Both metagenomics and single-cell sequencing can detect unknown genes from uncultured microbial strains in environments, and either method may find the significant potential metabolites and roles of these strains. However, such gene/genome-based techniques do not allow detailed investigations that are possible with cultures. To solve this problem, various approaches for cultivation of uncultured bacteria have been developed, but there are still difficulties in maintaining pure cultures by subculture.
KEYWORDS: cultivation, intensive soil extract medium (ISEM), isolation, low-molecular-weight organic substances (LMWOS), new soil extract (NSE), new taxonomic candidates, subculture, uncultured bacteria
ABSTRACT
Here, a new medium, named intensive soil extract medium (ISEM), based on new soil extract (NSE) using 80% methanol, was used to efficiently isolate previously uncultured bacteria and new taxonomic candidates, which accounted for 49% and 55% of the total isolates examined (n = 258), respectively. The new isolates were affiliated with seven phyla (Proteobacteria, Acidobacteria, Firmicutes, Actinobacteria, Verrucomicrobia, Planctomycetes, and Bacteroidetes). The result of chemical analysis showed that NSE included more diverse components of low-molecular-weight organic substances than two conventional soil extracts made using distilled water. Cultivation of previously uncultured bacteria is expected to extend knowledge through the discovery of new phenotypic, physiological, and functional properties and even roles of unknown genes.
IMPORTANCE Both metagenomics and single-cell sequencing can detect unknown genes from uncultured microbial strains in environments, and either method may find the significant potential metabolites and roles of these strains. However, such gene/genome-based techniques do not allow detailed investigations that are possible with cultures. To solve this problem, various approaches for cultivation of uncultured bacteria have been developed, but there are still difficulties in maintaining pure cultures by subculture.
INTRODUCTION
Molecular tools revealed that prokaryotic species are very diverse and abundant in soil and contain numerous unexplored potential metabolites (1–4). Although these tools enable analysis of the broad range of metabolic diversity of microorganisms without the need to isolate species, many bacterial characteristics are unknown because of limitations of cultivation (5, 6). Since the establishment of solid culture media, secondary metabolites have been isolated from microorganisms cultured in laboratories. The lack of complex factors/conditions in the laboratory has contributed to the inability to isolate various species (7, 8). Since the concept of uncultured bacteria was published in 1990 (9) to refer to these bacteria not yet cultured in laboratories, several methods have been developed in an attempt to culture these bacteria. These methods involved transporting bacteria from their natural environment to the laboratory for growth in artificial media/conditions similar to those in the natural environment by modifying growth medium components (7) or growth conditions, such as pH and salt concentrations (9, 10), addition of inorganic compounds or metals lacking electron donors/acceptors (11, 12), use of various factors (13), coculture with helper bacteria (14, 15), performance of soil extracts using water (16, 17) or aqueous buffers (18, 19), use of diluted medium or serial dilution culture (20, 21), long incubation time (10, 22), etc. Furthermore, sophisticated techniques were developed that allowed analysis of individual cells in soil samples, such as iChip for in situ cultivation (23), the microbioreactor (24), optical tweezers (25), and the micromanipulator (26). However, new artificial media to maintain these cultures are needed. Although scientists can enrich slow-growing microorganisms using diffusion chambers (27, 28) or soil substrate membranes (29), most enriched bacteria do not grow on agar plates for isolation and further cultivation. Without successful cultivation, it is difficult to detect and identify novel organisms, obtain phenotypic and functional information, and determine the functions of unknown genes (30). The most important factors affecting the cultivation of uncultured bacteria and the most appropriate medium conditions remain unclear.
Here, we developed a simple culture method based on new soil extract (NSE) using 80% methanol without special equipment and successfully cultured many previously uncultured bacterial strains. To evaluate our method, we checked the proportion of uncultured strains among isolates as well as that of new taxa and analyzed chemical components of NSE for comparisons to two traditional soil extracts (TSEs) that are commonly used.
RESULTS
Composition of soil extracts utilized as culture supplements.
For nutrition and bacterial growth, heterotrophic soil bacteria depend on low-molecular-weight organic substances (LMWOS) and inorganic compounds (31). Thus, in this study, we compared the major LMWOS, such as amino acids, fatty acids, organic acids, and inorganic ions, in three different extraction methods (NSE, new soil extraction, developed in this study; TSE1, traditional soil extraction without autoclaving; TSE2, traditional soil extraction with autoclaving) (Table 1). To obtain more diverse LMWOS, we used a mixture of methanol and water to be consistent with 4:1 (80%) to extract bacterial nutrients from soil rather than water or aqueous buffers and named this extract NSE. Briefly, 500 g dry soil was prepared and shaken at 150 rpm with 1.3 liters of 80% methanol overnight at room temperature. The supernatant was transferred to a new flask, and a fresh 1.3 liters of 80% methanol was added to the remaining soil and mixed well for 1 h. The two supernatants were combined, filtered, and evaporated. The NSE was stored at 4°C until use. For amino acids, NSE showed a total yield of 18.50 mg · liter−1, which is much higher than that for the other two methods, which had values of 4.84 and 5.87 mg · liter−1, respectively (P < 0.004). Additionally, 21 amino acids were extracted, including four more amino acids (valine, pipecolic acid, serine, and threonine), and a very high concentration of tyrosine (9.29 mg · liter−1) compared to the other methods. This higher concentration and diversity of amino acids in the NSE than in the other two methods may cause better cultivability of uncultured soil bacteria. However, there was little difference between methods TSE1 and TSE2 (PTSE1 versus TSE2 < 0.03), indicating that autoclaving at 121°C had no significant effect on the extraction of more components of amino acids in soil. The results of fatty acid analysis can be compared, because the NSE method extracted a much higher total concentration (13.10 mg · liter−1 versus 0.79 mg · liter−1) and greater number (n = 16 versus n = 8) of fatty acids than the other two (Table 1). For organic acids, the results showed that the NSE method had a lower total yield of organic acids (25.14 mg · liter−1 versus 62.69 to 89.00 mg · liter−1; P < 0.03) but a greater total number (n = 16 versus n = 11), including lactic acid, glycolic acid, 2-hydroxybuyric acid, fumaric acid, and α-ketoglutaric acid (Table 1). The total amounts of organic acids obtained by methods TSE1 and TSE2 were significantly influenced by two major components, acetoacetic acid and oxaloacetic acid, but these did not significantly affect the total amount for method NSE.
TABLE 1.
Components of soil extracts prepared in three different waysa
| Ingredient | Amt (mg · liter−1) by soil extraction method |
||
|---|---|---|---|
| NSE | TSE1 | TSE2 | |
| Amino acids | |||
| Alanine | 0.47 | 0.10 | 0.18 |
| Glycine | 0.32 | 0.16 | 0.40 |
| Valine | 0.09 | ND | ND |
| Leucine | 0.47 | 0.05 | 0.06 |
| Isoleucine | 0.20 | 0.03 | 0.03 |
| Proline | 0.21 | 0.63 | 0.69 |
| γ-Aminobutric acid | 0.76 | 0.62 | 0.69 |
| Pipecolic acid | 0.09 | ND | ND |
| Pyroglutamic acid | 0.85 | 0.23 | 0.32 |
| Serine | 0.38 | ND | ND |
| Threonine | 1.38 | ND | ND |
| Phenylalanine | 0.22 | 0.03 | 0.04 |
| Cysteine | 0.22 | 0.12 | 0.29 |
| Aspartic acid | 0.27 | 0.08 | 0.31 |
| Glutamic acid | 0.55 | 0.40 | 0.39 |
| Asparagine | 0.57 | 0.41 | 0.52 |
| Ornithine | 0.77 | 0.46 | 0.45 |
| Glutamine | 0.55 | 0.67 | 0.66 |
| Lysine | 0.12 | 0.25 | 0.24 |
| Tyrosine | 9.29 | 0.10 | 0.09 |
| Tryptophane | 0.72 | 0.50 | 0.51 |
| Total | 18.50 | 4.84 | 5.87 |
| Fatty acids | |||
| Decanoic acid | 0.47 | ND | ND |
| Lauric acid | 0.48 | ND | ND |
| Myristoleic acid | 0.15 | ND | ND |
| Myristic acid | 1.18 | 0.07 | 0.08 |
| Isopentadecylic acid | 0.09 | ND | ND |
| Isopalmitic acid | 0.11 | ND | ND |
| Palmitoleic acid | 0.35 | 0.09 | 0.08 |
| Palmitic acid | 1.29 | 0.12 | 0.11 |
| Linoleic acid | 0.40 | ND | ND |
| Oleic acid | 0.40 | ND | ND |
| Stearic acid | 2.54 | 0.09 | 0.17 |
| Arachidic acid | 1.08 | 0.07 | 0.07 |
| Erucic acid | ND | 0.071 | 0.06 |
| Behenic acid | 1.80 | ND | ND |
| Nervonic acid | 0.07 | ND | ND |
| Lignoceric acid | 2.60 | 0.093 | 0.08 |
| Cerotic acid | 0.09 | 0.182 | 0.14 |
| Total | 13.10 | 0.79 | 0.79 |
| Organic acids | |||
| 3-Hydroxybutyric acid | 0.96 | 2.07 | 1.88 |
| Pyruvic acid | 0.40 | 4.31 | 5.75 |
| Acetoacetic acid | 1.47 | 28.75 | 27.25 |
| Lactic acid | 9.42 | ND | ND |
| Glycolic acid | 8.03 | ND | ND |
| 2-Hydroxybutyric acid | 0.09 | ND | ND |
| Malonic acid | 0.85 | 1.21 | 1.75 |
| Succinic acid | 1.43 | 2.18 | 1.78 |
| Fumaric acid | 0.05 | ND | ND |
| Oxaloacetic acid | 0.17 | 18.35 | 44.84 |
| α-Ketoglutaric acid | 0.11 | ND | ND |
| Malic acid | 0.73 | 3.32 | 3.33 |
| 2-Hydroxyglutaric acid | 0.50 | 0.93 | 0.72 |
| Cis-aconitic acid | 0.27 | 0.28 | 0.33 |
| Citric acid | 0.46 | 0.81 | 0.83 |
| Isocitric acid | 0.20 | 0.48 | 0.54 |
| Total | 25.14 | 62.69 | 89.00 |
| Inorganic compounds | |||
| Na+ | 58.87 | 83.65 | 85.05 |
| NH4+ | 6.93 | 17.76 | 19.45 |
| Mg2+ | 29.24 | 27.52 | 27.95 |
| K+ | 15.98 | 58.95 | 61.32 |
| Ca2+ | 87.93 | 114.59 | 119.49 |
| Cl− | 43.34 | 120.21 | 116.32 |
| NO3− | 489.67 | 398.43 | 384.51 |
| SO42− | 5.41 | 130.15 | 128.64 |
| PO42− | 10.66 | 4.74 | 4.18 |
| Total | 748.03 | 956.00 | 946.91 |
The NSE method was developed in this study; the TSE1 method involves autoclaving at 121°C for 1 h; the TSE2 method does not involve autoclaving. ND, not detected.
The total yields of inorganic compounds for each extraction were 748.03, 965.00, and 946.91 mg per liter of extract (Table 1). Although method NSE gave a lower concentration of total inorganic compounds than the other methods (P NSE versus TSE1 or TSE2 < 0.002), the methanol-water mixture appeared to dissolve substances similarly to water and recovered the same inorganic ions extracted with water (methods TSE1 and TSE2). In particular, NO3− and PO42− were higher in NSE than TSEs, and SO42− was much lower in NSE than TSEs. Autoclaving did not significantly affect the dissolution of inorganic or organic compounds in water between methods TSE1 and TSE2 (P > 0.3).
Method validation based on isolation rate of uncultured or new taxonomic bacteria.
To compare the three cultivation methods, the newly developed method for isolation of previously uncultured soil bacteria using ISEM (intensive soil extract medium; here simply called the new method), traditional soil extract culture medium (the traditional method), and modified transwell culture method (the modified method), the different bacterial strains (258, 243, and 252 for each method) were isolated from three soil samples and identified as described in Materials and Methods. The ratio of previously uncultured bacterial strains was significantly increased by 49% (126 isolated strains/total 258 isolated strains) for the new method, with values of 5% (11 isolated strains/total 243 isolated strains) for the traditional method and 15% (39 isolated strains/total 252 isolated strains) for the modified method (Fig. 1a; see also Tables S1 to S3 in the supplemental material).
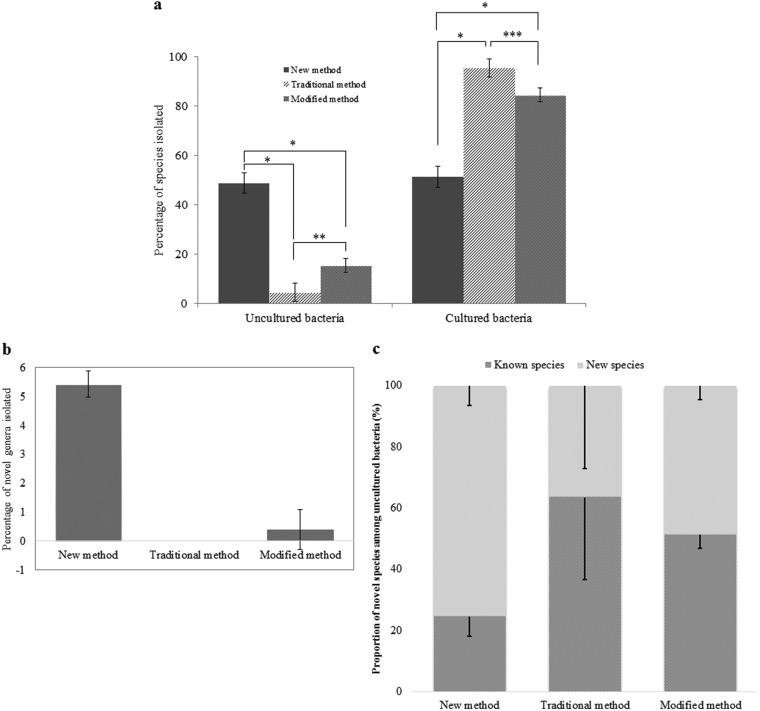
FIG 1.
Proportion of uncultured/cultured bacteria and new taxonomic candidates isolated from soil samples by using ISEM (newly developed method), traditional soil extract culture medium, and a modified transwell culture method. (a) Percentage of uncultured species (126 individual species among a total of 258, 11 of 243, and 39 of 252, respectively). Points of significance compared to two paired samples for means were determined by t test, as indicated by one (P < 0.05), two (P < 0.01), and three (P < 0.001) asterisks. (b) Percentage of novel genus candidates (14/258, 0/243, and 1/252, respectively). (c) Proportion of novel bacterial species and known species among 126, 11, and 39 previously uncultured species via the investigated methods. Error bars indicate standard deviations.
Taxonomic analysis also showed that this new method was much better than the two other methods in terms of new taxon isolation. In the new method, 142 new species candidates were found among isolates, including 13 at the genus level and 1 at the family level, showing 55% (142/258) efficiency compared to 13% (32/243) and 26% (65/252) for the traditional and modified methods, respectively (Tables S1 to S3). For the isolation efficiency for candidates at the genus level or higher, the new method showed a value of 5.4% (14/258), which is much higher than the 0.0% (0/243) and 0.4% (1/252) obtained for the other two methods (Fig. 1b). The new method isolated a family-level candidate, while the other two methods did not. Furthermore, the new method showed the highest ratio and largest number of new taxon candidates (at least at the species level) among uncultured isolated strains (75.4%, 95/126) compared to the other two methods, which showed values of 36.4% (4/11) and 48.7% (19/39), respectively (Fig. 1c). In addition, our method can directly isolate bacterial strains from a soil suspension without an enrichment culture step, saving time and labor.
Method validation through taxonomic analysis.
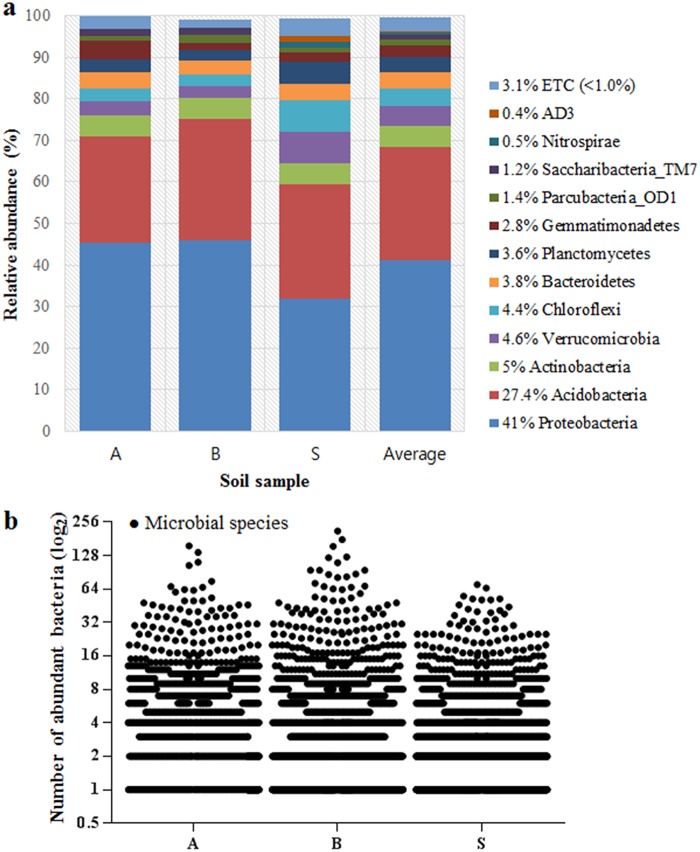
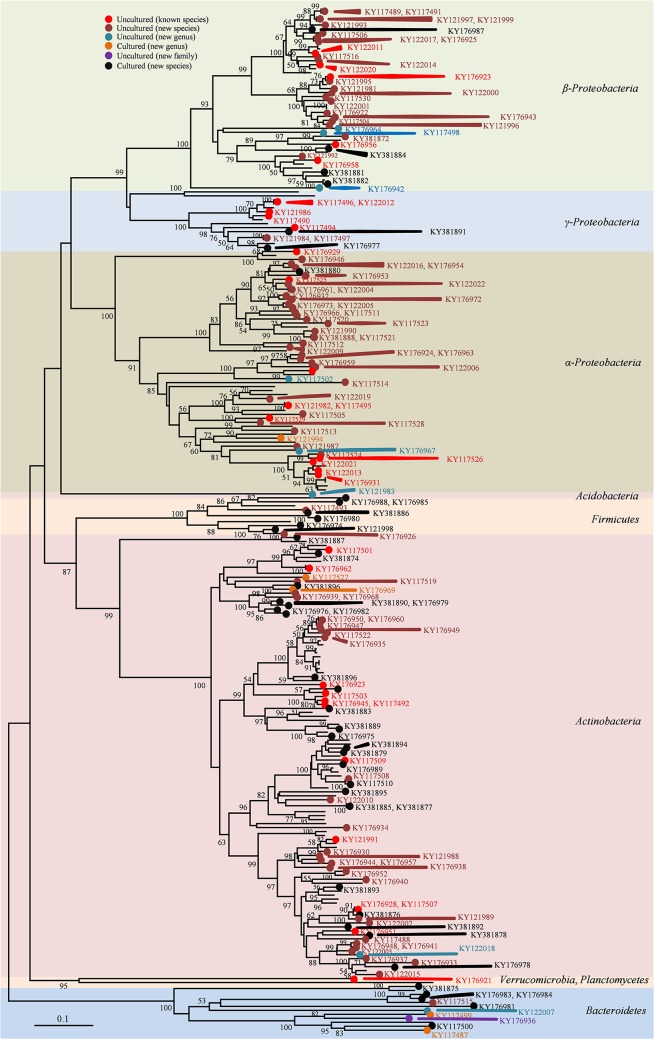
As a standard reference, this study tried to identify possible strains present in the same soil samples through a molecular technique, which can give some information important to evaluate the method developed. 16S amplicon sequencing data as a last generation method was analyzed according to Chao1 estimation at a 3% evolutionary distance and revealed the diversity of microbial community genomics in the soil samples, with 1,744 to 2,402 operational taxonomic units (Fig. S1a). Pyrosequencing analysis suggested that nine identified bacterial phyla commonly present in all soil samples were Chloroflexi, Planctomycetes, Verrucomicrobia, Bacteroidetes, Gemmatimonadetes, Actinobacteria, Acidobacteria, Proteobacteria, and Parcubacteria_OD1, and three unidentified phyla were Nitrospirae, Saccharibacteria_TM7, and AD3 (Fig. 2a). Proteobacteria and Acidobacteria were the most dominant phyla. Other remaining minor phyla (each <1% of the total, together comprising 3.1%, and referred to here as ETC) were major lineages that have cultured representatives (Chlorobi, Elusimicrobia, Armatimonadetes, Firmicutes, Chlamydiae, Tenericutes, Latescibacteria, Omnitrophica, and Hydrogenedentes), candidate phyla without cultured representatives (Kazan, Gracilibacteria, and Berkelbacteria), and other phyla. Additionally, the distribution and relative abundance of species identified by pyrosequencing in each soil sample were determined, showing that abundant species (more reads within a species) would be few and rare (fewer reads within a species would be many) (Fig. 2b). Phylogenetically, the new method achieved successful cultivation of strains from seven phyla among all bacteria present in the soils (Fig. 3): Proteobacteria (α, β, and γ) (46.9%), Actinobacteria (43.4%), Bacteroidetes (4.7%), Firmicutes (3.9%), Acidobacteria (0.4%), Verrucomicrobia (0.4%), and Planctomycetes (0.4%) (Fig. S1b). In contrast, the two other methods did not recover strains in three phyla: Acidobacteria, Verrucomicrobia, and Planctomycetes. Thus, the new method extended the taxonomic range of cultivation at the phylum level. Pyrosequencing analysis indicated that the three soil samples included 120 identified families, excluding 10% unclassified sequences, and 38, 22, and 29% of the identified families were recovered by the new, traditional, and modified methods, respectively (Fig. S2a). The isolates obtained using the new method represented 100 genera (86 known and 14 novel genera), which are compared with the 50 and 60 genera isolated using the traditional and modified methods, respectively (Fig. S2b). For the comparison at the species level, the new method independently cultivated soil bacteria compared to the other two methods, as only one species among the 258 species overlapped with the traditional method (none with the modified method), while the two other methods showed 31 species overlapping each other (Fig. S2c). This result indicated that the new method was more specific for the growth of uncultured bacteria than cultured bacteria. Until now, uncultured isolates from the new method were distributed in 53 genera of six phyla, while the other two methods showed a very limited taxonomic distribution: 5 genera in two phyla and 20 genera in three phyla, respectively (Fig. S2d).
FIG 2.
Abundance of bacteria in soil samples determined by pyrosequencing. (a) The abundance determined at a 1% ETC cutoff. (b) Distribution and relative abundance of identified species. Approximately 8,143, 8,314, and 6,836 individual species were detected in A, B, and S soil samples, respectively; scale of x axis, log2.
FIG 3.
Network topology tree for microbial cultivation based on full-length 16S rRNA gene sequencing. Only bootstrap support values of ≥50% are shown in the tree. Accession numbers for 16S rRNA gene sequences revealed close relationships with previously uncultured bacteria, and those cultured as new species and novel genera are shown in the tree.
Method validation through subculture of novel isolates.
We tested 7 different media to compare their ability to support subculture of newly isolated bacteria: basic salts (BS; negative control), BS plus micronutrients, BS plus vitamin B, BS plus d-amino acids, BS plus NSE, NSE only, and full ISEM (as a positive control). Here, 131 bacterial isolates (i.e., 131 species), including 126 previously uncultured bacteria and 5 novel genus candidates (Table 2 and Table S4), obtained using our developed culture method and three different soil samples, were used to determine the effectiveness of the various nutrient components by forming visible colonies on agar plates after streaking. While NSE and BS plus NSE showed 100% growth recovery (n = 131/131), BS plus d-amino acids showed a value of 8% (n = 11/131) and other components had a value of 0% (n = 0/131). Therefore, NSE and NSE-containing media can be more effective for isolating and subculturing uncultured soil bacteria than other media.
TABLE 2.
Number of bacterial isolates used to evaluate NSE and other media
| Soil sample type |
No. of isolates by level |
Total | ||||
|---|---|---|---|---|---|---|
| Species |
Genus |
Previously uncultured, novel family |
||||
| Previously uncultured, novel |
Previously uncultured, known |
Previously uncultured, novel |
Previously cultured, novel |
|||
| A | 24 | 12 | 2 | 3 | 41 | |
| B | 28 | 9 | 3 | 1 | 41 | |
| S | 34 | 10 | 3 | 1 | 1 | 49 |
| Total | 86 | 31 | 8 | 5 | 1 | 131 |
DISCUSSION
Soil contains elements necessary for living organisms. An aqueous soil extraction method was established previously (17) (the extract is included in, for example, ATCC medium 654 or DSMZ medium 80) and remains widely used. Most mineral or organic ingredients, such as ionic salts, vitamins, antibiotics, plant hormones, and plant-promoting growth factors, among others, can be dissolved in distilled water, while some organic components, such as nonpolar compounds, cannot be sufficiently dissolved in distilled water or aqueous buffers. Thus, we used 80% methanol to overcome this problem and achieved higher concentrations and more different types of organic ingredients than when we used distilled water (Table 1). Although methanol and water are polar protic solvents that easily solubilize polar molecules, methanol is less polar than water based on their polarity values of 5.1 and 10.2, respectively. Therefore, methanol may more easily dissolve or extract a greater amount of hydrophobic or amphipathic molecules in soil than water. In contrast, based on their dielectric constants according to Harris (32), water (approximately 80) is more likely to dissolve inorganic compounds than methanol (approximately 30).
NSE contained 21 amino acids, which was greater than the number obtained using water extraction methods, 17 in this study (Table 1), and even less in previous studies: 16 amino acids were obtained using 6 N HCl (33), and 14 amino acids were obtained using morpholinepropanesulfonic acid buffer (31). Fatty acids contain a polar carboxylic group and nonpolar hydrocarbon group of 4 to 36 carbons, making only short-chain fatty acids more or less water soluble. Thus, a combination of methanol and water improved their dissolution. This led to significant differences in the total number and amount of fatty acids between methods NSE and TSE1 (P < 0.002), but the total fatty acids obtained for the two comparative methods (methods TSE1 and TSE2) were similar to each other (P > 0.9) (Table 1). Greater amounts and larger numbers of fatty acids may improve the cultivability of uncultured soil bacteria. Organic acids are widely present in soil (31), and low-molecular-weight organic acids are typically miscible in water. Thus, the three extraction methods were relatively effective. Although methanol extracted lower concentrations than water, more diverse organic acids were extracted, increasing the spectrum of either carbon or electron donors/acceptors for microorganisms. Because inorganic substances typically dissolve well in water and even in pure methanol (34), 80% methanol can extract large amounts of inorganic compounds (5.41 to 489.67 mg/liter) from soil, although smaller amounts than those of the two water extraction methods. We supposed that the components present only in NSE, or present at greater concentrations than in the two TSEs, would stimulate growth of uncultured bacteria. Overall, NSE was superior to the two TSEs and other extraction methods, because greater concentrations and types of LMWOS were obtained, which may be required to support most soil bacteria, including uncultured bacteria.
Although an enhanced medium (traditional soil extract culture medium) derived from soil extract (TSE) containing yeast extract, tryptone, and salts was designed to support various soil bacteria (17), necessary elements for many uncultured bacteria may be absent, so that most isolates (∼96%) seemed to belong to previously cultured groups (Fig. 1a; also see Table S2 in the supplemental material). Additionally, the modified method with R2A, a complex efficient artificial media for cultivating heterotrophic bacteria, including fast-growing and slow-growing bacteria (35), showed better results and isolated a greater number of bacteria and new taxon candidates than the traditional method did (Fig. 1a and 2a and b; Table S3). Although this method is better than the traditional method, the isolation step shows limited recovery of various enriched uncultured soil bacteria. In particular, ISEM developed in this study allowed cultivation of large numbers of isolates of various uncultured bacteria and new taxa candidates compared to the numbers obtained using the other two methods. Thus, the new method more effectively isolated uncultured bacteria and new bacterial taxa present in soil. The most important aspect of this new method (ISEM) is the inclusion of NSE (described above). The direct isolation from a soil suspension using ISEM agar plates can be an effective method, because during the enrichment step, fast-growing microorganisms may overcome slow-growing bacteria. For any new cells to be formed, substrate complexes, including nutrients, supporting growth factors, etc., are required. Under in vitro conditions, artificially nutrient limited, most of the energy may be consumed by fast-growing bacteria, while slow-growing bacteria need longer times for the cell division process. When the fast-growing bacteria reach the highest growth rate, nutrient concentrations in culture media may shortly be too low or completely consumed. This leads to nutritional physiological stress (starvation) for slow-growing species (13). In addition, antibiotic produced by some fast-growing species may also be a growth-inhibiting factor for slower-growing species (13). As a result, the diversity of bacterial species can be reduced.
Among recently introduced methods, Kakumanu and Williams (36) developed a soil diffusion system and found uncultured bacteria in the phyla Proteobacteria, Bacteroidetes, Verrucomicrobia, Planctomycetes, and OP10 but only 8 uncultured bacteria at the species level. Furthermore, the study suggested conducting only enrichment culture, with no method for isolation of pure bacterial strains. Another study found that 27% of species belonged to 20 unnamed family-level groupings among 350 isolates (37), but these species were isolated from many different artificial media, not including soil extracts, and the effectiveness of each medium on the cultivability of uncultured soil bacteria was not determined. Various methods for cultivating uncultured soil bacteria have been developed: modification of growth media, modification of growth conditions, community culture, coculture, transwell plates with membranes, micromanipulator, optical tweezers, laser microdissection, high-throughput microbioreactor, simulated natural environments using diffusion chambers, single-cell encapsulation combined with flow cytometry, multiwell microbial culture chip (or iChip), and entrapped gelating agent coated with polymer (30). However, these methods exhibit low isolation efficiency of uncultured bacteria or lack strategies for subsequent pure culture. The new method developed in this study showed high isolation efficiency (49%), a 100% recovery rate of isolated uncultured soil bacteria, and easier application in laboratories than most previously developed methods.
Although Acidobacteria were the second most abundant phylum in pyrosequencing analysis for three soil samples, only one isolate was obtained by our new method, since the medium (ISEM) is not acidic (pH 6.8), while Acidobacteria subgroups 1, 2, 3, 12, 13, and 15 exhibit the most abundance at a soil pH of <6.5 (38–40). In the future, using ISEM with low pH or low/high temperature may lead to the successful isolation of more uncultured or novel Acidobacteria or other bacteria.
In summary, our new method showed a much higher isolation rate of new taxon candidates among uncultured isolates and greater isolation rate of uncultured soil bacteria and new taxon candidates than for traditional and modified methods tested in this study. Additionally, isolation was simpler, did not require enrichment culture, and could be directly subcultured to obtain more uncultured bacterial pure cultures for further experiments. Further, the variety of uncultured soil bacteria or new taxon candidates can be extended with ISEM by altering the cultivation conditions such as incubation temperature, pH, salt concentration, and anaerobic conditions or by using various soil samples and other samples.
MATERIALS AND METHODS
Soil sample used for making soil extracts.
Rhizosphere soil where Robinia pseudoacacia L. dominated was collected at Kyonggi University (154-42 Gwanggyosan-ro, Iui-dong, Yeongtong-gu, Suwon, Gyeonggi-do, South Korea; 37°30′04′′ N, 127°03′58′′ E) during June 2016. Fresh soil was dried at room temperature for 48 h by spreading soil samples on surface of aluminum foil and using an air conditioner in dry mode (25 to ∼30°C), and then any plant debris, gravel, and rocks were removed by a 0.2-mm sieve. To determine physicochemical properties of soil, the soil was dried at 110°C for 24 h and cooled at room temperature. Soil contained approximately 78% sand, 17% silt, and 5% clay. Its pH (5.7) was measured directly from fresh soil.
Preparing NSE in 1 liter of medium.
Approximately 1,000 g of the dry soil, sieved at room temperature, was divided into two equal parts (500 g each) in a 2-liter flask and then mixed with 1.3 liters of 80% methanol (494291; methanol [high-performance liquid chromatography grade of purity, ≥99.9%]; Sigma-Aldrich, St. Louis, MO, USA) in deionized water and shaken at 150 rpm overnight at room temperature (below 25°C). After settling for 30 min, the supernatant was transferred to a new flask, and then 1.3 liters of 80% methanol was added to the soil and mixed well for 1 h. The two supernatants were combined and filtered through Whatman paper (number 1001-150; ϕ, 150 mm; GE Healthcare, Little Chalfont, UK). Methanol was removed by a general rotary evaporator (∼40°C). The NSE was adjusted to a final volume of 200 ml with deionized water, sterilized through a 0.22-µm nitrocellulose filter (GSWP04700; Merck Millipore Ltd., Billerica, MA, USA) using a vacuum pump, stored in a dark Schott Duran bottle at 4°C, and used within 1 week.
Medium for isolation of soil bacteria.
The medium containing 0.23 g KH2PO4, 0.23 g K2HPO4, 0.23 g MgSO4·7H2O, 0.33 g NH4NO3, and 0.25 g NaHCO3 as a group of mineral salts and 15 g agar in 1 liter of water was sterilized at 121°C for 15 min. Fifteen grams of agar (A7049; Sigma-Aldrich) was treated several times with distilled water to discard any trace nutrients or elements before use (making purified agar). The following elements then were added to the medium: 5-mg quantities of various d-amino acids (d-valine, d-methionine, d-leucine, d-phenylalanine, d-threonine, and d-tryptophan), 1 ml vitamin B (vitamin stock solution containing 50 mg each thiamine hydrochloride, riboflavin, niacin, pyridoxine HCl, inositol, calcium pantothenate, and β-aminobenzoic acid and 25 mg biotin in 100 ml distilled water, sterilized through a 0.2-µm syringe filter, stored at 4°C in a dark Schott Duran bottle, and used within 1 month), 0.2 liters of NSE, 2 ml of selenite-tungstate solution (41) (composition in 1 liter of distilled water: 0.5 g NaOH, 3 mg Na2SeO3·5H2O, 4 mg Na2WO4·2H2O; the solution was filter sterilized, stored at 4°C, and used within 1 month), and 2 ml of trace element SL-10 (42) (ingredients included 10 ml of HCl [25%, vol/vol], 1.5 g of FeCl2·4H2O, 70 mg of ZnCl2, 100 mg of MnCl2·4H2O, 6 mg of H3BO3, 190 mg of CoCl2·6H2O, 2 mg of CuCl2·2H2O, 24 mg of NiCl2·6H2O, and 36 mg of Na2MoO4·2H2O in a final volume of 1 liter). This solution then was passed through a 0.2-μm filter and added directly in the medium after autoclaving). The final volume was 1 liter, and the pH was 6.8 ± 0.2. The complex medium was named intensive soil extract medium (ISEM). The medium should be prepared freshly and used within 1 week. In this study, we used 150- by 20-mm petri dishes (SPL Life Science Co., Ltd., Gyeonggi-do, South Korea). The larger dish allows for increased separation of colonies at high dilution concentrations during isolation.
Preparation of various media to recover previously uncultured soil bacterial isolates.
We used multiple combinations to find the best growth medium, as described above, to identify the most important elements for supporting the growth of previously uncultured soil bacteria. Several examinations were carried out based on the strains obtained in this study, namely, (i) basic salts (BS) only as a negative control, (ii) BS with selenite-tungstate solution and SL-10, (iii) BS plus d-amino acids, (iv) BS with vitamin B, (v) BS with NSE, (vi) NSE only, and a (vii) mixture of all components (ISEM) as a positive control, and then 15 g purified agar was added to each medium. Agar plates were incubated at 25°C for 4 weeks under aerobic conditions. To prevent the plates from drying out, two cups of distilled water was placed in the incubator.
Soil sampling sites and preparation of soil samples.
Three soil samples were acquired in South Korea in June 2016, including Ansan (sample A) (Il-dong, Sangnok-gu, Ansan, Gyeonggi-do; 37°17′58′′N, 126°53′57′′ E), Suwon (sample B) (Buksu-dong, Paldal-gu, Suwon, Gyeonggi-do; 37°16′42′′ N, 127°00′17′′ E), and Seoul (sample S) (Itaewon-ro, Yongsan-gu, Seoul; 37°31′06′′ N, 127°01′04′′ E). For each sample, approximately 10 g soil from ten different locations within a 150-m diameter was collected and mixed well. The sample was passed through a 0.1-mm sieve and isolated/enriched directly using three methods. A 25-g sieved soil sample was mixed with 250 ml sterile saline (0.9%, wt/vol, NaCl), stirred for 15 min, and allowed to separate between suspension and sediment before use.
Newly developed method for isolation of previously uncultured soil bacteria.
For the newly developed method, first 100 µl of each dilution of soil suspension was spread onto three agar plates of ISEM (to ensure uniformly distributed suspension on the surface of the medium, 100 μl of each the dilution plus 100 μl of ISEM liquid is recommended). These agar plates were incubated at 25°C for 6 weeks. A few colonies appeared after 1 week of incubation. The number of directly observable colonies was increased after 2 weeks, and tiny colonies were picked up and streaked onto fresh ISEM until morphologically pure colonies were obtained. Cells on fresh ISEM typically require at least 1 week of incubation. Uncultured bacteria generally showed weak growth; thus, in some cases pure colonies were activated in ISEM broth in a shaking incubator at 25°C, 150 rpm, for 1 to 2 weeks before being transferred onto the agar plate.
Traditional soil extract culture medium.
Approximately 1,000 g air-dried soil in 1.3 liters of deionized water was autoclaved at 121°C for 1 h and allowed to cool. The supernatant was filtered through Whatman paper before centrifugation in a 500-ml bottle at 5,009 × g for 30 min at room temperature. One liter of the supernatant (TSE) was obtained. Soil extract agar was enhanced by supplementation (17) with 0.04% K2HPO4, 0.005% MgSO4·7H2O, 0.01% NaCl, 0.001% FeCl3, 0.05% tryptone, 0.05% yeast extract, and 1.5% agar in 1 liter of soil extract liquid with a final pH of 6.8, and then 100 µl of each dilution of three soil samples was dispersed onto three soil extract agar plates and cultivated at 25°C for 6 weeks. Colonies were restreaked until pure colonies were obtained.
Modified transwell culture method.
A transwell plate system (35006; SPLInsert hanging; SPL Life Sciences) was used to enrich the bacterial community, especially for uncultured soil bacteria from soil samples, and contains 6 inserts with 6 wells in a plate. An insert has two different sized frames (upper, 28-mm outer diameter and 26.65-mm inner diameter; lower, 26.6-mm outer diameter and 23.3-mm inner diameter), with 28-mm height and 4.52-cm2 area of growth for each insert. The lower frame is covered with a 0.4-µm polycarbonate membrane. Its membrane specification is 25-mm diameter and 7- to ∼10-μm thickness. Approximately 3 g of soil sample was added to a transwell plate, 3 ml R2A medium (3.15 g of the powder in 1 liter of distilled water; MB-R2230; MB Cell, Los Angeles, CA, USA) was supplemented into the soil-containing wells, and then we put the insert on the wet soil. One hundred µl of the suspension and 1 ml R2A medium next was inoculated into the insert. The transwell culture system was covered with Parafilm to prevent evaporation. The system was shaken at 120 rpm and 25°C for 4 weeks. Sevenfold dilutions of the culture enriched were established in R2A broth medium; 100 µl of each dilution was spread onto three R2A agar plates and incubated at 25°C for 6 weeks. Colonies were subcultured on R2A medium to obtain individual colonies (see Table S5 in the supplemental material).
Identification of 16S rRNA gene sequences and accession numbers of 16S rRNA gene sequences.
Near-full-length 16S rRNA sequences were identified, and similarity to valid species was calculated using the EzTaxon Database Update (https://www.ezbiocloud.net) for comparison with published uncultured gene sequences via the nucleotide BLAST search at NCBI (https://www.ncbi.nlm.nih.gov/). In this study, based on full 16S rRNA similarity to validly published species, with four temporary divisions, candidates of novel species were defined by comparison of 16S rRNA similarity at a threshold of 98.7% (43), 95.3 to 90.0% novel genus level (44), and novel family level at an off-limit lower than 90.0%. All sequence data of the isolates were submitted to the GenBank database.
DNA extraction from soil.
Using a FastDNA SPIN kit for soil (116560-200; MP Biomedicals), soil DNA from 0.5 g of fresh soil was extracted and purified by following the manufacturer’s instructions. DNA quality was checked by 1.2% agarose gel electrophoresis in 0.5× Tris-acetate-EDTA (TAE) buffer, and DNA concentration was determined via MaestroNano spectrophotometer (Mastrogen). DNA samples then were held at −20°C until use.
PCR amplification and pyrosequencing.
Pure isolated DNA soil samples were subjected to amplification of the target V1 to V3 regions located in the 16S rRNA gene by PCR using the barcoding primers 27F 5′-CCTATCCCCTGTGTGCCTTGGCAGTCTCAGACGAGTTTGATCMTGGCTCAG-3′ and 518R 5′-CCATCTCATCCCTGCGTGTCTCCGACTCAGXACWTTACCGCGGCTGCTGG-3′ (X directs the unique barcode for each subject) (https://www.ezbiocloud.net). The reaction was conducted with initial denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s, and then final elongation at 72°C for 5 min. The amplicons next were evaluated by 2% agarose gel electrophoresis and observed with a Gel Doc system (Bio-Rad, Hercules, CA, USA). A QIAquick PCR purification kit (28106; Qiagen, Hilden, Germany) was used to purify the PCR products. An AMPure beads kit (Agencourt Bioscience, Beverly, MA, USA) was used to enhance the quality of the sample and remove nontarget products by following the manufacturer’s instructions. Quality and target size were estimated with a Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) using a DNA 7500 chip. PCR products next were mixed by emulsion PCR and deposited on picotiter plates. Target sequencing was conducted with a GS Junior sequencing system (Roche, Basel, Switzerland) according to the manufacturer’s instructions.
Analysis of pyrosequencing data.
Pyrosequencing results were analyzed as follows. Unique barcodes for each amplicon as a standard were sorted from distinctive samples, and readings were obtained. Removal of either of these nontarget sequences, including the barcode, linker, and primers, or more than two ambiguous nucleotides, resulted in low-quality score of less than 25 through Trimmomatic, version 0.321 (45), and reads shorter than 300 bp from the original sequencing reads. Additionally, the Bellerophon method was used to discard chimeric sequences, and then a full sequence was compared both in the forward and reverse directions via BLASTN (46). The similarity of each full sequence to valid published type strains was determined using the EzTaxon-e database (http://eztaxon-e.ezbiocloud.net) or an uncultured bacterium clone in the GenBank database (https://blast.ncbi.nlm.nih.gov). Chao1 estimation at a 3% distance (47) and Shannon diversity index (48) were used to confirm the levels of richness and diversity of each sample. Phylogenetic analysis of microbial communities was estimated via Fast UniFrac (49) combined with principle coordinate analysis. Finally, XOR analysis in the CLcommunity program (Chunlab Inc., Seoul, South Korea) was used to compare the number of operational taxonomic units among samples.
Determining and comparison of soil extract ingredients.
To determine the impacts of different ingredients, the rhizosphere soil (Robinia pseudoacacia L.) at Kyonggi University was collected and prepared via three methods. The first was NSE. The second was TSE1, where sample was autoclaved at 121°C for an hour and allowed to cool and settle, and then the supernatant was passed through a 0.22-µm-filter nitrocellulose membrane using a vacuum pump, followed by a rotary evaporator bath at 40°C to reduce water to 200 ml. The third method was TSE2, which is similar to TSE1 except sterilization (at 121°C) was replaced with shaking overnight at room temperature to compare variations in soil components at high temperature. Three samples obtained from the supernatant after soil extraction methods were lyophilized at −54°C to produce 6.36 g powder (NSE), 4.65 g for TSE1, and 4.73 g for TSE2. These powders were stored at 4°C until analysis. For inorganic composition and carbohydrates, soils were prepared and analyzed directly from a final volume of 1 liter of deionized water without adding any substrates. Experiments were repeated three times to ensure accuracy, and chemical data were prepared and analyzed individually in triplicate.
Sample preparation for simultaneous profiling analysis of amino acids, organic acids, and fatty acids in soil extract.
Amino acids, organic acids, and fatty acids were simultaneously profiled in soil samples as their ethoxycarbonylation (EOC), methoximation (MO), and tert-butyldimethylsilyl (TBDMS) derivatives as described previously (50, 51). Briefly, 2.5 mg soil extract was dissolved in distilled water containing 0.1 μg of norvaline, 3,4-dimethoxybenzoic acid, and pentadecanoic acid as internal standards. The solution pH was adjusted to ≥12 with 5.0 M sodium hydroxide and mixed with dichloromethane (2.0 ml) containing 40 μl ethyl chloroformate, which was converted to the EOC derivative. This was converted to the MO derivative via a reaction with methoxyamine hydrochloride at 60°C for 60 min. The aqueous phase as sequential EOC/MO derivatives was acidified (pH ≤2.0 with 10% sulfuric acid), saturated with sodium chloride, and extracted with diethyl ether (3 ml two times). The extracts were evaporated to dryness using a gentle nitrogen stream. Dry residues containing amino acids, organic acids, and fatty acids were reacted at 60°C for 30 min with trimethylamine (5 μl), toluene (15 μl), and N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (20 μl) to form the TBDMS derivative. All samples were prepared individually in triplicate and examined directly by gas chromatography-mass spectrometry (GC-MS) in selected ion monitoring (SIM) mode.
Inorganic ingredients.
The cations Li+, Na+, Mg2+, K+, Ca2+, and NH4+ were detected using a Dionex ICS3000 (Sunnyvale, CA, USA) with an IonPac CS12A column (4 by 250 mm; Dionex) and detector (suppressed conductivity, CSRS URTRA [4 mm], recycle mode). The oven temperature was 30°C, injection volume was 25 μl, samples were eluted with 20 mM methanesulfonic acid at a flow rate 1 ml/min, and run time was 20 min according to the IonPac CS12A manual (Thermo Fisher Scientific). A Dionex ICS3000 was also used to detect the anions F−, Cl−, Br−, NO2−, NO3−, SO42−, and PO42− with standards, and the column was an IonPac AS20 (4 by 250 mm; Dionex); the detector was a suppressed-conductivity ASRS URTRA II (4 mm) in recycle mode. Gradient elution was conducted for 0 to 8 min (12 mM KOH), 8 to 12 min (30 mM KOH), 12 to 17 min (30 mM KOH), 17 to 18 min (12 mM KOH), and 18 to 20 min (12 mM KOH) at a flow rate 1 ml/min. The oven temperature was 30°C, and injection volume was 25 μl. All processes were conducted as described in the IonPac AS20 anion-exchange column product manual (Thermo Fisher Scientific).
Accession number(s).
Newly determined sequence data have been deposited in GenBank under accession numbers KY117487 to KY117530, KY121981 to KY122022, KY176921 to KY176989, KY381872 to KY381896, KY445600 to KY445722, MH688754 to MH688835, MH698624 to MH698830, and MH699124 to MH699292.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A09916982).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01145-18.
REFERENCES
- 1.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman VA, Cohen DR, Felix CR, Fetterman KA, Millett WP, Nitti AG, Zullo AM, Chen C, Lewis K. 2015. A new antibiotic kills pathogens without detectable resistance. Nature 517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis K, Epstein S, D'Onofrio A, Ling LL. 2010. Uncultured microorganisms as a source of secondary metabolites. J Antibiot 63:468–476. doi: 10.1038/ja.2010.87. [DOI] [PubMed] [Google Scholar]
- 3.Doroghazi JR, Albright JC, Goering AW, Ju KS, Haines RR, Tchalukov KA, Labeda DP, Kelleher NL, Metcalf WW. 2014. A roadmap for natural product discovery based on large-scale genomics and metabolomics. Nat Chem Biol 10:963–968. doi: 10.1038/nchembio.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milshteyn A, Schneider JS, Brady SF. 2014. Mining the metabiome: identifying novel natural products from microbial communities. Chem Biol 21:1211–1223. doi: 10.1016/j.chembiol.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gawad C, Koh W, Quake SR. 2016. Single-cell genome sequencing: current state of the science. Nat Rev Genet 17:175–188. doi: 10.1038/nrg.2015.16. [DOI] [PubMed] [Google Scholar]
- 6.Heath JR, Ribas A, Mischel PS. 2016. Single-cell analysis tools for drug discovery and development. Nat Rev Drug Discov 15:204–216. doi: 10.1038/nrd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vartoukian SR, Palmer RM, Wade WG. 2010. Strategies for culture of “unculturable” bacteria. FEMS Microbiol Lett 309:1–7. doi: 10.1111/j.1574-6968.2010.02000.x. [DOI] [PubMed] [Google Scholar]
- 8.Stewart EJ. 2012. Growing unculturable bacteria. J Bacteriol 194:4151–4160. doi: 10.1128/JB.00345-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward DM, Weller R, Bateson MM. 1990. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature 345:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- 10.Stott MB, Crowe MA, Mountain BW, Smirnova AV, Hou S, Alam M, Dunfield PF. 2008. Isolation of novel bacteria, including a candidate division, from geothermal soils in New Zealand. Environ Microbiol 10:2030–2041. doi: 10.1111/j.1462-2920.2008.01621.x. [DOI] [PubMed] [Google Scholar]
- 11.Hobbie JE, Hobbie EA. 2013. Microbes in nature are limited by carbon and energy: the starving-survival lifestyle in soil and consequences for estimating microbial rates. Front Microbiol 4:1–11. doi: 10.3389/fmicb.2013.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierra M, Carmona-Martínez AA, Trably E, Godon JJ, Bernet N. 2015. Microbial characterization of anode-respiring bacteria within biofilms developed from cultures previously enriched in dissimilatory metal-reducing bacteria. Bioresour Technol 195:283–287. doi: 10.1016/j.biortech.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Davis KER, Joseph SJ, Peter H, Janssen PH. 2005. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Appl Environ Microbiol 71:826–834. doi: 10.1128/AEM.71.2.826-834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burmølle M, Johnsen K, Al-Soud WA, Hansen LH, Sørensen SJ. 2009. The presence of embedded bacterial pure cultures in agar plates stimulate the culturability of soil bacteria. J Microbiol Methods 79:166–173. doi: 10.1016/j.mimet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 15.D'Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K. 2010. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem Biol 17:254–264. doi: 10.1016/j.chembiol.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor CB. 1951. Nature of the factor in soil-extract responsible for bacterial growth-stimulation. Nature 168:115–116. doi: 10.1038/168115a0. [DOI] [PubMed] [Google Scholar]
- 17.Taylor CB. 1951. The nutritional requirements of the predominant bacterial flora of the soil. J Appl Microbiol 14:101–111. doi: 10.1111/j.1365-2672.1951.tb01999.x. [DOI] [Google Scholar]
- 18.Hamaki T, Suzuki M, Fudou R, Jojima Y, Kajiura T, Tabuchi A, Sen K, Shibai H. 2005. Isolation of novel bacteria and actinomycetes using soil-extract agar medium. J Biosci Bioeng 99:485–492. doi: 10.1263/jbb.99.485. [DOI] [PubMed] [Google Scholar]
- 19.Vilain S, Luo Y, Hildreth MB, Brozel VS. 2006. Analysis of the life cycle of the soil saprophyte Bacillus cereus in liquid soil extract and in soil. Appl Environ Microbiol 72:4970–4977. doi: 10.1128/AEM.03076-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen PH, Yates PS, Grinton BE, Taylor PM, Sait M. 2002. Improved culturability of soil bacteria and isolation in pure culture of novel members of the divisions Acidobacteria, Actinobacteria, Proteobacteria, and Verrucomicrobia. Appl Environ Microbiol 68:2391–2396. doi: 10.1128/AEM.68.5.2391-2396.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenborn L, Yates PS, Grinton BE, Hugenholtz P, Janssen PH. 2004. Liquid serial dilution is inferior to solid media for isolation of cultures representative of the phylum-level diversity of soil bacteria. Appl Environ Microbiol 70:4363–4366. doi: 10.1128/AEM.70.7.4363-4366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen PH. 2008. Accessing uncultured microorganisms: from the environment to organisms and genomes and back. ASM Press, Washington, DC. [Google Scholar]
- 23.Nichols D, Cahoon N, Trakhtenberg EM, Pham L, Mehta A, Belanger A, Kanigan T, Lewis K, Epstein SS. 2010. Use of iChip for high-throughput in situ cultivation of “uncultivable” microbial species. Appl Environ Microbiol 76:2445–2450. doi: 10.1128/AEM.01754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amanullah A, Otero JM, Mikola M, Hsu A, Zhang J, Aunins J, Schreyer HB, Hope JA, Russo AP. 2010. Novel micro-bioreactor high throughput technology for cell culture process development: reproducibility and scalability assessment of fed-batch CHO cultures. Biotechnol Bioeng 106:57–67. doi: 10.1002/bit.22664. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Liu KK. 2008. Optical tweezers for single cells. J R Soc Interface 5:671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fröhlich J, König H. 2000. New techniques for isolation of single prokaryotic cells. FEMS Microbiol Rev 24:567–572. doi: 10.1111/j.1574-6976.2000.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaeberlein T, Lewis K, Epstein SS. 2002. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 296:1127–1129. doi: 10.1126/science.1070633. [DOI] [PubMed] [Google Scholar]
- 28.Bollmann A, Lewis K, Epstein SS. 2007. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl Environ Microbiol 73:6386–6390. doi: 10.1128/AEM.01309-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrari BC, Binnerup SJ, Gillings M. 2005. Microcolony cultivation on a soil substrate membrane system selects for previously uncultured soil bacteria. Appl Environ Microbiol 71:8714–8720. doi: 10.1128/AEM.71.12.8714-8720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pham VHT, Kim J. 2012. Cultivation of unculturable soil bacteria. Trends Biotechnol 30:475–484. doi: 10.1016/j.tibtech.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Liebeke M, Brözel VS, Hecker M, Lalk M. 2009. Chemical characterization of soil extract as growth media for the ecophysiological study of bacteria. Appl Microbiol Biotechnol 83:161–173. doi: 10.1007/s00253-009-1965-0. [DOI] [PubMed] [Google Scholar]
- 32.Harris DC. 2010. Quantitative chemical analysis, 8th edition WH Freeman and Company, New York, NY. [Google Scholar]
- 33.Friedel JK, Scheller E. 2002. Composition of hydrolysable amino acids in soil organic matter and soil microbial biomass. Soil Biol Biochem 34:315–325. doi: 10.1016/S0038-0717(01)00185-7. [DOI] [Google Scholar]
- 34.Stenger VA. 1996. Solubilities of various alkali metal and alkaline earth metal compounds in methanol. J Chem Eng Data 41:1111–1113. doi: 10.1021/je960124k. [DOI] [Google Scholar]
- 35.Reasoner DJ, Geldreich EE. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakumanu ML, Williams MA. 2012. Soil diffusion system enriches the growth of diverse and previously uncultivated bacterial taxa. Soil Sci Soc Am J 76:463–474. doi: 10.2136/sssaj2011.0227. [DOI] [Google Scholar]
- 37.Joseph SJ, Hugenholtz P, Sangwan P, Osborne CA, Janssen PH. 2003. Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl Environ Microbiol 69:7210–7215. doi: 10.1128/AEM.69.12.7210-7215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sait M, Davis KE, Janssen PH. 2006. Effect of pH on isolation and distribution of members of subdivision 1 of the phylum Acidobacteria occurring in soil. Appl Environ Microbiol 72:1852–1857. doi: 10.1128/AEM.72.3.1852-1857.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer NA. 2009. Comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453. doi: 10.1038/ismej.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kielak AM, Barreto CC, Kowalchuk GA, van Veen JA, Kuramae EE. 2016. The ecology of Acidobacteria: moving beyond genes and genomes. Front Microbiol 7:744. doi: 10.3389/fmicb.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tschech A, Pfennig N. 1984. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch Microbiol 137:163–167. doi: 10.1007/BF00414460. [DOI] [Google Scholar]
- 42.Widdel F, Kohring GW, Mayer F. 1983. Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. Arch Microbiol 134:286–294. doi: 10.1007/BF00407804. [DOI] [PubMed] [Google Scholar]
- 43.Browne HP, Forster SC, Anonye BO, Kumar N, Neville BA, Stares MD, Goulding D, Lawley TD. 2016. Culturing of “unculturable” human microbiota reveals novel taxa and extensive sporulation. Nature 533:543–546. doi: 10.1038/nature17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glöckner FO, Rosselló-Móra R. 2008. The all-species living tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 31:241–250. doi: 10.1016/j.syapm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huber T, Faulkner G, Hugenholtz P. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319. doi: 10.1093/bioinformatics/bth226. [DOI] [PubMed] [Google Scholar]
- 47.Chao A. 1984. Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270. [Google Scholar]
- 48.Ludwig JA, Reynolds JF. 1988. Statistical ecology: a primer on methods and computing. Wiley, New York, NY. [Google Scholar]
- 49.Hamady M, Lozupone C, Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J 4:17–27. doi: 10.1038/ismej.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paik MJ, Kim KR. 2004. Sequential ethoxycarbonylation, methoximation and tert-butyldimethylsilylation for simultaneous determination of amino acids and carboxylic acids by dual-column gas chromatography. J Chromatogr A 1034:13–23. doi: 10.1016/j.chroma.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 51.Paik MJ, Lee HJ, Kim KR. 2005. Simultaneous retention index analysis of urinary amino acids and carboxylic acids for graphic recognition of abnormal state. J Chromatogr B Anal Technol Biomed Life Sci 821:94–104. doi: 10.1016/j.jchromb.2005.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.