ABSTRACT
Background
Research evidence outlines the benefits of intradialytic exercise (IDE), yet implementation into practice has been slow, ostensibly due to a lack of patient and staff engagement. The aim of this quality improvement project was to improve patient outcomes via the introduction of an IDE programme, evaluate patient uptake and sustainability and enhance the engagement of routine haemodialysis (HD) staff with the delivery of the IDE programme.
Methods
We developed and refined an IDE programme, including interventions designed to increase patient and staff engagement that were based on the Theoretical Domains Framework (TDF), using a series of ‘Plan, Do, Study, Act’ (PDSA) cycles. The programme was introduced at two UK National Health Service HD units. Process measures included patient uptake, withdrawals, adherence and HD staff involvement. Outcome measures were patient-reported functional capacity, anxiety, depression and symptomology. All measures were collected over 12 months.
Results
A total of 95 patients were enrolled in the IDE programme; 64 (75%) were still participating at 3 months, decreasing to 41 (48%) at 12 months. Adherence was high (78%) at 3 months, decreasing to 63% by 12 months. The provision of IDE by HD staff accounted for a mean of 2 (5%) sessions per 3-month time point. Patients displayed significant improvements in functional ability (P = 0.01) and a reduction in depression (P = 0.02) over 12 months, but the effects seen were limited to those who completed the programme.
Conclusions
A theory-based IDE programme is feasible and leads to improvement in functional capacity and depression. Sustaining IDE over time is complicated by high levels of patient withdrawal from the programme. Significant change at an organizational level is required to enhance sustainability by increasing HD staff engagement or access to professional exercise support.
Keywords: end-stage renal disease, exercise, haemodialysis, physical activity, quality improvement
INTRODUCTION
Problem
Within the UK, ∼58 968 people receive renal replacement therapy for end-stage renal disease (ESRD), of which 41% undertake haemodialysis (HD) [1]. HD is typically prescribed thrice weekly for 4 h throughout the patients lifespan or until transplantation and is associated with high levels of deconditioning and disability characterized by a reduction in exercise and functional capacity [2], significant levels of depression [3] and reduced quality of life [4].
Intradialytic exercise (IDE) may ameliorate many of these issues [5] and achieves greater adherence than home or outpatient programmes [6]. However, the implementation of IDE into practice has been slow nationally and internationally [7]. Numerous studies have reported multifactorial barriers and challenges to IDE uptake and engagement among both HD patients and staff [8–14], but few have developed and evaluated theory-based IDE programmes that address these barriers [15].
We wished to address low levels of physical function and quality of life locally via the introduction of an IDE programme, but, in common with previous research, we found that HD staff and patients experienced numerous barriers to participating in or engaging with IDE [16]. Interventions grounded in psychological theory may be more effective than those that are intuitively developed [17, 18], and earlier work indicated that the Theoretical Domains Framework (TDF) may be a useful model to guide the selection of intervention components [16, 19, 20]. This article describes the development, refinement and evaluation of a theory-based IDE programme structured according to Standards for Quality Improvement Reporting Excellence guidelines [21]. Our objectives were to improve patient outcomes via the introduction of an IDE programme, to evaluate patient uptake and sustainability and to enhance the engagement of HD staff with IDE delivery.
MATERIALS AND METHODS
Context
The project took place at two UK National Health Service (NHS) Trusts, namely, the Hamilton dialysis unit in Leicester and Addenbrooks Hospital in Cambridge. Leicester has an ethnically diverse population of 330 000 [22], including 917 HD patients, served by three dialysis units [1]. The Hamilton unit treats 114 patients and staffing ratios are four qualified nurses and one assistant per 19 patients [23]. Cambridge has a population of 124 000 [22], including 583 HD patients [1]. The Addenbrooks unit treats 228 patients and staffing ratios are one qualified nurse per four patients. Both units are operationally run by Fresenius Medical Care but clinically managed by their respective NHS Trusts.
Developing the interventions
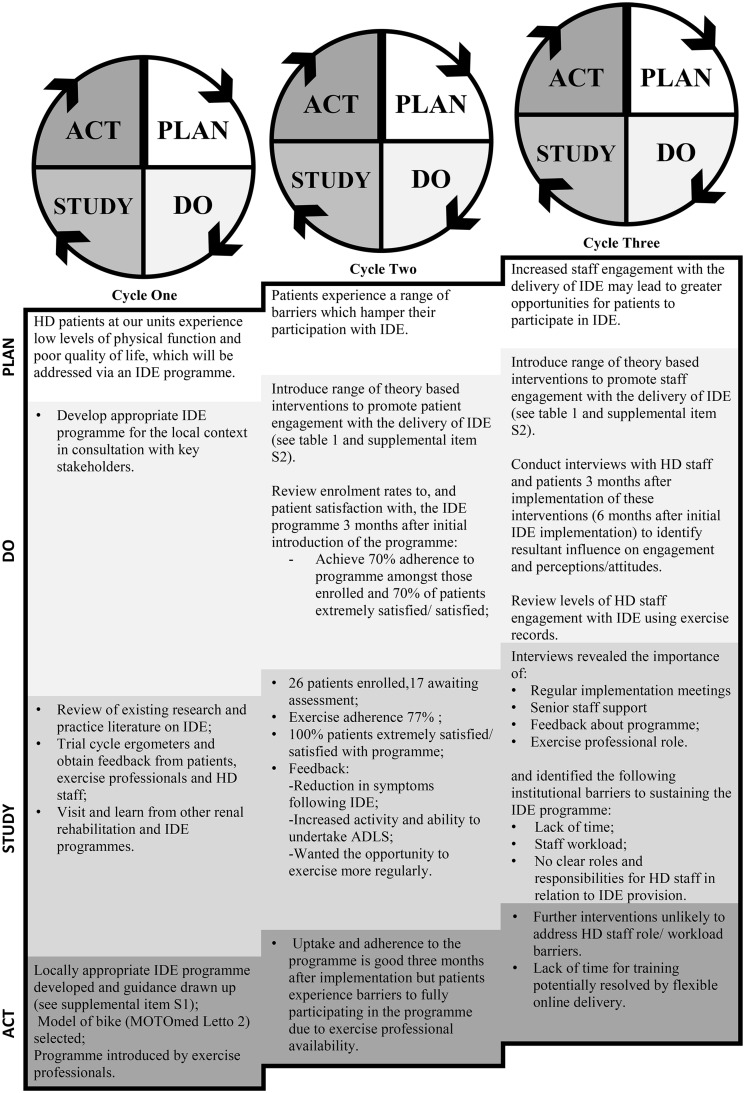
Figure 1 summarizes the three successive ‘Plan, Do, Study, Act’ (PDSA) cycles used. PDSA is a pragmatic method of testing implementation initiatives that advocates learning and adaptation from one cycle to the next, so that larger-scale implementation is more likely to be effective [24]. The IDE programme was initially implemented at the Hamilton dialysis unit in September 2011 and then introduced at Addenbrooks in 2015.
FIGURE 1.
Details of the three successive PDSA cycles. ADLS, activities of daily living; HD, haemodialysis; IDE, intradialytic exercise.
Cycle one focused on developing an appropriate IDE programme for local use. A literature review indicated that aerobic training, delivered by means of a bespoke cycle ergometer, could impart significant benefits [25]. It was also considered a simpler approach to deliver to a large number of patients when compared with combined aerobic and resistance training, thereby increasing the likelihood of long-term adoption into practice. A range of other activities were also undertaken (Figure 1) and, following this, a locally appropriate IDE programme was developed.
Full details of the programme are provided in Supplementary Item S1. Briefly, patients were offered thrice weekly supervised IDE, except the evening and Saturday shifts due to exercise staff availability. The initial sessions of training used a graded approach. Patients were encouraged to progress until they could achieve at least 30 min of moderately intense, continuous cycling (using a MOTOmed Letto, RECK-Technik, Betzenweiler, Germany) at a speed of up to 70 revolutions per minute (rpm). Progressive interval training was allowed if required and participants were encouraged to complete longer sessions if possible. Gears were used to make the exercise progressively more challenging. The IDE programme was initially delivered by a 0.3 Whole Time Equivalent (WTE) Band Six Physiotherapist (a senior therapist with at least 2 years postgraduate experience) and 0.2 WTE Exercise Physiologist at the Leicester site and 1 WTE Band Five (newly qualified) Physiotherapist with 0.3 WTE oversight from a Band 7 Physiotherapist (a senior therapist with at least 5 years postgraduate experience) at Addenbrooks.
In PDSA 2, we focused on patient uptake of IDE. Based on previous work, we used the TDF to develop a range of interventions, outlined in Table 1, designed to address patient-identified barriers and utilize facilitators [16]. Details of these and how they were incorporated into the IDE programme are included in Supplementary Item S2 An audit and patient survey at 3 months found good levels of uptake but a desire for more regular exercise sessions. PDSA 3 was therefore devoted to maximizing HD staff engagement with the IDE programme, again by using previous work to develop theory-based interventions designed to enhance HD staff roles engagement and delivery of IDE (see Table 1 and Supplementary Item S2). Roles relating to the delivery of IDE included assessing exercise suitability at each session; patient encouragement; setting up, monitoring progression and recording exercise and liaising with exercise staff as required.
Table 1.
Components of programme design and implementation strategy selected according to HD staff with patient barriers and facilitators identified and consideration of local context from observation
| Barrier/facilitator | Domains of TDF | Intervention component selected to overcome barrier or utilise facilitator (technique, mode)* | |
|---|---|---|---|
| Patients | |||
| Beliefs about positive consequences of participating in IDE on health and use of dialysis time | Beliefs about consequences | Technique: Persuasive communication, information regarding outcomes, feedback, monitoring | |
| Mode: Patient information leaflet, | |||
| reassessment | |||
| Beliefs about negative consequences of participating in IDE including injury, disruption to dialysis and safety | Beliefs about consequences | Technique: Persuasive communication | |
| Mode: Exercise assessment | |||
| Low awareness of the benefits of IDE and what participation would involve | Knowledge | Technique: Information provision | |
| Mode: Patient information leaflet, exercise bulletin board, newsletters, opportunity to try the bike, exercise assessment, initial exercise sessions | |||
| Patients, beliefs about capabilities to participate in IDE considering comorbidities and age, which were perceived to be important determinants of the ability to exercise | Beliefs about capabilities | Technique: Graded tasks, social process, feedback, motivational interviewing, goal setting | |
| Mode: Exercise assessment, initial exercise sessions, during the course of the programme | |||
| Patients’ perception that HD staff were negative about IDE | Social influences | Technique: Demonstration, encouragement and support from nursing staff of all levels | |
| Mode: During the course of the programme | |||
| Skills relating to participation in IDE | Beliefs about capabilities | Technique: Modelling, self-monitoring, decision making, social process, feedback | |
| Mode: Initial exercise sessions, during the course of the programme, exercise reassessment | |||
| Staff | |||
| HD staffs’ perceptions of patients’ capabilities to participate in IDE | Beliefs about (patients) capabilities | Technique: Feedback, social process | |
| Mode: Staff handovers, during the course of the programme | |||
| Low awareness of the benefits of IDE and exercise prescription and rehabilitation in general | Knowledge | Technique: Information provision | |
| Mode: Training programme, monthly reports and patient feedback, local IDE guidance and reminder prompts | |||
| Skills and beliefs about capabilities related to running an IDE programme, particularly setting up, operating the bikes and encouraging patient participation | Skills | Technique: Monitoring, problem solving, decision making, rehearsal of skills, demonstration | |
| Mode: Training programme | |||
| Beliefs about negative consequences of IDE on staff workload | Beliefs about consequences | Technique: Self-monitoring (patients), information regarding behaviour and outcome | |
| Mode: Exercise assessment and initial exercise sessions, implementation group | |||
| Beliefs about the role of HD staff (nurses) in the provision of IDE (with discrepancies in beliefs about this depending on the seniority of staff) | Social/professional role and identity | Technique: Modelling of IDE provision by nursing staff, encouragement and support | |
| Mode: During the course of the programme, posters | |||
| Limited time and busy workloads | Environmental context and resources | Technique: Changes to the environment to facilitate the behaviour | |
| Mode: Implementation group | |||
Technique, component description; mode, how the component was delivered; content, what was delivered; IDE, intradialytic exercise; TDF, theoretical domains framework.
Three months later, HD staff and patients participated in one-on-one interviews as part of a qualitative study [20]. Results indicated that although many initial barriers to implementation had been overcome, those relating to staff time and workload—coupled with unclear professional role boundaries—that could not be easily resolved without the addition of further resources or formal adaptation of job descriptions remained. These notable barriers precluded HD staff from providing IDE and accessing the IDE training offered.
Study of the interventions
To evaluate our intervention, we utilized an uncontrolled design with data collection at baseline and 3, 9 and 12 months.
Process measures
Demographic data were collected from patient records. Information on adherence, withdrawal and HD staff engagement with exercise delivery were collected via exercise records.
Outcome measures
The potential effect of the IDE programme was evaluated using a range of measures selected on the basis of their importance to patients from earlier work [16]. The Hospital Anxiety and Depressions Scale (HADS) measures anxiety and depression, and a decrease in score indicates improvement [26]. The Leicester Uraemic Symptoms Score (LUSS) assesses the frequency and intrusiveness of 11 uraemic symptoms, with greater scores indicating an increased severity and number of symptoms [4]. The Dukes Activity Status Index (DASI) assesses perceived functional ability [27]. The weighting assigned to each question is based on the approximate energy requirement of the activity (in metabolic equivalent units) and can be used to estimate the oxygen consumption (VO2) peak [27], with an increase in scores indicating improvement. All questionnaire data were collected during HD by the exercise staff.
Analysis
Normality was checked using the Shapiro–Wilk test. As data violated assumptions for parametric tests, Friedman’s analysis of variance (ANOVA) was used to analyse repeated measures and Kruskal–Wallis ANOVA was used for unpaired data for those who completed 12 months of training.
Ethical considerations
Our project was a quality improvement initiative and, in line with national research ethics guidance, ethical approval was not required. The project was registered with the clinical audit team at University Hospitals of Leicester NHS Trust (8604e). Patients provided informed consent prior to participation.
RESULTS
A total of 95 patients enrolled in the IDE programmes over 12 months, 57 in the Leicester unit and 38 in the Addenbrooks unit. This represents an uptake of 50% of the overall population at the Leicester unit and 33% at the Addenbrooks unit, although it is accepted that a proportion of these patients may not be fit to exercise. The baseline characteristics of patients enrolled are described in Table 2. There were no clinically or statistically significant differences between groups and results were pooled for subsequent analyses.
Table 2.
Demographics of patients enrolled in IDE at the two units
| Leicester (n = 57) | Addenbrooks (n = 38) | Total (N = 95) | |
|---|---|---|---|
| Age (years) | 61 (50–73) | 75 (65–82) | 67 (53–77) |
| HD vintage (time on HD in months) | 28 (13–34) | 43 (26–85) | 32 (18–47) |
| Gender, n (%) | |||
| Male | 34 (59.6) | 29 (76.3) | 63 (66.3) |
| Female | 23 (40.4) | 9 (23.7) | 32 (33.7) |
| Ethnicity, n (%) | |||
| White | 26 (45.6) | 33 (86.8) | 59 (62.0) |
| BAME | 29 (50.9) | 1 (2.60) | 30 (32.0) |
| Other | 2 (3.50) | 1 (2.60) | 3 (3.00) |
| Not stated | 0 | 3 (7.90) | 3 (3.00) |
| Aetiology, n (%) | |||
| Chronic glomerulonephritis | 14 (24.6) | 3 (7.90) | 17 (17.9) |
| Diabetes | 9 (15.8) | 8 (21.0) | 17 (17.9) |
| Polycystic kidney disease | 8 (14.0) | 2 (5.30) | 10 (10.5) |
| Renal vascular disease | 3 (5.30) | 2 (5.30) | 5 (5.3) |
| Pyelonephritis | 4 (7.00) | 0 | 4 (4.2) |
| Other | 2 (3.50) | 7 (18.4) | 9 (9.5) |
| Uncertain | 17 (29.8) | 7 (18.4) | 24 (25.3) |
| Unknown | 0 | 9 (23.7) | 9 (9.5) |
Continuous variables are presented as median with lower and upper quartiles.
BAME, Black, Asian and minority ethnic backgrounds.
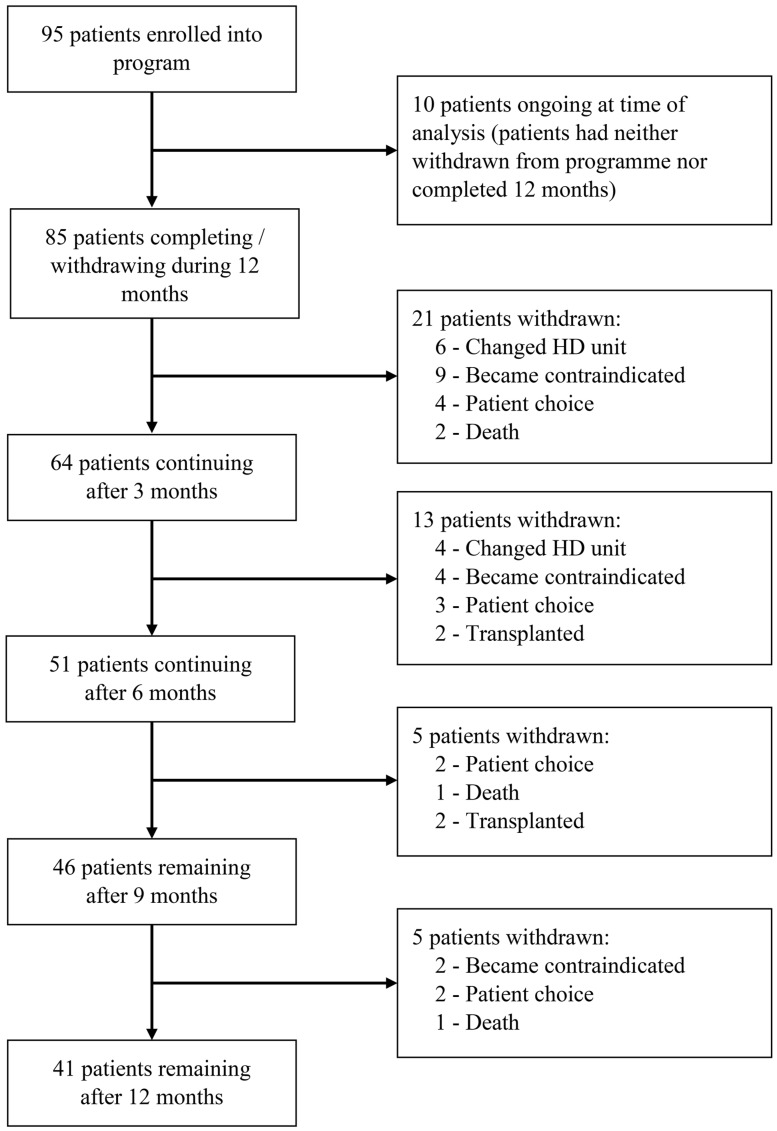
Figure 2 shows the number of patients enrolled in the programme at three monthly increments, including reasons for attrition at each stage. At 3 months, 64 (75%) were still participating in the programme, 51 (60%) at 6 months and 46 (54%) at 9 months. By 12 months, 41 (48%) patients were still participating. Seventy-five per cent of withdrawals were largely due to factors beyond patients’ control (e.g. exercise became contraindicated, transplant). Only 11 (25%) patients volitionally withdrew over 12 months. The baseline characteristics of these patients were not clinically or statistically significantly different from those who completed the programme.
FIGURE 2.
Flow diagram of participation. Patients reported as ongoing at time of analysis remained in the programme but had not yet reached their assessment point. Contraindications to exercise are outlined in Supplementary Item S1 and were assessed by a senior physiotherapist or nephrologist.
Adherence to the programme based on sessions offered was 78% at 3 months, decreasing to 63% by 12 months (Table 3). This may reflect the high levels of motivation in patients who remained in the programme. The availability of exercise professionals allowed patients to participate in IDE for 61% of their total HD sessions. HD staff provision of IDE accounted for only 2 (5%) sessions per 3-month time point and was predominantly during shifts when the exercise professionals were not present.
Table 3.
The number of HD sessions available over 3 months, sessions where IDE was offered to the patient and where IDE was completed
| Adherence, % ± SD |
|||||
|---|---|---|---|---|---|
| HD sessions available, mean ± SD | HD sessions IDE offered, n ± SD (% ± SD) | HD sessions with IDE completed, mean ± SD | To available sessions | To offered sessions | |
| 3 months | 40 ± 3 | 26 ± 7 (65 ± 17) | 20 ± 7 | 51 ± 19 | 78 ± 18 |
| 6 months | 35 ± 9 | 22 ± 9 (59 ± 21) | 15 ± 9 | 41 ± 23 | 66 ± 27 |
| 9 months | 36 ± 9 | 23 ± 9 (61 ± 20) | 16 ± 7 | 44 ± 18 | 70 ± 23 |
| 12 months | 37 ± 7 | 23 ± 9 (59 ± 22) | 15 ± 10 | 42 ± 25 | 63 ± 28 |
Adherence to the programme is expressed both as a percentage of the total number of HD sessions available and HD sessions where IDE was offered. SD, standard deviation.
Perceived exertion was light to moderate {rating of perceived exertion 12 [interquartile range (IQR) 11–13]} across 12 months. Median exercise duration significantly increased from 25 to 30 min between 3 and 6 months (P = 0.03) and was maintained thereafter. A non-significant increase in median distance cycled, which increased from 4.9 (IQR 3.4–7.5) to 6.8 (4.1–9.4) miles (P = 0.2) and estimated energy expenditure increased from 34.8 (IQR 24.3–77.4) to 54.4 (IQR 31.2–90.8) kcals (P = 0.3) after 6 months. Average power output did not change between 0 and 9 months [7.1 (IQR 4.6–13.7) to 11.3 (IQR 6.3–20) Watts; P = 0.2] and plateaued at 12 months.
Multiple reasons for not exercising were noted during the evaluation period (data not shown). Being unwell and declining to exercise were most prevalent at the start of the programme, decreasing over 12 months. The number of times fatigue, pain and falls were cited as reasons for non-adherence remained static over 12 months, while episodes of overload and hypotension increased.
Changes in outcome scores over 12 months are shown in Table 4. The low baseline DASI scores and corresponding estimated VO2 peak confirm the low levels of physical functioning reported in this population. Patients displayed significant (P = 0.01) improvement in median DASI scores and a reduction in median HADS depression scores (P = 0.02) over 12 months, with the greatest successive change occurring from baseline to 3 months. No change was seen in uraemic symptoms (P = 0.6) or the anxiety subscale of the HADS (P = 0.3) over 12 months.
Table 4.
Changes in outcome measure scores over 12 months of IDE
| Median (lower–upper quartile) |
Friedman’s ANOVA |
|||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 9 months | 12 months | χ2 (df) | P-value | |
| DASI (n = 33) | 19.2 | 23.5 | 25.0 | 26.7 | 0.01 | |
| (9.50–27.4) | (14.5–29.5) | (16.2–37.5) | (16.9–36.1) | |||
| Estimated VO2 peak, (mL−1kg−1min) | 17.86 | 19.71 | 20.35 | 21.08 | 11.59 (3) | |
| (from DASI) | (13.69–21.38) | (15.49–22.29) | (16.57–19.18) | (16.87–25.12) | ||
| HADS Anxiety score (n = 32) | 6 | 5 | 5 | 5 | 4.05 | 0.3 |
| (3–11) | (4–9) | (2–8) | (2–8) | (3) | ||
| HADS Depression score (n = 32) | 8 | 5 | 6 | 5 | 10.20 (3) | 0.02 |
| (4 -11) | (2 -9) | (3 -9) | (3–8) | |||
| LUSS (n = 33) | 34 | 34 | 33 | 36 | 2.50 (3) | 0.5 |
| (21–54) | (20–41) | (20–47) | (21–43) | |||
DASI, Dukes Activity Status Index; df, degrees of freedom; HADS, Hospital Anxiety and Depression Scale; LUSS, Leicester Uraemic Symptoms Scale.
DISCUSSION
Summary
This project is the first to provide a detailed description of the theory and processes underpinning the development and implementation of an IDE programme that aimed to engage patients and HD staff and improve patient outcomes. Our findings indicate the programme was feasible and led to potential improvement in patient outcomes. Interventions designed to increase staff engagement and sustain long-term participation were more challenging and further research into the implementation of different programmes of IDE and rehabilitation for HD patients is warranted.
Interpretation
Between 33% and 50% of all HD patients enrolled in the programme across both sites, but only 48% completed 12 months of IDE. Our enrolment rates are comparable with trials of IDE, reported to be ∼52% of all eligible HD patients [28], but lower than the 62% reported in a non-NHS clinical setting [24], potentially because of higher levels of exercise professional availability within this service. Our work supports evidence that suggests IDE can lead to statistically significant increases in function and exercise capacity [5] and a reduction in depression [3] and extends these findings to a real-world setting. Minimum clinically important differences (MCIDs) have yet to be established for the HD population, but comparison with evidence from other chronic diseases suggests IDE does not achieve the MCID of 3.5 mL/kg/min for VO2 peak (estimated using the DASI) but exceeds the 1.5-point change in HADS scores that is indicative of clinically relevant change [25, 29].
Existing reviews of IDE advocate 30 min of moderate intensity training thrice weekly for a minimum of 8 weeks, with 6 months of training conferring additional increases in exercise capacity [5]. Our findings suggest that a progressive, light to moderately intense IDE programme of 3 months’ duration, delivered at least twice weekly, may still convey benefit. Dropouts were also lowest and adherence levels highest within the first 3 months. Although improvements in exercise capacity and depression were maintained over 12 months with continued adherence, there was marked attrition of patients over this time period. Whether maintenance of effects would have been sustained without ongoing intervention is uncertain, due to lack of a control group, but it is important to acknowledge that a balance may need to be struck between what is ‘optimal’ and what is practically possible within a clinical setting. HD centres may struggle to provide programmes for an extended period because of a lack of resources and staffing. Shorter programmes of IDE may be more sustainable and different models of rehabilitation (e.g. home or web-based) that may provide additional benefits, as progression and outcomes plateau with IDE, could be explored.
No change in anxiety or uraemic symptoms was observed during our programme. Several studies have indicated that IDE has a role in reducing anxiety, but methodological limitations make it difficult to draw firm conclusions [30, 31]. Research into the effects of IDE on symptoms is limited. IDE is unlikely to impact all symptoms experienced and in choosing to measure global symptomology, a more nuanced understanding of its potential role in symptom management may have been missed. Restless leg syndrome and fatigue are the most widely studied symptoms experienced by HD patients; data for the former being altered with exercise training are inconclusive [32] but fatigue may improve [33]. Fatigue was a key reason for non-adherence in our project. Fatigue in ESRD is associated with ‘all or nothing’ or avoidant behaviours, perpetuating fatigue and functional impairment [33]. Training staff to target unhelpful beliefs may help patients to overcome negative perceptions of fatigue, promote consistent exercise adherence and improve outcomes, and may be as important as training them to deliver the more practical aspects of IDE.
Our programme may not have been intensive or progressive enough to realize the full benefits of IDE, as indicated by the non-significant increases in training data. Other authors have noted limitations to the volume and intensity of exercise that can be provided during HD [34]. In support of this, even patients who participated in thrice weekly IDE still failed to meet physical activity guidance [35]. To achieve these levels, HD patients need to be encouraged to participate in physical activity in the interdialytic period. Participation in IDE does not seem to influence physical activity levels outside of the unit [36], possibly because it does not help patients to build the skills required to incorporate physical activity into their lives. Research is currently lacking, but examining how an IDE programme can be adapted to promote greater physical activity outside of the dialysis period may further improve outcomes.
The sustainability and efficacy of IDE may be enhanced by adopting a multidisciplinary approach that utilizes the existing workforce. Many studies of IDE utilize exercise professionals, who are not part of routine HD staff and incur additional costs, but also bring expertise and dedicated time, cited as important to both the implementation and maintenance of IDE programmes [37]. The involvement of the equivalent of one WTE Physiotherapist at each unit was a driving force in the implementation of the programme, but provided patients with the opportunity to exercise in only 61% of available HD sessions. Further investment would be required to deliver equitable care across shifts and increase access to IDE. To address this, our programme was specifically tailored to promote delivery by HD staff. Despite a change in perceptions towards IDE following PDSA 3 [16], the barriers HD staff continued to identify were primarily related to lack of time and unclear roles in relation to IDE provision. In support of this, our project demonstrated low levels of HD staff engagement. Numerous studies have reported a lack of staff engagement with IDE as a major barrier to implementation [7–10, 14]. One programme that has successfully engaged HD staff has included the provision of IDE within their job descriptions and workloads [38] and a similar approach may be required to sustain IDE programmes. Greater involvement of the wider multidisciplinary team, such as nephrologists and dieticians, may also better address issues related to non-adherence, for example, due to fluid overload.
Without a formal change in the roles of HD staff, an alternative approach may be to demonstrate the cost-effectiveness of an entirely exercise professional–run programme. A cost break-down was not collated for our IDE programme but would provide useful information for those wishing to implement a similar programme. The use of alternative forms of cycle ergometer and non-qualified exercise professionals (e.g. physiotherapy technicians or assistants) may further reduce the cost of the programme and extend its reach. The costs of a programme should also be considered against the potential savings. A recent study suggests that IDE may be associated with reduced hospitalization and length of stay [39], but further robust prospective economic evaluations are required.
LIMITATIONS
This project was a quality improvement initiative rather than a research study. The uncontrolled before/after design makes it difficult to directly attribute change to the intervention [40]. HD patients encounter many health care professionals and services. These, as well as the influence of increased interaction with the exercise professionals, may have influenced the outcomes observed. While our programme demonstrated potential benefit from participation, this analysis includes only patients who completed 12 months of IDE. Future studies should aim to follow-up those patients who withdrew.
It was not possible to establish which parts of the programme were the most effective and those that should be abandoned or modified. Implementation is known to be context dependent, and therefore our findings may not be generalizable to other programmes. Further work examining the implementation of different programmes in a range of contexts, including detailed process evaluation, may lead to more effective widespread implementation [17].
CONCLUSIONS
This is the first publication to describe in detail the theory-based implementation of an IDE programme. The interventions used were feasible and lead to potential improvement in patient outcomes. The efficacy of IDE within a clinical environment may be enhanced by considering the design of the programme in relation to available resources, and programmes designed to encourage physical activity outside of the unit merit further exploration. Sustaining an IDE programme in practice is more challenging, particularly due to a reduction in patient participation and adherence over time. The implementation of IDE in practice requires significant changes at an organizational level to overcome existing constraints on HD staff involvement or increase access to exercise professional support.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the staff and patients at both units particularly Dr James Medcalf and Dr Richard Baines, Fresenius Medical Care, the Leicester Kidney Care Appeal and the Addenbrooks Kidney Patients Association.
FUNDING
The research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre and partly funded by the Stoneygate Trust. H.M.L.Y. and J.O.B. are supported by grants from the NIHR (grant award numbers DRF-2016-09-015 and CS-2013-13-014). S.J.S. is supported by the Collaboration for Leadership in Applied Health Research and Care East Midlands. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
CONFLICT OF INTEREST STATEMENT
Outside the submitted work Reck United Kingdom (manufacturer of the exercise cycle) funded H.M.L.Y., M.D. and J.O.B. to attend the 2012 BMJ Awards. All other authors declared no conflicts of interest.
REFERENCES
- 1. MacNeill SJ, Casula A, Shaw C. et al. UK Renal Registry 18th annual report: chapter 2 UK renal replacement therapy prevalence in 2014: national and centre-specific analyses. Nephron 2016; 132(Suppl 1): 41–68 [DOI] [PubMed] [Google Scholar]
- 2. Kosmadakis GC, Bevington A, Smith AC. et al. Physical exercise in patients with severe kidney disease. Nephron Clin Pract 2010; 115: c7–c16 [DOI] [PubMed] [Google Scholar]
- 3. Chung Y, Yeh M, Liu Y.. Effects of intradialytic exercise on the physical function, depression, and quality of life for hemodialysis patients: a systematic review and meta-analysis of randomized controlled trials. J Clin Nurs 2016; 26: 1801–1813 [DOI] [PubMed] [Google Scholar]
- 4. Pugh‐Clarke K, Naish P, Mercer T.. Quality of life in chronic kidney disease. J Renal Care 2006; 32: 156–159 [DOI] [PubMed] [Google Scholar]
- 5. Sheng K, Zhang P, Chen L. et al. Intradialytic exercise in hemodialysis patients: a systematic review and meta-analysis. Am J Nephrol 2014; 40: 478–490 [DOI] [PubMed] [Google Scholar]
- 6. Konstantinidou E, Koukouvou G, Kouidi E. et al. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med 2002; 34: 40–45 [DOI] [PubMed] [Google Scholar]
- 7. Greenwood SA, Koufaki P, Rush R. et al. Exercise counselling practices for patients with chronic kidney disease in the UK: a renal multidisciplinary team perspective. Nephron Clin Pract 2014; 128: 67–72 [DOI] [PubMed] [Google Scholar]
- 8. Painter P, Carlson L, Carey S. et al. Determinants of exercise encouragement practices in hemodialysis staff. Nephrol Nurs J 2004; 31: 67–74 [PubMed] [Google Scholar]
- 9. Painter P, Clark L, Olausson J.. Physical function and physical activity assessment and promotion in the hemodialysis clinic: a qualitative study. Am J Kid Dis 2014; 64: 425–433 [DOI] [PubMed] [Google Scholar]
- 10. Kontos PC, Miller K-L, Brooks D. et al. Factors influencing exercise participation by older adults requiring chronic hemodialysis: a qualitative study. Int Urol Nephrol 2007; 39: 1303–1311 [DOI] [PubMed] [Google Scholar]
- 11. Heiwe S, Tollin H.. Patients’ perspectives on the implementation of intra-dialytic cycling—a phenomenographic study. Implement Sci 2012; 7: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman ED, Ballou MB.. Perceived barriers and motivators to exercise in hemodialysis patients. Nephrol Nurs J 2004; 31: 23. [PubMed] [Google Scholar]
- 13. Delgado C, Johansen KL.. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant 2012; 27: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansen KL, Sakkas GK, Doyle J. et al. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis 2003; 41: 171–178 [DOI] [PubMed] [Google Scholar]
- 15. Abdulnassir L, Egas-Kitchener S, Whibley D. et al. Captivating a captive audience: a quality improvement project increasing participation in intradialytic exercise across five renal dialysis units. Clin Kidney J 2017: 10: 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young HML, Hudson N, Clarke AL. et al. Patient and staff perceptions of intradialytic exercise before and after implementation: a qualitative study. PLoS One 2015; 10: e0128995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Craig P, Dieppe P, Macintyre S. et al. Developing and evaluating complex interventions: the new medical research council guidance. BMJ 2008; 337: a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Michie S, Johnston M, Abraham C. et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care 2005; 14: 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. French SD, Green SE, O’Connor DA. et al. Developing theory-informed behaviour change interventions to implement evidence into practice: a systematic approach using the theoretical domains framework. Implement Sci 2012; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michie S, Johnston M, Francis J. et al. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Appl Psychol 2008; 57: 660–680 [Google Scholar]
- 21. Ogrinc G, Davies L, Goodman D. et al. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Contin Educ Nurs 2015; 46: 501–507 [DOI] [PubMed] [Google Scholar]
- 22. Office for National Statistics. 2011. census. https://www.ons.gov.uk/census/2011census (14 June 2018, date last accessed)
- 23. Care Quality Commission. Hamilton renal dialysis unit. http://www.cqc.org.uk/location/1-124257952 (14 June 2018, date last accessed)
- 24. Anding K, Bär T, Trojniak-Hennig J, Kuchinke S. et al. Long-term follow-up of chronic kidney disease patients performing a structured physical exercise program (SPEP) during hemodialysis. Nephrol Dial Transplant 2015; 30(Suppl 3): iii15 [Google Scholar]
- 25. Puhan MA, Frey M, Büchi S. et al. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zigmond AS, Snaith RP.. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370 [DOI] [PubMed] [Google Scholar]
- 27. Hlatky MA, Boineau RE, Higginbotham M. et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989; 64: 651–654 [DOI] [PubMed] [Google Scholar]
- 28. Smart N, Steele M.. Exercise training in haemodialysis patients: a systematic review and meta‐analysis. Nephrology 2011; 16: 626–632 [DOI] [PubMed] [Google Scholar]
- 29. Koufaki P, Greenwood S, Painter P. et al. The BASES expert statement on exercise therapy for people with chronic kidney disease. J Sports Sci 2015; 33: 1902–1907 [DOI] [PubMed] [Google Scholar]
- 30. Moug SJ, Grant S, Creed G. et al. Exercise during haemodialysis: West of Scotland pilot study. Scott Med J 2004; 49: 14–17 [DOI] [PubMed] [Google Scholar]
- 31. Mi Rye Suh R, Hyuk Jung H, Bae Kim S. et al. Effects of regular exercise on anxiety, depression, and quality of life in maintenance hemodialysis patients. Ren Fail 2002; 24: 337–345 [DOI] [PubMed] [Google Scholar]
- 32. Gopaluni S, Sherif M, Ahmadouk NA.. Interventions for chronic kidney disease-associated restless legs syndrome. Cochrane Database Syst Rev 2016; 11: CD010690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chilcot J, Moss-Morris R, Artom M. et al. Psychosocial and clinical correlates of fatigue in haemodialysis patients: the importance of patients’ illness cognitions and behaviours. Int J Behav Med 2016; 23: 271–281 [DOI] [PubMed] [Google Scholar]
- 34. Mendoza M, Han M, Meyring-Wosten A. et al. It's a non-dialysis day … do you know how your patient is doing? A case for research into interdialytic activity. Blood Purif 2015; 39: 74–83 [DOI] [PubMed] [Google Scholar]
- 35. Bull FC, Expert Working Groups. Physical activity guidelines in the U.K.: review and recommendations School of Sport, Exercise and Health Sciences, Loughborough University, May 2010. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/213743/dh_128255.pdf (14 June 2018, date last accessed)
- 36. Chang Y, Cheng S, Lin M. et al. The effectiveness of intradialytic leg ergometry exercise for improving sedentary life style and fatigue among patients with chronic kidney disease: a randomized clinical trial. Int J Nurs Stud 2010; 47: 1383–1388 [DOI] [PubMed] [Google Scholar]
- 37. Bennett PN, Breugelmans L, Barnard R. et al. Sustaining a hemodialysis exercise program: a review. Semin Dial 2010; 23: 62–73 [DOI] [PubMed] [Google Scholar]
- 38. Parker K. Intradialytic exercise is medicine for hemodialysis patients. Curr Sports Med Rep 2016; 15: 269–275 [DOI] [PubMed] [Google Scholar]
- 39. Parker K, Zhang X, Lewin A. et al. The association between intradialytic exercise and hospital usage among hemodialysis patients. Appl Physiol Nutr Metab 2014; 40: 371–378 [DOI] [PubMed] [Google Scholar]
- 40. Eccles M, Grimshaw J, Campbell M. et al. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care 2003; 12: 47–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.