Abstract
The current tumor node metastasis (TNM) staging system is inadequate for identifying high‐risk gastric cancer (GC) patients. Using a systematic and comprehensive‐biomarker discovery and validation approach, we attempted to build a microRNA (miRNA)‐recurrence classifier (MRC) to improve the prognostic prediction of GC. We identified 312 differentially expressed miRNAs in 446 GC tissues compared to 45 normal controls by analyzing high‐throughput data from The Cancer Genome Atlas (TCGA). Using a Cox regression model, we developed an 11‐miRNA signature that could successfully discriminate high‐risk patients in the training set (n = 372; P < 0.0001). Quantitative real‐time polymerase chain reaction‐based validation in an independent clinical cohort (n = 88) of formalin‐fixed paraffin‐embedded clinical GC samples showed that MRC‐derived high‐risk patients succumb to significantly poor recurrence‐free survival in GC patients (P < 0.0001). Cox and stratification analysis indicated that the prognostic value of this signature was independent of clinicopathological risk factors. Time‐dependent receiver operating characteristic (ROC) analysis revealed that the area under the curve of this signature was significantly larger than that of TNM stage in the TCGA (0.733 vs. 0.589 at 3 years, P = 0.004; 0.802 vs. 0.635 at 5 years, P = 0.005) and validation cohort (0.835 vs. 0.689 at 3 years, P = 0.003). A nomogram was constructed for clinical use, which integrated both MRC and clinical‐related variables (depth of invasion, lymph node status and distance metastasis) and did well in the calibration plots. In conclusion, this novel miRNA‐based signature is superior to currently used clinicopathological features for identifying high‐risk GC patients. It can be readily translated into clinical practice with formalin‐fixed paraffin‐embedded specimens for specific decision‐making applications.
Keywords: gastric cancer, miRNA signature, prediction, prognosis, recurrence‐free survival
Abbreviations
- AUC
area under receiver operating characteristic curve
- CI
confidence interval
- FDR
false discover rate
- FFPE
formalin‐fixed paraffin‐embedded
- GC
gastric cancer
- miRNA
microRNA
- MRC
miRNA‐recurrence classifier
- RFS
recurrence‐free survival
- ROC
receiver operating characteristic
- TCGA
The Cancer Genome Atlas
- TNM
tumor node metastasis
1. Introduction
Gastric cancer (GC) is the fourth most common malignancy and ranks as the second leading cause of cancer death worldwide (Siegel et al., 2017). Surgical resection with subsequent adjuvant chemoradiotherapy has been considered as a potentially curative treatment for patients with early‐stage GC (Bang et al., 2012; Cunningham et al., 2006; Macdonald et al., 2001; Sasako et al., 2011; Stiekema et al., 2015). However, recurrence occurs in up to 30–40% of patients within 5 years (Aoyama et al., 2011; Bang et al., 2012; Lee et al., 2012; Sakuramoto et al., 2007) and, in turn, 5‐year overall survival estimates range from 5% to 90%, depending largely on the stage of disease at presentation (Aoyama et al., 2011; Bang et al., 2012; Lee et al., 2012; Sakuramoto et al., 2007). GC is a clinically heterogeneous disease and it is difficult to accurately predict outcomes even within the same stage. The ability to predict the precise prognosis of an individual patient is critical for the selection of an appropriate treatment plan and follow‐up strategies, whereas the current staging system for GC has shown insufficient prediction for prognosis of patients (Choi et al., 2017; Edge and Compton, 2010; Son et al., 2012). Hence, the identification of novel markers that could predict survival and relapse in GC would greatly optimize the treatment planning and benefit patients.
Recent advancements in transcriptome profiling have provided compelling evidence of small non‐coding RNA [such as microRNA (miRNA), Piwi‐interacting RNA and small nucleolar RNA] dysregulation in cancers, highlighting the potential of these molecules as diagnostic and prognostic biomarkers (Romano et al., 2017). miRNAs, in particular, have shown promising prognostic associations with major cancer outcomes (Nair et al., 2012). A growing number of studies have indicated that differentially expressed miRNAs in tumor tissues are key players in oncogenesis and have an impact on the prognosis for GC patients (Kogo et al., 2011; Li et al., 2015; Nishida et al., 2011). Recent findings on the mechanisms of miRNA‐mediated gene regulation in GC also support the development of biomarkers for the precise evaluation of cancer progression (Ishimoto et al., 2016). However, because the limited number of miRNAs or patients involved, or different miRNA expression profiling platforms in a GC study, such studies lack a normalized standard.
The Cancer Genome Atlas (TCGA) provides a foundation for systematic analysis of large‐scale miRNA expression data. Most recently, a comprehensive study based on the TCGA and other data platforms has successfully identified an 8‐miRNA signature that significantly predicted recurrence‐free interval in stage II and III colorectal cancer (Kandimalla et al., 2018). In the present study, we employed a large cohort of GC patients from the TCGA project and identified a novel miRNA‐based signature for predicting recurrence‐free survival (RFS) in patients with GC, followed by validation of its clinical significance in an independent clinical cohort. Additionally, we assessed the prognostic and predictive value of this signature in the TCGA and validation datasets.
2. Materials and methods
2.1. Candidate miRNA selection and miRNA signature identification using TCGA data
Data for selected samples of 446 GC patients and 45 normal controls were downloaded from The Cancer Genome Atlas Cancer Genome (https://portal.gdc.cancer.gov). The dataset acquired above contained 1881 noted miRNA expression data. The downloaded clinicopathological information and follow‐up data were matched with the miRNA expression profiles. The RFS events included the first recurrence of GC at a local, regional or distant site, and death from any cause. Patients without events or death were censored at the time of last follow‐up.
The TCGA GC patients were used as the training cohort for identifying prognostic miRNAs and building the miRNA‐recurrence classifier (MRC). First, TCGA miRNA data were log2 transformed and the miRNA expression levels between non‐cancer and cancer were compared using the criteria: absolute log2 fold‐change > 1, false discovery rate (FDR) < 0.05 and relatively high expression levels of miRNAs (count per million > 1). Subsequently, differentially expressed miRNAs were subjected to univariate Cox proportional hazards regression analysis. The miRNAs with P < 0.05 were considered as the candidate prognostic miRNAs of RFS and entered into multivariate Cox proportional hazards regression. To identify the independent predictors that significantly contributed to RFS, we used the least value of Akaike information criterion (AIC) with respect to miRNA selection and the established MRC. The risk score of each patient was calculated to predict RFS of GC, with the regression coefficients of multivariate Cox regression model being used to weight each miRNA expression level in the prognostic classifier:
Using the optimum cut‐off value obtained from x‐tile plots (x‐tile, version 3.6.1; Yale University School of Medicine, New Haven, CT, USA), patients were categorized into high‐risk and low‐risk groups.
2.2. Patient and sample collection
In total, 88 formalin‐fixed paraffin‐embedded (FFPE) specimens were collected from GCs patients who underwent radical surgery at Qilu Hospital, Shandong University between 2012 and 2014. All samples were evaluated by two pathologists in accordance with the American Joint Committee on Cancer TNM grading system (7th edition) (https://cancerstaging.org). All procedures performed in the study involving human participants were conducted in accordance with the ethical standards of the Clinical Research Ethics Committee of Qilu Hospital, Shandong University and the Declaration of Helsinki. The experiments were undertaken with the understanding and written consent of each subject.
2.3. RNA isolation, cDNA synthesis and quantitative real‐time polymerase chain reaction
Total RNA extraction from 10‐μm thick FFPE specimens was performed using miRNA isolation Kits (Bioteke, Beijing, China). All RNA manipulations were carried out under RNase‐free conditions and cDNA was synthesized using miRNA‐specific Bugle‐Loop primers (Ribobio, Guangzhou, China) and the M‐MLV RT kit in accordance with the manufacturer's recommendations (Invitrogen, Carlsbad, CA, USA). miRNA expression was assessed by a quantitative real‐time polymerase chain reaction using ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The relative levels of miRNA expression were determined using the method with the U6 small nuclear RNA (U6) as the reference gene to normalize the data. The normalized values were further log2 transformed. All primers for miRNAs used in this part of the study were purchased from Ribobio.
2.4. Statistical analysis
prism, version 7.0 (GraphPad Software Inc., San Diego, CA, USA) and r, version 3.4.0 (http://www.Rproject.org) were used to analyze all the data. P < 0.05 was considered statistically significant. Differential expression analysis of miRNAs between non‐cancer and cancer groups was performed using the edgeR package of r (Robinson et al., 2010). In survival analyses, we used the Kaplan–Meier method to draw survival curves, which were compared by log‐rank tests. A Cox proportional hazard regression model was applied for the univariate analysis and multivariate analysis of prognostic factors. The prognostic or predictive accuracy of each variable was investigated using time‐dependent receiver operating characteristic (ROC) analysis in the survivalROC package and the bootstrap method was applied to test the significance of differences between the ROC curves. The regression coefficients in multivariable Cox regression model were used to generate the nomogram. A calibration plot was used to explore the agreement of nomogram between predictions and observations. Nomogram and calibration plot were performed using the rms package of r software.
3. Results
3.1. Identification of GC‐specific miRNAs by analyzing the TCGA dataset
Based on the miRNA expression data from the TCGA dataset, we compared miRNA expression profiles between 446 GC and normal 45 control groups and found 312 miRNAs with an absolute fold‐change differences of 2 and a FDR < 0.05 (Table S1). These significantly differentially expressed miRNAs were considered as candidate prognostic biomarkers for GC patients, among which 260 miRNAs were identified as upregulated and 52 as downregulated in GC compared to normal control (Fig. S1).
3.2. Identification of the prognostic miRNAs from the training cohort
To single out the prognostic miRNAs, 312 GC‐specific miRNAs were initially subjected to univariate Cox proportional hazards regression analysis in 372 patients for whom complete miRNA data, clinicopathological characteristics and follow‐up information were available. In total, 24 miRNAs were found to be significantly associated with the GC patient RFS (P < 0.05) (Table S2) and were subsequently entered into a multivariate Cox regression analysis. For the purpose of identifying the best predictors that significantly contributed to patient RFS, we used the lowest value of the Akaike information criterion for variable selection and built a prognostic classifier, which consisted of 11 miRNAs (miR‐365a, miR‐145, miR‐181b, miR‐549a, miR‐708, miR‐7‐3, miR‐378i, miR‐466, miR‐3923, miR‐4793 and miR‐3144). Among these miRNAs, seven (miR‐145, miR‐549a, miR‐7‐3, miR‐378i, miR‐466, miR‐4793 and miR‐3144) with a negative coefficient were protective factors as a result of the close association between their high expression and a longer patient RFS, whereas the remaining four (miR‐365a, miR‐181b, miR‐708 and miR‐3923) were risk factors.
3.3. Construction of a miRNA prognostic risk model and its predictability assessment in the training cohort
Using the regression coefficients of multivariate Cox regression model to weight each miRNA expression level in the MRC, we developed a risk score formula to predict patient survival: risk score = miR‐365a × 0.15853 + miR‐145 × (−0.14044) + miR‐181b × 0.22993 + miR‐549a × (−0.15682) + miR‐708 × 0.13047 + miR‐7‐3 × (−0.1562) + miR‐378i × (−0.37045) + miR‐466 × (−0.23334) + miR‐3923 × 0.25761 + miR‐4793 × (−0.6004 6) + miR‐3144 × (−0.25687). We then calculated the risk scores for all GC patients using this formula. Using x‐tile plots to generate the optimum cut‐off value (Fig. S2A), we included those patients with a risk score of 1.30 or lower in the group of patients at low risk of disease recurrence (low‐risk group) and also those with a risk score higher than 1.30 in the high‐risk group. Patients with a higher risk score generally had poorer survival than those with lower risk score and the distribution of risk scores and survival status is shown in Fig. 1A. Kaplan–Meier survival analysis demonstrated that patients with high‐risk scores had a shorter RFS than those with low‐risk scores (log‐rank test, P < 0.001) (Fig. 1B). Figure 1C shows the predictive potential of MRC using time‐dependent ROC curves. The area under the ROC curve (AUC) of the prognostic model for RFS was 0.733 at 3 years and 0.802 at 5 years. In the univariate Cox regression model of RFS, the high‐risk group showed a 2.492‐fold increased risk of recurrence [95% confidence interval (CI) 1.867–3.326, P = 5.78 × 10–10] compared to the low‐risk group (Table 1).
Figure 1.
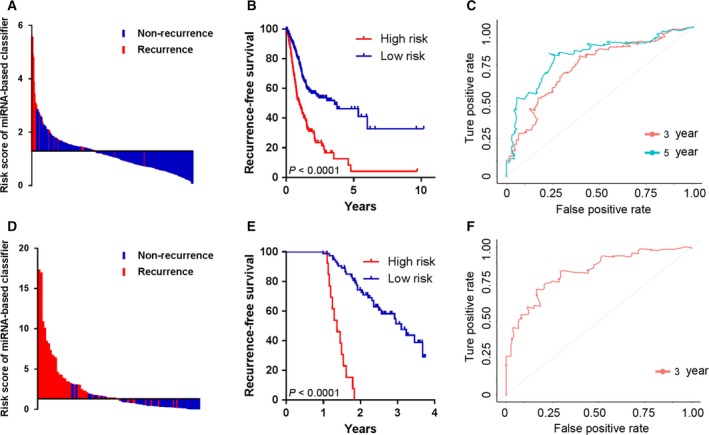
Risk score by the 11‐miRNA classifier, time‐dependent ROC curves and Kaplan–Meier survival in the training set (A–C) and validation set (D–F).
Table 1.
Variables associated with RFS according to the Cox proportional hazards regression model
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Age | 1.01 | 0.997–1.024 | 0.148 | |||
| Sex | ||||||
| Male vs. female | 1.272 | 0.935–1.731 | 0.126 | |||
| Helicobacter pylori infection | ||||||
| Yes vs. no | 0.435 | 0.175–1.079 | 0.072 | |||
| Histologic grade | ||||||
| G1 | Ref | – | – | |||
| G2 | 0.5742 | 0.180–1.838 | 0.350 | |||
| G3 | 0.8426 | 0.268–2.652 | 0.770 | |||
| Stage | ||||||
| Stage I | Ref | – | – | |||
| Stage II | 1.383 | 0.784–2.441 | 0.263 | |||
| Stage III | 2.005 | 1.180–3.408 | 0.010 | |||
| Stage IV | 3.447 | 1.839–6.462 | 0.000 | |||
| T | ||||||
| T1 | Ref | – | – | Ref | – | – |
| T2 | 4.014 | 1.240–13.00 | 0.020 | 2.869 | 0.880–9.349 | 0.080 |
| T3 | 4.173 | 1.322–13.18 | 0.015 | 2.378 | 0.740–7.648 | 0.146 |
| T4 | 4.055 | 1.265–13.00 | 0.018 | 2.107 | 0.643–6.900 | 0.218 |
| N | ||||||
| N0 | Ref | – | – | Ref | – | – |
| N1 | 1.86 | 1.237–2.797 | 0.003 | 1.593 | 1.049–2.421 | 0.029 |
| N2 | 1.739 | 1.115–2.711 | 0.015 | 1.586 | 1.009–2.494 | 0.046 |
| N3 | 2.676 | 1.769–4.047 | 0.000 | 2.114 | 1.371–3.261 | 0.001 |
| M | ||||||
| M1 vs. M0 | 1.968 | 1.158–3.344 | 0.012 | 2.048 | 1.174–3.573 | 0.012 |
| MRC | ||||||
| High vs. low | 2.492 | 1.867–3.326 | 0.000 | 2.327 | 1.731–3.129 | 0.000 |
3.4. Validation of the miRNA classifier for RFS prediction in the validation cohort
To determine whether the MRC derived from the TCGA cohort was robust, we measured its performance in an independent validation cohort, which comprised 88 FFPE tissues from GC patients. We examined the expression levels of all 11 miRNAs in GC tissues and constructed a prognostic classifier using a Cox proportional hazard model. Based upon the Cox‐model derived risk scores, patients in the validation cohort were dichotomized into low and high‐risk groups, using the x‐tile derived cut‐off threshold (Fig. S2B). In line with the results from the TCGA cohort, more patients with recurrence fell into the high‐risk group (Fig. 1D), in which the RFS was shorter than that in low‐risk group (Fig. 1E). Furthermore, the MRC achieved an AUC of 0.835, which was clinically interesting (Fig. 1F).
3.5. Prognostic value of the miRNA classifier
To investigate whether the prognostic value of MRC was independent of other clinicopathological variables, the univariable and multivariable Cox regression analyses were initially performed in the TCGA cohort. We found that risk score was significantly associated with RFS even when adjusted by other clinical factors (Table 1). We also observed that clinical stages and clinicopathologic classifications (T, N and M) were significant in Cox regression analyses. Therefore, stratification analysis was introduced to determine the independence of MRC according to clinical stages and T, N and M classifications. As shown in Fig. 2, the high‐risk survival curves were below the low‐risk curves in all subgroups, including TNM stage (Stage I and II, Fig. 2A; Stage III and IV, Fig. 2B); T stage (T1 and T2, Fig. 2C; T3 and T4, Fig. 2D); lymph node status (LN−, Fig. 2E; LN+, Fig. 2F); and M stage (M−, Fig. 2G; M+, Fig. 2H). A log‐rank test showed that MRC was still a clinically and statistically significant prognostic signature in all subgroups except for distant metastasis group (M+ group). For the M+ subgroup, the difference was marginal (P = 0.0694). This was probably because the sample size was too small (only 21 patients) to draw any firm conclusions. Stratification analysis yielded similar results in the validation cohort (Fig. 3). We also performed ROC analysis to compare the prognostic accuracy of MRC with tumor stage. Figure 4A,B shows that the 11‐miRNA risk score model possessed a stronger predictive power than TNM stage for the prognostic evaluation of GC patients in the TCGA cohort (0.733 vs. 0.589, 95% CI = 0.613–0.853 vs. 0.514–0.664 at 3 years; 0.802 vs. 0.635, 95% CI = 0.652–0.952 vs. 0.548–0.722 at 5 years; P = 0.005). When the MRC was combined with the TNM stage, no significant difference was found between the combined model and the MRC (P > 0.05). Subsequent analysis in the FFPE tissues produced similar results (Fig. 4C). The results from the validation dataset further confirmed the reliable predictive ability of MRC.
Figure 2.
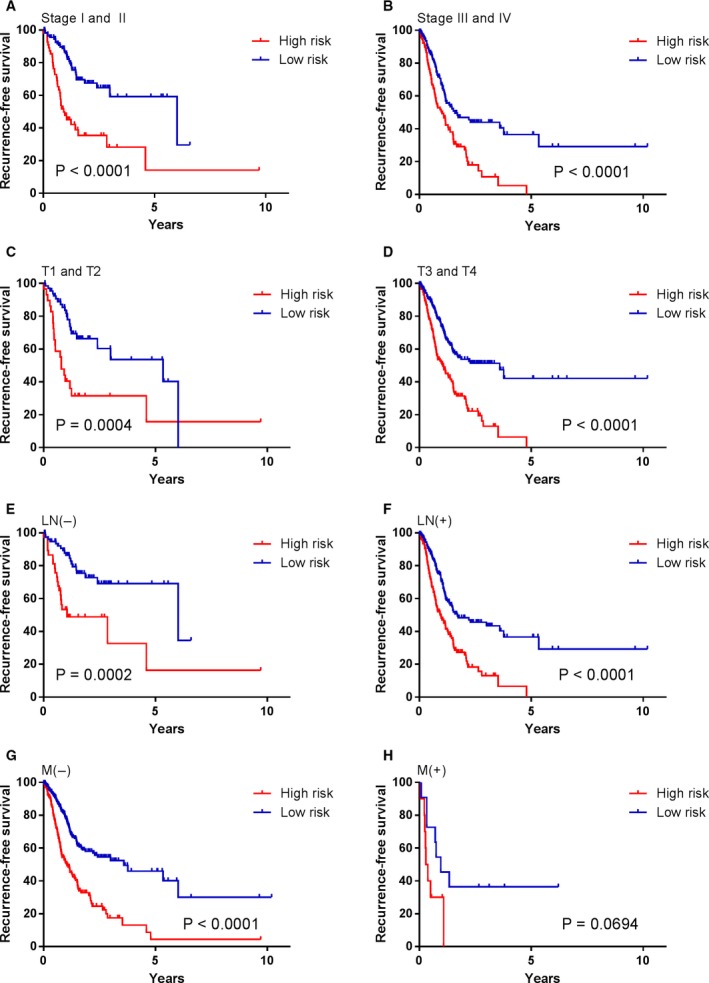
Kaplan–Meier survival analysis according to the 11‐miRNA classifier stratified by clinicopathological risk factors in the TCGA cohort. (A, B) TNM stage. (C, D) T stage. (E, F) lymph node status. (G, H) M stage. P values were calculated using the log‐rank test.
Figure 3.
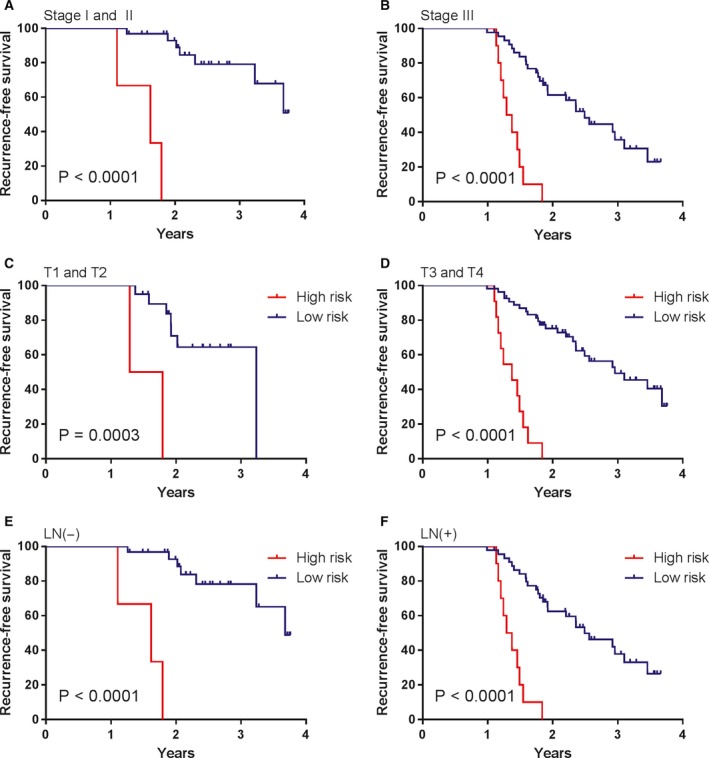
Kaplan–Meier survival analysis according to the 11‐miRNA classifier stratified by clinicopathological risk factors in the validation cohort. (A, B) TNM stage. (C, D) T stage. (E, F) lymph node status.
Figure 4.
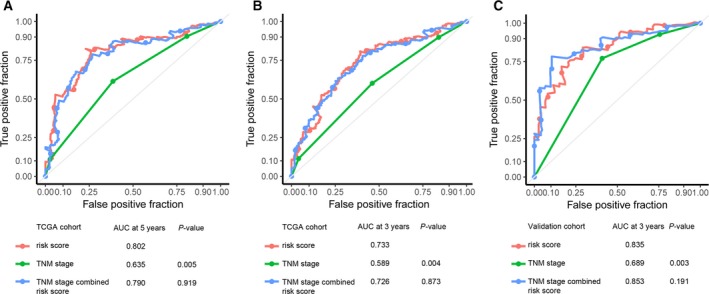
Time‐dependent ROC curves to compare the prognostic accuracy of the 11‐miRNA classifier with tumor stage in the training cohort (A, B) and validation cohort (C).
3.6. Construction of nomogram based on the miRNA classifier
To provide the clinician with a quantitative method for predicting the individual probability of cancer recurrence, we built a nomogram that integrated the MRC and clinicopathological independent risk factors for RFS (Fig. 5A). The bias‐corrected line in the calibration plot was found to be closer to the ideal curve (the 45° line), which indicated good agreement between prediction and observation (Fig. 5B). The predictive accuracy of the nomogram was calculated via ROC analysis: the AUC of nomogram was 0.754, which implied that the discrimination performance was favorable (Fig. 5C).
Figure 5.
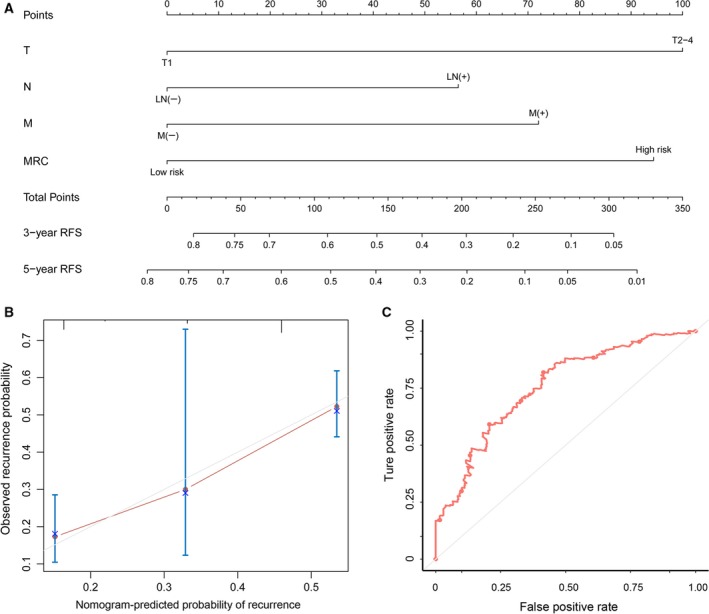
The nomogram to predict probability of RFS for CRC patients in the training set. (A) The nomogram for predicting the proportion of patients with RFS. (B) The calibration plot of the nomogram for the probability of RFS at 3 years. (C) Time‐dependent ROC based on the nomogram for recurrence probability. AUC = 0.754. Nomogram‐predicted probability of recurrence is plotted on the x‐axis and observed recurrence is plotted on the y‐axis. The red line represents our nomogram and the vertical bars represent the 95% CI.
4. Discussion
An effective molecular‐based method for predicting prognosis in cancer patients is urgently needed to optimize tailored treatment in the era of precision medicine. In the present study, we identified an 11‐miRNA signature that is associated with tumor recurrence in GC patients using high‐throughput data from the TCGA database. We confirmed these findings in an independent clinical cohort of GC patients. Patients with a high‐risk score for this 11‐miRNA signature had increased tumor recurrence, even after stratifying patients by clinicopathological risk factors.
Previous studies on miRNA expression profiling have consistently revealed that the miRNA‐based signature is a potential predictor for progression or relapse in patients with various types of cancers, including GC (Li et al., 2010; Ueda et al., 2010). Moreover, the TCGA has profiled and analyzed large numbers of human cancers to identify molecular aberrations at the DNA, RNA, proteomic and epigenetic levels (Weinstein et al., 2013). Recently, Sohn et al. (2017) developed a model based on four molecular subtypes of GC from the TCGA project to predict survival and adjuvant chemotherapy outcomes. However, to date, the miRNA expression signatures from the TCGA database with respect to the survival prognosis of GC have not been investigated systematically. The miRNA predictive signature for survival meets some crucial standards: (i) its expression must be specific in cancer and non‐cancer; (ii) it is correlated with patient survival; and (iii) it has a synergized effect in the survival prognosis. Based on the genome‐wide discovery of GC‐specific miRNAs in the TCGA dataset, we identified 11 miRNAs that were strongly related to RFS in GC patients. In agreement with our findings, similar studies have already been carried out for the identification of prognostic miRNA signatures from the TCGA in other types of cancers, including bladder cancer, glioblastoma, colon cancer, etc. (Gonzalez‐Vallinas et al., 2018; Hermansen et al., 2017; Liu et al., 2017a; Wong et al., 2016; Xu et al., 2016; Zhou et al., 2015).
Among the identified miRNAs in the present study, we found that four miRNAs, including miR‐365a, miR‐181b‐1, miR‐708 and miR‐3923, were risk factors, whereas the other seven, including miR‐145, miR‐549a, miR‐7‐3, miR‐378i, miR‐466, miR‐4793 and miR‐3144, were protective factors. High levels of risk factors and low levels of protective factors were independent negative prognostic factors according to our multivariate analysis. These results are consistent with previous research showing that miR‐181b was involved in transforming growth factor beta‐induced epithelial‐to‐mesenchymal transition and GC metastasis (Zhou et al., 2016). Considering protective factors, we noted that miR‐145 could inhibit the malignant phenotypes and suppress metastasis of GC via different molecular mechanisms (Gao et al., 2013; Lei et al., 2017; Tong et al., 2018; Xing et al., 2015; Xue et al., 2016; Zheng et al., 2013). In another study, miR‐7‐3 was confirmed to be an independent prognostic indicator for stomach adenocarcinoma (Huo, 2017). Two miRNAs, miR‐466 and miR‐3144, have been indicated as tumor suppressors in other types of cancers (Colden et al., 2017; Lin et al., 2015; Tong et al., 2018), although there are no data available regarding their roles in GC. Other miRNAs, miR‐365a, miR‐3923, miR‐549a, miR‐378i and miR‐4793, are also reported for the first time in GC in the present study. Although it appears that miR‐708 as a risk factor was inconsistent with previous studies reporting its anti‐oncogenic role in GC (Li et al., 2018), increased miR‐708 expression and its association with poor survival in lung adenocarcinoma has been demonstrated by other researchers (Jang et al., 2012). Thus, further studies are required to comprehensively assess the exact contribution of these miRNAs in tumor progression.
The combined analysis of a panel of multiple factors, rather than a single biomarker, will have more power to provide clinically useful information. An ideal prognostic classifier for GC risk prediction should be robust and potentially feasible in FFPE samples that would overcome barriers of sample collection and storage. A validation from FFPE samples was performed and the combined index of the 11 miRNAs showed a significant association with survival in GC patients. The results of multivariate analysis showed that the 11‐miRNA signature is independent of traditional clinical risk factors. When the stratification analysis was performed, we found the 11‐miRNA signature could discriminate patients at high‐risk from those at low‐risk within all subgroups. Time‐independent ROC analysis showed that our 11‐miRNA signature was superior to TNM stage for prognostic evaluation. To improve the ability of prognostic prediction, we combined the 11‐miRNA signature with TNM stage. However, there was no significant difference between the combined model and our miRNA signature, indicating that our 11‐miRNA signature could yield reliable predictive ability by itself.
It has been reported that miRNAs are sufficiently stable to be detected in both FFPE and blood samples (Hall et al., 2012; Mitchell et al., 2008). Circulating miRNAs might enable successful close monitoring for early signs of cancer relapse because serum molecules are easily detectable and are also acceptable for patients. Previously, a 7‐miRNA classifier within plasma was reported to predict tumor recurrence in stage II and III GC (Liu et al., 2017b); however, our miRNA classifier illustrated its ability to predict recurrence in all stages of GC and performed well. Although we did not have access to blood specimens, it is very likely that our miRNA signatures may eventually be translated into a blood‐based surveillance assay.
Prognostic nomograms comprise the visualization of statistical models specifically developed to optimize the predictive accuracy of individuals, enabling a more individualized prediction of outcome based on a combination of variables (Balachandran et al., 2015; Iasonos et al., 2008; Shariat et al., 2008). Regarding GC, some models based on clinical‐associated factors, such as age, tumor size, tumor invasion depth and lymph node involvement, were demonstrated to be useful for prognosis prediction in patients with GC (Hirabayashi et al., 2014; Kim et al., 2015). However, an optimal approach that combines multiple miRNA biomarkers and clinical risk factors as a predictive model has yet to be developed. In the present study, we built a nomogram that integrated the miRNA‐based classifier and clinicopathological independent risk factors for RFS. The nomogram performed well in the calibration plot, which indicated good agreement between prediction and observation. The ROC for the prediction nomogram was 0.754, which implied that the discrimination performance was favorable. Therefore, our nomogram may be an important tool for risk stratification and prognosis prediction in GC patients, aiding in individualized treatment decisions and postoperative counseling, and ultimately contributing to improved survival.
5. Conclusions
In conclusion, the present study revealed a novel, robust 11‐miRNA classifier for tumor recurrence prediction in patients with GC. This approach can be readily deployed in clinical practice with FFPE samples and achieved superior predictive accuracy compared to currently used clinicopathological risk factors. Moreover, the 11‐miRNA classifier was an independent prognostic factor for, and had a better prognostic ability than, clinical risk factors. Also, these findings should be validated in large‐scale multicenter clinical trials.
Author contributions
YZ conceived and designed the experiments. YY, AQ, XZ and ZD performed all of the experiments. YY, AQ, RZ, GZ, HP, HW and XY analyzed the data. YY and AQ wrote the manuscript. MH provided the pathological samples. All authors read and approved the final manuscript submitted for publication.
Supporting information
Fig. S1. Volcano plot showing the differentially expressed miRNAs between gastric tumors and normal tissue samples from the TCGA cohort.
Fig. S2. x‐tile plots of the miRNA‐recurrence classifier and the risk score in the training (A) and validation (B) sets.
Table S1. Summary of 312 differentially expressed microRNAs.
Table S2. Identification of the prognostic miRNAs from the TCGA cohort.
Acknowledgements
This project was supported by grants from National Natural Science Foundation of China (No. 81601846, 81300297, 81702084, 81401709 and 81572070); Natural Science Foundation of Shandong Province (No. ZR2017MH044 and ZR20170215018); Shandong Key Research and Development Program (No. 2016GSF201122, 2016CYJS01A02, 2016GSF201124 and 2018GSF118104); Science and Technology Development Project in Jinan (No. 201805084); Fundamental Research Funds of Shandong University (No. 2014QLKY03); Science Foundation of Qilu Hospital of Shandong University (No. 2015QLMS51); and Taishan Scholar Program of Shandong Province.
References
- Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H and Tsuburaya A (2011) Survival and prognosticators of gastric cancer that recurs after adjuvant chemotherapy with S‐1. Gastric Cancer 14, 150–154. [DOI] [PubMed] [Google Scholar]
- Balachandran VP, Gonen M, Smith JJ and Dematteo RP (2015) Nomograms in oncology: more than meets the eye. Lancet Oncol 16, e173–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY et al (2012) Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open‐label, randomised controlled trial. Lancet 379, 315–321. [DOI] [PubMed] [Google Scholar]
- Choi KH, Kim BS, Oh ST, Yook JH and Kim BS (2017) Comparison the sixth and seventh editions of the AJCC staging system for T1 gastric cancer: a long‐term follow‐up study of 2124 patients. Gastric Cancer 20, 43–48. [DOI] [PubMed] [Google Scholar]
- Colden M, Dar AA, Saini S, Dahiya PV, Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S (2017) MicroRNA‐466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis 8, e2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355, 11–20. [DOI] [PubMed] [Google Scholar]
- Edge SB and Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol, 17, 1471–1474. [DOI] [PubMed] [Google Scholar]
- Gao P, Xing AY, Zhou GY, Zhang TG, Zhang JP, Gao C, Li H and Shi DB (2013) The molecular mechanism of microRNA‐145 to suppress invasion‐metastasis cascade in gastric cancer. Oncogene 32, 491–501. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Vallinas M, Rodriguez‐Paredes M, Albrecht M, Sticht C, Stichel D, Gutekunst J, Pitea A, Sass S, Sanchez‐Rivera FJ, Lorenzo‐Bermejo J et al (2018) Epigenetically regulated chromosome 14q32 miRNA cluster induces metastasis and predicts poor prognosis in lung adenocarcinoma patients. Mol Cancer Res 16, 390–402. [DOI] [PubMed] [Google Scholar]
- Hall JS, Taylor J, Valentine HR, Irlam JJ, Eustace A, Hoskin PJ, Miller CJ and West CM (2012) Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br J Cancer 107, 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen SK, Sorensen MD, Hansen A, Knudsen S, Alvarado AG, Lathia JD and Kristensen BW (2017) A 4‐miRNA signature to predict survival in glioblastomas. PLoS One 12, e188090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi S, Kosugi S, Isobe Y, Nashimoto A, Oda I, Hayashi K, Miyashiro I, Tsujitani S, Kodera Y, Seto Y et al (2014) Development and external validation of a nomogram for overall survival after curative resection in serosa‐negative, locally advanced gastric cancer. Ann Oncol 25, 1179–1184. [DOI] [PubMed] [Google Scholar]
- Huo Q (2017) Analysis of expression profile of miRNA in stomach adenocarcinoma. J BUON 22, 1154–1159. [PubMed] [Google Scholar]
- Iasonos A, Schrag D, Raj GV and Panageas KS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26, 1364–1370. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Baba H, Izumi D, Sugihara H, Kurashige J, Iwatsuki M and Tan P (2016) Current perspectives toward the identification of key players in gastric cancer microRNA dysregulation. Int J Cancer 138, 1337–1349. [DOI] [PubMed] [Google Scholar]
- Jang JS, Jeon HS, Sun Z, Aubry MC, Tang H, Park CH, Rakhshan F, Schultz DA, Kolbert CP, Lupu R et al (2012) Increased miR‐708 expression in NSCLC and its association with poor survival in lung adenocarcinoma from never smokers. Clin Cancer Res 18, 3658–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla R, Gao F, Matsuyama T, Ishikawa T, Uetake H, Takahashi N, Yamada Y, Becerra CR, Kopetz S, Wang X et al (2018) Genome‐wide discovery and identification of a novel miRNA signature for recurrence prediction in stage II and III colorectal cancer. Clin Cancer Res 24, 3867–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Spolverato G, Ejaz A, Squires MH, Poultsides G, Fields RC, Bloomston M, Weber SM, Votanopoulos K, Acher AW et al (2015) A nomogram to predict overall survival and disease‐free survival after curative resection of gastric adenocarcinoma. Ann Surg Oncol 22, 1828–1835. [DOI] [PubMed] [Google Scholar]
- Kogo R, Mimori K, Tanaka F, Komune S and Mori M (2011) Clinical significance of miR‐146a in gastric cancer cases. Clin Cancer Res 17, 4277–4284. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH et al (2012) Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 30, 268–273. [DOI] [PubMed] [Google Scholar]
- Lei C, Du F, Sun L, Li T, Li T, Min Y, Nie A, Wang X, Geng L, Lu Y et al (2017) miR‐143 and miR‐145 inhibit gastric cancer cell migration and metastasis by suppressing MYO6. Cell Death Dis 8, e3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Zhang Y, Ding J, Wu K and Fan D (2010) Survival prediction of gastric cancer by a seven‐microRNA signature. Gut 59, 579–585. [DOI] [PubMed] [Google Scholar]
- Li X, Zhong X, Pan X and Ji Y (2018) Tumor suppressive microRNA‐708 targets Notch1 to suppress cell proliferation and invasion in gastric cancer. Oncol Res. 10.3727/096504018x15179680859017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y, Mao XH, Wu C, Yang SM, Zeng H, Zou QM et al (2015) MicroRNA‐25 promotes gastric cancer migration, invasion and proliferation by directly targeting transducer of ERBB2, 1 and correlates with poor survival. Oncogene 34, 2556–2565. [DOI] [PubMed] [Google Scholar]
- Lin L, Cai Q, Zhang X, Zhang H, Zhong Y, Xu C and Li Y (2015) Two less common human microRNAs miR‐875 and miR‐3144 target a conserved site of E6 oncogene in most high‐risk human papillomavirus subtypes. Protein Cell 6, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang H, Fu JD, Liu JY, Yan AG and Guan YY (2017a) A five‐miRNA expression signature predicts survival in hepatocellular carcinoma. APMIS 125, 614–622. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang X, Zhang Z, Chang J, Wang Z, Wu Z, Wang C, Sun Z, Ge X, Geng R et al (2017b) Plasma microRNA‐based signatures to predict 3‐year postoperative recurrence risk for stage II and III gastric cancer. Int J Cancer 141, 2093–2102. [DOI] [PubMed] [Google Scholar]
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345, 725–730. [DOI] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova‐Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A et al (2008) Circulating microRNAs as stable blood‐based markers for cancer detection. Proc Natl Acad Sci USA 105, 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair VS, Maeda LS and Ioannidis JP (2012) Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst 104, 528–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Mimori K, Fabbri M, Yokobori T, Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y and Mori M (2011) MicroRNA‐125a‐5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res 17, 2725–2733. [DOI] [PubMed] [Google Scholar]
- Robinson MD, Mccarthy DJ and Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano G, Veneziano D, Acunzo M and Croce CM (2017) Small non‐coding RNA and cancer. Carcinogenesis 38, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H et al (2007) Adjuvant chemotherapy for gastric cancer with S‐1, an oral fluoropyrimidine. N Engl J Med 357, 1810–1820. [DOI] [PubMed] [Google Scholar]
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and Ohashi Y (2011) Five‐year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S‐1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29, 4387–4393. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Karakiewicz PI, Suardi N and Kattan MW (2008) Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res 14, 4400–4407. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD and Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67, 7–30. [DOI] [PubMed] [Google Scholar]
- Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH et al (2017) Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas Project. Clin Cancer Res 23, 4441–4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son T, Hyung WJ, Lee JH, Kim YM, Kim HI, An JY, Cheong JH and Noh SH (2012) Clinical implication of an insufficient number of examined lymph nodes after curative resection for gastric cancer. Cancer 118, 4687–4693. [DOI] [PubMed] [Google Scholar]
- Stiekema J, Trip AK, Jansen EP, Aarts MJ, Boot H, Cats A, Ponz OB, Gradowska PL, Verheij M and van Sandick JW (2015) Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an r1 resection? Results from a dutch cohort study. Ann Surg Oncol 22, 581–588. [DOI] [PubMed] [Google Scholar]
- Tong F, Ying Y, Pan H, Zhao W, Li H and Zhan X (2018) MicroRNA‐466 (miR‐466) functions as a tumor suppressor and prognostic factor in colorectal cancer (CRC). Bosn J Basic Med Sci 18, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Volinia S, Okumura H, Shimizu M, Taccioli C, Rossi S, Alder H, Liu CG, Oue N, Yasui W et al (2010) Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol 11, 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C and Stuart JM (2013) The Cancer Genome Atlas Pan‐Cancer analysis project. Nat Genet 45, 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong N, Khwaja SS, Baker CM, Gay HA, Thorstad WL, Daly MD, Lewis JJ and Wang X (2016) Prognostic microRNA signatures derived from The Cancer Genome Atlas for head and neck squamous cell carcinomas. Cancer Med 5, 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing AY, Wang YW, Su ZX, Shi DB, Wang B and Gao P (2015) Catenin‐delta1, negatively regulated by miR‐145, promotes tumour aggressiveness in gastric cancer. J Pathol 236, 53–64. [DOI] [PubMed] [Google Scholar]
- Xu J, Zhao J and Zhang R (2016) Four microRNAs signature for survival prognosis in colon cancer using TCGA data. Sci Rep 6, 38306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Zhao L, Yang F, Li Z and Li G (2016) MicroRNA145 inhibits the malignant phenotypes of gastric carcinoma cells via downregulation of fascin 1 expression. Mol Med Rep 13, 1033–1039. [DOI] [PubMed] [Google Scholar]
- Zheng L, Pu J, Qi T, Qi M, Li D, Xiang X, Huang K and Tong Q (2013) miRNA‐145 targets v‐ets erythroblastosis virus E26 oncogene homolog 1 to suppress the invasion, metastasis, and angiogenesis of gastric cancer cells. Mol Cancer Res 11, 182–193. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tang K, Xiao H, Zeng J, Guan W, Guo X, Xu H and Ye Z (2015) A panel of eight‐miRNA signature as a potential biomarker for predicting survival in bladder cancer. J Exp Clin Cancer Res 34, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zheng X, Chen L, Xu B, Yang X, Jiang J and Wu C (2016) Smad2/3/4 pathway contributes to TGF‐beta‐induced MiRNA‐181b expression to promote gastric cancer metastasis by targeting Timp3. Cell Physiol Biochem 39, 453–466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Volcano plot showing the differentially expressed miRNAs between gastric tumors and normal tissue samples from the TCGA cohort.
Fig. S2. x‐tile plots of the miRNA‐recurrence classifier and the risk score in the training (A) and validation (B) sets.
Table S1. Summary of 312 differentially expressed microRNAs.
Table S2. Identification of the prognostic miRNAs from the TCGA cohort.


