyses.
β (SE) represents effect for outcomes per 1 decrease in log-transformed ΔTMAO, Δcholine, or ΔL-carnitine after adjusted for age, sex, ethnicity, diet group, parental history of diabetes, body mass index at baseline, value for the respective outcome amino acids at baseline, and either TMAO, choline, or L-carnitine at baseline.
RESULTS
Characteristics of the study participants at the baseline examination according to baseline TMAO, choline, or L-carnitine levels are shown in Supplemental Table 1. Before the dietary intervention, elevated levels of L-carnitine were significantly associated with elevated levels of fasting glucose (p <0.001), HbA1c (p=0.007), fasting insulin (p=0.018), and HOMA-IR (p=0.004) (Supplemental Table 1). Individuals with higher levels of TMAO had a lower dietary intake of carbohydrate (p=0.007) and were more likely to have a higher intake of dietary fat at baseline before the intervention. Elevated TMAO and choline levels were associated with higher citrulline levels (p=0.03 and p=0.0004, respectively) at the baseline examination. Also, both choline and L-carnitine were significantly (p <0.05) associated with BCAAs and AAAs at the baseline examination (Supplemental Table 2A, 2B, and 2C).
When we examined associations of ΔTMAO, Δcholine, or ΔL-carnitine after the diet intervention with changes in amino acids at 6 months (Table 1 and Supplemental Figure 2), participants with a greater reduction in choline showed larger reductions of alanine (p=0.02), total AAAs (p=0.0006), and glutamic acid (p=0.03) at 6 months. A greater decrease in L-carnitine was significantly associated with larger reductions of alanine (p=0.0007), citrulline (p=0.02), total BCAAs (p=0.002), total AAAs (p=0.0002), and glutamic acid (p=0.04) at 6 months. There were no significant relationships between ΔTMAO and changes in amino acids among the total participants.
We then investigated associations of ΔTMAO, Δcholine, and ΔL-carnitine with improvements in glycemia and insulin sensitivity considering concurrent changes in the amino acids (Table 2). We found that decreases in choline and L-carnitine were significantly associated with improvements in fasting insulin concentrations and HOMA-IR at 6 months in the multivariate-adjusted model 1 including covariates of age, sex, ethnicity, parental history of diabetes, diet group, BMI at baseline, value for the respective outcome traits at baseline, and either choline or L-carnitine at baseline. Each 1 log-transformed decrease in choline was associated with reductions of log-transformed fasting insulin (β [standard error, SE] –0.25 [0.09], p=0.009) and log-transformed HOMA-IR (β [SE] –0.26 [0.1], p=0.01) at 6 months. Also, each 1 log-transformed decrease in L-carnitine was significantly associated with reductions of log-transformed fasting insulin (β [SE] –0.36 [0.12], p=0.002) and log-transformed HOMA-IR (β [SE] –0.4 [0.13], p=0.002) at 6 months. The associations remained significant when we adjusted for concurrent changes in alanine, BCAAs, AAAs, and glutamic acid in model 2. When we further adjusted for concurrent changes in body weight in the model, the associations of changes in choline and L-carnitine with insulin resistance became non-significant (p >0.05). On the other hand, for TMAO changes, each 1 log-transformed decrease in TMAO was slightly associated with reductions of log-transformed fasting insulin (β [SE] –0.06 [0.03], p=0.03) and log-transformed HOMA-IR (β [SE] –0.07 [0.03], p=0.03) at 6 months in the model adjusted for body weight changes. The decrease in TMAO was related to improvement in insulin sensitivity particularly among participants who achieved more weight loss at 6 months (Supplemental Figure 3).
Table 2.
Changes (Δ) in glycemic and insulin measurements at 6 months per 1 log-transformed decreases in trimethylamine N-oxide (TMAO), choline, and L-carnitine levels
| N of participants | ΔTMAO
|
ΔCholine
|
ΔL-carnitine
|
||||
|---|---|---|---|---|---|---|---|
| Outcomes | β (SE) | P | β (SE) | β (SE) | P | β (SE) | |
| Model 1 | |||||||
| ΔFasting glucose, mmol/L | 504 | –0.03 (0.03) | 0.4 | –0.12 (0.1) | 0.23 | –0.19 (0.12) | 0.11 |
| ΔHbA1c, % | 504 | 0.02 (0.02) | 0.45 | –0.01 (0.07) | 0.85 | –0.07 (0.08) | 0.42 |
| Δlog-transformed fasting insulin | 490 | –0.03 (0.03) | 0.32 | –0.25 (0.09) | 0.009 | –0.36 (0.12) | 0.002 |
| Δlog-transformed HOMA-IR | 490 | –0.04 (0.04) | 0.3 | –0.26 (0.1) | 0.01 | –0.4 (0.13) | 0.002 |
| Model 2 | |||||||
| ΔFasting glucose, mmol/L | 504 | –0.03 (0.03) | 0.39 | –0.09 (0.1) | 0.36 | –0.15 (0.12) | 0.2 |
| ΔHbA1c, % | 504 | 0.02 (0.02) | 0.48 | –0.01 (0.07) | 0.85 | –0.07 (0.08) | 0.41 |
| Δlog-transformed fasting insulin | 490 | –0.04 (0.03) | 0.26 | –0.22 (0.09) | 0.02 | –0.31 (0.12) | 0.008 |
| Δlog-transformed HOMA-IR | 490 | –0.04 (0.04) | 0.24 | –0.23 (0.1) | 0.03 | –0.34 (0.13) | 0.009 |
Hemoglobin A1c, HbA1c; HOMA-IR, homeostasis model assessment-of-insulin resistance.
β (SE) represents changes in outcomes when the circulating metabolite levels were decreased during the intervention.
Model 1: data after adjusted for age, sex, ethnicity, parental history of diabetes, diet group, body mass index at baseline, value for the respective outcome traits at the baseline examination, and either TMAO, choline, or L-carnitine at baseline.
Model 2: data after adjusted for covariates in the model 1 and 6-month changes in alanine, branched-chain amino acids, aromatic amino acids, and glutamic acid.
In results of our sensitivity analyses to examine independent associations of the metabolites with the outcomes, Δcholine and ΔL-carnitine were significantly associated with improvements in fasting insulin and HOMA-IR independently of ΔTMAO. When Δcholine and ΔL-carnitine were concurrently included in a model, ΔL-carnitine, but not Δcholine, was significantly associated with the improvements in fasting insulin and HOMA-IR (detailed data not shown).
Since we found significant associations of 6-month changes in choline or L-carnitine with insulin sensitivity, but not with fasting glucose or HbA1c (Table 2), we then further examined trajectories of changes in insulin and HOMA-IR over 2 years according to tertile (T) categories of Δcholine (Figure 1, panels A and B) and ΔL-carnitine (panels C and D). Among the 504 individuals, median (25th, 75th) values of Δcholine were –1.9 (–2.9, –1.4) μM in the lowest tertile (T1); –0.2 (–0.5, 0.2) μM in T2, and 1.6 (1.0, 2.1) μM in the highest tertile (T3). For ΔL-carnitine, median (25th, 75th) values were T1: –5.0 (–7.5, –3.4) μM, T2: 0.2 (–0.9, 1.1) μM, and T3: 4.6 (3.2, 6.7) μM. Individuals in the lowest tertile group of Δcholine or ΔL-carnitine had consistently improved fasting insulin and HOMA-IR over 2 years, as compared to those with less of a decrease in the metabolite levels. In particular, the initial decrease in choline was significantly associated with improvements in fasting glucose (p=0.004), log-transformed fasting insulin (p <0.001), and log-transformed HOMA-IR (p <0.001) at 2 years (Supplemental Table 3). Again, the associations were independent of changes in alanine, BCAAs, AAAs, and glutamic acid. There was no significant association of TMAO, choline, or L-carnitine with changes in HbA1c over 2 years.
Figure 1.
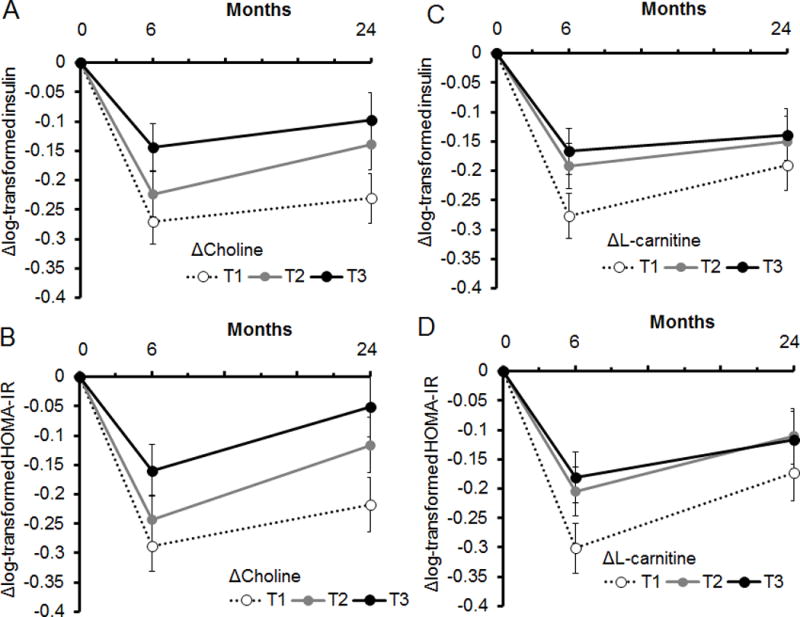
Trajectories of changes in insulin resistance over 2 years according to tertile (T) categories of 6-month changes in choline (panels A and B) and L-carnitine (panels C and D). Data are means ± SE values after adjusted for age, sex, ethnicity, diet group, parental history of diabetes, body mass index, value for the respective outcome traits at the baseline examination, and either choline or L-carnitine levels at baseline.
For Δcholine, median (25th, 75th) values were T1: –1.9 (–2.9, –1.4) μM; T2: –0.2 (–0.5, 0.2) μM; T3: 1.6 (1.0, 2.1) μM, respectively.
For ΔL-carnitine, median (25th, 75th) values were T1: –5.0 (–7.5, –3.4) μM, T2: 0.2 (–0.9, 1.1) μM, and T3: 4.6 (3.2, 6.7) μM, respectively.
We also investigated whether associations of ΔTMAO, Δcholine, and ΔL-carnitine with improving glycemia and insulin sensitivity were modified by different macronutrient intake. We found significant interactions between ΔTMAO and dietary fat intake for changes in fasting glucose, HbA1c, fasting insulin, and HOMA-IR (Pinteraction <0.05 for all) (Figure 2 and Supplemental Table 4). In response to the high-fat diet, individuals with a decrease of TMAO (T1 group: median (25th, 75th), –2.0 (–3.5, –1.2) μM) showed reductions in fasting glucose (Figure 2, panel A), HbA1c (panel B), fasting insulin (panel C), and HOMA-IR (panel D) at 6 months. Among individuals who consumed the high-fat diet, those with an increase in TMAO (T3 group: 1.9 (1.3, 4.0) μM) showed increased levels of fasting glucose and HbA1c, and a less improvement in insulin sensitivity. Conversely, among individuals who consumed the low-fat diet, those with greater increases in TMAO had larger reductions in the outcomes at 6 months. The associations were independent of concurrent body weight changes and amino acid changes (Supplemental Table 4, model 2). There were no significant interactions between different diet groups and changes in choline or L-carnitine for the outcomes.
Figure 2.
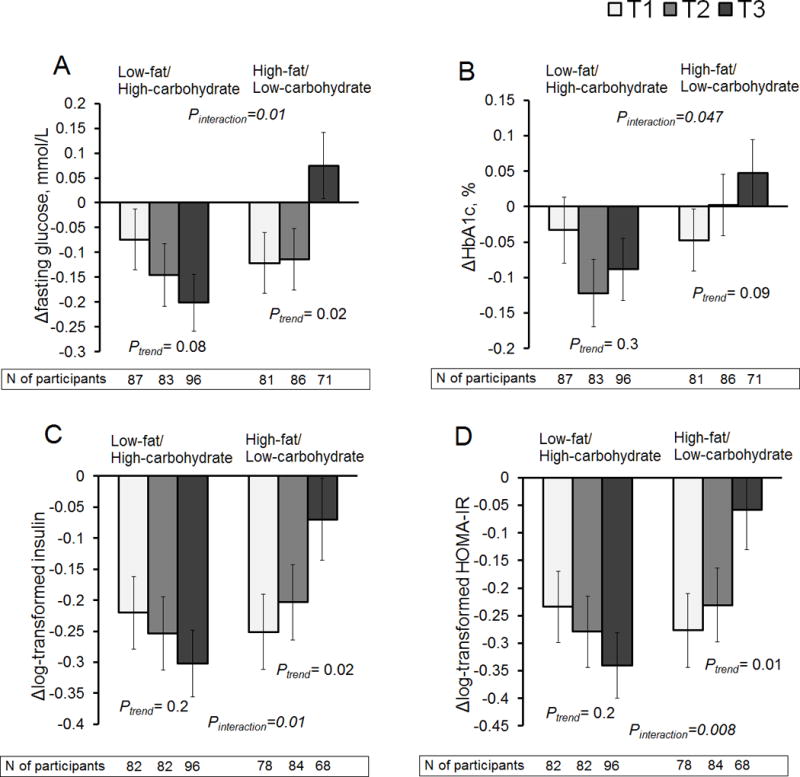
Changes in glycemic and insulin measures at 6 months according to tertile (T) categories of changes in trimethylamine N-oxide (TMAO) in low- or high-fat diet group. Panel A: changes in fasting glucose; panel B: changes in hemoglobin A1c (HbA1c); panel C: changes in log-transformed fasting insulin; panel D: changes in log-transformed homeostasis model assessment-of-insulin resistance (HOMA-IR).
Data are means ± SE values after adjusted for age, sex, ethnicity, and parental history of diabetes.
For ΔTMAO, median (25th, 75th) values were T1 (white): –2.0 (–3.5, –1.2) μM, T2 (gray): 0 (–0.3, 0.3) μM, and T3 (black): 1.9 (1.3, 4.0) μM, respectively among the total participants.
DISCUSSION
We showed that diet-induced changes in TMAO, choline, and L-carnitine were significantly related to the improvement in insulin sensitivity among overweight and obese participants. Interestingly, dietary fat intake significantly modified the associations of changes in TMAO with improved glycemia and insulin sensitivity. Among participants who consumed a high-fat diet, greater increases in TMAO were related to lesser improvements in glycemia and insulin sensitivity. The findings on changes in TMAO and its precursors with the improvements in glycemia and insulin sensitivity were independent of concurrent alterations in insulin-resistance-related or diabetes-related amino acids.
Several lines of evidence suggest that circulating TMAO and TMAO-generating pathways may contribute to the regulation of glucose metabolism and insulin sensitivity.36–38 The TMAO-producing enzyme, FMO3, has been reported as a necessary factor in the development of diabetes-related phenotypes.37 The TMAO-generating pathway involving FMO3 may be a novel target for the improvements of insulin resistance and whole-body insulin sensitivity.7,37 Of note, we found that the decrease in TMAO was weakly but significantly associated with the improvement in insulin resistance among total participants after we controlled for concurrent weight changes in the multivariate-adjusted analysis. Also, the achievement of successful weight loss influenced the association. As to the potential explanations for these findings, the TMAO-generating FMO3 plays roles in regulating obesity37 and the beiging of white adipose tissue.7 Changes in TMAO levels might also be affected by degrees of weight loss in our study participants.26 Additionally, the FMO3 is suppressed by insulin, and also increased by glucagon37 which is secreted from pancreatic α-cells to promote the elevation of blood glucose.39 Both the suppression of glucagon and insulin resistance are in parallel with weight loss and act together to improve the glucose homeostasis following a weight-loss diet intervention.40 These previous studies support our observation that the association of TMAO with insulin sensitivity might be partially affected by adiposity.
Interestingly, we found significant interactions between dietary fat intake and changes in TMAO (but not between changes in choline or L-carnitine) for diabetes-related traits. It has been reported that a high-fat diet led to alterations in gut microbial composition16–18 that have been associated with insulin resistance.18,41 In an experimental study of non-obese adults, a high-fat diet increased postprandial plasma TMAO levels (after 4 hours of the high-fat meal challenge) whereas postprandial plasma choline or L-carnitine levels did not change after eating the high-fat diet.20 On the other hand, the study did not find a significant increase in fasting plasma TMAO concentrations following the short-term (5-day) high-fat diet intervention in the non-obese men.20 Another study of normal-weight individuals showed that a 4-week high-fat diet significantly increased plasma TMAO concentrations, whereas plasma L-carnitine or choline concentrations did not increase in the high-fat diet group.21 In the present study, differences in TMAO changes between low-/high-fat diet groups were not statistically significant, probably due to different characteristics of participants and study design (such as obesity status, weight changes, and duration of the diet intervention) between our study and the previous studies. Nonetheless, according to a study of mice fed a high-fat diet, dietary supplementation of TMAO increased plasma TMAO concentrations which exacerbated impaired glucose tolerance and insulin resistance;38 the study also suggested a biological mechanism that TMAO aggravated blockage of the insulin signaling pathway in the high-fat-fed mice.38 Taken together, previous studies have provided a biological basis for the potential linkages between dietary fat intake and TMAO for the improvements in glucose metabolism and insulin sensitivity, as we found in the present study. Nonetheless, the high-fat diet was the same as low-carbohydrate diet in this study, so that we could not determine whether either dietary fat or carbohydrate, or both nutrients influenced our findings. Our results suggest that the effectiveness of an energy-reduced high-fat/low-carbohydrate diet intervention might be affected by changes in TMAO, a metabolite of the gut-microbiota leading to improved glycemia and insulin sensitivity.
Our study also found associations of changes in choline and L-carnitine with insulin sensitivity independently of concurrent changes in TMAO. One study showed that elevated levels of circulating choline were significantly associated with type 2 diabetes.7 In animal studies, a choline-deficient-diet lowered fasting insulin concentrations and improved glucose tolerance,14 and the choline metabolism may play an active role in the development of insulin resistance.13 Our results indicated in particular that a decrease in choline was associated with long-term improvements in glycemia and insulin sensitivity during the weight-loss intervention. On the other hand, the association of choline with the outcomes became null if we controlled for concurrent changes in L-carnitine. Although both choline and L-carnitine are dietary precursors of TMAO, different pathways are involved in the formation of trimethylamine,42 which might be a potential explanation for our different findings between choline and L-carnitine. L-carnitine is abundant in red meat,9 and contributes to trimethylamine formation directly, via the action of carnitine (Rieske-type) oxygenase of the microbiota,43 or indirectly via formation of γ-Butyrobetaine by the microbiota.44 Circulating L-carnitine may be related to cardiovascular risk through the TMAO production,9,45 and one study showed a positive relationship between plasma L-carnitine and glucose concentrations in elderly women.23 On the other hand, L-carnitine supplementation has been suggested to improve glycemic control in patients with type 2 diabetes.46 We have recently reported that changes in choline and L-carnitine were significantly associated with improvements in adiposity measurements.26 In the present study, we found that the adjustment for concurrent weight changes weakened the associations of choline or L-carnitine with insulin resistance. The diet-induced changes in choline and L-carnitine may be related to improving insulin resistance along with achieving successful weight loss.
Epidemiological studies have shown significant associations of the gluconeogenic substrates, BCAAs, and AAAs with insulin resistance or dysglycemia.31–35 We showed positive relationships between TMAO precursors and amino acids, such as BCAAs and AAAs, at the baseline examination before the intervention. The diet-induced changes in choline and L-carnitine were associated with changes in these amino acids in the present study. These findings are supported by results of more recent studies that showed significant associations between the gut microbiota and amino acids.2,27,47,48 A recent study has shown that altered human gut microbiota may impact the metabolism of BCAAs and insulin resistance, suggesting that gut microbiota may be another independent contributor for elevating levels of BCAAs in the insulin resistant state.2 However, our results indicated that the associations of gut microbiota metabolites with diabetes-related outcomes were independent of changes in BCAAs and AAAs, suggesting other pathways were likely to be involved. Since our study focused on the selected diabetes-related amino acids, further investigations including other serum metabolites and gut microbiota metagenomics would be of value.
Our study has several strengths. We introduced data on changes in TMAO, choline, and L-carnitine as well as changes in amino acids in one of the largest and longest dietary interventions to comprehensively examine our hypothesis rather than using measurements of metabolites at a single time point. Our randomized intervention study has major advantages over observational studies to suggest an effect of weight-loss diets on changes in gut microbial metabolites in the development and prevention of metabolic abnormalities. Nonetheless, we could not determine a causal effect of the metabolites for the outcomes, and further investigations are warranted to understanding biological pathways to explain our findings. The findings on the relationship between dietary fat and TMAO changes were consistent for different diabetes-related outcomes which strengthens our conclusion. We also acknowledge several potential limitations. The present study did not include data on the gut microbiota itself. There might be endogenous or exogenous nutrients that might affect the metabolite levels. Our study participants were overweight or obese adults who were majority white, and mainly well-educated. Further research would be necessary to confirm our findings, especially in a population that is more representative of the US population with overweight and obesity. Our study participants were free of diabetes with medical treatment so that our results may not be applicable for patients with diagnosed diabetes to improve glycemic control, since medical treatment for diabetes may affect the gut microbiome and its related metabolism.49,50 Also, we did not have data on oral glucose tolerance test, and further research would be warranted to investigate whether changes in gut microbial metabolites are related to improvements in impaired glucose tolerance and postprandial insulin secretion using data on repeated glucose tolerance tests.
In conclusion, diet-induced changes in TMAO, choline, and L-carnitine were significantly associated with the improvements in glycemia and insulin sensitivity. These improvements modulated by gut microbial metabolites were possibly through a different mechanism of diabetes-related amino acids. Dietary fat intake may modify the associations of TMAO with the improvements of glucose metabolism and insulin sensitivity.
Supplementary Material
Significance of this study.
1. What is already known about this subject?
Evidence has linked the gut microbiome to host insulin sensitivity, glucose metabolism, and impaired diabetes-related amino acid metabolism.
A gut microbiota-dependent metabolite, trimethylamine N-oxide (TMAO), has been a novel predictor of type 2 diabetes. It remains unclarified how changes in TMAO and its precursors are related to improvements in glucose metabolism and diabetes-related amino acids.
2. What are the new findings?
Changes in gut microbial metabolites after consuming a weight-loss diet were significantly related to improvement in insulin sensitivity and glucose metabolism. Among participants who consumed a high-fat diet, greater increase in TMAO was related to lesser improvements in the diabetes-related outcomes. Further, changes in the precursors of TMAO were significantly associated with changes in diabetes-related amino acids including branched-chain and aromatic amino acids.
Nonetheless, our results indicated that the relations between gut microbiota metabolites and diabetes-related outcomes were independent of changes in branched-chain and aromatic amino acids, suggesting other pathways were likely to be involved.
3. How might it impact on clinical practice in the foreseeable future?
It has been consistently demonstrated that various weight-loss diets are effective for the improvement in glucose metabolism and the prevention of type 2 diabetes, and our results add to the evidence that changes in microbial metabolites may play a significant role in protective effects among overweight and obese adults.
Acknowledgments
The authors thank all of the participants in the study for their dedication and contribution to the research. The authors also thank the Prevention Research Laboratory and Laboratory Diagnostic Core, Cleveland Clinic for the measurements.
Funding: The study is supported by National Institutes of Health (NIH) grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States– Israel Binational Science Foundation Grant 2011036. LQ was a recipient of the American Heart Association Scientist Development Award (0730094N). YH is a recipient of a Grant-in-Aid for Scientific Research and Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. The sponsors had no role in the design or conduct of the study.
Abbreviations
- TMAO
Trimethylamine N-oxide
- FMO3
Flavin-containing enzyme monooxygenase 3
- HOMA-IR
Homeostasis model assessment-of-insulin resistance
- AAAs
Aromatic amino acids
- BCAAs
Branched-chain amino acids
- POUNDS
Preventing Overweight Using Novel Dietary Strategies
- BMI
Body mass index
- HbA1c
Hemoglobin A1c
- SE
Standard error
- T
Tertile
Footnotes
Conflicts of Interest: The authors declare no conflict of interest associated with this publication.
Author Contributions: YH contributed to the study concept and design, analysis and interpretation of data, drafting and revising the manuscript, statistical analysis and study supervision. DS and XL contributed analysis and interpretation of data, and drafting and revising the manuscript. JD contributed measurements and interpretation of data, and drafting and revising the manuscript. GB and FS contributed to acquisition of data, interpretation of data, and drafting and revising the manuscript. LQ contributed to the study concept and design, acquisition of data, analysis and interpretation of data, drafting and revising the manuscript, statistical analysis, and funding and study supervision. LQ had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–81. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
- 3.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 4.Dambrova M, Latkovskis G, Kuka J, et al. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp Clin Endocrinol Diabetes. 2016;124:251–6. doi: 10.1055/s-0035-1569330. [DOI] [PubMed] [Google Scholar]
- 5.Obeid R, Awwad HM, Rabagny Y, et al. Plasma trimethylamine N-oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–11. doi: 10.3945/ajcn.115.121269. [DOI] [PubMed] [Google Scholar]
- 6.Shan Z, Sun T, Huang H, et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am J Clin Nutr. 2017;106:888–94. doi: 10.3945/ajcn.117.157107. [DOI] [PubMed] [Google Scholar]
- 7.Schugar RC, Shih DM, Warrier M, et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19:2451–61. doi: 10.1016/j.celrep.2017.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motika MS, Zhang J, Cashman JR. Flavin-containing monooxygenase 3 and human disease. Expert Opin Drug Metab Toxicol. 2007;3:831–45. doi: 10.1517/17425255.3.6.831. [DOI] [PubMed] [Google Scholar]
- 12.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56:22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–6. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raubenheimer PJ, Nyirenda MJ, Walker BR. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–20. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Wang Y, Hao F, et al. Human serum metabonomic analysis reveals progression axes for glucose intolerance and insulin resistance statuses. J Proteome Res. 2009;8:5188–95. doi: 10.1021/pr900524z. [DOI] [PubMed] [Google Scholar]
- 16.Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fava F, Gitau R, Griffin BA, et al. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes (Lond) 2013;37:216–23. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 18.Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care. 2015;18:515–20. doi: 10.1097/MCO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CA, Corbin KD, da Costa KA, et al. Effect of egg ingestion on trimethylamine-N-oxide production in humans: a randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100:778–86. doi: 10.3945/ajcn.114.087692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutagy NE, Neilson AP, Osterberg KL, et al. Short-term high-fat diet increases postprandial trimethylamine-N-oxide in humans. Nutr Res. 2015;35:858–64. doi: 10.1016/j.nutres.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Boutagy NE, Neilson AP, Osterberg KL, et al. Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 2015;23:2357–63. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 22.Rohrmann S, Linseisen J, Allenspach M, et al. Plasma Concentrations of Trimethylamine-N-oxide Are Directly Associated with Dairy Food Consumption and Low-Grade Inflammation in a German Adult Population. J Nutr. 2016;146:283–9. doi: 10.3945/jn.115.220103. [DOI] [PubMed] [Google Scholar]
- 23.Malinowska AM, Szwengiel A, Chmurzynska A. Dietary, anthropometric, and biochemical factors influencing plasma choline, carnitine, trimethylamine, and trimethylamine-N-oxide concentrations. Int J Food Sci Nutr. 2017;68:488–95. doi: 10.1080/09637486.2016.1256379. [DOI] [PubMed] [Google Scholar]
- 24.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merlotti C, Morabito A, Ceriani V, et al. Prevention of type 2 diabetes in obese at-risk subjects: a systematic review and meta-analysis. Acta Diabetol. 2014;51:853–63. doi: 10.1007/s00592-014-0624-9. [DOI] [PubMed] [Google Scholar]
- 26.Heianza Y, Sun D, Smith SR, et al. Changes in Gut Microbiota-Related Metabolites and Long-term Successful Weight Loss in Response to Weight-Loss Diets: The POUNDS Lost Trial. Diabetes Care. 2018;41:413–19. doi: 10.2337/dc17-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–68. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. doi: 10.1093/eurheartj/ehu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y, Ceglarek U, Huang T, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103:505–11. doi: 10.3945/ajcn.115.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurtz P, Tiainen M, Makinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care. 2012;35:1749–56. doi: 10.2337/dc11-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wurtz P, Soininen P, Kangas AJ, et al. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care. 2013;36:648–55. doi: 10.2337/dc12-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guasch-Ferré M, Hruby A, Toledo E, et al. Metabolomics in prediabetes and diabetes: A systematic review and meta-analysis. Diabetes Care. 2016;39:833–46. doi: 10.2337/dc15-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y, Ceglarek U, Huang T, et al. Plasma Taurine, Diabetes Genetic Predisposition, and Changes of Insulin Sensitivity in Response to Weight-Loss Diets. J Clin Endocrinol Metab. 2016;101:3820–26. doi: 10.1210/jc.2016-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumas ME, Rothwell AR, Hoyles L, et al. Microbial-Host Co-metabolites Are Prodromal Markers Predicting Phenotypic Heterogeneity in Behavior, Obesity, and Impaired Glucose Tolerance. Cell Rep. 2017;20:136–48. doi: 10.1016/j.celrep.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao X, Liu X, Xu J, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Campbell JE, Drucker DJ. Islet alpha cells and glucagon–critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11:329–38. doi: 10.1038/nrendo.2015.51. [DOI] [PubMed] [Google Scholar]
- 40.Silvestre MP, Goode JP, Vlaskovsky P, et al. The role of glucagon in weight loss-mediated metabolic improvement: a systematic review and meta-analysis. Obes Rev. 2018;19:233–53. doi: 10.1111/obr.12631. [DOI] [PubMed] [Google Scholar]
- 41.Caricilli AM, Saad MJ. The role of gut microbiota on insulin resistance. Nutrients. 2013;5:829–51. doi: 10.3390/nu5030829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fennema D, Phillips IR, Shephard EA. Trimethylamine and Trimethylamine N-Oxide, a Flavin-Containing Monooxygenase 3 (FMO3)-Mediated Host-Microbiome Metabolic Axis Implicated in Health and Disease. Drug Metab Dispos. 2016;44:1839–50. doi: 10.1124/dmd.116.070615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111:4268–73. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koeth RA, Levison BS, Culley MK, et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20:799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heianza Y, Ma W, Manson JE, et al. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidal-Casariego A, Burgos-Pelaez R, Martinez-Faedo C, et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. 2013;121:234–8. doi: 10.1055/s-0033-1333688. [DOI] [PubMed] [Google Scholar]
- 47.Org E, Blum Y, Kasela S, et al. Relationships between gut microbiota, plasma metabolites, and metabolic syndrome traits in the METSIM cohort. Genome Biology. 2017;18:70. doi: 10.1186/s13059-017-1194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dodd D, Spitzer MH, Van Treuren W, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–52. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, et al. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 50.Gu Y, Wang X, Li J, et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8:1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


