Abstract
Objective
Gastrointestinal (GI) tract, like other mucosal surface, is colonized with a microbial population known as gut microbiota. Outer membrane vesicles (OMVs) which are produced by gram negative bacteria could be sensed by Toll like receptors (TLRs). The interaction between gut microbiota and TLRs affects homeostasis and immune responses. In this study, we evaluated TLR2, TLR4 genes expression and cytokines concentration in Caco-2 cell line treated with Bacteroides fragilis (B. fragilis) and its OMVs.
Materials and Methods
In this experimental study, OMVs were extracted using sequential centrifugation and their physicochemical properties were evaluated as part of quality control assessment. Caco-2 cells were treated with B. fragilis and its OMVs (180 and 350 µg/ml). Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) was performed to assess TLR2 and TLR4 mRNA expression levels. Pro-inflammatory (IFNᵧ) and anti-inflammatory (IL- 4 and IL-10) cytokines were evaluated by ELISA.
Results
B. fragilis significantly decreased TLR2 and slightly increased TLR4 mRNA levels in Caco-2 cell line. The TLR2 mRNA level was slightly increased at 180 and 350 µg/ml of OMVs. Conversely, the TLR4 mRNA level was decreased at 180 µg/ml of OMVs, while it was significantly increased at 350 µg/ml of OMVs. Furthermore, B. fragilis and its OMVs significantly increased and decreased IFNᵧ concentration, respectively. Anti-inflammatory cytokines were increased by B. fragilis and its OMVs.
Conclusion
B. fragilis and its OMVs have pivotal role in the cross talk between gut microbiota and the host especially in the modulation of the immune system. Based on the last studies on immunomodulatory effect of B. fragilis derived OMVs on immune cells and our results, we postulate that B. fragilis derived OMVs could be possible candidates for the reduction of immune responses.
Keywords: Bacteroides fragilis, Gut Microbiota, Membrane Vesicles, Toll Like Receptors
Introduction
Gastrointestinal (GI) tract is colonized by a variety, complex and dynamic microbial community referring as gut microbiota. This microbial community also consists of bacteria, fungi, archea, protozoa and viruses (1). Gut microbiota constantly interacts with the epithelium of GI tract. This putative cross talk has potential role in both host functions (locally and systemically) and establishment of gut microbiota pattern. Thus, host functions and gut microbiota pattern regulate health and diseases status (2).
Gut microbiota is considered as a reservoir for immune system stimulatory molecules due to the presence of immunogenic compounds such as lipopolysaccharides (LPS), peptidoglycans (PG) and extracellular vesicles (EVs) (3, 4). These bacterial components are encountered in the gut barrier (epithelial layer) as the first line of gut innate immunity. The gut barrier is also composed of intestinal epithelial cells, mucus layer that is produced by goblet cells, innate and adaptive immune factors (i.e. antimicrobial peptides and immunoglobulins, mainly including IgA). Indeed, the gut barrier shapes gut microbiota and its interaction to host (5, 6). Moreover, the gut barrier functions are under the control of pattern recognition receptors (PRRs) including toll like receptors (TLRs), nucleotide binding domain leucine rich repeat containing receptors (NLRs), retinoic acid inducible gene like receptors (RLRs), C-type lectin receptors (CLRs) and absent in melanoma 2 (AIM2)-like receptors (ALRs) (7, 8). PRRs sense pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs), trigger various signaling cascades and induce different responses (9). Various cell types including immune and intestinal epithelial cells express TLRs that are belonged to type I transmembrane receptors (10). The expression patterns of TLRs among GI epithelial cell are different and the interaction between gut microbiota and TLRs affects local and systemic immunity (8). Disrupted homeostasis, considered as dysbiosis, results from the imbalance between gut microbiota and immune responses. It is considered as a turning point to induce many disorders including metabolic syndrome (11). This condition which is characterized by impaired permeability of gut barrier, known as leaky gut syndrome, causes a great activation of TLRs in intestinal epithelial cells (IEPCs) (12). Consequently, increased cytokines and chemokines trigger low grade inflammation. Increased inflammatory cytokines disrupt insulin signaling cascade and may cause insulin resistance (IR), ultimately promoting metabolic syndrome and obesity (13).
Bacteroides spp. such as B. fragilis have significant roles in gut microbiota-host interactions, especially on metabolic and immune system (14). Similarly, Bacteroides spp. derived outer membrane vesicles (OMVs) are key players in gut microbiota host interactions (15). OMVs are nanosized and spherical vesicles which could affect metabolic and immune system since they contain bacterial components including LPS, outer membrane proteins, phospholipids, periplasmic components, DNA, RNA, hydrolytic enzymes and signaling molecules (16).
B. fragilis also secretes capsular polysaccharide A(PSA) containing OMVs. These OMVs interact with dendritic cells (DCs) through TLR2 signaling pathway, resulting in CD4+ and regulatory T- cells (Tregs) induction. The latter one is crucial for host immune tolerance towards commensal intestinal bacteria. Therefore, B. fragilis derived OMVs contribute to maintain gut microbiota homeostasis (17, 18). In this regard, we evaluated and compared the effects of B. fragilis and its OMVs on TLR2, TLR4 genes expression and cytokines concentration on Caco-2 cell line as a IEPCs model.
Materials and Methods
Bacterial growth conditions
In this experimental study, B. fragilis ATCC 23745 was grown on blood agar plates containing 5% sheep blood or brain heart infusion (BHI) broth supplemented with 5 µg/ ml hemin (Sigma-Aldrich, USA) and 1 µg/ml menadione (Sigma-Aldrich, USA), while they were incubated at 37°C, in 80% N2, 10% CO2 and 10% H2 atmosphere (19).
Outer membrane vesicles extraction
OMVs were isolated as described previously (20). Briefly, after an overnight cultivation, the medium was centrifuged at 6000 g, 4°C. The pellets were washed twice with phosphate buffer solution (PBS) and re-suspended in 9% sodium chloride solution. Then the suspension was centrifuged for 1 hour at 6000 g, 4°C. OMVs were extracted through sequential centrifugation for 90 minutes at 20000 g, 4°C using Tris-ethylene diamine tetra acetic acid (EDTA)-sodium deoxycholate (Sigma-Aldrich, USA) buffers. Finally, OMVs were stored at -20°C (20).
Scanning electron microscopy
The OMVs were fixed in PBS containing 2.5% glutaraldehyde and 2% paraformaldehyde. Following PBS washing, the samples were air-dried and coated with gold by sputter coater (KYKY Technology, China (using physical vapor deposition method. The prepared samples were examined by SEM (KYKY Technology, China) (21).
Cell culture and treatment
The human epithelial cell line, IBRC C10094 Caco-2, was obtained from Iranian Biological Resource Center. The cells were grown in Dulbecco’s modified eagle medium (DMEM/high glucose; Gibco™, USA), supplemented with 10% fetal bovine serum (FBS, Gibco™, USA) and 1% penicillin/streptomycin (Gibco™, USA) and incubated at 37°C in a 5% CO2 atmosphere (22). The cells were treated with B. fragilis and OMVs (180 and 350 µg/ ml) and incubated overnight.
RNA isolation and cDNA synthesis
Total RNA was isolated using RNX-Plus (CinnaGen, Iran). RNA quantity and quality were respectively evaluated by NanoDrop 2000 (Thermo Fisher Scientific, USA) and gel electrophoresis. cDNAs were synthesized by RevertAid first strand cDNA synthesis kit (Thermo Scientific, USA) according to manufacturers’ instructions.
Quantitative reverse transcriptase polymerase chain reaction analysis
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using LightCycler® 96 SW 1.1 instrument (Roche, Germany). Each reaction mixture was composed of SYBR Premix Ex Taq II (Takara, China), specific primers (Table 1) and DNA template. GAPDH was used as housekeeping gene. The amplification program was consisted of 1 cycle at 95°C for 60 seconds, followed by 40 cycles of denaturation at 95°C for 5 seconds, annealing at 55°C for 30 seconds and extension at 72°C for 30 seconds.
Table 1.
List of primers for quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analysis
| Gene | Prime sequence (5ˊ-3ˊ) |
|---|---|
| GAPDH | F: GGAGCGAGATCCCTCCAAAAT |
| R: GGCTGTTGTCATACTTCTCATGG | |
| TLR2 | F: TTATCCAGCACACGAATACACAG |
| R: AGGCATCTGGTAGAGTCATCAA | |
| TLR4 | F: AGACCTGTCCCTGAACCCTAT |
| R: CGATGGACTTCTAAACCAGCCA | |
Cytokines concentration assay
Following overnight incubation of Caco-2 cells with B. fragilis and its OMVs, the supernatants were collected and stored at -20°C. The IFNγ, IL-10 and IL-4 concentrations were measured using enzyme-linked immunosorbent assay (ELISA) kit (Human cytokine ELISAPRO kit, MABTECH, Swedish biotech, Sweden), according to manufacturer’s instructions.
Statistical analyses
Data were analyzed by independent sample t test and one- way ANOVA using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA). All results demonstrate as mean ± standard deviation (SD). In all experiments, P<0.05 was considered statistically significant.
Results
Properties of B. fragilis derived outer membrane vesicles
B. fragilis produced OMVs in BHI broth. The morphology and size of OMVs were examined by SEM. Diameter of spherical shaped OMVs was in the range of 30-110 nm (Fig .1). Mean dimension of OMVs was 85.7 ± 15.3 nm.
Fig.1.
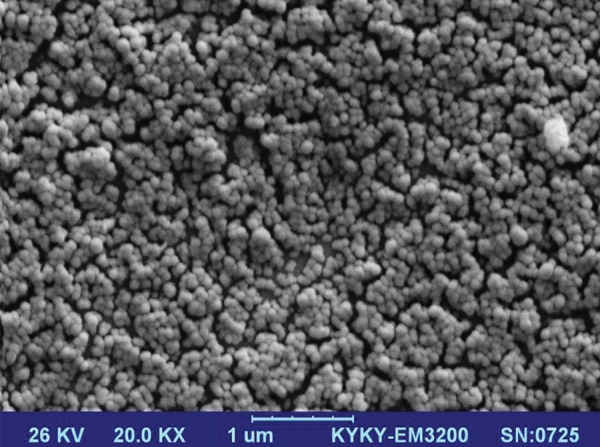
B. fragilis produces outer membrane vesicles (OMVs) with a mean dimention of 85.7 ± 15.3 nm: scanning electron microscopy of B. fragilis derived-OMVs (magnification: ×20K).
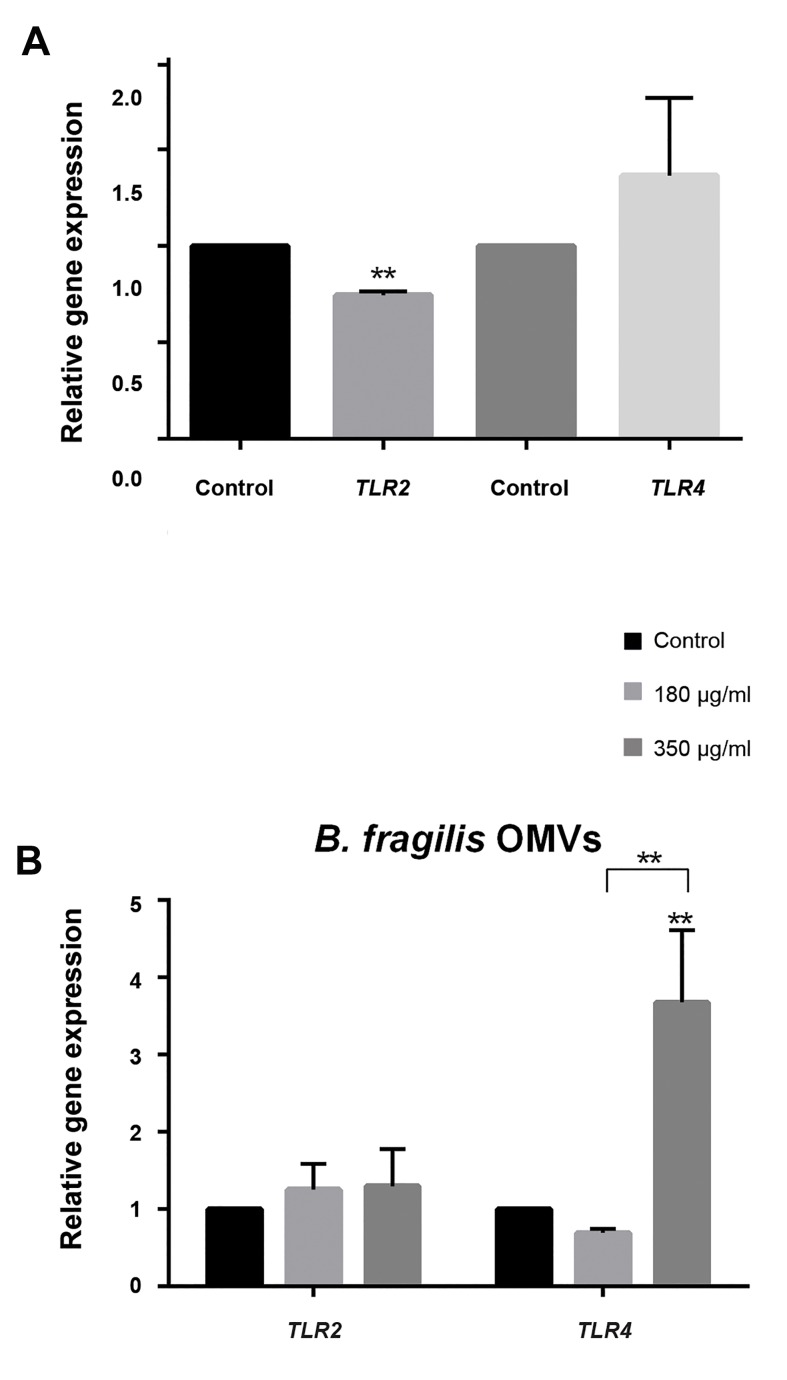
Effect of B. fragilis and outer membrane vesicles on TLR gene expressions
Human intestinal epithelial cell line Caco-2 was used to study the effects of B. fragilis and its OMVs on TLR2 and TLR4 gene expressions using qRT-PCR. B. fragilis significantly decreased TLR2 gene expression. TLR4 gene expression was slightly increased by this bacterium (Fig .2A). The cells were treated with B. fragilis derived OMVs in two concentrations, 180 and 350 µg/ml. The mRNA levels of TLR2 were slightly increased in both of OMVs concentrations. Interestingly, TLR4 gene expression was decreased and significantly increased at 180 and 350 µg/ml of OMVs, respectively (Fig .2B).
Fig.2.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) analyzes of B. fragilis and its outer membrane vesicles (OMVs) on TLR genes expressions. A. The cells were initially deprived of serum and then treated with either B. fragilis or phosphate buffer solution (PBS) overnight and B. In the same condition, the other group cells were treated with either B. fragilis derived OMVs (350 and 180 µg/ml) or sucrose, overnight. Values of triplicate experiments are demonstrated as mean ± SD. Significant results are presented as ** based on P<0.01.
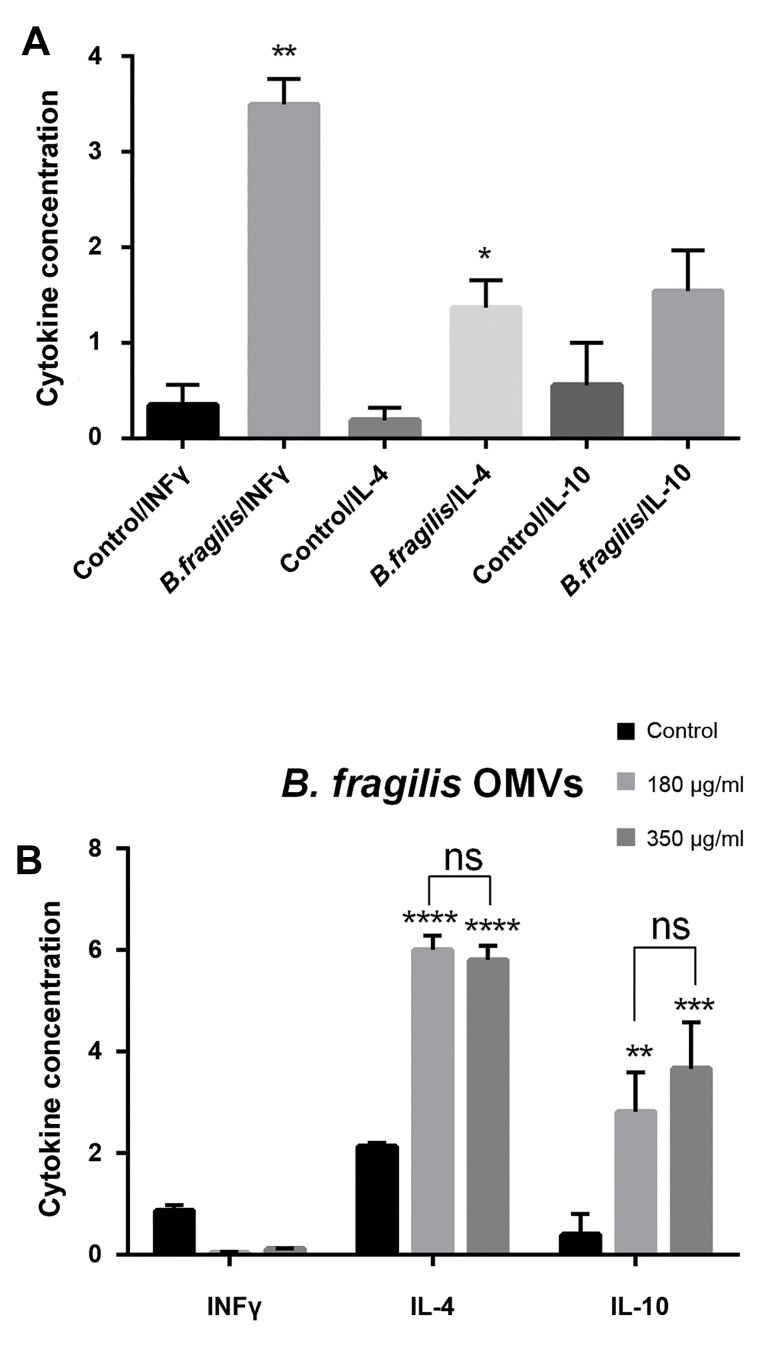
Effect of B. fragilis and outer membrane vesicles on cytokines concentration
After overnight stimulation of Caco-2 cells by B. fragilis and its OMVs, the concentration of pro- inflammatory (IFNγ) and anti-inflammatory (IL-4 and IL-10) cytokines were measured by ELISA. B. fragilis significantly elevated IFNγ concentration (Fig .3A). Interestingly, IFNγ concentration was decreased by 180 and 350 µg/ml of OMVs (Fig .3B). B. fragilis was able to increase IL-4 and IL-10 concentrations (Fig .3A). In addition, the related OMVs of this bacterium (180 and 350 µg/ml) significantly enhanced IL-4 and IL-10 concentrations (Fig .3B).
Fig.3.
ELISA analyzes of B. fragilis and its outer membrane vesicles (OMVs) on cytokines concentration. A. Cells were initially deprived of serum and then treated with either B. fragilis or phosphate buffer solution (PBS), overnight and B. In the same condition, the other group cells were treated with either B. fragilis derived OMVs (350 and 180 µg/ml) or sucros, for overnight. Values of triplicate experiments are demonstrated as mean ± SD. Significant results are presented as *, **, ***, **** based on P<0.05, P<0.01, P<0.001, and P<0.0001.
Discussion
The epithelial layer of GI tract is continuously exposed to huge amount of immunogenic stimulatory molecules, derived from gut microbiota, nutrient and pathogenic microorganisms (3). IEPCs are the interface between gut microbiota and immune system via lamina propria cells. The potential of IEPCs to modulate immunity depends on PRRs gene expression (5). Additionally, the gut microbiota has immunomodulation potential in host. In this regard, B. fragilis and its OMVs affect gut microbiota-host interactions (15). Therefore, we aimed to study in more details the effects of B. fragilis and its OMVs on TLR genes expression and cytokines concentration in Caco-2 cell line as a human IEPCs model.
It has been found that TLRs play a crucial role in immune responses and B. fragilis influences homeostasis and immunity (14). In other words, B. fragilis activate CD4+ T cells responses through TLR2 signaling in DCs. B. fragilis has anti-inflammatory effects through mediation of Th1/Th2 balanced ratio, as well as CD4+ T cells differentiation into Tregs and Th17 limited responses (17). Moreover, TLRs signaling in GI epithelium triggers the cross talk between gut microbiota and the host, locally and systemically (6). TLRs signaling is involved in proliferation, differentiation of IEPCs alongside with induction of pro- and anti-inflammatory cytokines responses. As IEPCs are located in frontline of gut environment, their TLRs signaling has critical role in immune tolerance to gut microbiota and defense against pathogens (8). Expression patterns and induction mode of TLRs are different throughout GI epithelium. IEPCs have relatively low expression of TLR2 and TLR4, which are the main receptors for gram positive and negative bacterial MAMPs (9). In this regard, Furrie et al. (23) reported that B. fragilis does not change the TLR1-4 expression levels in Caco-2 cell line. In our study, although B. fragilis significantly decreased TLR2 , but increased TLR4 gene expression. Perhaps, differences in bacterial quantity and incubation time could justify this discrepancy.
As mentioned above, gut microbiota could intervene with cytokines secretion. For instance, B. fragilis has immune-modulatory effect through induction of IL10 and reduction of IL-17 production during intestinal inflammation (17). Bahrami et al. (24) studied the influence of intestinal commensal bacteria (i.e. B. fragilis) on pro- and anti-inflammatory cytokine productions. Their data showed that B. fragilis did not affect cytokine concentration. However, we noticed that IFNγ, IL-4 and IL-10 concentrations were increased after corresponding treatment.
It has been demonstrated that B. fragilis releasing OMVs is an influential factor for mediation of immune responses. Since B. fragilis apparently does not have well established secretory system, immunogenic components (PSA) delivery is facilitated through OMVs production. Shen et al. have shown that B. fragilis has protective role against intestinal inflammatory disease in animal model via OMVs production. Indeed, B. fragilis OMVs induce Treg development and IL-10 production thorough TLR2 signaling in DCs (17, 18). We believe that this is the first study reporting the effects of B. fragilis-derived OMVs on TLR2 and TLR4 genes expression, as well as the concentration of IFNγ, IL-10 and IL-4 on Caco-2 cell line. Taken together, our results depicted that TLR2 mRNA levels were not altered by B. fragilis derived OMVs. However, these vesicles significantly changed TLR4 gene expression. Interestingly, B. fragilis derived OMVs had stimulatory effect on anti-inflammatory cytokines (IL-4 and IL-10) while it decreased IFNγ concentration as a pro-inflammatory cytokine.
Conclusion
Based on immunomodulatory effects of B. fragilis derived OMVs on immune system and our current findings, we suggest that these OMVs may have a substantial role in the improvement of the inflammatory responses and it may have yet no recognized and understudied function in the inter-kingdom modulation of host genes.
Acknowledgments
This research was funded by Iran Biotech Fund grant 94/10243 and Pasteur Institute of Iran. The authors would like to thank Fatemeh Ettehad Marvasti for her help and our colleagues at Pasteur Institute of Iran. There is no conflict of interest in this study.
Author’s Contributions
S.A.B., S.D.S.; Contributed to conception and design. S.A.B., S.D.S., S.K., S.I.; Contributed to all experimental work, data and statistical analysis, and interpretation of data. S.D.S.; Was responsible for overall supervision. All authors read and approved the final manuscript.
References
- 1.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 2.Ho JT, Chan GC, Li JC. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015;16:21–21. doi: 10.1186/s12865-015-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraca M, Putignani L, Fierabracci A, Teti A, Perilongo G. Gut microbiota-derived outer membrane vesicles: under-recognized major players in health and disease. Discov Med. 2015;19(106):343–348. [PubMed] [Google Scholar]
- 5.König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, et al. Human intestinal barrier function in Health and Disease. Clin Transl Gastroenterol. 2016;7(10):e196–e196. doi: 10.1038/ctg.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vereecke L, Beyaert R, van Loo G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol Med. 2011;17(10):584–593. doi: 10.1016/j.molmed.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Gourbeyre P, Berri M, Lippi Y, Meurens F, Vincent‐Naulleau S, Laffitte J, Rogel‐Gaillard C, Pinton P, Oswald IP. Pattern recognition receptors in the gut: analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol Rep. 2015;3(2) doi: 10.14814/phy2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013;6(3):451–463. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu S, Gao N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci. 2015;72(17):3343–3353. doi: 10.1007/s00018-015-1931-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woting A, Blaut M. The intestinal microbiota in metabolic disease. Nutrients. 2016;8(4):202–202. doi: 10.3390/nu8040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42–42. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73(1):147–162. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients. 2014;7(1):45–73. doi: 10.3390/nu7010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Y. Interkingdom communication of a bacterial mutualist and its mammalian host (Doctoral dissertation, California Institute of Technology).Presented for the Ph.D., California.California Institute of Technology. California Institute of Technology; 2012. [Google Scholar]
- 16.Ahmadi Badi S, Moshiri A, Fateh A, Rahimi Jamnani F, Sarshar M, Vaziri F, et al. Microbiota-derived extracellular vesicles as new systemic regulators. Front Microbiol. 2017;8:1610–1610. doi: 10.3389/fmicb.2017.01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci (Landmark Ed) 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12(4):509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and 278 proteases into Bacteroides outer membrane vesicles. MBio. 2014;5(2):e00909–e00914. doi: 10.1128/mBio.00909-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siadat SD, Vaziri F, Eftekhary M, Karbasian M, Moshiri A, Aghasadeghi MR, et al. Preparation and evaluation of a new lipopolysaccharide- based conjugate as a vaccine candidate for brucellosis. Osong Public Health Res Perspect. 2015;6(1):9–13. doi: 10.1016/j.phrp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakao R, Hasegawa H, Ochiai K, Takashiba S, Ainai A, Ohnishi M, et al. Outer membrane vesicles of Porphyromonas gingivalis elicit a mucosal immune response. PLoS One. 2011;6(10):e26163–e26163. doi: 10.1371/journal.pone.0026163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato K, Kumita W, Ode T, Ichinose S, Ando A, Fujiyama Y, et al. OmpA variants affecting the adherence of ulcerative colitis-derived Bacteroides vulgatus. J Med Dent Sci. 2010;57(1):55–64. [PubMed] [Google Scholar]
- 23.Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Microbiology & Gut Biology Group; Tayside Tissue & Tumour Bank.Toll‐like receptors‐ 2,‐3 and‐4 expression patterns on human colon and their regulation by mucosal‐associated bacteria. Immunology. 2005;115(4):565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bahrami B, Macfarlane S, Macfarlane GT. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J Appl Microbiol. 2011;110(1):353–363. doi: 10.1111/j.1365-2672.2010.04889.x. [DOI] [PubMed] [Google Scholar]