The widespread use of antibiotics in therapy and in the prevention of Streptococcus suis infection in the swine industry raises concerns for the emergence of a resistant strain. The use of antivirulence agents has potential benefits, mainly because of the reduced selective pressure for the development of bacterial resistance. In this study, we found that amentoflavone is an effective agent against S. suis serotype 2 (SS2) infection both in vitro and in vivo. Our results demonstrated that amentoflavone is a promising anti-infective therapeutic for S. suis infections, due to its antivirulence and anti-inflammatory effects without antibacterial activity, with fewer side effects than conventional antibacterial agents.
KEYWORDS: Streptococcus suis, amentoflavone, anti-inflammation, antivirulence, suilysin
ABSTRACT
Streptococcus suis, an important zoonotic pathogen, has caused considerable economic losses in the swine industry and severe public health issues worldwide. The development of a novel effective strategy for the prevention and therapy of S. suis is urgently needed. Here, amentoflavone, a natural biflavonoid compound isolated from Chinese herbs that has negligible anti-S. suis activity, was identified as a potent antagonist of suilysin (SLY)-mediated hemolysis without interfering with the expression of SLY. Amentoflavone effectively inhibited SLY oligomerization, which is critical for its pore-forming activity. The treatment with amentoflavone reduced S. suis-induced cytotoxicity in macrophages (J774 cells). Furthermore, S. suis-infected mice that received amentoflavone exhibited lower mortality and bacterial burden. Additionally, amentoflavone significantly decreased the production of tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 in an S. suis-infected cell model. Analyses of signaling pathways demonstrated that amentoflavone reduced S. suis-induced inflammation in S. suis serotype 2 (SS2)-infected cells by regulating the p38, Jun N-terminal protein kinase 1 and 2 (JNK1/2), and NF-κB pathways. The antivirulence and anti-inflammatory properties of amentoflavone against S. suis infection provide the possibility for future pharmaceutical application of amentoflavone in the treatment of S. suis infection.
IMPORTANCE The widespread use of antibiotics in therapy and in the prevention of Streptococcus suis infection in the swine industry raises concerns for the emergence of a resistant strain. The use of antivirulence agents has potential benefits, mainly because of the reduced selective pressure for the development of bacterial resistance. In this study, we found that amentoflavone is an effective agent against S. suis serotype 2 (SS2) infection both in vitro and in vivo. Our results demonstrated that amentoflavone is a promising anti-infective therapeutic for S. suis infections, due to its antivirulence and anti-inflammatory effects without antibacterial activity, with fewer side effects than conventional antibacterial agents.
INTRODUCTION
Streptococcus suis is an important zoonotic pathogen that is responsible for considerable economic losses in the swine industry worldwide (1). S. suis infection can cause septicemia, pneumonia, endocarditis, arthritis, or even severe systemic diseases such as meningitis and toxic shock-like syndrome (2). S. suis, an emerging infectious pathogen, has attracted much attention primarily because of its high zoonotic potential in humans by close contact with infected pigs and contaminated raw pork products or the consumption of undercooked pork products (3). More than 30 serotypes of S. suis have been identified, and they vary in their distribution according to the geographic location (3). Among them, serotype 2 of S. suis is historically considered the most frequent and virulent type because of its close correlation to clinical infections in both swine and humans worldwide (4). Since S. suis was first discovered, approximately 1,600 S. suis-infected cases have been reported in humans worldwide (5). In addition to two large-scale outbreaks of S. suis in China, the pathogen (S. suis serotype 2 [SS2]) is considered the primary cause of adult meningitis in Vietnam, the second in Thailand, and the third in Hong Kong (2, 6). The survivors of S. suis-induced meningitis often suffer from irreversible sequelae such as deafness (7). S. suis infections have caused severe public health issues. There is an urgent need to better understand the factors associated with the pathology of S. suis infection and to implement effective strategies that minimize the infection-induced society burdens.
Among the virulence factors of S. suis, suilysin (SLY) is a secreted extracellular pore-forming toxin which belongs to the cholesterol-dependent cytolysin family (2). SLY, similarly to other members of the cholesterol-dependent cytolysin family, can cause necrosis, apoptosis, and cell lysis in various host cells (8). SLY was shown to contribute to the development of bacterial meningitis and the increased mortality in mouse infection models (9). S. suis with an increased SLY production caused higher mortality in intraperitoneally infected mice than a nonvirulent strain, indicating that an increase in SLY production was associated with an enhanced S. suis virulence (10). Although the role of SLY in its contribution to human SS2 infections is not fully understood, SLY-positive strains have been reported to result in more severe symptoms than SLY-negative strains (11, 12). Furthermore, the virulence of SS2 can be enhanced through the upregulation of the sly gene at the transcriptional level (11, 13, 14). In addition, SLY was also reported as the sole stimulatory factor for platelet activation and aggregation in infections caused by S. suis (15). Therefore, SLY plays an important role during the pathogenesis of S. suis infection (16), and SLY may be a potential new target for the treatment of SS2 infection.
The severity and outcome of SS2 infections are closely related to the host innate immune response—the first line of defense against invading microorganisms. Innate immune cells (natural killers, macrophages, and dendritic cells) recognize the pathogens by using pattern-recognition receptors (PRRs) and subsequently proceed with a tightly regulated pathogen-specific immune response (17). Both NF-κB and mitogen-activated protein kinase (MAPK) are activated by PRRs, resulting in inflammatory responses. S. suis infection triggers the release of several proinflammatory cytokines and chemokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 (18, 19). Furthermore, excessive inflammation is closely associated with some clinical manifestations, including meningitis, septicemia, septic shock, and sudden death (20). Therefore, an alleviation of the exaggerated inflammatory response is suggested as one of the methods to improve the outcome of SS2 infection.
Amentoflavone (4′,4‴,5,5″,7,7″-hexahydroxy-3‴,8-biflavone) is a biflavonoid compound that has been widely used in traditional Chinese medicine. Amentoflavone has been identified and isolated from Selaginella tamariscina, Ginkgo biloba, Hypericum perforatum, and other plants. The chemical structure of amentoflavone is depicted in Fig. 1A. Amentoflavone has been shown to have antioxidant (21), anticancer (22), anti-inflammatory (21), antibacterial (23), antiviral (24), and antiradiation (25) activities. In addition, we recently found that amentoflavone exhibits strong antagonizing effects against pneumolysin by interacting with the toxin and reducing the oligomerization of wild-type PLY, suggesting that amentoflavone is an effective antivirulence agent (26). In this study, we investigated the antivirulence and anti-inflammatory activities of amentoflavone and investigated its mechanism of action on S. suis-induced infection.
FIG 1.
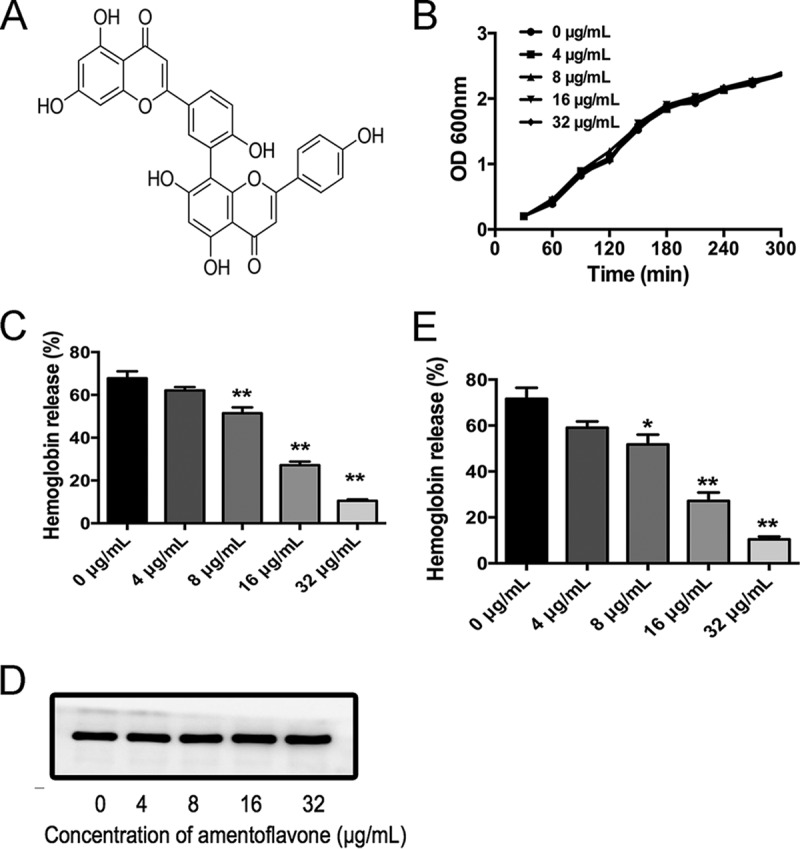
Amentoflavone inhibits SLY pore-forming activity. (A) Chemical structure of amentoflavone. (B) Growth curves of SS2 cultured in Todd-Hewitt broth containing 2% yeast extract and 5% FBS and treated with different concentrations of amentoflavone. (C) Hemolytic activity of supernatants from SS2 and amentoflavone coculture system. (D) Western blot of SLY expression in the culture supernatants with or without amentoflavone. (E) Inhibitory effect of amentoflavone on hemolytic activity of purified SLY (100 ng/ml). The data shown are representative of three independent experiments. *, P < 0.05; **, P < 0.01 versus 0 μg/ml.
RESULTS
Amentoflavone inhibits SLY pore-forming activity without interfering with SS2 growth.
The MIC of amentoflavone that was tested against SS2 was >256 μg/ml, which indicated that amentoflavone had little antimicrobial activity against SS2. Growth curve assays were performed to confirm that increasing the amentoflavone concentration from 4 to 32 μg/ml had no significant inhibitory effect on the growth of SS2 (Fig. 1B). As shown in Fig. 1C, the supernatants from SS2 culture medium showed hemolysis activity, as previously reported (27). The supernatants from an SS2 and amentoflavone (8 to 32 μg/ml) coculture system showed significantly lower hemolysis activity than that from SS2, suggesting that amentoflavone attenuated the hemolytic activity of SS2 culture supernatants. The percentage of hemoglobin released was also dose dependent at test concentrations of amentoflavone ranging from 4 to 32 μg/ml. SLY in the supernatants was the main component with hemolytic activity. Therefore, a decrease in hemolysis activity induced by amentoflavone was likely due to a reduction in the expression of SLY or in the pore-forming activity of SLY in the culture supernatants. Furthermore, the culture supernatants were subjected to Western blot analysis. There was no significant change in the expression of SLY in the supernatants of cultures treated with increasing amentoflavone concentrations, ranging from 0 to 32 μg/ml (Fig. 1D), indicating that amentoflavone reduced the pore-forming activity of SLY rather than SLY expression. To better determine the neutralization activity of amentoflavone on SLY itself, a separate experiment was carried out, using purified SLY incubated with amentoflavone to verify that amentoflavone alone can inhibit SLY protein hemolysis activity. The activity of purified SLY was significantly decreased with increased amentoflavone concentrations, ranging from 8 to 32 μg/ml (Fig. 1E), suggesting that amentoflavone directly inhibited SLY pore-forming activity. Four trials were conducted to confirm that amentoflavone directly neutralized SLY activity without interfering with SS2 growth with the tested concentrations.
Amentoflavone changed the secondary structure of SLY.
Circular dichroism (CD) spectroscopy is an excellent method to detect the secondary structures and to monitor the structural changes of proteins under diverse conditions (28). In this study, a CD spectrum analysis was performed to evaluate the conformational change of SLY incubated with different concentrations of amentoflavone. The resulting secondary structures were calculated and are listed in Table 1. Amentoflavone treatment caused some alterations of SLY conformation. The percentage of SLY in an α-helix conformation was reduced after incubation with amentoflavone, whereas the proportions of β-sheets, β-turns, and other structures increased. These results indicate that amentoflavone treatment caused the conformational changes in SLY molecules.
TABLE 1.
The secondary structural contents of SLY with different concentration of amentoflavone
| Concn of amentoflavone (μg/ml) |
Content (%) |
NRMSDa | |||
|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turns | Others | ||
| 0 (control) | 10.7 | 39.2 | 12.1 | 38 | 0.03675 |
| 4 | 0 | 44.6 | 13.6 | 41.8 | 0 |
| 8 | 0 | 42.4 | 16 | 41.7 | 0.09307 |
| 16 | 0 | 44.2 | 15.6 | 40.2 | 0.11475 |
| 32 | 0 | 40.9 | 20.4 | 38.7 | 0.13482 |
NRMSD, normalized root-mean-square deviation.
Amentoflavone inhibits the oligomerization of SLY.
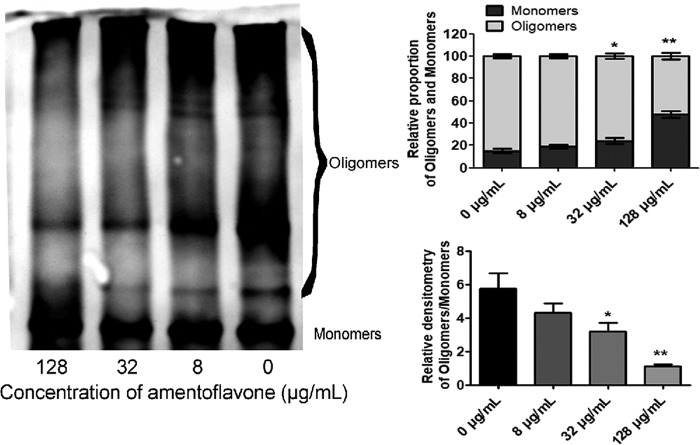
The SLY secreted by SS2 comprises water-soluble monomers in the original form, which self-associate into oligomers that subsequently form pores in the membranes of host cells (16). Oligomerization is essential for the pore-forming activity of SLY. Purified SLY was incubated with amentoflavone, and then oligomerization was induced in a high-salt-concentration environment. Figure 2 shows the significant inhibition of SLY oligomerization by amentoflavone. Amentoflavone treatment decreased the amount of SLY oligomers but increased the corresponding SLY monomer content. The amounts of SLY oligomers decreased with increasing amentoflavone concentrations, ranging from 8 to 128 μg/ml. Amentoflavone had a significant inhibitory effect on the formation of SLY oligomers, resulting in a reduction in the pore-forming activity of SLY.
FIG 2.
Inhibitory effect of amentoflavone on oligomerization of suilysin. Purified SLY (500 μg/ml) was incubated with amentoflavone (8 to 128 μg/ml), and the oligomerization of SLY was analyzed by Western blot. *, P < 0.05; **, P < 0.01 versus 0 μg/ml.
Amentoflavone decreases SS2-induced proinflammatory cytokine production within J774 cells.
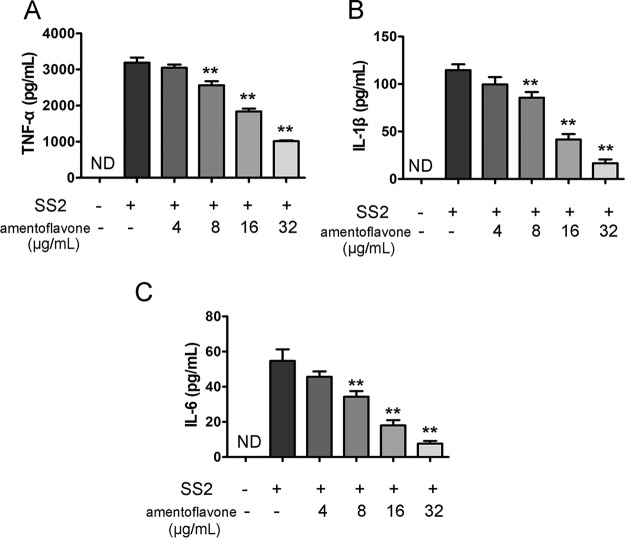
S. suis infection can trigger the release of several proinflammatory cytokines, including TNF-α, IL-1β, and IL-6. It has been reported that the overproduction of proinflammatory cytokines is closely associated with some clinical manifestations, such as meningitis and streptococcal toxic shock-like syndrome (STSLS) (20). To investigate whether amentoflavone exhibited immunomodulatory activity on macrophages, J774 cells were incubated with SS2 (multiplicity of infection [MOI] = 10:1) and various concentrations of amentoflavone for 6 h. As shown in Fig. 3, TNF-α, IL-6, and IL-1β were secreted from J774 cells in response to the SS2 challenge as measured by enzyme-linked immunosorbent assays (ELISAs). The levels of TNF-α (Fig. 3A), IL-1β (Fig. 3B), and IL-6 (Fig. 3C) in supernatants were significantly lower in SS2-infected cells treated with amentoflavone (8 to 32 μg/ml) than in untreated SS2-infected ones. Furthermore, amentoflavone treatment suppressed the secretion of TNF-α, IL-6, and IL-1β resulting from the SS2 infection in a dose-dependent manner.
FIG 3.
Amentoflavone inhibited SS2-mediated cytokine production within J774 cells. Amentoflavone inhibited the secretion of TNF-α (A), IL-1β (B), and IL-6 (C). Cells were incubated with SS2 (MOI = 10:1) and various concentrations of amentoflavone for 6 h. TNF-α, IL-1β, and IL-6 concentrations in culture medium were assayed by ELISA. ND, nondetectable; *, P < 0.05; **, P < 0.01 versus SS2 alone.
Amentoflavone decreases inflammation in SS2-infected cells by regulating the p38, JNK1/2, and NF-κB pathways.
MAPK and NF-κB signaling pathways play important roles in the regulation of inflammatory mediator production in response to SS2 challenge (29). To investigate the anti-inflammatory mechanism of amentoflavone in J774 macrophage-like cells infected by SS2, the expression of inflammation-related protein p38 mitogen-activated protein kinase (p38 MAPK), Jun N-terminal kinase (JNK), and NF-κB p65 was examined. As shown in Fig. 4, SS2 strongly induced the activation of p38, JNK1/2, and NF-κB p65 within J774 macrophages. Amentoflavone treatment for 6 h significantly repressed the activation of p38 MAPK, JNK1/2, and NF-κB p65, suggesting that amentoflavone inhibited the SS2-mediated inflammation by downregulating the p38, JNK1/2, and NF-κB pathways.
FIG 4.
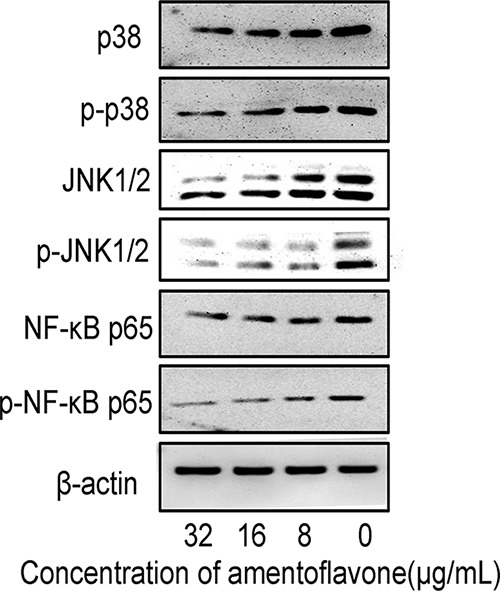
Inhibition effect of amentoflavone on SS2-induced MAPK/NF-κB activation. J774 cells were infected with SS2 at different concentrations of amentoflavone (MOI = 10:1) for 6 h, and protein samples were analyzed by Western blotting. Immunoblots showing expression levels of phospho-p38, p38, phospho-JNK1/2, JNK1/2, phospho-NF-κB p65, and NF-κB p65. β-Actin was used as an internal control.
Amentoflavone alleviates SS2-induced injury of J774 cells.
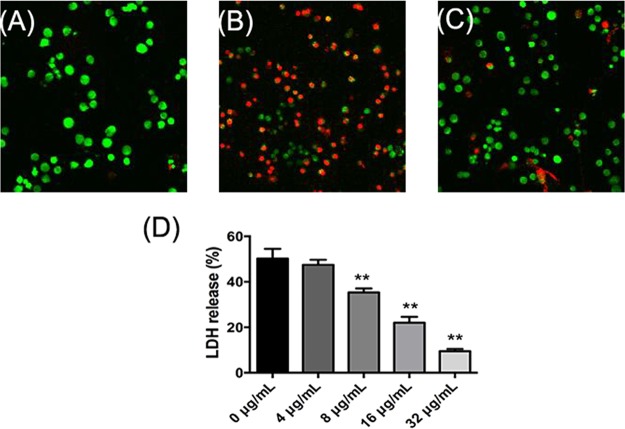
The uninfected J774 cells displayed green fluorescence when stained with a LIVE/DEAD (green/red) staining reagent (Fig. 5A). After infection with SS2 for 5 h, the transformations in cellular morphology and increases in the number of red fluorescent dead cells were observed in J774 cells (Fig. 5B), indicating that SS2 caused cell injury and death. The addition of amentoflavone (32 μg/ml) to infected J774 cells protected against SS2-mediated cell injury, as demonstrated by the significant reduction in red fluorescence (Fig. 5C). To quantify the protective effect of amentoflavone on SS2-mediated J774 cell injury, a cell viability analysis was performed using a lactate dehydrogenase (LDH) release assay kit. As shown in Fig. 5D, SS2 induced the release of approximately 50.21% of the LDH in amentoflavone-free culture supernatants. The LDH release was significantly decreased with increasing amentoflavone concentrations, ranging from 8 to 32 μg/ml, suggesting that amentoflavone protected J774 cells against SS2-induced cell injury.
FIG 5.
Protective effect of amentoflavone against SS2-mediated cell damage in J774 cells. LIVE/DEAD (green/red)-stained J774 cells were imaged with a laser scanning confocal microscope. Uninfected cells (A), SS2-infected cells (B), and SS2-infected cells treated with 32 μg/ml amentoflavone (C). (D) LDH release from J774 cells with increasing amentoflavone concentrations, from 0 to 32 μg/ml. *, P < 0.05 and **, P < 0.01 versus 0 μg/ml.
Amentoflavone protects mice from SS2 infection.
A mouse model was established to investigate the protective effect of amentoflavone against SS2 infection in vivo. As shown in Fig. 6A, 95% of infected mice died within 84 h after intraperitoneal injections of 2 × 109 CFU of bacteria per mouse (Fig. 6A). However, 60% of the infected mice that were treated with amentoflavone died by 96 h postinfection (Fig. 6A). The mortality of infected mice that received amentoflavone was significantly lower than that of mice treated with dimethyl sulfoxide (DMSO), which died within 4 days. The treatment with amentoflavone significantly reduced the bacterial burden in the liver and spleen tissues in the infected mice. Furthermore, the mice treated only with amentoflavone survived, indicating that amentoflavone itself was not toxic to the mice (Fig. 6A). The CFUs on Todd-Hewitt broth (THB) agar plates were counted to evaluate the bacterial burden in liver or spleen tissues isolated from SS2-infected mice treated with or without amentoflavone. The numbers of viable bacteria colonized in the livers and spleens of the SS2-infected mice treated with amentoflavone were significantly lower than those of infected mice treated with DMSO (Fig. 6B). These results indicate that amentoflavone treatment had a protective effect against SS2 infection in mice.
FIG 6.
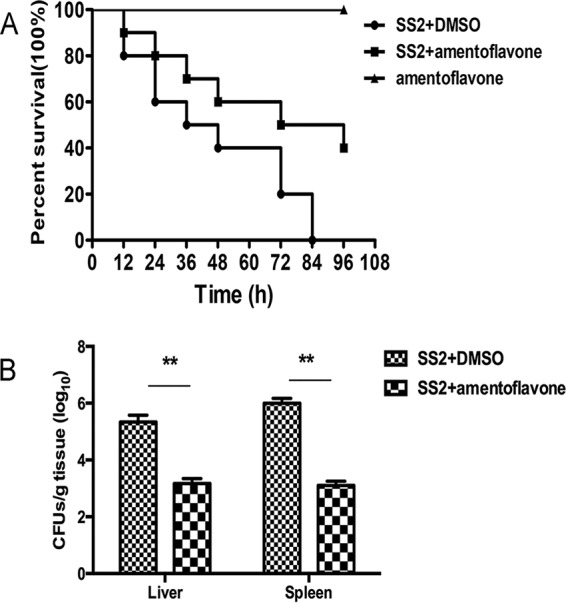
Amentoflavone protects mice from SS2 infection. Mice were infected with SS2 by intraperitoneal injections and treated with 100 mg/kg of amentoflavone or DMSO as a control. The bacterial burden was determined from the sacrificed mice at 48 h after infection. (A) Survival analysis of infected or uninfected mice treated with amentoflavone over a 96-h period. (B) Bacterial burdens in the livers and spleens of the infected mice. The results shown are representative of three independent experiments. **, P < 0.01.
DISCUSSION
S. suis has caused considerable economic losses in the swine industry and severe public health issues over the past decades. The widespread use of antibiotics in the therapy and prevention of S. suis infection in the swine industry raises concerns for the emergence of a resistant strain. Therefore, effective control of S. suis infections has become a major challenge, as with many other pathogen infections (1). The use of antivirulence agents has potential benefits, mainly because of the reduced selective pressure for the development of bacterial resistance (30). In this study, we explored alternatives from natural products for the prevention and treatment of S. suis infections.
Amentoflavone, a biflavonoid widely used in traditional Chinese medicine, was identified as an efficient inhibitor of SLY, by decreasing its hemolytic activity regardless of whether the SLY was purified or in culture supernatants. Meanwhile, amentoflavone did not affect the growth of SS2 at concentrations effective for inhibiting SLY pore-forming activity, suggesting that treatment with amentoflavone against SS2 infection would apply a milder selective pressure than conventional antibacterial agents. In a coculture system, amentoflavone exhibited antivirulence via interactions with SLY itself rather than inhibiting SLY expression. A CD spectrum analysis was performed to evaluate the secondary structures of SLY upon amentoflavone treatment. The change of secondary structure is reportedly related to the interactions between the protein and other components (31). Therefore, a change in the secondary structure of SLY might be ascribed to the molecular interactions between SLY and amentoflavone. Finally, amentoflavone interferes with the formation of high-molecular-weight complexes (also called oligomers) of SLY, which is critical for its pore-forming activity, similar to that for other cholesterol-dependent cytolysin family members, including listeriolysin, pneumolysin, and Hla (16). The data from clinical cases and murine models support a role for SLY in the pathogenesis of meningitis both in mice and in humans infected with S. suis ST1 strains, which commonly cause meningitis (9). Here, we found that amentoflavone produced its antivirulence effect by directly targeting SLY, without putting selective pressure on SS2. Therefore, amentoflavone is expected to be an effective candidate to alleviate S. suis-induced infections. To prove this hypothesis, some trials were then subsequently conducted in laboratory animals and at the cellular level. Consistent with the above-mentioned expectation, the cell injury upon SS2 infection was significantly alleviated by amentoflavone treatment at a concentration that did not inhibit SS2 growth. Therefore, amentoflavone provided effective protection to macrophages against SS2-mediated cytotoxicity. Furthermore, amentoflavone exerted protective effects against SS2 infection in a mouse model, observed as reduced mortality and reduced CFU counts in the livers and spleens of infected mice. These results indicated that amentoflavone could be an effective anti-infective agent against SS2 infection both in vitro and in vivo.
It is well known that inflammation is a normal response to protect the human body from various pathogen infections. However, uncontrolled exaggerated inflammation is harmful and can cause serious diseases (32). For example, the severity and outcome of SS2 infections is closely related to the host innate immune response. SS2 can stimulate the host immune system to produce massive amounts of proinflammatory cytokines, including TNF-α, gamma interferon (IFN-γ), IL-1β, IL-6, IL-12, and monocyte chemoattractant protein 1 (MCP-1) (20, 33). Furthermore, excessive inflammation plays an important role in some clinical manifestations of SS2 infection, including meningitis, septicemia, septic shock, and sudden death (34). Therefore, the alleviation of the exaggerated inflammatory response is suggested as one of the possible means to improve the outcome of SS2 infection. Amentoflavone was previously reported to have anti-inflammatory activity in various models (35, 36). In this study, we investigated the anti-inflammatory activity of amentoflavone in response to SS2 infection. The results demonstrated that amentoflavone significantly decreased the levels of TNF-α, IL-1β, and IL-6 in supernatants of SS2-infected J774 cells in a dose-dependent manner. Furthermore, we sought to determine the possible signaling pathway involved in the regulation of this inflammatory response. The MAPK/NF-κB pathway has been considered to play a pivotal role in the inflammatory response of SS2 infection (37). Amentoflavone treatment significantly inhibited SS2-induced phosphorylation of JNK1/2, p38, and NF-κB in J774 cells. Our results suggest that amentoflavone suppresses the MAPK-JNK1/2-p38 pathway, as well as NF-κB signaling, both of which mediate the production of proinflammatory cytokines in J774 cells, thus contributing to the anti-inflammatory effects in response to an SS2 challenge.
In summary, our data suggest that amentoflavone attenuated Streptococcus suis infection by targeting SLY and the inflammation response, which indicates that amentoflavone is a promising candidate for treating S. suis infections.
MATERIALS AND METHODS
Bacterial strain, reagents, culture conditions.
Amentoflavone (>99% purity) and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA). The bacterial strain ZY05719 (highly virulent S. suis serotype 2) was kindly provided by Hongjie Fan (Key Lab of Animal Bacteriology, Ministry of Agriculture, Nanjing Agricultural University, Nanjing, China). SS2 strain ZY05719 was cultured in THB (Qingdao Hope Biol-Technology Co., Ltd., Qingdao, China) supplemented with or without amentoflavone at 37°C. High-glucose and l-glutamine Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were purchased from Thermo Fisher Scientific Co. (Waltham, MA, USA). The cytotoxicity detection (LDH) kit was purchased from Roche (Basel, Switzerland). IL-1β, IL-6, and TNF-α ELISA kits were purchased from BioLegend (San Diego, CA, USA).
MIC testing.
The MIC of amentoflavone for S. suis cells (5 × 105 CFU/ml) was determined using the broth microdilution method in THB at 37°C (38). The final concentrations of amentoflavone used ranged from 0.2 to 1,024 μg/ml. The MICs were defined as the lowest drug concentrations that inhibited bacterial growth.
Growth curve.
The growth of S. suis cells cultured with the indicated concentrations of amentoflavone was tested by determining the optical density of each sample at 600 nm (OD600) every 30 min.
Hemolysis assay.
The hemolytic activity of bacterial culture supernatants was assessed as described previously (39). Briefly, overnight cultures of SS2 were inoculated in fresh THB (1:50, containing 2% yeast extract and 5% FBS) for 2 h with shaking and then treated with various concentrations of amentoflavone until the OD600 reached 2.4, at which point they were harvested by centrifugation (10,000 rpm for 4 min). The bacterial culture supernatants were incubated with rabbit erythrocytes (2.5%) in 0.1 M phosphate-buffered saline (PBS; pH 7.4) at 37°C for 30 min and then subjected to centrifugation (10,000 rpm for 1 min). The hemolytic activity of each sample was determined by measuring the release of hemoglobin in supernatants at an OD543. Triton X-100 (1%) was used as the positive control, and PBS served as the negative control. The percentage of hemolysis was calculated by comparing each sample to the positive control in both assays.
To examine the direct effect of amentoflavone on hemolysis activity induced by SLY, purified SLY was obtained according to our previous study (27). The hemolysis activity of purified SLY incubated with or without amentoflavone was determined as described above.
Western blotting assay.
The bacterial culture supernatants with or without amentoflavone described above were collected by centrifugation (10,000 rpm for 1 min). SDS-PAGE was performed under reducing conditions using a Mini-Protean Tetra Cell (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The samples were separated on a 12% separating gel at 120 V and then transferred to polyvinylidene difluoride (PVDF) membranes. After blocking in 5% nonfat dry milk, the membranes were incubated with the primary rabbit anti-SLY antibody diluted 1:1,000 and the horseradish peroxidase-conjugated secondary antibody (Proteintech) diluted 1:8,000. Protein bands on the membranes were visualized with Amersham ECL Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK).
Circular dichroism analysis.
Purified SLY (0.5 mg/ml) was incubated with different concentrations of amentoflavone for 1 h at 37°C. The secondary structure of the purified SLY was determined by a CD spectrophotometer (MOS-500; Bio-Logic, France) using a quartz cuvette of 1-mm optical path length at room temperature (25°C). The scanning wavelengths for measurement were 190 to 250 nm, with the scanning rate at 50 nm/min and a bandwidth of 1.0 nm. The secondary structures of SLY were analyzed using the BeStSel web server (40).
Oligomeric inhibition assay.
Purified SLY (0.4 mg/ml) was incubated with different concentrations of amentoflavone for 30 min at 37°C and then maintained in saturated potassium chloride for 5 min. Then, 2.5 μl rabbit red blood cells was added, and the mixture was kept on ice for 5 min. The oligomerization level of SLY was detected by Western blotting. Briefly, the sample was collected and mixed with SDS-PAGE loading buffer without 2-hydroxy-1-ethanethiol and then placed in a water bath at 55°C for 10 min. The protein was separated on a 6% separating gel at 125 V.
Cell culture and infection.
The J774 macrophage-like cells were incubated in high-glucose and l-glutamine DMEM supplemented with 1% (vol/vol) penicillin/streptomycin and 10% (vol/vol) FBS. The cells were infected by SS2 at an MOI of 10 CFU/cell.
LIVE/DEAD and cytotoxicity assays.
The J774 cells were seeded at a density of 2 × 104 cells/well in 96-well plates and incubated at 37°C and 5% CO2 for 24 h. The culture medium was removed, and SS2 was diluted with cell culture medium with or without amentoflavone. After 5 h of incubation, the cells were subjected to LIVE/DEAD and cytotoxicity analyses. For the LIVE/DEAD assay, cells were treated with LIVE/DEAD (green/red) reagent (Invitrogen) and observed with a laser scanning confocal microscope (FV1000; Olympus). Cell viability was determined by measuring LDH released into the supernatants of the coculture system by using a cytotoxicity detection kit (LDH; Roche, Basel, Switzerland) as previously described (41).
Cytokine and Western blot assays.
The J774 cells were seeded at a density of 1 × 106 cells/well in 6-well plates and incubated for 12 h. The cells were infected with SS2 with or without amentoflavone for 6 h. The levels of inflammatory cytokines TNF-α, IL-1β, and IL-6 in the culture supernatants were determined by ELISA kits (BioLegend, San Diego, CA, USA) according to the manufacturer’s instructions.
The cells were washed with 1 ml PBS/well three times. Total protein from J774 cells was extracted with M-PER mammalian protein extraction reagent (Thermo). The proteins were separated by SDS-PAGE on 10% gels. The separated proteins were transferred to PVDF membranes, blocked, and incubated with primary antibodies (p38 [1:1,000], p-p38 [1:1,000], JNK1/2 [1:800], phospho-JNK1/2 [1:800], phospho-NF-κB p65 [1:1,000], NF-κB p65 [1:1,000], and β-actin [1:1,000]; Proteintech) for 2 h at room temperature. Subsequently, the membranes were treated with secondary antibodies (horseradish peroxidase [HRP]-conjugated anti-rabbit or anti-mouse IgG antibody) for 90 min at room temperature. Protein bands on the membranes were visualized with Amersham ECL Western blotting detection reagents (GE Healthcare, Buckinghamshire, UK).
Mouse infections.
Female 6- to 8-week-old C57BL/6J mice were purchased from the Experimental Animal Center of Jilin University (Changchun, China). To establish an infection model, C57BL/6J mice received intraperitoneal injections (100 μl) of a prepared bacterial suspension. The concentration of injected SS2 was 2 × 109 CFU/ml for the survival analysis. The infected mice were administered 100 mg/kg of amentoflavone subcutaneously 2 h after infection and again at 8-h intervals up to 96 h. The dose of amentoflavone was chosen on the basis of the amount of bacteria injected in our preliminary experiments (data not shown). The positive group was injected with equal volumes of DMSO at the same time. Meanwhile, the toxicity of amentoflavone was also examined in a group of healthy mice. Each group had 10 mice, and the survival statistics were collected. To analyze the bacterial burden in tissues, the liver and spleen tissues were collected from the mice, lysed in 2% Triton X-100, and inoculated on THB agar plates at 37°C overnight.
Statistical analysis.
All experimental data are expressed as the means ± standard deviations (SDs). GraphPad Prism 5.0 was used for statistical analyses. For the survival statistics, the data were analyzed using log rank tests, and Student’s t tests were used for the other assays. P values of <0.05 and <0.01 were considered significant and are indicated in the figures.
ACKNOWLEDGMENTS
This project was funded by The National Key Technology R&D program (no. 2016YFD05013) and the National Natural Science Foundation of China (grant 31602109).
REFERENCES
- 1.Haas B, Grenier D. 2018. Understanding the virulence of Streptococcus suis: a veterinary, medical, and economic challenge. Med Mal Infect 48:159–166. doi: 10.1016/j.medmal.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Fittipaldi N, Segura M, Grenier D, Gottschalk M. 2012. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol 7:259–279. doi: 10.2217/fmb.11.149. [DOI] [PubMed] [Google Scholar]
- 3.Segura M, Fittipaldi N, Calzas C, Gottschalk M. 2017. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol 25:585–599. doi: 10.1016/j.tim.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. 2014. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 3:e45. doi: 10.1038/emi.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, Wang C. 2014. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5:477–497. doi: 10.4161/viru.28595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerdsin A, Akeda Y, Takeuchi D, Dejsirilert S, Gottschalk M, Oishi K. 2018. Genotypic diversity of Streptococcus suis strains isolated from humans in Thailand. Eur J Clin Microbiol Infect Dis 37:917–925. doi: 10.1007/s10096-018-3208-8. [DOI] [PubMed] [Google Scholar]
- 7.Hughs JM, Wilson ME, Wertheim HFL, Nghia HDT, Taylor W, Schultsz C. 2009. Streptococcus suis: an emerging human pathogen. Clin Infect Dis 48:617–625. doi: 10.1086/596763. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy S, O'Riordan M. 2013. More than a pore: the cellular response to cholesterol–dependent cytolysins. Toxins 5:618–636. doi: 10.3390/toxins5040618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi D, Akeda Y, Nakayama T, Kerdsin A, Sano Y, Kanda T, Hamada S, Dejsirilert S, Oishi K. 2014. The contribution of suilysin to the pathogenesis of Streptococcus suis meningitis. J Infect Dis 209:1509–1519. doi: 10.1093/infdis/jit661. [DOI] [PubMed] [Google Scholar]
- 10.He Z, Pian Y, Ren Z, Bi L, Yuan Y, Zheng Y, Jiang Y, Wang F. 2014. Increased production of suilysin contributes to invasive infection of the Streptococcus suis strain 05ZYH33. Mol Med Rep 10:2819–2826. doi: 10.3892/mmr.2014.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk M, Segura M, Xu J. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev 8:29–45. doi: 10.1017/S1466252307001247. [DOI] [PubMed] [Google Scholar]
- 12.Allgaier A, Goethe R, Wisselink HJ, Smith HE, Valentin-Weigand P. 2001. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J Clin Microbiol 39:445–453. doi: 10.1128/JCM.39.2.445-453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Liu L, Qiu D, Chen H, Zhou R. 2010. Identification of Streptococcus suis serotype 2 genes preferentially expressed in the natural host. Int J Med Microbiol 300:482–488. doi: 10.1016/j.ijmm.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Tan C, Liu M, Jin M, Liu J, Chen Y, Wu T, Fu T, Bei W, Chen H. 2008. The key virulence-associated genes of Streptococcus suis type 2 are upregulated and differentially expressed in vivo. FEMS Microbiol Lett 278:108–114. doi: 10.1111/j.1574-6968.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Wang J, Chen S, Yin J, Pan Z, Liu K, Li L, Zheng Y, Yuan Y, Jiang Y. 2016. Effects of suilysin on Streptococcus suis-induced platelet aggregation. Front Cell Infect Microbiol 6:128. doi: 10.3389/fcimb.2016.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenenbaum T, Asmat TM, Seitz M, Schroten H, Schwerk C. 2016. Biological activities of suilysin: role in Streptococcus suis pathogenesis. Future Microbiol 11:941–954. doi: 10.2217/fmb-2016-0028. [DOI] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Al-Numani D, Segura M, Doré M, Gottschalk M. 2003. Up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 on human THP-1 monocytes stimulated by Streptococcus suis serotype 2. Clin Exp Immunol 133:67–77. doi: 10.1046/j.1365-2249.2003.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Segura M, Stankova J, Gottschalk M. 1999. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect Immun 67:4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segura M, Vanier G, Al-Numani D, Lacouture S, Olivier M, Gottschalk M. 2006. Proinflammatory cytokine and chemokine modulation by Streptococcus suis in a whole-blood culture system. FEMS Immunol Med Microbiol 47:92–106. doi: 10.1111/j.1574-695X.2006.00067.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim HK, Son KH, Chang HW, Kang SS, Kim HP. 1998. Amentoflavone, a plant biflavone: a new potential anti-inflammatory agent. Arch Pharm Res 21:406–410. doi: 10.1007/BF02974634. [DOI] [PubMed] [Google Scholar]
- 22.Guruvayoorappan C, Kuttan G. 2008. Inhibition of tumor specific angiogenesis by amentoflavone. Biochemistry (Mosc) 73:209–218. doi: 10.1134/S0006297908020132. [DOI] [PubMed] [Google Scholar]
- 23.Pegnyemb DE, Mbing JN, de Théodore Atchadéa A, Tih RG, Sondengam BL, Blond A, Bodo B. 2005. Antimicrobial biflavonoids from the aerial parts of Ouratea sulcata. Phytochemistry 66:1922–1926. doi: 10.1016/j.phytochem.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Lin YM, Anderson H, Flavin MT, Pai YH, Mata-Greenwood E, Pengsuparp T, Pezzuto JM, Schinazi RF, Hughes SH, Chen FC. 1997. In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J Nat Prod 60:884–888. doi: 10.1021/np9700275. [DOI] [PubMed] [Google Scholar]
- 25.Lee CW, Choi HJ, Kim HS, Kim DH, Chang IS, Moon HT, Lee SY, Oh WK, Woo ER. 2008. Biflavonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fibroblasts. Bioorg Med Chem 16:732–738. doi: 10.1016/j.bmc.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Liu B, Liu S, Wang L, Wang J. 2017. Anticytotoxin effects of amentoflavone to pneumolysin. Biol Pharm Bull 40:61. doi: 10.1248/bpb.b16-00598. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Lu G, Qi Z, Li H, Wang L, Wang Y, Liu B, Niu X, Deng X, Wang J. 2017. Morin attenuates Streptococcus suis pathogenicity in mice by neutralizing suilysin activity. Front Microbiol 8:460. doi: 10.3389/fmicb.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikani BA, Singh SP. 2015. Enzyme stability, thermodynamics and secondary structures of α-amylase as probed by the CD spectroscopy. Int J Biol Macromol 81:450–460. doi: 10.1016/j.ijbiomac.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 29.Alva-Murillo N, Ochoa-Zarzosa A, Lopez-Meza JE. 2017. Sodium octanoate modulates the innate immune response of bovine mammary epithelial cells through the TLR2/P38/JNK/ERK1/2 pathway: implications during Staphylococcus aureus internalization. Front Cell Infect Microbiol 7:78. doi: 10.3389/fcimb.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasko DA, Sperandio V. 2010. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov 9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 31.Mizutani Y, Matsumura Y, Imamura K, Nakanishi K, Mori T. 2003. Effects of water activity and lipid addition on secondary structure of zein in powder systems. J Agric Food Chem 51:229–235. doi: 10.1021/jf0205007. [DOI] [PubMed] [Google Scholar]
- 32.Martinon F, Tschopp J. 2005. NLRs join TLRs as innate sensors of pathogens. Trends Immunol 26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Ye C, Zheng H, Zhang J, Jing H, Wang L, Xiong Y, Wang W, Zhou Z, Sun Q, Luo X, Du H, Gottschalk M, Xu J. 2009. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J Infect Dis 199:97–107. doi: 10.1086/594370. [DOI] [PubMed] [Google Scholar]
- 34.Domínguez-Punaro MDLC, Segura M, Radzioch D, Rivest S, Gottschalk M. 2008. Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect Immun 76:3901–3910. doi: 10.1128/IAI.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bais S, Abrol N, Prashar Y, Kumari R. 2017. Modulatory effect of standardised amentoflavone isolated from Juniperus communis L. against Freund's adjuvant induced arthritis in rats (histopathological and X ray analysis). Biomed Pharmacother 86:381–392. doi: 10.1016/j.biopha.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Sakthivel KM, Guruvayoorappan C. 2013. Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis. Int Immunopharmacol 17:907–916. doi: 10.1016/j.intimp.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Tanabe S, Gottschalk M, Grenier D. 2008. Hemoglobin and Streptococcus suis cell wall act in synergy to potentiate the inflammatory response of monocyte-derived macrophages. Innate Immun 14:357–363. doi: 10.1177/1753425908098388. [DOI] [PubMed] [Google Scholar]
- 38.Langfield RD, Scarano FJ, Heitzman ME, Kondo M, Hammond GB, Neto CC. 2004. Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J Ethnopharmacol 94:279–281. doi: 10.1016/j.jep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Liu B, Teng Z, Zhou X, Wang X, Zhang B, Lu G, Niu X, Yang Y, Deng X. 2017. Phloretin attenuates Listeria monocytogenes virulence both in vitro and in vivo by simultaneously targeting listeriolysin O and sortase A. Front Cell Infect Microbiol 7:9. doi: 10.3389/fcimb.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Micsonai A, Wien F, Kernya L, Lee YH, Goto Y, Réfrégiers M, Kardos J. 2015. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc Natl Acad Sci U S A 112:E3095–E3103. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Qiu J, Tan W, Zhang Y, Wang H, Zhou X, Liu S, Feng H, Li W, Niu X, Deng X. 2015. Fisetin inhibits Listeria monocytogenes virulence by interfering with the oligomerization of listeriolysin O. J Infect Dis 211:1376–1387. doi: 10.1093/infdis/jiu520. [DOI] [PubMed] [Google Scholar]