Highlights
-
•
The threshold tracking transcranial magnetic stimulation [TMS] technique provides valuable insights into ALS pathogenesis.
-
•
Cortical hyperexcitability identifies the disease site origin and subsequent spread.
-
•
Threshold tracking TMS holds great promise as an ALS biomarker.
Keywords: Amyotrophic lateral sclerosis, Cortical hyperexcitability, Threshold tracking TMS
Abstract
Upper motor neuron [UMN] and lower motor neuron [LMN] dysfunction, in the absence of sensory features, is a pathognomonic feature of amyotrophic lateral sclerosis [ALS]. Although the precise mechanisms have yet to be elucidated, one leading hypothesis is that UMN precede LMN dysfunction, which is induced by anterograde glutamatergic excitotoxicity. Transcranial magnetic stimulation (TMS) is a neurophysiological tool that provides a non-invasive and painless assessment of cortical function. Threshold tracking methodologies have been recently adopted for TMS, whereby changes in threshold rather than motor evoked potential (MEP) amplitude serve as outcome measures. This technique is reliable and provides a rapid assessment of cortical function in ALS. Utilisng the threshold tracking TMS technique, cortical hyperexcitability was demonstrated as an early feature in sporadic ALS preceding the onset of LMN dysfunction and possibly contributing to disease spread. Separately, cortical hyperexcitability was reported to precede the clinical onset of familial ALS. Of further relevance, the threshold tracking TMS technique was proven to reliably distinguish ALS from mimicking disorders, even in the presence of a comparable degree of LMN dysfunction, suggesting a diagnostic utility of TMS. Taken in total, threshold tracking TMS has provided support for a cortical involvement at the earliest detectable stages of ALS, underscoring the utility of the technique for probing the underlying pathophysiology. The present review will discuss the physiological processes underlying TMS parameters, while further evaluating the pathophysiological and diagnostic utility of threshold tracking TMS in ALS.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by dysfunction of the upper and lower motor neuron compartments of the human nervous system, with relentless disease progression (Ravits et al., 2007, Ravits and La Spada, 2009, Kiernan et al., 2011, Geevasinga et al., 2016c, Eisen et al., 2017). Fundamental to the understanding of ALS pathophysiology is the relationship between upper and lower motor neuron dysfunction. In terms of disease origin, it has been hypothesised that ALS originates at a cortical level, with corticomotoneurons mediating lower motor neuron degeneration through a glutamatergic excitotoxic mechanism (Eisen et al., 1992, Vucic et al., 2008; Kiernan et al., 2011, Menon et al., 2014, Menon et al., 2015b, Eisen et al., 2017). In contrast, others have proposed a primacy of lower motor neuron dysfunction with upper motor neuron dysfunction occurring as a secondary phenomenon (Williamson and Cleveland, 1999, Fischer et al., 2004, Boillee et al., 2006, Pun et al., 2006). A third school of thought proposed that the upper and lower motor neuron degeneration occurs independently with disease spread evolving in a contiguous and random pattern, conforming to the underlying neuronal anatomy (Ravits et al., 2007, Ravits and La Spada, 2009).
Resolving the relationship between upper and lower motor neuron dysfunction, particularly the site of disease onset, could be important for the understanding of ALS pathogenesis. In turn, such concepts would potentially enable identification of novel therapeutic targets and lead to development of treatment strategies for ALS. This review will critically analyse the physiological processes underlying TMS, incorporating discussion about the pathophysiological and diagnostic utility of threshold tracking TMS in ALS, focussing on the potential of the technique as an outcome biomarker in a clinical trial setting.
1.1. Pathophysiological insights provided by paired-pulse threshold tracking TMS
Paired-pulse TMS techniques are invaluable for assessing cortical excitability in neurological diseases (Vucic et al., 2013b, Rossini et al., 2015, Ziemann et al., 2015, Vucic and Kiernan, 2017). With implementation of the paired-pulse TMS paradigm the following TMS biomarkers have emerged; short interval intracortical inhibition (SICI), intracortical facilitation (ICF), short interval intracortical facilitation (SICF) and long interval intracortical inhibition (LICI). It has been reported that the above mentioned TMS biomarkers exhibit utility in the understanding of ALS pathogenesis, diagnosis and clinical research (Vucic et al., 2013b, Rossini et al., 2015).
1.2. Physiology of SICI and ICF
Short interval intracortical inhibition and ICF are generated by delivering a subthreshold conditioning stimulus at pre-determined time interval before a test stimulus (Kujirai et al., 1993, Nakamura et al., 1997, Hanajima et al., 1998, Vucic et al., 2006). In the original technique, the strength of the conditioning and test stimuli were kept constant and changes in the motor evoked potential (MEP) amplitude were measured (Kujirai et al., 1993, Ziemann et al., 1996c, Nakamura et al., 1997). With interstimulus interval (ISI) set between 1 and 5 ms, the test MEP response is inhibited, a phenomenon referred to as SICI. Increasing the ISI to between 7 and 30 ms increases the test MEP amplitude, termed ICF (Chen et al., 2008).
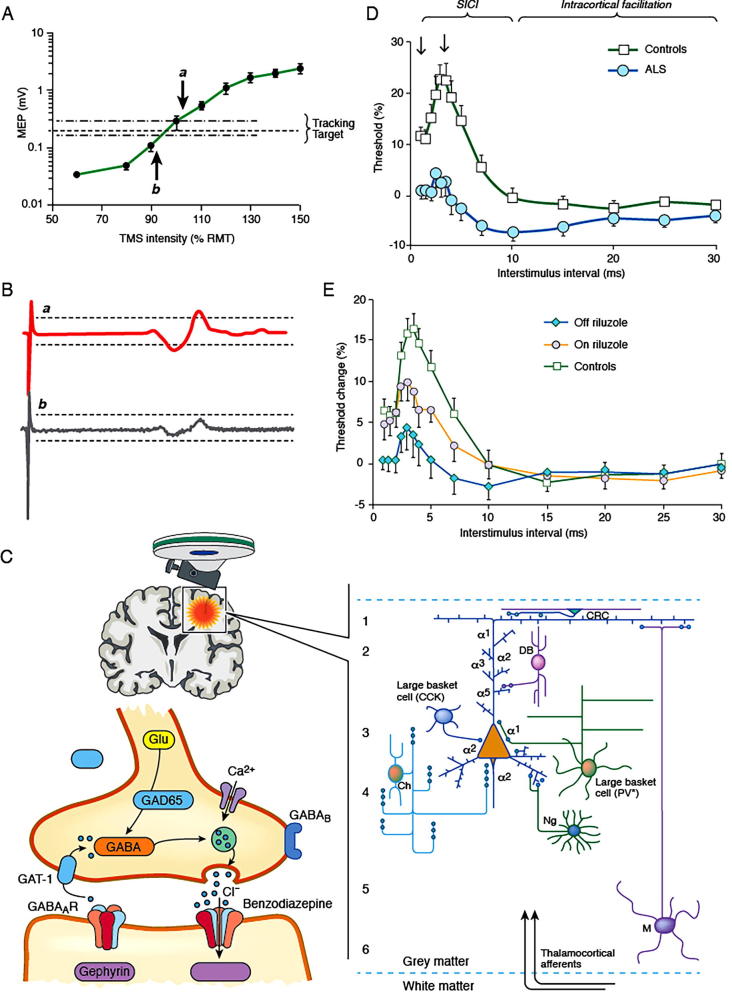
A potential limitation of the “constant stimulus” technique pertains to marked variability in MEP amplitudes with consecutive stimuli (Kiers et al., 1993, Hanajima et al., 1998). In order to overcome this limitation, a threshold tracking technique was developed, whereby an MEP response of fixed amplitude (0.2 mV ± 20%) is tracked by a test stimulus (Fig. 1A, B) (Fisher et al., 2002, Vucic et al., 2006). Consequently, SICI is reflected by an increase in test stimulus current required to produce and maintain the fixed MEP target when compared to the unconditioned stimulus, while ICF is represented by the converse [Fig. 1D]. Utility of the threshold tracking technique has been well established in healthy subjects and human disease (Vucic et al., 2013b, Vucic and Kiernan, 2017). Moreover, a recent study has reported a greater reliability of the threshold tracking technique when compared to the constant stimulus method (Samusyte et al., 2018).
Fig. 1.
(A) The tracking target of 0.2 mV ( ± 20%) lies in the steepest portion of the stimulus response curve (green color), such that a large variation in motor evoked potential (MEP) amplitude translates to a small variation in transcranial magnetic stimulation (TMS) intensity. (B) When the MEP amplitude is larger than the tracking target (a) the TMS intensity is reduced on subsequent stimulus, while when MEP is smaller (b) the TMS intensity is increased. (C) SICI is mediated by inhibitory GABAergic interneurons located within the primary motor cortex, mostly layers 2 and 3, projecting onto pyramidal cells. (D) Short interval intracortical inhibition (SICI) is reflected by conditioned-test stimulus intensity being greater than zero, while the converse is true for intracortical facilitation (ICF). In amyotrophic lateral sclerosis (ALS) there is a significant reduction in SICI and an increase in ICF, indicative of cortical hyperexcitability. (E) Treatment with the anti-glutamatergic agent riluzole partially normalizes SICI in ALS patients.
Although the precise physiological processes underlying SICI and ICF remain to be fully elucidated, it is now widely accepted that these TMS parameters reflect a balance between inhibitory and facilitatory cortical circuits projecting onto the pyramidal output tracts (Di Lazzaro et al., 2008, Ni and Chen, 2008, Rusu et al., 2014). Recordings of descending corticospinal volleys by high cervical epidural electrodes have provided evidence for a cortical basis of both SICI and ICF (Nakamura et al., 1997, Di Lazzaro et al., 1998). The development of SICI was associated with a reduction in frequency and amplitude of later indirect (I3) waves, but not earlier I1 waves, suggesting that SICI is mediated by activation of intracortical circuits rather than direct excitability of pyramidal neurons (Hanajima et al., 1998). Neuropharmacological studies have suggested that SICI is predominantly mediated by the activity of GABAergic interneuronal circuits acting via GABAA receptors [Fig. 1C] (Ziemann et al., 1996b). In addition, dopamine agonists increase while dopamine antagonists and noradrenergic agonists reduced SICI (Ziemann et al., 1996a, Ziemann et al., 1997a, Ilic et al., 2003, Korchounov and Ziemann, 2011). Recently, cortical modelling studies have indicated that interneuronal circuits located in layers 2 and 3 of the motor cortex were important for SICI generation (Rusu et al., 2014).
Two phases of SICI have been identified using the threshold tracking technique, a smaller phase at ISI ≤ 1 ms and larger peak at ISI “2.5–3 ms” [Fig. 1C] (Fisher et al., 2002, Vucic et al., 2006, Vucic et al., 2009, Vucic et al., 2011a). Synaptic processes acting via GABAergic neuronal circuits (GABAA receptor mediated) were postulated to mediate the second phase of SICI (Ziemann et al., 1996b, Di Lazzaro et al., 2000, Ilic et al., 2002, Di Lazzaro et al., 2006). The precise mechanisms underlying the first phase of SICI remains uncertain. Relative refractoriness of cortico-cortical axons (Chan et al., 2002, Fisher et al., 2002, Hanajima et al., 2003), as well as synaptic processes distinct to those that mediating the later phases of SICI (Roshan et al., 2003, Vucic et al., 2009), have been proposed.
Intracortical facilitation is measured using an identical conditioning-test stimulus paradigm as SICI, although the interstimulus intervals range from 8 to 30 ms (Kujirai et al., 1993, Vucic et al., 2006). While the physiological process underlying ICF remain to be fully clarified, ICF appears to reflect the activity of excitatory motor cortical circuits that are physiologically distinct from those mediating SICI (Ziemann et al., 1996c). This notion is supported by a higher threshold for activation and the dependence of ICF on the direction of current flow in the motor cortex (Ziemann et al., 1996c). In contrast, others have suggested that the tail end of SICI contributes to net facilitation of ICF (Hanajima et al., 1998). At a neuropharmacological level, ICF is reduced by GABA agonists and glutamatergic antagonists, while noradrenergic agonists increase ICF (Inghilleri et al., 1996, Ziemann et al., 1996b, Schwenkreis et al., 1999, Boroojerdi et al., 2001, Plewnia et al., 2001, Plewnia et al., 2002, Ziemann et al., 2015).
1.3. Physiology of SICF
Short interval intracortical facilitation is generated by a conditioning stimulus set to near or above threshold, preceding a test stimulus set to threshold intensity (Tokimura et al., 1996, Ziemann et al., 1996c). The SICF develops over ISIs of 1–5 ms with three distinct peaks reported at 1–1.5 ms, 2.4–2.9 and greater than 4.5 ms (Tokimura et al., 1996, Ziemann et al., 1996c, Chen and Garg, 2000). The threshold tracking TMS technique was recently adapted to generate SICF, revealing two distinct peaks at ISIs of 1.5 and 3 ms (Van den Bos et al., 2018b). A cortical origin for SICF has been proposed, with a facilitatory interaction of I-waves produced by conditioning and test stimuli postulated as a likely mechanism (Ziemann et al., 1998, Di Lazzaro et al., 1999). Separately, disinhibition of inhibitory neuronal circuits has also been suggested as a potential physiological mechanism (Wagle-Shukla et al., 2009, Ziemann et al., 2015). A recent study argued against the latter possibility and suggested that the discordant conclusions may relate to differences in sample size, methodologies or variations in the anatomical arrangements of interneuronal circuits underlying SICF and SICI (Van den Bos et al., 2018b).
1.4. Physiology of LICI
Long interval intracortical inhibition is generated by delivering a suprathreshold conditioning stimulus 50–200 ms before a test stimulus (Valls-Sole et al., 1992, Nakamura et al., 1997). The magnitude of LICI correlates with the strength of the conditioning stimulus (Valls-Sole et al., 1992). LICI can also be assessed by using the threshold tracking technique: LICI then develops over ISIs 50–300 ms and peaks at 150 ms (Vucic et al., 2006).
Neuropharmacological studies have suggested that LICI is mediated by long latency inhibitory post synaptic potentials acting via GABAB receptors (Werhahn et al., 1999). Although LICI and SICI appear to me mediated by distinct cell populations, LICI exerts a suppressive effect on SICI through presynaptic GABAB receptors (Sanger et al., 2001).
1.5. Cortical inhibition and facilitation in ALS
In sporadic ALS patients, a reduction or absence of SICI and an increase in ICF [Fig. 1C] have been reported, all indicative cortical hyperexcitability (Hanajima et al., 1996, Yokota et al., 1996, Ziemann et al., 1997b, Sommer et al., 1999, Stefan et al., 2001, Zanette et al., 2002b, Vucic et al., 2006, Vucic et al., 2008, Blair et al., 2010). The reduction of SICI has been reported as an early and specific feature of ALS, correlating with biomarkers of lower motor neuron dysfunction (Vucic et al., 2006), and preceding the onset of detectable LMN dysfunction in sporadic ALS cohorts (Menon et al., 2015b). Furthermore, reduction of SICI was associated with specific clinical features of ALS such as the split hand and split hand-plus signs (Bae et al., 2014, Menon et al., 2014), as well as contributing to the pattern of disease spread (Menon et al., 2017). Importantly, the reduction of SICI was reported to be an adverse prognostic factor in ALS (Shibuya et al., 2016), suggesting a potential role for SICI as a prognostic biomarker in ALS.
Separately, SICI is partially normalized by riluzole (Vucic et al., 2013a), an anti-glutamatergic agent exhibiting modest clinical effectiveness in ALS [Fig. 1E] (Bensimon et al., 1994, Lacomblez et al., 1996). This modulating effect lasts approximately 3 months (Geevasinga et al., 2016b), paralleling the clinical efficacy of the drug, and may be related to overexpression of efflux pumps at the blood brain barrier during the disease course (Kuo, 2009). Irrespective of the underlying mechanisms, riluzole studies have suggested a utility of threshold tracking TMS in assessing biological effectiveness of compounds at an early stage of drug development so as to avoid unnecessary and costly Phase 3 trials.
In addition to the pathophysiological insights provided in classical ALS, threshold tracking TMS has established the presence of cortical hyperexcitability in atypical ALS phenotypes. Specifically, reduced SICI and increased ICF have been documented in the flail arm and leg variants of ALS, as well as primary lateral sclerosis (Vucic and Kiernan, 2007, Geevasinga et al., 2015b, Menon et al., 2016). Importantly, cortical hyperexcitability correlated with neurodegeneration, underscoring its importance in the pathogenesis of atypical ALS phenotypes.
Features of cortical hyperexcitability have also been reported in familial ALS cohorts, including phenotypes linked to mutations in the superoxide dismutase-1 (Vucic et al., 2008), fused in sarcoma (Williams et al., 2013) and c9orf72 genes (Geevasinga et al., 2015a), and were comparable to findings in sporadic ALS patients. Significant correlations between cortical hyperexcitability and peripheral neurodegeneration have also been established (Vucic et al., 2010, Geevasinga et al., 2015a). Interestingly, asymptomatic mutation carriers exhibited normal cortical function (Vucic et al., 2008, Geevasinga et al., 2015a), with features of hyperexcitability preceding the clinical development of familial ALS by months (Vucic et al., 2008).
Findings in sporadic and familial ALS cohorts have supported the notion that ALS is mediated by a multistep process (Al-Chalabi et al., 2014), with cortical hyperexcitability (and specifically dysfunction of cortical interneuronal circuits) being potentially an important step in ALS pathogenesis. This view is supported by studies in the TDP-43A315T mouse model whereby hyperactivity of excitatory cortical interneurons underlies the development of hyperexcitability in cortical output tracts (Zhang et al., 2016). From a therapeutic perspective, focal ablation of excitatory interneuronal circuits may lead to normalization of excitability and potentially neuroprotection, a hypothesis that needs to be further assessed.
The notion that cortical dysfunction is a compensatory mechanism in response to motor neuron degeneration in ALS has been suggested (Zanette et al., 2002b). Given that ALS mimicking disorders, such as Kennedys disease, acquired neuromyotonia, demyelinating sensorimotor radiculopolyneuropathy and multifocal motor neuropathy with conduction block, exhibit normal cortical function, despite a comparable peripheral disease burden (Vucic et al., 2008, Vucic et al., 2010, Menon et al., 2015a), potentially argued against such a notion.
There has been a paucity of studies assessing the SICF changes in sporadic ALS. Recently, a significant increase in SICF was reported in ALS, and this increase was accompanied by reduction in SICI (Van den Bos et al., 2018a). The index of excitation, a novel neurophysiological biomarker of cortical excitability, was increased in ALS patients suggesting that overactivity of facilitatory circuits contribute to hyperexcitability. There was a significant correlation between this index of excitation and functional disability, underscoring the pathogenic importance of facilitatory circuit overactivity in ALS, thereby providing a potential therapeutic target.
Reduction or absence of LICI has been previously reported in sporadic ALS using the constant stimulus TMS technique (Zanette et al., 2002b, Zanette et al., 2002a). The reduction in LICI was accompanied by reductions in SICI, and both correlated with disease severity and the degree of upper motor neuron dysfunction. Degeneration of GABAergic cortical neurons was postulated to underlie these abnormalities of cortical inhibition and to form the pathogenic basis of ALS.
2. Single-pulse threshold tracking TMS
2.1. Physiology of motor thresholds (MT)
Motor thresholds reflect the excitability of corticomotoneurons located in the motor cortex (Rossini et al., 2015). Originally, the MT was defined as the minimum stimulus intensity required to elicit a small motor evoked potential (usually peak-peak ≥ 50 μV) in 50% of trials utilizing the constant stimulus technique (Rossini et al., 2015). Threshold-tracking TMS defines MT as the stimulus intensity required to elicit and maintain a target motor evoked potential (MEP) response of 200 μV (Awiszus et al., 1999, Fisher et al., 2002, Vucic et al., 2006, Groppa et al., 2012). From a physiological perspective, motor thresholds reflect the density of corticomotoneuronal (CM) projections onto the spinal or bulbar motor neuron, with MTs being lowest for intrinsic hand muscles due to a higher density of CM projections (Brouwer and Ashby, 1990, Macdonell et al., 1991, Chen et al., 1998). In addition, MTs are influenced by the TMS pulse width and waveform, such that longer and monophasic TMS pulse-widths, directed in a posterior-anterior direction, lead to lower MTs. These findings suggest that neuronal populations contributing to MT predominantly reside in the anterior bank of the central sulcus (Casula et al., 2018).
In addition to density of corticomotoneuronal projections, MTs are also modulated by a variety of pharmacological agents (Ziemann et al., 2015). Specifically, voltage-gated Na+ channel blocking agents, such as tegretol, dilantin or lamotrigine, increase MT, (Mavroudakis et al., 1994, Boroojerdi et al., 2001, Sommer et al., 2012, Lang et al., 2013), suggesting the importance of Na+ channel-mediated axonal excitability in determining motor thresholds. MT is decreased by enhancing glutamatergic neurotransmission via AMPA receptors (Di Lazzaro et al., 2003), but the effects on MT of other neurotransmitter systems and of voltage gated Ca2+ channels have been inconsistent (Ziemann et al., 2015). This underscores the complex physiology of motor thresholds.
2.2. Pathophysiological implications of motor thresholds in ALS
Although some studies have reported normal MTs (Caramia et al., 1991, Kohara et al., 1996, Mills and Nithi, 1997, Zanette et al., 2002b, Vucic et al., 2006, Vucic et al., 2008, Menon et al., 2017), a reduction in threshold may occur in ALS patients, being more prominent over the dominant motor cortex and the cortex that is contralateral to site of disease onset (Menon et al., 2017). Increased MTs or inexcitable motor cortices have also been documented in ALS (Eisen et al., 1990, Berardelli et al., 1991, Triggs et al., 1992, Miscio et al., 1999, Triggs et al., 1999, Urban et al., 2001, de Carvalho et al., 2003, Attarian et al., 2005). The variable findings may relate to the clinical and pathological heterogeneity of ALS, the stage at which the disease is assessed and rate of disease progression. Reduction in MTs have been documented in the early stages of ALS, and this reduction in MTs was associated with clinical features of profuse fasciculations, preserved muscle bulk and hyper-reflexia (Mills and Nithi, 1997, Eisen and Weber, 2001). These findings could also reflect the presence of spinal motor neuron hyperexcitability, with the reduction in cortical thresholds reflecting a smaller corticospinal volley required to activate the lower motor neuronal pool. With disease progression, a progressive increase in MTs ensues, eventually leading to motor cortex inexcitability (Mills and Nithi, 1997). However, a decrease in MT was shown to be an independent predictor of cognitive dysfunction in ALS (Agarwal et al., 2018).
2.3. Physiology of MEP amplitude
The MEP amplitude is measured from MEPs evoked by suprathreshold TMS stimuli and reflect a summation of descending corticospinal volleys consisting of D (direct) and I-waves (Amassian et al., 1987, Di Lazzaro et al., 1998, Rossini et al., 2015). The MEP amplitude increases with higher TMS stimulus intensity and the resulting stimulus-response curve follows a sigmoid function (Devanne et al., 1997). As with MT, the MEP amplitude reflects the density of corticomotoneuronal projections onto motor neurons, provided that spinal mechanisms are controlled (Ziemann, 2004). Typically, the MEP is expressed as a percentage of the maximum compound muscle action potential (CMAP) amplitude in order to control for peripheral nerve conduction, thereby providing insight into the percentage of the motor neurone pool activated in the MEP (Rossini et al., 2015). When compared to MT, the MEP amplitude may give insight into neurons that are less excitable or further away from the centre of the TMS induced electrical field (Chen et al., 2008).
Large inter-subject variability has been reported for MEP amplitudes, an observation attributed to fluctuations in neuronal excitability at the cortical and spinal cord levels (Rossini et al., 2015). The MEP amplitudes correlate with the power and phase of EMG and EEG in a frequency band around 18 Hz in wakeful states and up-phase of slow oscillations in non-REM sleep (Bergmann et al., 2012, Ferreri et al., 2014, Keil et al., 2014). In addition, the MEP amplitude is increased by muscle contraction, underscoring its role as an important biomarker of state dependency of cortical excitability.
As with motor thresholds, a variety of CNS neurotransmitter systems modulate MEP amplitudes (Ziemann et al., 2015). Specifically, GABAergic agonists reduce MEP amplitudes, while glutamatergic and noradrenergic agonists increase MEP amplitudes (Inghilleri et al., 1996, Boroojerdi et al., 2001, Plewnia et al., 2001, Plewnia et al., 2002, Di Lazzaro et al., 2003, Ilic et al., 2003). In contrast, modulators of voltage-gated Na+ channels did not exert any significant effects on MEP amplitude, indicating that the physiological processes mediating MEP amplitudes are independent to those underlying MT.
2.4. MEP amplitude and pathophysiological implications in ALS
Greater MEP amplitudes have been reported in sporadic and familial forms of ALS, being most prominent in early stages of the disease (Vucic et al., 2006, Vucic and Kiernan, 2007, Vucic et al., 2008, Menon et al., 2014, Geevasinga et al., 2015a, Menon et al., 2015a, Menon et al., 2015b, Geevasinga et al., 2017). The increase in MEP amplitude appears to be more prominent over the dominant motor cortex which is contralateral to the side of disease onset (Menon et al., 2017). This increase in MEP amplitude correlates with surrogate biomarkers of motor neuron degeneration (Vucic et al., 2006, Vucic et al., 2010). While cortical hyperexcitability could account for the bigger MEP amplitudes, the state of the spinal cord circuitry may also be an important modulator of MEP amplitudes in ALS patients. A descending TMS-induced volley would elicit a greater discharge in the setting of spinal motor neuron hyperexcitability. Consequently, the higher MEP amplitudes and lower cortical thresholds evident in ALS patients could also be mediated by spinal motor neuronal hyperexcitability. Separately, MEP amplitudes were normal in non-ALS mimic disorders, despite a comparable degree of lower motor neuron dysfunction, thereby arguing against the notion that cortical plasticity was responsible for increased MEP amplitudes in ALS (Vucic et al., 2008, Vucic et al., 2010, Menon et al., 2017).
2.5. Physiology of the cortical silent period (CSP) duration
The CSP refers to a period of electrical inactivity in the background voluntary EMG signal that occurs immediately after a TMS stimulus delivered over the contralateral motor cortex (Cantello et al., 1992). The CSP duration is measured from onset of the facilitated MEP to resumption of voluntary EMG activity and may vary from 100 to 300 ms, being dependent on the strength of the TMS stimulus (Cantello et al., 1992, Inghilleri et al., 1993, Triggs et al., 1993, Ziemann, 2004, Rossini et al., 2015). CSP duration appears to be determined by a combination of spinal inhibitory mechanisms and long latency inhibitory cortical processes. Specifically, the early part of the CSP duration (∼the first 50 ms) appears to be spinal in origin, mediated by activation of Renshaw cells and Ia inhibitory interneurons, as well as refractoriness of spinal motor neurons (Rossini et al., 2015). The long duration of the CSP is probably due to activation of cortical inhibitory neurons acting predominantly via GABAB receptors (Cantello et al., 1992, Inghilleri et al., 1993, Siebner et al., 1998, Chen et al., 1999, Werhahn et al., 1999), although a role for GABAA receptor function has also been reported at lower stimulus intensities (Werhahn et al., 1999, Stetkarova and Kofler, 2013). Separately, the CSP duration is longer in the upper limbs, suggesting an importance of the density of corticomotoneuronal projections onto motor neurons in regulating CSP (Chen et al., 2008).
2.6. Pathophysiological implication of reduced CSP duration in ALS
A reduction of CSP duration has been documented in sporadic and familial ALS phenotypes, and is most prominent in the early stages of the disease (Prout and Eisen, 1994, Desiato and Caramia, 1997, Siciliano et al., 1999, Zanette et al., 2002b, Mills, 2003, Vucic et al., 2006, Wittstock et al., 2007, Vucic et al., 2008, Vucic et al., 2010, Geevasinga et al., 2015a, Geevasinga et al., 2015b, Grieve et al., 2015, Geevasinga et al., 2017). Reduction in CSP duration has also been established in atypical ALS phenotypes, such as the flail arm and leg variants of ALS (Vucic and Kiernan, 2007, Menon et al., 2016). This reduction of CSP duration most likely represents degeneration and dysfunction of GABAergic inhibitory neurotransmission. As with other TMS parameters, the reduction of CSP duration is a specific feature of ALS in the context of neuromuscular diseases (Vucic et al., 2008, Vucic et al., 2010, Vucic et al., 2011b, Menon et al., 2015a).
2.7. Threshold tracking TMS, site of disease onset and patterns of disease spread
The site of ALS onset remains an important and unresolved issue in the understanding of ALS pathogenesis. Resolution of this quandary has implications for identifying appropriate therapeutic targets and treatment strategies (Geevasinga et al., 2016c). Jean Martin Charcot first published on the importance of UMN dysfunction in ALS pathogenesis (Charcot and Joffroy, 1869), a theory that was crystalized in the dying forward hypothesis some 100 years later (Eisen et al., 1992). Eisen and colleagues proposed that motor neuron degeneration was mediated by an anterograde excitotoxic mechanism (Eisen et al., 1992). This hypothesis was supported by clinical observations of dissociated wasting of intrinsic hand muscles (split-hand and split-hand plus phenomenon) in ALS (Wilbourn, 2000, Kuwabara et al., 2008, Menon et al., 2013, Menon et al., 2014). Importantly, cortical hyperexcitability appears to underlie the development of both the split-hand and split-hand plus phenomenon (Bae et al., 2014, Menon et al., 2014), underscoring the primacy of UMN dysfunction in ALS pathogenesis.
Cortical hyperexcitability has been identified as an early feature of sporadic ALS, correlating with biomarkers of motor neuron degeneration (Vucic et al., 2006). Furthermore, cortical hyperexcitability was shown to precede the development of detectible LMN dysfunction in sporadic ALS and the clinical onset of familial ALS phenotypes (Vucic et al., 2008, Menon et al., 2015b). While there remains a possibility that upper and lower motor neurons degenerate independently or that ALS begins at a peripheral level (muscle or neuromuscular junction as suggested by the dying-back hypothesis), threshold tracking TMS provides indirect support for the primacy of corticomotoneurons in ALS pathogenesis, namely the dying forward mechanism.
Separately, upper motor neuron dysfunction has been implicated in the patterns of disease spread in ALS. This notion is supported by clinical observations that limb dominance is a significant factor in underlying the site of disease onset in ALS (Turner et al., 2011, Devine et al., 2014). In addition, cortical hyperexcitability is more prominent over the motor cortex contralateral to the side of disease onset (Menon et al., 2017). Separately, a comparable degree of cortical hyperexcitability was evident in patients exhibiting contiguous and non-contiguous patterns of spread, suggesting the importance of cortical processes in mediating disease evolution in ALS.
The precise mechanisms by which cortical hyperexcitability contributes to ALS spread remain to be fully elucidated, although it’s likely to be complex and multifactorial (Geevasinga et al., 2016c). A number of previously implicated molecular processes, including oxidative stress, paracrine signalling, mitochondrial dysfunction, neuroimmunotoxicity, abnormalities of protein folding and transmembrane signalling, along with non-neuronal cell dysfunction and prion-like spread could be operative at the central nervous system level (Ravits and La Spada, 2009, Eisen et al., 2014). ALS appears to be a multi-step disease requiring 6 critical events for development (Al-Chalabi et al., 2014). Genetic mutations, epigenetic and environmental factors as well as dysfunction of molecular processes, appear to be potential steps (Al-Chalabi et al., 2014, Chio et al., 2018). Cortical hyperexcitability may be a critical final step in the pathogenic process for ALS. Contiguous and non-contiguous patterns of disease spread could be explained by development of hyperexcitability in specific corticomotoneurons and the motor cortex, with disease spread evolving along the neocortex and under direct control of corticofugal projections, namely the neurons that project outward from the primary motor cortex (Eisen et al., 2017). Future studies should assess the evolution of cortical excitability changes in sporadic and familial ALS cohorts, relating these cortical changes to clinical and anatomical patterns of disease spread by combining the threshold tracking TMS with TMS-EEG, quantitative and functional MRI techniques.
2.8. Diagnostic utility of threshold tracking TMS in ALS
The diagnosis of ALS relies on identifying concomitant upper and lower motor neuron signs, with evidence of rapid disease progression (Kiernan et al., 2011). Given the absence of a diagnostic test, clinically based criteria have been developed in order to facilitate an earlier diagnosis of ALS (Brooks, 1994, Brooks et al., 2000). The clinically based criteria have exhibited poor sensitivity, particularly in early stages of the disease (Turner et al., 2009, Costa et al., 2012, Geevasinga et al., 2016a, Geevasinga et al., 2016d). In order to increase the diagnostic yield, a neurophysiologically based Awaji-Shima criteria were proposed in 2006 (de Carvalho et al., 2008). While the Awaji criteria have a greater sensitivity (Costa et al., 2012, Geevasinga et al., 2016a, Geevasinga et al., 2016d), the major limitation pertained to the reliance on clinical assessment for identifying UMN dysfunction. In part, this limitation relates to difficulties in identifying UMN signs in ALS patients, especially in the setting of weak muscle and wasting (Higashihara et al., 2012, Swash, 2012).
The threshold tracking TMS technique has proven to be a robust and objective biomarker of UMN dysfunction in ALS (Vucic et al., 2011b, Geevasinga et al., 2014, Menon et al., 2015a). The presence of cortical dysfunction, as heralded by reduction of SICI or motor cortex inexcitability, reliably differentiated ALS from neuromuscular mimicking disorders, hastening the diagnosis of ALS by ∼8 months when compared to clinical criteria (Vucic et al., 2011b). Importantly, identification of cortical dysfunction enhances the diagnostic utility of the Awaji criteria by 34%, irrespective of disease stage or site of onset (Menon et al., 2015a). Furthermore, sub-clinical UMN dysfunction has been identified in atypical ALS phenotypes by utilising the threshold tracking TMS technique (Vucic and Kiernan, 2007, Menon et al., 2016), and thereby further aiding the diagnosis of ALS.
Combining threshold tracking TMS with quantitative neuroimaging (Magnetic Resonance imaging) techniques has increased the sensitivity of detecting UMN dysfunction in ALS (Grieve et al., 2015). Notably, the presence of cortical thinning in the precentral gyrus (motor cortex) along with features of cortical hyperexcitability, identified UMN dysfunction in 88% of ALS patients (Grieve et al., 2015). Future ALS diagnostic criteria may consider incorporating functional (TMS) and structural (MRI) biomarkers of UMN dysfunction as a means of enhancing the diagnosis. Such an approach may result in an earlier recruitment of patients into clinical trials, at a stage when neuroprotective therapies may be more efficacious (Eisen et al., 2014).
3. Conclusion
In conclusion, the threshold tracking TMS technique has provided invaluable pathophysiological insights in sporadic and familial ALS. Cortical hyperexcitability has been identified as an important feature in ALS, preceding the onset of neurodegeneration and correlating with biomarkers of motor neuronal loss. In addition, the site of disease onset and patterns of disease spread appear to be mediated by corticomotoneuronal hyperexcitability. Separately, the threshold tracking TMS technique has yielded a robust and objective biomarker of upper motor neuron dysfunction in ALS, leading to earlier diagnosis. Future studies incorporating threshold tracking TMS along with other physiological techniques, such as TMS-EEG, neuroimaging as well molecular and genetic techniques may shed further insights into the mechanisms triggering cortical dysfunction and potentially identifying much needed therapeutic targets.
Acknowledgments
Acknowledgements
Funding support from the National Health and Medical Research Council of Australia [Project grant numbers 510233, 1024915, 1055778, Program Grant #1037746] and Motor Neuron Disease Research Institute of Australia is gratefully acknowledged.
Declaration of interest
(i) SV report receiving honoraria from Merck Australia Pty Ltd, Sanofi-Aventis and CSL for work unrelated to the current manuscript; (ii) MvB, Pm JH and TD declare no conflict of interest; (iii) MCK declares editor responsibilities for Journal of Neurology, Neurosurgery and Psychiatry.
References
- Agarwal S., Highton-Williamson E., Caga J., Matamala J.M., Dharmadasa T., Howells J. Primary lateral sclerosis and the amyotrophic lateral sclerosis-frontotemporal dementia spectrum. J. Neurol. 2018;265:1819–1828. doi: 10.1007/s00415-018-8917-5. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A., Calvo A., Chio A., Colville S., Ellis C.M., Hardiman O. Analysis of amyotrophic lateral sclerosis as a multistep process: a population-based modelling study. Lancet Neurol. 2014;13:1108–1113. doi: 10.1016/S1474-4422(14)70219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian V.E., Stewart M., Quirk G.J., Rosenthal J.L. Physiological basis of motor effects of a transient stimulus to cerebral cortex. Neurosurgery. 1987;20:74–93. [PubMed] [Google Scholar]
- Attarian S., Azulay J.P., Lardillier D., Verschueren A., Pouget J. Transcranial magnetic stimulation in lower motor neuron diseases. Clin. Neurophysiol. 2005;116:35–42. doi: 10.1016/j.clinph.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Awiszus F., Feistner H., Urbach D., Bostock H. Characterisation of paired-pulse transcranial magnetic stimulation conditions yielding intracortical inhibition or I-wave facilitation using a threshold-hunting paradigm. Exp. Brain Res. 1999;129:317–324. doi: 10.1007/s002210050901. [DOI] [PubMed] [Google Scholar]
- Bae J.S., Menon P., Mioshi E., Kiernan M.C., Vucic S. Cortical hyperexcitability and the split-hand plus phenomenon: pathophysiological insights in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:250–256. doi: 10.3109/21678421.2013.872150. [DOI] [PubMed] [Google Scholar]
- Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Inghilleri M., Cruccu G., Mercuri B., Manfredi M. Electrical and magnetic transcranial stimulation in patients with corticospinal damage due to stroke or motor neurone disease. Electroencephalogr. Clin. Neurophysiol. 1991;81:389–396. doi: 10.1016/0168-5597(91)90028-v. [DOI] [PubMed] [Google Scholar]
- Bergmann T.O., Mölle M., Schmidt M.A., Lindner C., Marshall L., Born J. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J. Neurosci. 2012;32:243–253. doi: 10.1523/JNEUROSCI.4792-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair I.P., Williams K.L., Warraich S.T., Durnall J.C., Thoeng A.D., Manavis J. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J. Neurol. Neurosurg. Psychiatry. 2010;81:1286–1288. doi: 10.1136/jnnp.2009.194399. [DOI] [PubMed] [Google Scholar]
- Boillee S., Vande Velde C., Cleveland D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B., Battaglia F., Muellbacher W., Cohen L.G. Mechanisms influencing stimulus-response properties of the human corticospinal system. Clin. Neurophysiol. 2001;112:931–937. doi: 10.1016/s1388-2457(01)00523-5. [DOI] [PubMed] [Google Scholar]
- Brooks B. El escorial world federation of neurology criteria for the diagnosis of amyotrophic lateral sclerosis. subcommittee on motor neuron diseases/amyotrophic lateral sclerosis of the world federation of neurology research group on neuromuscular diseases and the el escorial “clinical limits of amyotrophic lateral sclerosis” workshop contributors. J. Neurol. Sci. 1994;124:96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brouwer B., Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr. Clin. Neurophysiol. 1990;76:509–519. doi: 10.1016/0013-4694(90)90002-2. [DOI] [PubMed] [Google Scholar]
- Cantello R., Gianelli M., Civardi C., Mutani R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 1992;42:1951–1959. doi: 10.1212/wnl.42.10.1951. [DOI] [PubMed] [Google Scholar]
- Caramia M.D., Cicinelli P., Paradiso C., Mariorenzi R., Zarola F., Bernardi G. 'Excitability changes of muscular responses to magnetic brain stimulation in patients with central motor disorders. Electroencephalogr. Clin. Neurophysiol. 1991;81:243–250. doi: 10.1016/0168-5597(91)90009-m. [DOI] [PubMed] [Google Scholar]
- Casula E.P., Rocchi L., Hannah R., Rothwell J.C. Effects of pulse width, waveform and current direction in the cortex: a combined cTMS-EEG study. Brain Stimul. 2018;11:1063–1070. doi: 10.1016/j.brs.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Chan J.H., Lin C.S., Pierrot-Deseilligny E., Burke D. Excitability changes in human peripheral nerve axons in a paradigm mimicking paired-pulse transcranial magnetic stimulation. J. Physiol. 2002;542:951–961. doi: 10.1113/jphysiol.2002.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charcot J., Joffroy A. Deux cas d'atrophie musculaire progressive avec lesion de la substance grise et des faisceaux antero-lateraux de la moelle epiniere. Arch. Physiol. Neurol. Pathol. 1869;2:744–754. [Google Scholar]
- Chen R., Cros D., Curra A., Di Lazzaro V., Lefaucheur J.P., Magistris M.R. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chen R., Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J. Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R., Lozano A.M., Ashby P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp. Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Chen R., Tam A., Butefisch C., Corwell B., Ziemann U., Rothwell J.C. Intracortical inhibition and facilitation in different representations of the human motor cortex. J. Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chio A., Mazzini L., D'Alfonso S., Corrado L., Canosa A., Moglia C. The multistep hypothesis of ALS revisited: the role of genetic mutations. Neurology. 2018;91:e635–e642. doi: 10.1212/WNL.0000000000005996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J., Swash M., de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis:a systematic review. Arch. Neurol. 2012;69:1410–1416. doi: 10.1001/archneurol.2012.254. [DOI] [PubMed] [Google Scholar]
- de Carvalho M., Dengler R., Eisen A., England J.D., Kaji R., Kimura J. Electrodiagnostic criteria for diagnosis of ALS. Clin. Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- de Carvalho M., Turkman A., Swash M. Motor responses evoked by transcranial magnetic stimulation and peripheral nerve stimulation in the ulnar innervation in amyotrophic lateral sclerosis: the effect of upper and lower motor neuron lesion. J. Neurol. Sci. 2003;210:83–90. doi: 10.1016/s0022-510x(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Desiato M.T., Caramia M.D. Towards a neurophysiological marker of amyotrophic lateral sclerosis as revealed by changes in cortical excitability. Electroencephalogr. Clin. Neurophysiol. 1997;105:1–7. doi: 10.1016/s0924-980x(96)96582-0. [DOI] [PubMed] [Google Scholar]
- Devanne H., Lavoie B.A., Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Devine M.S., Kiernan M.C., Heggie S., McCombe P.A., Henderson R.D. Study of motor asymmetry in ALS indicates an effect of limb dominance on onset and spread of weakness, and an important role for upper motor neurons. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:481–487. doi: 10.3109/21678421.2014.906617. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Meglio M., Cioni B., Tamburrini G., Tonali P. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin. Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Oliviero A., Profice P., Pennisi M.A., Pilato F., Zito G. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J. Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V., Pilato F., Dileone M., Ranieri F., Ricci V., Profice P. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J. Physiol. 2006;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V., Restuccia D., Oliviero A., Profice P., Ferrara L., Insola A. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp. Brain Res. 1998:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Rothwell J.C., Oliviero A., Profice P., Insola A., Mazzone P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to human motor cortex. Exp. Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V., Ziemann U., Lemon R.N. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 2008;1:345–362. doi: 10.1016/j.brs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Eisen A., Braak H., Del Tredici K., Lemon R., Ludolph A.C., Kiernan M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2017;88:917–924. doi: 10.1136/jnnp-2017-315573. [DOI] [PubMed] [Google Scholar]
- Eisen A., Kiernan M., Mitsumoto H., Swash M. Amyotrophic lateral sclerosis: a long preclinical period? J. Neurol. Neurosurg. Psychiatry. 2014;85:1232–1238. doi: 10.1136/jnnp-2013-307135. [DOI] [PubMed] [Google Scholar]
- Eisen A., Kim S., Pant B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron? Muscle Nerve. 1992;15:219–224. doi: 10.1002/mus.880150215. [DOI] [PubMed] [Google Scholar]
- Eisen A., Shytbel W., Murphy K., Hoirch M. Cortical magnetic stimulation in amyotrophic lateral sclerosis. Muscle Nerve. 1990;13:146–151. doi: 10.1002/mus.880130211. [DOI] [PubMed] [Google Scholar]
- Eisen A., Weber M. The motor cortex and amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:564–573. doi: 10.1002/mus.1042. [DOI] [PubMed] [Google Scholar]
- Ferreri F., Vecchio F., Ponzo D., Pasqualetti P., Rossini P.M. Time-varying coupling of EEG oscillations predicts excitability fluctuations in the primary motor cortex as reflected by motor evoked potentials amplitude: an EEG-TMS study. Hum. Brain Mapp. 2014;35:1969–1980. doi: 10.1002/hbm.22306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L.R., Culver D.G., Tennant P., Davis A.A., Wang M., Castellano-Sanchez A. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fisher R.J., Nakamura Y., Bestmann S., Rothwell J.C., Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp. Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Korgaonkar M.S., Menon P., Van den Bos M., Gomes L., Foster S. Brain functional connectome abnormalities in amyotrophic lateral sclerosis are associated with disability and cortical hyperexcitability. Eur. J. Neurol. 2017;24:1507–1517. doi: 10.1111/ene.13461. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Loy C.T., Menon P., de Carvalho M., Swash M., Schrooten M. Awaji criteria improves the diagnostic sensitivity in amyotrophic lateral sclerosis: a systematic review using individual patient data. Clin. Neurophysiol. 2016;127:2684–2691. doi: 10.1016/j.clinph.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Ng K., Van Den Bos M., Byth K., Kiernan M.C. Riluzole exerts transient modulating effects on cortical and axonal hyperexcitability in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:1–9. doi: 10.1080/21678421.2016.1188961. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Nicholson G.A., Ng K., Howells J., Kril J.J. Cortical Function in Asymptomatic Carriers and Patients With C9orf72 Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015;72:1268–1274. doi: 10.1001/jamaneurol.2015.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Ozdinler P.H., Kiernan M.C., Vucic S. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat. Rev. Neurol. 2016;12:651–661. doi: 10.1038/nrneurol.2016.140. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Scherman D.B., Simon N., Yiannikas C., Henderson R.D. Diagnostic criteria in amyotrophic lateral sclerosis A multicenter prospective study. Neurology. 2016;87:684–690. doi: 10.1212/WNL.0000000000002988. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Sue C.M., Kumar K.R., Ng K., Yiannikas C. Cortical excitability changes distinguish the motor neuron disease phenotypes from hereditary spastic paraplegia. Eur. J. Neurol. 2015;22:826–831. doi: 10.1111/ene.12669. [DOI] [PubMed] [Google Scholar]
- Geevasinga N., Menon P., Yiannikas C., Kiernan M.C., Vucic S. Diagnostic utility of cortical excitability studies in amyotrophic lateral sclerosis. Eur. J. Neurol. 2014;21:1451–1457. doi: 10.1111/ene.12422. [DOI] [PubMed] [Google Scholar]
- Grieve S.M., Menon P., Korgaonkar M.S., Gomes L., Foster S., Kiernan M.C. Potential structural and functional biomarkers of upper motor neuron dysfunction in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2015;17:1–8. doi: 10.3109/21678421.2015.1074707. [DOI] [PubMed] [Google Scholar]
- Groppa S., Oliviero A., Eisen A., Quartarone A., Cohen L.G., Mall V. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin. Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R., Furubayashi T., Iwata N.K., Shiio Y., Okabe S., Kanazawa I. Further evidence to support different mechanisms underlying intracortical inhibition of the motor cortex. Exp. Brain Res. 2003;151:427–434. doi: 10.1007/s00221-003-1455-z. [DOI] [PubMed] [Google Scholar]
- Hanajima R., Ugawa Y., Terao Y., Ogata K., Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J. Neurol. Sci. 1996:109–116. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Hanajima R., Ugawa Y., Terao Y., Sakai K., Furubayashi T., Machii K. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J. Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashihara M., Sonoo M., Imafuku I., Fukutake T., Kamakura K., Inoue K. Fasciculation potentials in amyotrophic lateral sclerosis and the diagnostic yield of the Awaji algorithm. Muscle Nerve. 2012;45:175–182. doi: 10.1002/mus.22299. [DOI] [PubMed] [Google Scholar]
- Ilic T.V., Korchounov A., Ziemann U. Methylphenidate facilitates and disinhibits the motor cortex in intact humans. NeuroReport. 2003;14:773–776. doi: 10.1097/00001756-200304150-00023. [DOI] [PubMed] [Google Scholar]
- Ilic T.V., Meintzschel F., Cleff U., Ruge D., Kessler K.R., Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J. Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M., Berardelli A., Cruccu G., Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J. Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M., Berardelli A., Marchetti P., Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp. Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- Keil J., Timm J., Sanmiguel I., Schulz H., Obleser J., Schonwiesner M. Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J. Neurophysiol. 2014;111:513–519. doi: 10.1152/jn.00387.2013. [DOI] [PubMed] [Google Scholar]
- Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- Kiers L., Cros D., Chiappa K.H., Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1993;89:415–423. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Kohara N., Kaji R., Kojima Y., Mills K.R., Fujii H., Hamano T. Abnormal excitability of the corticospinal pathway in patients with amyotrophic lateral sclerosis: a single motor unit study using transcranial magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1996;101:32–41. doi: 10.1016/0013-4694(95)00166-2. [DOI] [PubMed] [Google Scholar]
- Korchounov A., Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology. 2011;36:1894–1902. doi: 10.1038/npp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T., Caramia M.D., Rothwell J.C., Day B.L., Thompson P.D., Ferbert A. Corticocortical inhibition in human motor cortex. J. Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.T. Redox regulation of multidrug resistance in cancer chemotherapy: molecular mechanisms and therapeutic opportunities. Antioxid. Redox Signal. 2009;11:99–133. doi: 10.1089/ars.2008.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara S., Sonoo M., Komori T., Shimizu T., Hirashima F., Inaba A. Dissociated small hand muscle atrophy in amyotrophic lateral sclerosis: frequency, extent, and specificity. Muscle Nerve. 2008;37:426–430. doi: 10.1002/mus.20949. [DOI] [PubMed] [Google Scholar]
- Lacomblez L., Bensimon G., Leigh P.N., Guillet P., Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- Lang N., Rothkegel H., Peckolt H., Deuschl G. Effects of lacosamide and carbamazepine on human motor cortex excitability: a double-blind, placebo-controlled transcranial magnetic stimulation study. Seizure. 2013;22:726–730. doi: 10.1016/j.seizure.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Macdonell R.A., Shapiro B.E., Chiappa K.H., Helmers S.L., Cros D., Day B.J. Hemispheric threshold differences for motor evoked potentials produced by magnetic coil stimulation. Neurology. 1991;41:1441–1444. doi: 10.1212/wnl.41.9.1441. [DOI] [PubMed] [Google Scholar]
- Mavroudakis N., Caroyer J.M., Brunko E., Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Menon P., Bae J.S., Mioshi E., Kiernan M.C., Vucic S. Split-hand plus sign in ALS: Differential involvement of the flexor pollicis longus and intrinsic hand muscles. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:315–318. doi: 10.3109/21678421.2012.734521. [DOI] [PubMed] [Google Scholar]
- Menon P., Geevasinga N., van den Bos M., Yiannikas C., Kiernan M.C., Vucic S. Cortical hyperexcitability and disease spread in amyotrophic lateral sclerosis. Eur. J. Neurol. 2017;24:816–824. doi: 10.1111/ene.13295. [DOI] [PubMed] [Google Scholar]
- Menon P., Geevasinga N., Yiannikas C., Howells J., Kiernan M., Vucic S. The sensitivity and specificity of threshold-tracking transcranial magnetic stimulation for the diagnosis of amyotrophic lateral sclerosis: a prospective study. Lancet Neurol. 2015;14:478–484. doi: 10.1016/S1474-4422(15)00014-9. [DOI] [PubMed] [Google Scholar]
- Menon P., Geevasinga N., Yiannikas C., Kiernan M.C., Vucic S. Cortical contributions to the flail leg syndrome: Pathophysiological insights. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:389–396. doi: 10.3109/21678421.2016.1145232. [DOI] [PubMed] [Google Scholar]
- Menon P., Kiernan M.C., Vucic S. Cortical dysfunction underlies the development of the split-hand in amyotrophic lateral sclerosis. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0087124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon P., Kiernan M.C., Vucic S. Cortical hyperexcitability precedes lower motor neuron dysfunction in ALS. Clin. Neurophysiol. 2015;126:803–809. doi: 10.1016/j.clinph.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Mills K.R. The natural history of central motor abnormalities in amyotrophic lateral sclerosis. Brain. 2003;126:2558–2566. doi: 10.1093/brain/awg260. [DOI] [PubMed] [Google Scholar]
- Mills K.R., Nithi K.A. Corticomotor threshold is reduced in early sporadic amyotrophic lateral sclerosis. Muscle Nerve. 1997;20:1137–1141. doi: 10.1002/(sici)1097-4598(199709)20:9<1137::aid-mus7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Miscio G., Pisano F., Mora G., Mazzini L. Motor neuron disease: usefulness of transcranial magnetic stimulation in improving the diagnosis. Clin. Neurophysiol. 1999;110:975–981. doi: 10.1016/s1388-2457(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Kitagawa H., Kawaguchi Y., Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J. Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z., Chen R. Short-interval intracortical inhibition: a complex measure. Clin. Neurophysiol. 2008;119:2175–2176. doi: 10.1016/j.clinph.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Plewnia C., Bartels M., Cohen L., Gerloff C. Noradrenergic modulation of human cortex excitability by the presynaptic alpha(2)-antagonist yohimbine. Neurosci. Lett. 2001;307:41–44. doi: 10.1016/s0304-3940(01)01921-8. [DOI] [PubMed] [Google Scholar]
- Plewnia C., Hoppe J., Hiemke C., Bartels M., Cohen L.G., Gerloff C. Enhancement of human cortico-motoneuronal excitability by the selective norepinephrine reuptake inhibitor reboxetine. Neurosci. Lett. 2002;330:231–234. doi: 10.1016/s0304-3940(02)00803-0. [DOI] [PubMed] [Google Scholar]
- Prout A.J., Eisen A. The cortical silent period and ALS. Muscle Nerve. 1994;17:217–223. doi: 10.1002/mus.880170213. [DOI] [PubMed] [Google Scholar]
- Pun S., Santos A.F., Saxena S., Xu L., Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Ravits J., Paul P., Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68:1571–1575. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- Ravits J.M., La Spada A.R. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009;73:805–811. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshan L., Paradiso G.O., Chen R. Two phases of short-interval intracortical inhibition. Exp. Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Rossini P.M., Burke D., Chen R., Cohen L.G., Daskalakis Z., Di Iorio R. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee Clin. Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu C.V., Murakami M., Ziemann U., Triesch J. A model of TMS-induced I-waves in motor cortex. Brain Stimul. 2014;7:401–414. doi: 10.1016/j.brs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Samusyte G., Bostock H., Rothwell J., Koltzenburg M. Short-interval intracortical inhibition: comparison between conventional and threshold-tracking techniques. Brain Stimul. 2018;11:806–817. doi: 10.1016/j.brs.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger T.D., Garg R.R., Chen R. Interactions between two different inhibitory systems in the human motor cortex. J. Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P., Witscher K., Janssen F., Addo A., Dertwinkel R., Zenz M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci. Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Shibuya K., Park S.B., Geevasinga N., Menon P., Howells J., Simon N.G. Motor cortical function determines prognosis in sporadic ALS. Neurology. 2016;87:513–520. doi: 10.1212/WNL.0000000000002912. [DOI] [PubMed] [Google Scholar]
- Siciliano G., Manca M.L., Sagliocco L., Pastorini E., Pellegrinetti A., Sartucci F. Cortical silent period in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 1999;169:93–97. doi: 10.1016/s0022-510x(99)00223-3. [DOI] [PubMed] [Google Scholar]
- Siebner H.R., Dressnandt J., Auer C., Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sommer M., Gileles E., Knappmeyer K., Rothkegel H., Polania R., Paulus W. Carbamazepine reduces short-interval interhemispheric inhibition in healthy humans. Clin. Neurophysiol. 2012;123:351–357. doi: 10.1016/j.clinph.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Sommer M., Tergau F., Wischer S., Reimers C.D., Beuche W., Paulus W. Riluzole does not have an acute effect on motor thresholds and the intracortical excitability in amyotrophic lateral sclerosis. J Neurol. 1999;246(Suppl 3):III22–III26. doi: 10.1007/BF03161086. [DOI] [PubMed] [Google Scholar]
- Stefan K., Kunesch E., Benecke R., Classen J. Effects of riluzole on cortical excitability in patients with amyotrophic lateral sclerosis. Ann. Neurol. 2001;49:536–539. [PubMed] [Google Scholar]
- Stetkarova I., Kofler M. Differential effect of baclofen on cortical and spinal inhibitory circuits. Clin. Neurophysiol. 2013;124:339–345. doi: 10.1016/j.clinph.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Swash M. Why are upper motor neuron signs difficult to elicit in amyotrophic lateral sclerosis? J. Neurol. Neurosurg. Psychiatry. 2012;83:659–662. doi: 10.1136/jnnp-2012-302315. [DOI] [PubMed] [Google Scholar]
- Tokimura H., Ridding M.C., Tokimura Y., Amassian V.E., Rothwell J.C. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr. Clin. Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Triggs W.J., Kiers L., Cros D., Fang J., Chiappa K.H. Facilitation of magnetic motor evoked potentials during the cortical stimulation silent period. Neurology. 1993;43:2615–2620. doi: 10.1212/wnl.43.12.2615. [DOI] [PubMed] [Google Scholar]
- Triggs W.J., Macdonell R.A., Cros D., Chiappa K.H., Shahani B.T., Day B.J. Motor inhibition and excitation are independent effects of magnetic cortical stimulation. Ann. Neurol. 1992;32:345–351. doi: 10.1002/ana.410320307. [DOI] [PubMed] [Google Scholar]
- Triggs W.J., Menkes D., Onorato J., Yan R.S., Young M.S., Newell K. Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology. 1999;53:605–611. doi: 10.1212/wnl.53.3.605. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Kiernan M.C., Leigh P.N., Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8:94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Wicks P., Brownstein C.A., Massagli M.P., Toronjo M., Talbot K. Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2011;82:853–854. doi: 10.1136/jnnp.2010.208413. [DOI] [PubMed] [Google Scholar]
- Urban P., Wicht S., Hopf H. Sensitivity of transcranial magnetic stimulation of cortico-bulbar vs. cortico-spinal tract involvement in ALS. J. Neurol. 2001;248:850–855. doi: 10.1007/s004150170068. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J., Pascual-Leone A., Wassermann E.M., Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr. Clin. Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- van den Bos M.A.J., Menon P., Geevasinga N., Kiernan M.C., Vucic S. 6. ALS disability is predicted by a shift in the balance between short latency facilitatory and inhibitory circuits. Clin. Neurophysiol. 2018;129 [Google Scholar]
- Van den Bos M.A.J., Menon P., Howells J., Geevasinga N., Kiernan M.C., Vucic S. Physiological processes underlying short interval intracortical facilitation in the human motor cortex. Front. Neurosci. 2018;12:240. doi: 10.3389/fnins.2018.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Cheah B.C., Kiernan M.C. Dissecting the mechanisms underlying short-interval intracortical inhibition using exercise. Cereb. Cortex. 2011;21:1639–1644. doi: 10.1093/cercor/bhq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Cheah B.C., Krishnan A.V., Burke D., Kiernan M.C. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009;1273:39–47. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Vucic S., Cheah B.C., Yiannikas C., Kiernan M.C. Cortical excitability distinguishes ALS from mimic disorders. Clin. Neurophysiol. 2011;122:1860–1866. doi: 10.1016/j.clinph.2010.12.062. [DOI] [PubMed] [Google Scholar]
- Vucic S., Howells J., Trevillion L., Kiernan M.C. Assessment of cortical excitability using threshold tracking techniques. Muscle Nerve. 2006;33:477–486. doi: 10.1002/mus.20481. [DOI] [PubMed] [Google Scholar]
- Vucic S., Kiernan M.C. Abnormalities in cortical and peripheral excitability in flail arm variant amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2007;78:849–852. doi: 10.1136/jnnp.2006.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Kiernan M.C. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics. 2017;14:91–106. doi: 10.1007/s13311-016-0487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S., Lin C.S.-Y., Cheah B.C., Murray J., Menon P., Krishnan A.V. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361–1370. doi: 10.1093/brain/awt085. [DOI] [PubMed] [Google Scholar]
- Vucic S., Nicholson G.A., Kiernan M.C. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- Vucic S., Nicholson G.A., Kiernan M.C. Cortical excitability in hereditary motor neuronopathy with pyramidal signs: comparison with ALS. J. Neurol. Neurosurg. Psychiatry. 2010;81:97–100. doi: 10.1136/jnnp.2008.157537. [DOI] [PubMed] [Google Scholar]
- Vucic S., Ziemann U., Eisen A., Hallett M., Kiernan M.C. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J. Neurol. Neurosurg. Psychiatry. 2013;84:1161–1170. doi: 10.1136/jnnp-2012-304019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle-Shukla A., Ni Z., Gunraj C.A., Bahl N., Chen R. Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J. Physiol. 2009;587:5665–5678. doi: 10.1113/jphysiol.2009.181446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J., Kunesch E., Noachtar S., Benecke R., Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J. Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbourn A.J. The “split hand syndrome”. Muscle Nerve. 2000;23:138. doi: 10.1002/(sici)1097-4598(200001)23:1<138::aid-mus22>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Williams K.L., Fifita J.A., Vucic S., Durnall J.C., Kiernan M.C., Blair I.P. Pathophysiological insights into ALS with C9ORF72 expansions. J. Neurol. Neurosurg. Psychiatry. 2013;84:931–935. doi: 10.1136/jnnp-2012-304529. [DOI] [PubMed] [Google Scholar]
- Williamson T.L., Cleveland D.W. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- Wittstock M., Wolters A., Benecke R. Transcallosal inhibition in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2007;118:301–307. doi: 10.1016/j.clinph.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Yokota T., Yoshino A., Inaba A., Saito Y. Double cortical stimulation in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 1996;61:596–600. doi: 10.1136/jnnp.61.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G., Tamburin S., Manganotti P., Refatti N., Forgione A., Rizzuto N. Changes in motor cortex inhibition over time in patients with amyotrophic lateral sclerosis. J. Neurol. 2002;249:1723–1728. doi: 10.1007/s00415-002-0926-7. [DOI] [PubMed] [Google Scholar]
- Zanette G., Tamburin S., Manganotti P., Refatti N., Forgione A., Rizzuto N. Different mechanisms contribute to motor cortex hyperexcitability in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2002;113:1688–1697. doi: 10.1016/s1388-2457(02)00288-2. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang L., Liang B., Schroeder D., Zhang Z., Cox G.A. Hyperactive somatostatin interneurons contribute to excitotoxicity in neurodegenerative disorders. Nat. Neurosci. 2016;19:557–559. doi: 10.1038/nn.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. Cortical threshold and excitability measurements. In: Eisen A., editor. Clinical Neurophysiology of Motor Neuron Diseases. Handbook of Clinical Neurophysiology. Elsevier; Amsterdam: 2004. pp. 317–335. [Google Scholar]
- Ziemann U., Bruns D., Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neurosci. Lett. 1996;208:187–190. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Lonnecker S., Steinhoff B.J., Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp. Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Reis J., Schwenkreis P., Rosanova M., Strafella A., Badawy R. TMS and drugs revisited 2014. Clin. Neurophysiol. 2015;126:1847–1868. doi: 10.1016/j.clinph.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Rothwell J.C., Ridding M.C. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U., Tergau F., Bruns D., Baudewig J., Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr. Clin. Neurophysiol. 1997;105:430–437. doi: 10.1016/s0924-980x(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Tergau F., Wischer S., Hildebrandt J., Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr. Clin. Neurophysiol. 1998;109:321–330. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Ziemann U., Winter M., Reimers C.D., Reimers K., Tergau F., Paulus W. Impaired motor cortex inhibition in patients with amyotrophic lateral sclerosis. Evidence from paired transcranial magnetic stimulation. Neurology. 1997;49:1292–1298. doi: 10.1212/wnl.49.5.1292. [DOI] [PubMed] [Google Scholar]