ABSTRACT
Background
The Kidney Disease: Improving Global Outcomes guidelines have cautioned against administering intravenous (IV) iron to hemodialysis patients with high serum ferritin levels due to safety concerns, but prior research has shown that the association between high ferritin and mortality could be attributed to confounding by malnutrition and inflammation. Our goal was to better understand the ferritin–mortality association and relative influence of IV iron and inflammation in the USA, where ferritin levels have recently increased dramatically, and in Europe and Japan, where ferritin levels are lower and anemia management practices differ.
Methods
Data from 18 261 patients in Phases 4 and 5 (2009–15) of the international Dialysis Outcomes and Practice Patterns Study, a prospective cohort study, were analyzed. Using Cox regression, we modeled the association between baseline ferritin and 1-year mortality with restricted cubic splines and assessed the impact of potential confounders.
Results
Median ferritin levels were 718 ng/mL in the USA, 405 in Europe and 83 in Japan. High ferritin levels were associated with elevated mortality (relative to region-specific medians) in all three regions. The strength of this association was attenuated more by adjustment for malnutrition and inflammation than by IV iron and erythropoiesis-stimulating agent dose in each region.
Conclusion
The utility of high ferritin as a biomarker for clinical risk due to excess iron stores may be limited, although caution regarding IV iron dosing to higher upper ferritin targets remains warranted. Research to resolve biomarker criteria for iron dosing, and whether optimal anemia management strategies differ internationally, is still needed.
Keywords: anemia, ferritin, hemodialysis, inflammation, iron, mortality
INTRODUCTION
Most hemodialysis (HD) patients require treatment with erythropoiesis-stimulating agents (ESAs) and/or intravenous (IV) iron to maintain hemoglobin levels within target ranges [1, 2]. Striking the right balance of ESAs and IV iron use in the context of safety concerns continues to generate extensive discussion in the nephrology community [3–6]. Large cohort studies investigating the association between IV iron dosing and adverse events in HD patients have yielded mixed results [7–11]. Higher mortality risk with larger doses of IV iron was observed by Bailie et al. [7] (≥300 mg/month) and Kalantar-Zadeh et al. [8] (>400 mg/month). In contrast, no association between IV iron dose and all-cause mortality was observed by Miskulin et al. [9] and Feldman et al. [10]. Serum ferritin is one marker of iron stores, along with transferrin saturation (TSAT), commonly used to guide IV iron dosing practices in dialysis. However, the value of using high ferritin levels to limit IV iron dosing due to safety concerns is uncertain.
A recent study of dialysis patients, excluding those with overt inflammation or malnutrition, found that ferritin was the best marker of iron stores based on hepatic magnetic resonance imaging and recommended that target values should be lowered to avoid iron overload [12]. However, others have argued that ferritin has several disadvantages as an index of iron status and is inadequate for guiding iron repletion therapy [13–17]. Serum ferritin is elevated when patients are inflamed, leading to strong correlations with C-reactive protein (CRP) and other markers of acute illness, such as recent hospitalization [18–21]. The utility of a single measurement of serum ferritin as a marker of iron stores may be further limited by extreme within-patient variability over time [22–23]. These issues complicate attempts to interpret studies seeking to estimate the effect of ferritin on adverse events.
Bazeley et al. [21] reported that serum ferritin, in contrast to CRP and serum albumin, did not improve the prediction of mortality risk beyond that of other markers of inflammation. Kalantar-Zadeh et al. [8] showed that while high ferritin levels were strongly associated with elevated mortality in a crude analysis, this association was almost eliminated after adjustment for patient characteristics and malnutrition–inflammation complex syndrome. Since the analysis of this 2001–03 cohort [8], ferritin levels have increased dramatically among US HD patients [24–26], largely driven by acceptance of higher ferritin and/or TSAT targets at many centers following the introduction of the Centers for Medicare & Medicaid Services bundled payment system in 2011 [27]. In the current setting, a large proportion of US patients have serum ferritin levels >800 ng/mL [24, 25]. These levels far exceed 2012 guidelines [2] released by the Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group (which recommended stopping IV iron dosing when ferritin is >500 ng/mL) and a 2013 response from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) recommending IV iron treatment when ferritin is <800 ng/mL [28].
A reassessment of the potential risk of high ferritin levels is warranted for two reasons: (i) the safety of the very high ferritin levels observed in the USA in the postbundle [27] era is unknown, as anemia management practices may plausibly drive the ferritin–mortality relation, in addition to inflammation and (ii) a comparison of the ferritin–mortality association in the USA versus Europe and Japan, where differing anemia management practices, financial incentives and/or guidelines [29–31] keep ferritin levels much lower than in the USA [32–34], could provide further clarification of the mechanism(s) behind the ferritin–mortality association in different international settings. Our primary objectives were to characterize the association between high ferritin levels and mortality in three international regions and to assess the impact of potential confounders, namely IV iron dosing and inflammation, on that association.
MATERIALS AND METHODS
Data source
The Dialysis Outcomes and Practice Patterns Study (DOPPS) is a large international prospective cohort study of patients ≥18 years of age treated with in-center HD (see http://www.dopps.org for details). Patients were randomly selected from national samples of dialysis facilities in each country [35, 36]. Study approval and patient consent were obtained as required by national and local ethics committee regulations. In this analysis, patient data from nine countries in three regions were used: the USA, Europe (Belgium, France, Germany, Italy, Spain, Sweden, UK) and Japan. Data on demographics (baseline), comorbid conditions (baseline), laboratory values (monthly) and prescriptions (monthly) were abstracted from medical records using uniform and standardized data collection tools. Transfusion data were not collected prior to 2011, and not at all in most US facilities; CRP was not routinely measured in US facilities.
The primary analysis of ferritin and mortality included patients in DOPPS Phase 4 (2009–11) and Phase 5 (2012–15). The exposure of interest was each patient’s first serum ferritin measured >4 months after study entry, allowing for a 3- to 4-month window to collect information on potential confounders (e.g. IV iron and ESA dose) prior to baseline ferritin measurement. For the outcome of all-cause mortality, follow-up started at baseline ferritin measurement and continued for 1 year or until death, study phase end, loss to follow-up, transplantation, switch to home dialysis or 7 days after leaving the facility (whichever occurred first). Figure 1 summarizes the timing of data collection for variables included in the Cox models. The number of patients in our primary analysis was 8510 in the USA, 6757 in Europe and 2994 in Japan. A separate analysis of facility ferritin targets used a question from the annual (2009–14) DOPPS Medical Director Survey: ‘For clinical protocols relating to serum ferritin at your unit, indicate the current upper limit of target used for patients in your dialysis unit’.
FIGURE 1.
Timing of variables included in models. aExcluded in Japan due to near-uniform distributions (black race, catheter use) or high missingness (bicarbonate). bHemoglobin measured at the beginning and end of the interval. cBlood transfusions and CRP excluded in USA due to limited data collection. RBC, red blood cell.
Statistical analysis
We summarized patient characteristics by region-specific ferritin categories. To estimate the association between ferritin and all-cause mortality in each region, we used Cox regression stratified by calendar year and by dialysis organization size in the USA or by country in Europe. We accounted for facility clustering using robust sandwich covariance estimators. Anticipating a nonlinear association with mortality, serum ferritin was parameterized using a restricted cubic spline with three knots at the 10th, 50th and 90th percentiles within each region [37], treating region-specific medians as reference levels. Results outside of cohort-specific 5th and 95th percentiles were suppressed to limit tenuous extrapolation. To avoid cluttering the figures with several sets of confidence intervals (CIs), the precision of the hazard ratio (HR) estimates from these models are presented as 95% CIs at selected ferritin levels in Table 1. The proportional hazards assumption was confirmed via log-log survival plots and by testing the interaction between log-time and ferritin in each region.
Table 1.
Ferritin and mortality, by region and level of adjustment
| HR (95% CI) of ferritin (ng/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| Region | 20 | 100 | 200 | 400 | 700 | 1000 | 1400 |
| USA (Ref = 718 ng/mL) | |||||||
| Model 1 | – | – | 0.92 (0.77–1.09) | 0.94 (0.85–1.04) | 1.00 (0.99–1.00) | 1.10 (1.05–1.14) | 1.29 (1.19–1.40) |
| Model 2 | – | – | 0.87 (0.73–1.04) | 0.92 (0.83–1.01) | 0.99 (0.99–1.00) | 1.09 (1.05–1.13) | 1.24 (1.14–1.35) |
| Model 3 | – | – | 0.83 (0.68–1.01) | 0.89 (0.80–1.00) | 0.99 (0.99–1.00) | 1.07 (1.03–1.12) | 1.16 (1.06–1.27) |
| Europe (Ref = 405 ng/mL) | |||||||
| Model 1 | – | 1.06 (0.90–1.25) | 1.03 (0.93–1.14) | 1.00 (1.00–1.00) | 1.09 (1.02–1.15) | 1.28 (1.18–1.40) | – |
| Model 2 | – | 1.03 (0.87–1.22) | 1.01 (0.91–1.13) | 1.00 (1.00–1.00) | 1.08 (1.02–1.15) | 1.25 (1.15–1.36) | – |
| Model 3 | – | 1.05 (0.89–1.25) | 1.03 (0.92–1.15) | 1.00 (1.00–1.00) | 1.06 (0.99–1.12) | 1.19 (1.08–1.30) | – |
| Model 4 | – | 1.09 (0.92–1.30) | 1.05 (0.94–1.18) | 1.00 (1.00–1.00) | 1.03 (0.97–1.09) | 1.14 (1.04–1.25) | – |
| Model 5 | – | 1.10 (0.93–1.31) | 1.06 (0.95–1.18) | 1.00 (1.00–1.00) | 1.02 (0.96–1.09) | 1.12 (1.02–1.23) | – |
| Japan (Ref = 83 ng/mL) | |||||||
| Model 1 | 1.00 (0.80–1.25) | 1.00 (0.96–1.05) | 1.06 (0.87–1.28) | 1.27 (1.01–1.60) | – | – | – |
| Model 2 | 0.97 (0.78–1.21) | 1.01 (0.96–1.06) | 1.07 (0.88–1.29) | 1.23 (0.98–1.53) | – | – | – |
| Model 3 | 1.04 (0.83–1.32) | 0.99 (0.95–1.04) | 1.00 (0.81–1.22) | 1.12 (0.89–1.40) | – | – | – |
| Model 4 | 1.07 (0.85–1.36) | 0.99 (0.94–1.04) | 0.97 (0.79–1.19) | 1.07 (0.85–1.34) | – | – | – |
| Model 5 | 1.06 (0.84–1.35) | 0.99 (0.94–1.04) | 0.98 (0.80–1.20) | 1.07 (0.85–1.34) | – | – | – |
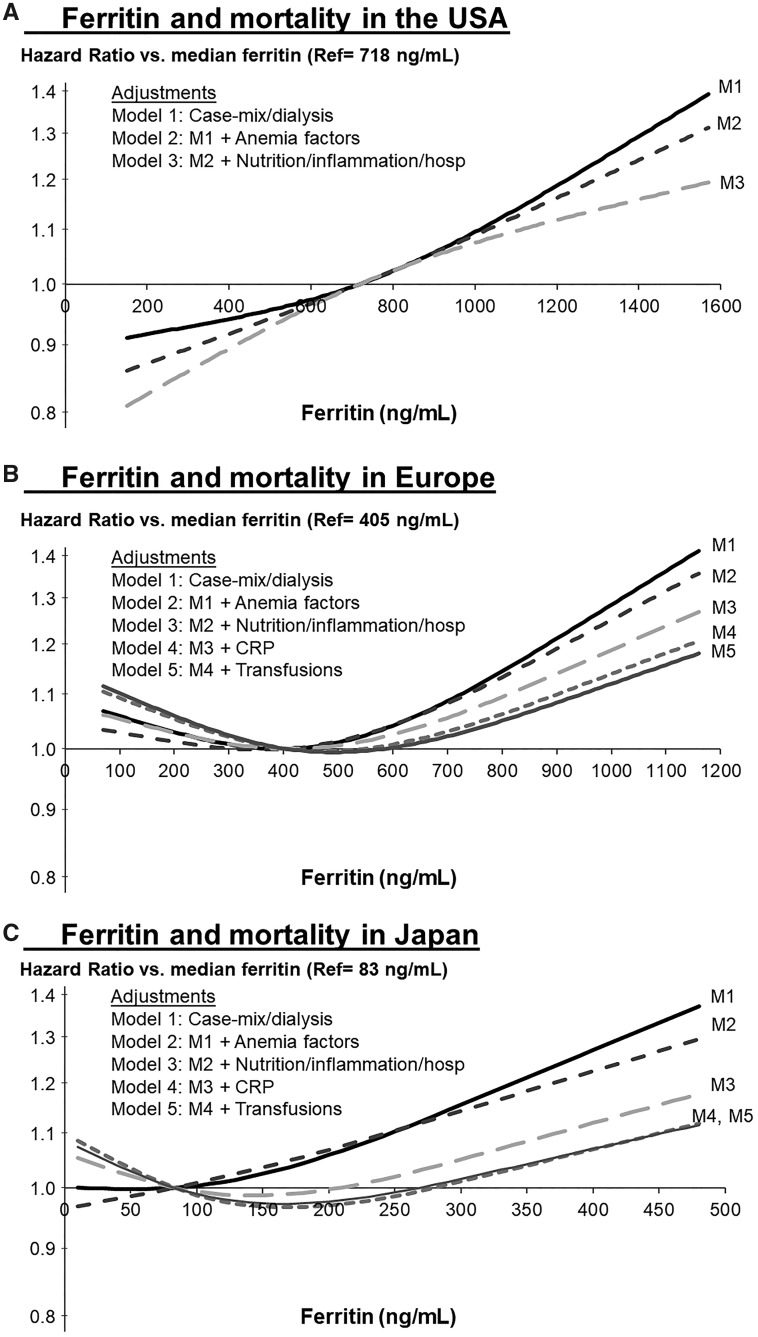
HR and 95% CIs of Figure 3A–C models at specified ferritin levels.
To investigate the degree to which the unadjusted association between high ferritin and mortality could be attributed to certain mortality risk factors associated with ferritin level (e.g. IV iron dose, inflammatory markers) in each region, we used a series of five progressively adjusted models to observe changes in the shape and magnitude of the ferritin–mortality association, as described in Figures 3A–C. The adjustments were as follows: Model 1 (case mix): age, sex, black race, vintage, 13 comorbidities (listed in Table 2), catheter use, and Kt/V at study entry; Model 2 (anemia factors): Model 1 plus hemoglobin, average IV iron and ESA dose prescribed during the 3–4 months prior to ferritin measurement, an interaction between IV iron dose and vintage and an indicator for whether the patient received a bolus IV iron dose, defined as at least 1 of the 3–4 prior months with at least 500 mg IV iron; Model 3 (nutrition and inflammation): Model 2 plus body mass index (BMI) and normalized protein catabolic rate (nPCR) at study entry, any hospitalization during the prior 3–4 months, albumin, creatinine, white blood cell (WBC) count, potassium, bicarbonate, phosphorus and phosphorus squared; Model 4: Model 3 plus CRP (not measured routinely in the US); Model 5: Model 4 plus an indicator for any blood transfusion in the prior 3–4 months (only limited data collected in the USA). Progressive model adjustments are sensitive to the order in which covariates are added; since we hypothesized that the crude association between high ferritin and mortality is principally explained by inflammation rather than anemia measures and treatments, we added anemia management variables first to provide a more conservative estimate of the impact of inflammation. In analyses of Japanese patients, covariates with near-uniform distributions (race, catheter use) or high missingness (serum bicarbonate) were excluded from the model.
FIGURE 3.
Association of ferritin level with mortality, shown by progressive adjustment in (A) USA, (B) Europe and (C) Japan (reference point = region-specific median). Restricted cubic splines with three knots used to model ferritin and mortality in Cox regression models, stratified by calendar year and by large dialysis organization in the USA or by country in Europe. Results outside of cohort-specific 5th and 95th percentiles were suppressed to limit tenuous extrapolation. Adjustments in Model 1 (case mix): age, sex, black race, vintage, 13 comorbidities, catheter use and Kt/V at study entry; Model 2 (anemia factors): Model 1 plus hemoglobin, average IV iron and ESA dose prescribed during the 3–4 months prior to ferritin measurement, an interaction between IV iron dose and vintage and an indicator for whether the patient received a bolus IV iron dose, defined as at least 1 of the 3–4 prior months with at least 500 mg IV iron; Model 3 (nutrition and inflammation): Model 2 plus BMI and nPCR at study entry, any hospitalization during the prior 3–4 months, albumin, creatinine, WBC count, potassium, bicarbonate, phosphorus and phosphorus squared; Model 4: Model 3 plus CRP (not measured routinely in the USA); Model 5: Model 4 plus indicator for any blood transfusion in the prior 3–4 months (only limited data collected in USA). Table 1 provides CIs for all models in panels A–C at selected ferritin increments.
Table 2.
Patient characteristics by serum ferritin in US DOPPS (2009–15)
| Patient characteristic | All | Serum ferritin (ng/mL) |
||||
|---|---|---|---|---|---|---|
| <200 | 200–499 | 500–799 | 800–1199 | ≥1200 | ||
| Patients, n (%) | 8510 | 666 (8) | 1910 (22) | 2316 (27) | 2261 (27) | 1357 (16) |
| Demographics | ||||||
| Age (years), mean ± SD | 62.4 ± 15.1 | 59.1 ± 15.9 | 60.7 ± 15.1 | 62.2 ± 14.8 | 63.4 ± 14.9 | 65.1 ± 14.8 |
| Sex (male), % | 56 | 63 | 60 | 56 | 53 | 51 |
| Race (black), % | 32 | 25 | 32 | 32 | 34 | 35 |
| Vintage (years), mean ± SD | 3.6 ± 3.9 | 3.2 ± 4.2 | 2.9 ± 3.7 | 3.5 ± 3.9 | 4.1 ± 3.9 | 4.2 ± 4.0 |
| HD characteristics | ||||||
| Catheter use, % | 28 | 39 | 38 | 28 | 22 | 22 |
| Single pool Kt/V, mean ± SD | 1.54 ± 0.31 | 1.49 ± 0.35 | 1.47 ± 0.32 | 1.53 ± 0.31 | 1.58 ± 0.28 | 1.60 ± 0.29 |
| Anemia treatments (past 3–4 months) | ||||||
| IV iron (with any dose), % | 77 | 64 | 82 | 80 | 77 | 72 |
| IV iron (mg/week) among treated, mean ± SD | 75 ± 52 | 74 ± 50 | 72 ± 49 | 68 ± 46 | 75 ± 51 | 94 ± 62 |
| Bolusa dose of IV iron among treated, % | 38 | 41 | 35 | 32 | 39 | 52 |
| ESA (with any dose), % | 93 | 84 | 93 | 94 | 93 | 94 |
| ESA (1000 units/week) among treated, mean ± SD | 15 ± 15 | 22 ± 20 | 16 ± 15 | 15 ± 14 | 13 ± 14 | 14 ± 15 |
| Transfused, % | – | – | – | – | – | – |
| Anemia labs, mean ± SD | ||||||
| Ferritin (ng/mL) | 774 ± 467 | 119 ± 54 | 363 ± 84 | 650 ± 86 | 975 ± 112 | 1, 552 ± 417 |
| TSAT (%) | 31.3 ± 12.7 | 24.6 ± 11.2 | 28.8 ± 11.2 | 30.9 ± 11.5 | 33.1 ± 12.7 | 36.0 ± 15.0 |
| Hemoglobin (g/dL) | 11.2 ± 1.2 | 11.5 ± 1.5 | 11.4 ± 1.2 | 11.2 ± 1.1 | 11.0 ± 1.2 | 10.9 ± 1.2 |
| Nutrition and inflammation markers, mean ± SD | ||||||
| Body mass index (kg/m2) | 28.7 ± 7.0 | 28.9 ± 6.6 | 28.9 ± 7.0 | 28.8 ± 7.2 | 28.7 ± 7.1 | 28.0 ± 6.8 |
| Normalized PCR (g/kg/day) | 0.95 ± 0.26 | 0.91 ± 0.26 | 0.93 ± 0.26 | 0.95 ± 0.26 | 0.97 ± 0.25 | 0.97 ± 0.26 |
| Serum phosphorus (mg/dL) | 5.2 ± 1.5 | 5.3 ± 1.5 | 5.2 ± 1.5 | 5.2 ± 1.5 | 5.1 ± 1.4 | 5.1 ± 1.5 |
| Serum bicarbonate (mEq/L) | 23.6 ± 3.3 | 23.3 ± 3.4 | 23.3 ± 3.2 | 23.5 ± 3.3 | 23.7 ± 3.3 | 23.9 ± 3.3 |
| Serum potassium (mEq/L) | 4.7 ± 0.7 | 4.7 ± 0.7 | 4.7 ± 0.7 | 4.7 ± 0.7 | 4.8 ± 0.6 | 4.7 ± 0.7 |
| Serum creatinine (mg/dL) | 8.2 ± 3.1 | 8.1 ± 3.2 | 8.2 ± 3.1 | 8.2 ± 3.1 | 8.3 ± 2.9 | 8.2 ± 3.1 |
| Serum albumin (g/dL) | 3.84 ± 0.40 | 3.79 ± 0.42 | 3.84 ± 0.40 | 3.86 ± 0.39 | 3.87 ± 0.40 | 3.81 ± 0.43 |
| WBC count (1000 cells/mm3) | 6.9 ± 2.3 | 6.8 ± 2.3 | 6.7 ± 2.1 | 6.8 ± 2.1 | 7.1 ± 2.3 | 7.4 ± 2.5 |
| CRP (mg/L) | – | – | – | – | – | – |
| Hospitalized in past 3–4 months, % | 16 | 19 | 17 | 14 | 15 | 19 |
| Comorbid conditions, % | ||||||
| Coronary artery disease | 32 | 32 | 31 | 33 | 31 | 30 |
| Congestive heart failure | 33 | 34 | 34 | 34 | 33 | 32 |
| Cerebrovascular disease | 10 | 9 | 9 | 9 | 10 | 11 |
| Peripheral vascular disease | 17 | 17 | 17 | 18 | 18 | 17 |
| Other cardiovascular disease | 17 | 19 | 16 | 17 | 16 | 17 |
| Hypertension | 81 | 84 | 80 | 80 | 82 | 82 |
| Diabetes | 61 | 56 | 62 | 63 | 62 | 58 |
| Neurologic disease | 7 | 8 | 7 | 7 | 7 | 8 |
| Psychiatric disorder | 13 | 17 | 12 | 13 | 12 | 13 |
| Lung disease | 11 | 13 | 12 | 11 | 11 | 11 |
| Cancer (non-skin) | 8 | 7 | 7 | 7 | 9 | 11 |
| Gastrointestinal bleeding | 3 | 3 | 3 | 3 | 3 | 4 |
| Recurrent cellulitis, gangrene | 8 | 8 | 9 | 8 | 8 | 5 |
Data on transfusions and CRP were not available in the USA.
Defined as at least 1 month (of prior 3–4 months) with IV iron dose ≥500 mg.
To deal with missing covariate data, we used multiple imputation, assuming data were missing at random. Missing covariate values were multiply imputed using the Sequential Regression Multiple Imputation Method by IVEware [38]. Results from 20 such imputed data sets were combined for the final analysis using Rubin’s formula [39]. The proportion of missing data was <20% for all variables used for covariate adjustment, with the exception of blood transfusions (52% in Europe, 43% in Japan), nPCR (33% in Europe), Kt/V (33% in Europe), CRP (27% in Japan, 21% in Europe) and serum bicarbonate (26% in Europe). A full summary of missing data is provided in Supplementary data, Table S1. All analyses were conducted using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Distribution of ferritin levels by region
Median ferritin levels were 718 ng/mL [interquartile range (IQR) 439–1026)] in the USA, 405 (224–640) in Europe and 83 (36–176) in Japan. Since these levels were markedly different by region, all analyses were conducted separately for the USA, Europe and Japan.
Patient characteristics by ferritin level
Table 2 shows that 8% of US patients had ferritin <200 ng/mL; these patients tended to be younger, more likely male and dialyzing with a catheter and had received larger ESA doses than patients with ferritin ≥200 ng/mL. Forty-three percent of US patients had ferritin ≥800 ng/mL and 16% had ferritin ≥1200 ng/mL; patients with higher ferritin (≥800 ng/mL) appeared to be older, with more years on dialysis (vintage), higher WBC count and nPCR and lower hemoglobin levels and were more likely to have received a bolus dose of IV iron than patients with lower (<800 ng/mL) ferritin levels.
Table 3 shows that in Europe, 21% of patients had ferritin <200 ng/mL, while 15% had ferritin ≥800 ng/mL, including 4% with ferritin ≥1200 ng/mL. Patients with ferritin <200 ng/mL were younger and more likely male than those with ferritin ≥200 ng/mL. Patients with ferritin ≥800 ng/mL had higher CRP and WBC count, were more likely to have recently received a transfusion and had received higher IV iron doses than patients with ferritin <800 ng/mL.
Table 3.
Patient characteristics by serum ferritin in Europe DOPPS (2009–15)
| Patient characteristic | All | Serum ferritin (ng/mL) |
|||
|---|---|---|---|---|---|
| <200 | 200–499 | 500–799 | ≥800 | ||
| Patients, n (%) | 6757 | 1443 (21) | 2676 (40) | 1636 (24) | 1002 (15) |
| Demographics | |||||
| Age (years), mean ± SD | 65.9 ± 14.9 | 64.6 ± 15.0 | 66.1 ± 15.2 | 66.8 ± 14.5 | 65.8 ± 14.6 |
| Sex (male), % | 62 | 68 | 62 | 60 | 55 |
| Race (black), % | 3 | 3 | 3 | 4 | 3 |
| Vintage (years), mean ± SD | 4.5 ± 5.8 | 4.5 ± 6.1 | 4.5 ± 5.7 | 4.5 ± 5.8 | 4.7 ± 5.7 |
| HD characteristics | |||||
| Catheter use, % | 28 | 31 | 28 | 28 | 27 |
| Single pool Kt/V, mean ± SD | 1.50 ± 0.33 | 1.44 ± 0.33 | 1.52 ± 0.34 | 1.50 ± 0.33 | 1.53 ± 0.32 |
| Anemia treatments (past 3–4 months) | |||||
| IV iron (with any dose), % | 79 | 73 | 81 | 81 | 77 |
| IV iron (mg/week) among treated, mean ± SD | 74 ± 47 | 72 ± 46 | 68 ± 44 | 77 ± 47 | 90 ± 53 |
| Bolusa dose of IV iron among treated, % | 22 | 23 | 17 | 22 | 31 |
| ESA (with any dose), % | 90 | 84 | 91 | 93 | 93 |
| ESA (1000 units/week) among treated, mean ± SD | 10 ± 8 | 11 ± 9 | 10 ± 8 | 9 ± 8 | 9 ± 8 |
| Transfused, % | 5 | 6 | 3 | 5 | 10 |
| Anemia labs, mean ± SD | |||||
| Ferritin (ng/mL) | 486 ± 380 | 116 ± 54 | 343 ± 85 | 628 ± 85 | 1, 166 ± 438 |
| TSAT (%) | 28.0 ± 12.5 | 22.7 ± 10.6 | 27.2 ± 10.7 | 29.9 ± 12.4 | 35.1 ± 15.3 |
| Hemoglobin (g/dL) | 11.5 ± 1.4 | 11.6 ± 1.5 | 11.5 ± 1.3 | 11.4 ± 1.3 | 11.3 ± 1.4 |
| Nutrition and inflammation markers, mean ± SD | |||||
| Body mass index (kg/m2) | 26.1 ± 5.5 | 26.1 ± 5.3 | 26.4 ± 5.6 | 25.9 ± 5.3 | 25.6 ± 5.5 |
| Normalized PCR (g/kg/day) | 1.00 ± 0.25 | 1.01 ± 0.25 | 1.00 ± 0.26 | 0.99 ± 0.23 | 0.99 ± 0.25 |
| Serum phosphorus (mg/dL) | 4.8 ± 1.5 | 5.0 ± 1.5 | 4.7 ± 1.4 | 4.8 ± 1.4 | 4.9 ± 1.5 |
| Serum bicarbonate (mEq/L) | 23.2 ± 3.3 | 22.7 ± 3.4 | 23.2 ± 3.2 | 23.2 ± 3.3 | 23.5 ± 3.5 |
| Serum potassium (mEq/L) | 5.0 ± 0.8 | 5.1 ± 0.8 | 5.0 ± 0.8 | 5.0 ± 0.8 | 5.1 ± 0.9 |
| Serum creatinine (mg/dL) | 8.0 ± 2.6 | 8.3 ± 2.8 | 8.0 ± 2.6 | 8.0 ± 2.6 | 7.7 ± 2.4 |
| Serum albumin (g/dL) | 3.72 ± 0.47 | 3.73 ± 0.47 | 3.72 ± 0.46 | 3.68 ± 0.48 | 3.72 ± 0.48 |
| WBC count (1000 cells/mm3) | 7.0 ± 2.2 | 7.0 ± 2.2 | 7.0 ± 2.3 | 7.0 ± 2.2 | 7.3 ± 2.3 |
| CRP (mg/L), median (IQR) | 6 (3–14) | 5 (2–12) | 5 (3–12) | 6 (3–14) | 8 (3–21) |
| Hospitalized in past 3–4 months, % | 17 | 17 | 16 | 18 | 19 |
| Comorbid conditions, % | |||||
| Coronary artery disease | 35 | 36 | 34 | 35 | 35 |
| Congestive heart failure | 19 | 17 | 19 | 18 | 20 |
| Cerebrovascular disease | 16 | 17 | 16 | 16 | 17 |
| Peripheral vascular disease | 30 | 34 | 30 | 28 | 30 |
| Other cardiovascular disease | 30 | 29 | 31 | 30 | 33 |
| Hypertension | 86 | 85 | 87 | 85 | 86 |
| Diabetes | 36 | 35 | 37 | 35 | 37 |
| Neurologic disease | 11 | 10 | 12 | 11 | 12 |
| Psychiatric disorder | 17 | 17 | 17 | 16 | 17 |
| Lung disease | 13 | 14 | 14 | 13 | 12 |
| Cancer (nonskin) | 17 | 15 | 16 | 19 | 19 |
| Gastrointestinal bleeding | 4 | 5 | 4 | 4 | 5 |
| Recurrent cellulitis, gangrene | 9 | 7 | 9 | 9 | 11 |
Defined as at least 1 month (of prior 3–4 months) with IV iron dose ≥500 mg.
Table 4 shows that only 21% of Japanese patients had ferritin ≥200 ng/mL, while 34% had ferritin levels <50 ng/mL. Patients with very low ferritin (<50 ng/mL) had the highest levels of phosphorus and creatinine. Patients with ferritin ≥200 ng/mL (versus <200 ng/mL) tended to be older and were more likely to have recently received IV iron—albeit still less likely (48%) than in the USA and Europe, where more than three-quarters of sampled patients received IV iron in the past 3–4 months. In each region, minimal differences in comorbidities were observed across ferritin categories.
Table 4.
Patient characteristics by serum ferritin in Japan DOPPS (2009–15)
| Patient characteristic | All | Serum ferritin (ng/mL) |
|||
|---|---|---|---|---|---|
| <50 | 50–99 | 100–199 | ≥200+ | ||
| Patients, n (%) | 2994 | 1012 (34) | 666 (22) | 682 (23) | 634 (21) |
| Demographics | |||||
| Age (years), mean ± SD | 64.9 ± 12.0 | 63.8 ± 11.8 | 64.5 ± 12.5 | 65.6 ± 11.7 | 66.2 ± 12.1 |
| Sex (male), % | 66 | 67 | 65 | 65 | 66 |
| Race (black), % | 0 | 0 | 0 | 0 | 0 |
| Vintage (years), mean ± SD | 7.0 ± 7.4 | 7.6 ± 7.5 | 7.3 ± 7.6 | 6.5 ± 7.6 | 6.0 ± 6.6 |
| HD characteristics | |||||
| Catheter use, % | 1 | 0 | 2 | 1 | 2 |
| Single pool Kt/V, mean ± SD | 1.34 ± 0.30 | 1.36 ± 0.28 | 1.36 ± 0.30 | 1.33 ± 0.31 | 1.31 ± 0.32 |
| Anemia treatments (past 3–4 months) | |||||
| IV iron (with any dose), % | 33 | 21 | 32 | 39 | 48 |
| IV iron (mg/week) among treated, mean ± SD | 32 ± 21 | 29 ± 17 | 32 ± 25 | 31 ± 19 | 35 ± 20 |
| Bolusa dose of IV iron among treated, % | 17 | 22 | 18 | 15 | 15 |
| ESA (with any dose), % | 90 | 83 | 90 | 94 | 95 |
| ESA (1000 units/week) among treated, mean ± SD | 6 ± 5 | 6 ± 5 | 5 ± 4 | 5 ± 4 | 6 ± 5 |
| Transfused, % | 2 | 2 | 2 | 2 | 3 |
| Anemia labs, mean ± SD | |||||
| Ferritin (ng/mL) | 145 ± 205 | 26 ± 12 | 72 ± 15 | 141 ± 29 | 415 ± 311 |
| TSAT (%) | 26.1 ± 12.0 | 21.1 ± 11.0 | 26.3 ± 10.6 | 28.6 ± 10.7 | 31.4 ± 13.6 |
| Hemoglobin (g/dL) | 10.7 ± 1.2 | 10.8 ± 1.2 | 10.8 ± 1.1 | 10.7 ± 1.1 | 10.4 ± 1.3 |
| Nutrition and inflammation markers, mean ± SD | |||||
| Body mass index (kg/m2) | 21.5 ± 3.5 | 21.8 ± 3.5 | 21.5 ± 3.7 | 21.4 ± 3.4 | 21.0 ± 3.1 |
| Normalized PCR (g/kg/day) | 0.92 ± 0.21 | 0.93 ± 0.19 | 0.92 ± 0.21 | 0.92 ± 0.22 | 0.92 ± 0.22 |
| Serum phosphorus (mg/dL) | 5.3 ± 1.3 | 5.5 ± 1.3 | 5.3 ± 1.3 | 5.1 ± 1.3 | 5.2 ± 1.3 |
| Serum bicarbonate (mEq/L) | – | – | – | – | – |
| Serum potassium (mEq/L) | 4.8 ± 0.8 | 4.9 ± 0.7 | 4.8 ± 0.8 | 4.8 ± 0.8 | 4.8 ± 0.8 |
| Serum creatinine (mg/dL) | 10.4 ± 2.9 | 10.9 ± 3.0 | 10.4 ± 2.8 | 10.1 ± 2.8 | 9.9 ± 3.0 |
| Serum albumin (g/dL) | 3.70 ± 0.41 | 3.71 ± 0.40 | 3.70 ± 0.40 | 3.72 ± 0.39 | 3.67 ± 0.45 |
| WBC count (1000 cells/mm3) | 5.9 ± 1.9 | 6.0 ± 1.9 | 5.8 ± 1.9 | 5.8 ± 1.9 | 5.9 ± 2.0 |
| CRP (mg/L), median (IQR) | 1 (1–3) | 1 (1–3) | 1 (0–3) | 1 (0–3) | 1 (1–5) |
| Hospitalized in past 3–4 months, % | 10 | 10 | 9 | 9 | 11 |
| Comorbid conditions, % | |||||
| Coronary artery disease | 27 | 26 | 28 | 27 | 29 |
| Congestive heart failure | 19 | 19 | 17 | 20 | 19 |
| Cerebrovascular disease | 12 | 12 | 12 | 12 | 14 |
| Peripheral vascular disease | 16 | 16 | 17 | 18 | 14 |
| Other cardiovascular disease | 25 | 24 | 26 | 24 | 26 |
| Hypertension | 80 | 78 | 80 | 80 | 85 |
| Diabetes | 40 | 41 | 38 | 40 | 38 |
| Neurologic disease | 7 | 5 | 7 | 8 | 7 |
| Psychiatric disorder | 5 | 5 | 5 | 5 | 6 |
| Lung disease | 4 | 3 | 4 | 3 | 5 |
| Cancer (nonskin) | 11 | 10 | 9 | 11 | 14 |
| Gastrointestinal bleeding | 5 | 6 | 4 | 3 | 5 |
| Recurrent cellulitis, gangrene | 4 | 4 | 4 | 3 | 4 |
Serum bicarbonate was not measured in 80% of Japanese patients.
Defined as at least 1 month (of prior 3–4 months) with IV iron dose ≥500 mg.
Trends in upper ferritin target levels
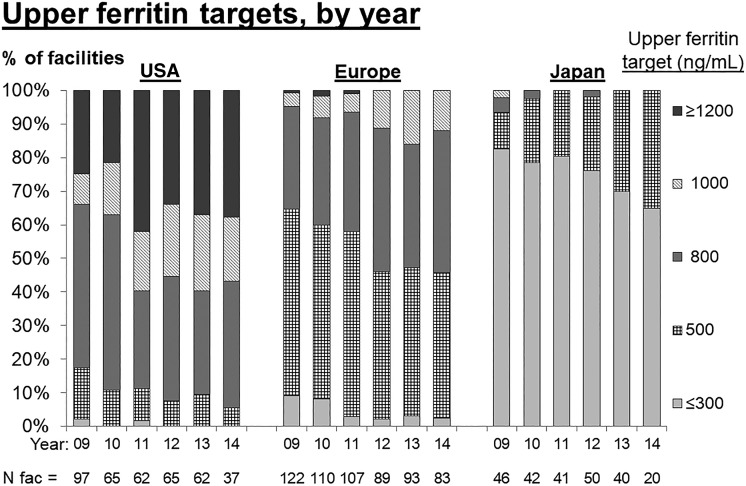
Figure 2 shows upper ferritin targets by region and calendar year (2009–14) as reported by dialysis facility medical directors. In the USA, the percentage of facilities with an upper ferritin target ≥1200 ng/mL increased from ∼20% to 40% from 2010 to 2011. More than 90% of US facilities had an upper ferritin target ≥800 ng/mL in 2014. While ferritin targets in Europe increased from 2009 to 2014, upper targets of 500 ng/mL remained common and no European facility had an upper ferritin target ≥1200 ng/mL in 2014. In Japan, upper ferritin targets were even lower, with most facilities targeting upper limits of ≤300 ng/mL.
FIGURE 2.
Trend in facility upper ferritin target, by year (Medical Director Survey data, 2009–14). Results were rounded to the five most common upper target values.
Ferritin and mortality by level of adjustment
In the worldwide eligible sample of 18 261 patients, 1856 (10%) died within 1 year and 10 954 (60%) survived 1 year; among patients censored within 1 year, 501 (3%) received a transplant, 109 (1%) changed modality to peritoneal dialysis or home HD, 1164 (6%) transferred to another facility, 3410 (19%) reached administrative study phase end and 267 (1%) were otherwise lost to follow-up. The crude all-cause mortality rate during the 1-year follow-up period was 0.146/year (962 deaths) in the USA, 0.139/year (757 deaths) in Europe and 0.051/year (137 deaths) in Japan. Figures 3A–C illustrate the shape of the observed association between ferritin and mortality by region and level of covariate adjustment.
In the USA we observed a positive monotonic association between ferritin and mortality after adjusting only for case mix, Kt/V and catheter use (Figure 3A, Model 1). We observed relatively small changes in the shape and magnitude of this association after additional adjustment for IV iron dose, ESA dose and hemoglobin (Model 2). Adjustment for nutrition, inflammation and recent hospitalizations had a greater impact in attenuating the association between high (versus median) ferritin and mortality (Model 3).
In Europe we observed an elevated mortality rate at high levels of ferritin in Figure 3B, Model 1, with minimal impact of adjustment for anemia factors (Model 2). Adjustment for recent hospitalization and markers of nutrition and inflammation (Model 3), particularly CRP (Model 4), attenuated this association between high (versus median) ferritin and mortality. Further adjustment for blood transfusions had minimal impact (Model 5). Regarding low versus median ferritin, Models 3 and 4 adjustments pushed the HR slightly >1, resulting in a weak J-shaped association.
In Japan we observed a positive monotonic association between ferritin and mortality in Figure 3C, Model 1, again with minimal impact on adjusting for anemia factors (Model 2). Similar to Europe, adjustment for recent hospitalization, nutrition and inflammation (including CRP) resulted in attenuation of the high (versus median) ferritin and mortality association and a weak J-shape with slightly elevated mortality at the lowest ferritin levels (Model 5).
DISCUSSION
In this international study of >18 000 HD patients, we observed that both facility ferritin targets and patient ferritin levels were highest in the USA, followed by Europe and Japan. Relatively high ferritin levels in each region were associated with higher mortality rates in a model minimally adjusted for case mix. In each region, adjustment for markers of nutrition and inflammation (Model 3), particularly for CRP in Europe and Japan (Model 4), attenuated this association more than adjustment for anemia measures and treatments (Model 2). After adjustment for all measured confounders, the ferritin–mortality association was positive monotonic in the USA and somewhat J-shaped in Europe (nadir ∼500 ng/mL) and Japan (nadir ∼175 ng/mL), where patients with the lowest ferritin levels had slightly higher (∼10%) mortality rates compared with the region-specific medians.
While nutrition, inflammation and recent prior hospitalization appear to play a large role in explaining the ferritin–mortality relation, it was not to the degree observed by Kalantar-Zadeh et al. [8]. Discrepancies between that study [8], using a 2001–03 cohort, and our US results may be due in part to differences in anemia management practices across time, patient mix, exposure measurement (baseline versus time-varying ferritin) and/or covariate adjustment.
In our European cohort, the positive monotonic association between ferritin and mortality at ferritin levels >500 ng/mL was comparable to our US results at the same levels of adjustment. Because adjustment for CRP and blood transfusions (not available in US data) further attenuated this association, we speculate that the observed ferritin–mortality relation in the USA may be exaggerated by our inability to more fully account for inflammation as in Europe and Japan, with the true association likely flatter than that shown in Figure 3A, Model 3.
Three recent multicenter studies of Japanese HD patients have explored the association between ferritin and mortality [40–42]. Maruyama et al. [40] found an elevated mortality rate only among HD patients in their highest decile of serum ferritin (>496 ng/mL). Kuragano et al. [41] found a higher rate of infection and cerebrocardiovascular disease (but not all-cause mortality) among patients with high ferritin; however, because high ferritin was defined as >100 ng/mL, the observed elevated mortality rate may have been driven by patients with ferritin levels far exceeding 100 ng/mL. A recent analysis of Japan DOPPS data focused on effect modification of the ferritin–mortality association by markers of inflammation [42]. The results showed a U-shaped association between ferritin and all-cause mortality, with the 50–99 ng/mL ferritin group having the best survival, but only among noninflamed patients. Our findings showed the lowest adjusted mortality rate was at ∼100–250 ng/mL, though precision was low in Japan due to fewer events.
To bolster hemoglobin levels while avoiding the potential risk (and often higher cost) of high ESA doses, many HD facilities have increased IV iron dosing, often in the form of a bolus dose spread over 5–10 HD sessions or a single, large-dose iron preparation [43], raising the question of whether ESA toxicity is being replaced with iron toxicity [44]. Results of large multicenter studies of the effect of IV iron dosing on mortality have been mixed [7–11], and the ongoing Proactive IV irOn Therapy in haemodiALysis patients (PIVOTAL) clinical trial in the UK [45] compares clinical outcomes among dialysis patients assigned to higher-versus lower-dose IV iron regimens. If an IV iron effect on mortality exists, we would expect that the association between high ferritin levels, a marker of iron stores, and adverse events would be confounded by a high IV iron dose. However, we observed a minimal impact of adjustment for anemia management parameters on the ferritin–mortality association, suggesting that any effect of high IV iron dose on survival may be minimally related to body iron stores. Alternatively, serum ferritin may be an exceedingly poor marker of body iron stores in common clinical practice, particularly when only measured every 3 months. As others have suggested, the utility of high ferritin as a biomarker of any one particular condition may be limited [13–17, 22, 23]. Because ferritin levels are affected by a variety of clinical conditions and treatments, it is perhaps not surprising to observe differential associations with mortality across regions where the prevalence of these conditions and treatments vary widely.
A limitation of this study is that inflammation cannot be directly observed. We relied on proxy variables such as serum albumin, WBC count and CRP, which in combination may not fully reflect a patient’s inflammation status. Results may also be biased by other unmeasured confounders. Although this bias may be greatest in the USA, where transfusions and CRP data are missing, the impact of these variables observed in other regions can provide an approximation of what we might expect to observe in the USA. Another potential unmeasured confounder is cumulative IV iron dose on dialysis, which is unavailable for the majority of DOPPS patients due to the lack of data on IV iron dosing before DOPPS enrollment. If the impact of IV iron dosing is related more to cumulative than short-term exposure, then the effect may not be well captured in our 3- to 4-month window of observation. To help alleviate this potential bias, we adjusted for dialysis vintage, a strong correlate of cumulative IV iron dose, and the interaction between IV iron dose and vintage. Finally, a limitation of analyzing a single ferritin value for each patient is the potential for misclassification due to the high degree of within-patient variability of ferritin levels [22, 23]. This misclassification is most likely nondifferential, which would probably lead to misclassification bias toward the null.
To our knowledge, we are the first to explore the ferritin–mortality relation in the postbundle [27] era when ferritin levels >800 ng/mL are common in the USA. In total, >90% of US DOPPS facilities—and >50% of European DOPPS facilities—had upper ferritin targets exceeding the KDIGO guideline of 500 ng/mL. Other strengths of our study include a large sample size, international data collected with a standardized protocol, careful consideration of potential confounders and use of restricted cubic splines to explore the functional form of the ferritin–mortality association more accurately and precisely than prior publications.
In this study, high ferritin level was consistently associated with elevated mortality in each region in minimally adjusted models. While both inflammation and anemia management practices affect ferritin levels, the association between high ferritin and mortality was attenuated more by adjustment for markers of malnutrition and inflammation than by hemoglobin levels, IV iron and ESA doses. The residual associations between high ferritin and mortality may reflect effects of inflammation or iron not explained by measured variables. High serum ferritin is often used to limit iron repletion therapy, based on safety concerns [3], but our results suggest that the utility of high ferritin as a biomarker for clinical risk due to excess iron stores is limited. However, based on the positive ferritin–mortality association remaining after adjustment, caution regarding IV iron dosing to higher upper ferritin targets may remain warranted. Research to resolve criteria for iron dosing, and whether optimal anemia management strategies differ internationally, is still needed.
FUNDING
The DOPPS program is supported by Amgen, Kyowa Hakko Kirin and Baxter Healthcare. Additional support for specific projects and countries is provided by Amgen, AstraZeneca, European Renal Association–European Dialysis & Transplant Association, German Society of Nephrology, Hexal, Janssen, Japanese Society for Peritoneal Dialysis, Keryx, Proteon, Relypsa, Roche, Società Italiana di Nefrologia, Spanish Society of Nephrology and Vifor Fresenius Medical Care Renal Pharma. Public funding and support is provided for specific DOPPS projects, ancillary studies or affiliated research projects by Agence Nationale de la Recherche (France); Canadian Institutes of Health Research and Ontario Renal Network (Canada); National Health & Medical Research Council (Australia); National Institute for Health Research via the Comprehensive Clinical Research Network (UK); National Institutes of Health and Patient-Centered Outcomes Research Institute (USA) and Thailand Research Foundation, Chulalongkorn University Matching Fund, King Chulalongkorn Memorial Hospital Matching Fund and the National Research Council of Thailand (Thailand). All support is provided without restrictions on publications.
AUTHORS’ CONTRIBUTIONS
A.K., H.M., R.L.P., J.Z., L.C.L., F.K.P. and B.M.R. provided the research idea and study design. A.K., R.L.P., F.K.P., B.M.R. were responsible for data acquisition. A.K., H.M., R.L.P., J.Z., R.V., S.H.J., M.I., L.C.L., F.K.P. and B.M.R. were responsible for data analysis and interpretation. A.K., H.M., R.L.P., J.Z., F.K.P. and B.M.R. were responsible for statistical analysis. H.M., R.L.P., R.V., S.H.J., M.I., F.K.P., B.M.R. were responsible for supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
A.K. is a senior research analyst for the nonprofit research organization Arbor Research Collaborative for Health, which designed and carries out the DOPPS program. Grants are made to Arbor Research Collaborative for Health and not to individual investigators. H.M. is a consultant at the nonprofit research organization Arbor Research Collaborative for Health, which designed and carries out the DOPPS program. R.L.P. is a senior research scientist for the nonprofit research organization Arbor Research Collaborative for Health, which designed and carries out the DOPPS program. J.Z. is a research scientist for the nonprofit research organization Arbor Research Collaborative for Health, which designed and carries out the DOPPS program. M.I. has relationships with the following speakers bureaus: Bayer Yakuhin, Daiichi Snakyo, Kyowa-kirin, Mitsubishi Tanabe Pharma, AsahiKASEI Pharma, Chugai Pharmaceutical, Pfizer Japan, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, MSD Japan and Ono Pharmaceutical. L.C.L. is employed by Keryx Biopharmaceuticals. B.M.R. is the Vice President of Clinical Research for the nonprofit research organization Arbor Research Collaborative for Health, which designed and carries out the DOPPS program. All other authors have declared no conflicts of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Supplementary Material
REFERENCES
- 1. Locatelli F, Pisoni RL, Akizawa T. et al. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns Study (DOPPS) findings. Am J Kidney Dis 2004; 44: 27–33 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int 2017; 7: e1–335 [Google Scholar]
- 3. Macdougall IC, Bircher AJ, Eckardt K. et al. Iron management in chronic kidney disease: conclusions from a ‘Kidney Disease: Improving Global Outcomes’ (KDIGO) Controversies Conference. Kidney Int 2016; 89: 28–39 [DOI] [PubMed] [Google Scholar]
- 4. Dwyer JP. We give too much intravenous iron. Semin Dial 2016; 29: 309–311 [DOI] [PubMed] [Google Scholar]
- 5. Hung S, Tarng D.. ESA and iron therapy in chronic kidney disease: a balance between patient safety and hemoglobin target. Kidney Int 2014; 86: 676–678 [DOI] [PubMed] [Google Scholar]
- 6. Charytan DM, Pai AB, Chan CT. et al. Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol 2015; 26: 1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bailie GR, Larkina M, Goodkin DA. et al. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int 2015; 87: 162–168 [DOI] [PubMed] [Google Scholar]
- 8. Kalantar-Zadeh K, Regidor DL, McAllister CJ. et al. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol 2005; 16: 3070–3080 [DOI] [PubMed] [Google Scholar]
- 9. Miskulin DC, Tangri N, Bandeen-Roche K. et al. Intravenous iron exposure and mortality in patients on hemodialysis. Clin J Am Soc Nephrol 2014; 9: 1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feldman HI, Joffe M, Robinson B. et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc Nephrol 2004; 15: 1623–1632 [DOI] [PubMed] [Google Scholar]
- 11. Brookhart MA, Schneeweiss S, Avorn J. et al. Comparative mortality risk of anemia management practices in incident hemodialysis patients. JAMA 2010; 303: 857–864 [DOI] [PubMed] [Google Scholar]
- 12. Rostoker G, Griuncelli M, Loridon C. et al. Reassessment of iron biomarkers for prediction of dialysis iron overload: an MRI study. PLoS One 2015; 10: e0132006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH.. The fascinating but deceptive ferritin: to measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 2006; 1:(Suppl 1) S9–S18 [DOI] [PubMed] [Google Scholar]
- 14. Singh AK, Coyne DW, Shapiro W. et al. Predictors of the response to treatment in anemic hemodialysis patients with high serum ferritin and low transferrin saturation. Kidney Int 2007; 71: 1163–1171 [DOI] [PubMed] [Google Scholar]
- 15. Richardson D, Hodsman A, van Schalkwyk D. et al. Management of anaemia in haemodialysis and peritoneal dialysis patients (chapter 8). Nephrol Dial Transplant 2007; 22(Suppl 7): vii78–vii104 [DOI] [PubMed] [Google Scholar]
- 16. Ferrari P, Kulkarni H, Dheda S. et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hamano T, Fujii N, Hayashi T. et al. Thresholds of iron markers for iron deficiency erythropoiesis—finding of the Japanese nationwide dialysis registry. Kidney Int Suppl 2015; 5: 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalantar-Zadeh K, Don BR, Rodriguez RA. et al. Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis 2001; 37: 564–572 [PubMed] [Google Scholar]
- 19. Kalantar-Zadeh K, Rodriguez RA, Humphreys MH.. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant 2004; 19: 141–149 [DOI] [PubMed] [Google Scholar]
- 20. Rambod M, Kovesdy CP, Kalantar-Zadeh K.. Combined high serum ferritin and low iron saturation in hemodialysis patients: the role of inflammation. Clin J Am Soc Nephrol 2008; 3: 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bazeley J, Bieber BA, Li Y. et al. C-reactive protein and prediction of 1-year mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol 2011; 6: 2452–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ford BA, Coyne DW, Eby CS et al.. Variability of ferritin measurements in chronic kidney disease; implications for iron management. Kidney Int 2009; 75: 104–110 [DOI] [PubMed] [Google Scholar]
- 23. Van Wyck DB, Alcorn H Jr, Gupta R.. Analytical and biological variation in measures of anemia and iron status in patients treated with maintenance hemodialysis. Am J Kidney Dis 2010; 56: 540–546 [DOI] [PubMed] [Google Scholar]
- 24. Fuller DS, Pisoni RL, Bieber BA. et al. The DOPPS practice monitor for US dialysis care: trends through December 2011. Am J Kidney Dis 2013; 61: 342–348 [DOI] [PubMed] [Google Scholar]
- 25. Fuller DS, Pisoni RL, Bieber BA. et al. The DOPPS practice monitor for US dialysis care: update on trends in anemia management 2 years into the bundle. Am J Kidney Dis 2013; 62: 1213–1220 [DOI] [PubMed] [Google Scholar]
- 26. Karaboyas A, Zee J, Morgenstern H. et al. Understanding the recent increase in ferritin levels in U.S. dialysis patients: potential impact of changes in IV iron and ESA dosing. Clin J Am Soc Nephrol 2015; 10: 1814–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; end-stage renal disease prospective payment system. Final rule. Fed Regist 2010; 75: 49029–49214 [PubMed] [Google Scholar]
- 28. Kliger AS, Foley RN, Goldfarb DS. et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis 2013; 62: 849–859 [DOI] [PubMed] [Google Scholar]
- 29. Locatelli F, Covic A, Eckardt KU. et al. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant 2009; 24: 348–354 [DOI] [PubMed] [Google Scholar]
- 30. Locatelli F, Bárány P, Covic A. et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 31. Tsubakihara Y, Nishi S, Akiba T. et al. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial 2010; 14: 240–275 [DOI] [PubMed] [Google Scholar]
- 32. Bailie GR, Larkina M, Goodkin DA. et al. Variation in intravenous iron use internationally and over time: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2013; 28: 2570–2579 [DOI] [PubMed] [Google Scholar]
- 33. Birnie K, Caskey F, Ben-Shlomo Y. et al. Erythropoiesis-stimulating agent dosing, haemoglobin and ferritin levels in UK haemodialysis patients 2005–13. Nephrol Dial Transplant 2017; 32: 692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.2012 Annual Report of the Dialysis Outcomes and Practice Patterns Study: Hemodialysis Data 1997-2011. Arbor Research Collaborative for Health, Ann Arbor, MI. dopps.org/annualreport/ (29 July 2016, date last accessed)
- 35. Young EW, Goodkin DA, Mapes DL. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 2000; 57(Suppl 74): S74–S81 [DOI] [PubMed] [Google Scholar]
- 36. Pisoni RL, Gillespie BW, Dickinson DM. et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 2004; 44: 7–15 [DOI] [PubMed] [Google Scholar]
- 37. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer, 2001 [Google Scholar]
- 38. Raghunathan TE, Solenberger PW, Van Hoewyk J.. IVEware: Imputation and Variance Estimation Software: User Guide. Ann Arbor, MI: Institute for Social Research, University of Michigan, 2002 [Google Scholar]
- 39. Little RJA, Rubin DB.. Statistical Analysis with Missing Data. New York: Wiley, 1987 [Google Scholar]
- 40. Maruyama Y, Yokoyama K, Yokoo T. et al. The different association between serum ferritin and mortality in hemodialysis and peritoneal dialysis patients using Japanese nationwide dialysis registry. PLoS One 2015; 10: e0143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuragano T, Matsumura O, Matsuda A. et al. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int 2014; 86: 845–854 [DOI] [PubMed] [Google Scholar]
- 42. Shoji T, Niihata K, Fukuma S. et al. Both low and high serum ferritin levels predict mortality risk in hemodialysis patients without inflammation. Clin Exp Nephrol 2017; 21: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Airy M, Mandayam S, Mitani AA. et al. Comparative outcomes of predominant facility-level use of ferumoxytol versus other intravenous iron formulations in incident hemodialysis patients. Nephrol Dial Transplant 2015; 30: 2075–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slotki I, Cabantchi ZI.. The labile side of iron supplementation in CKD. J Am Soc Nephrol 2015; 26: 2612–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. European Union Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/trial/2013-002267-25/GB and http://www.kidneyresearchuk.org/pivotal (10 August 2016, date last accessed)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.