ABSTRACT
Renal injury is a common complication in multiple myeloma (MM). In fact, as many as 10% of patients with MM develop dialysis-dependent acute kidney injury related to increased free light chain (FLC) production by a plasma cell clone. Myeloma cast nephropathy (MCN) is the most prevalent pathologic diagnosis associated with renal injury, followed by light chain deposition disease and light chain amyloidosis. Several FLC removal techniques have been explored to improve kidney disease in MM but their impact on renal clinical outcomes remains unclear. According to the evidence, high cut-off haemodialysis should be restricted to MM patients on chemotherapy with histological diagnosis of MCN and haemodialysis requirements. From our perspective, more efforts are needed to improve kidney outcomes in patients with MM and renal failure.
Keywords: acute renal failure, free light chains, high cut-off haemodialysis, myeloma cast nephropathy, plasmapheresis
INTRODUCTION
Multiple myeloma (MM) is the result of a proliferation of a neoplastic clone of plasma cells in the bone marrow and encompasses 13% of all haematologic malignancies [1], showing an annual incidence of ∼60 cases per million population [2]. Clinical manifestations are heterogeneous, ranging from skeletal disease (70%), anaemia (40%), impaired humoural immunity (80%) and, as we will focus on this review, renal impairment (20–40%) [3, 4].
Since late last century, when immunofluorescence and electronic microscopy clarified the physiopathology of myeloma kidney, several therapeutic strategies have been tested to reduce the impact of myeloma paraproteins on kidney function, especially with extracorporeal techniques to remove free light chains (FLCs) [e.g. plasmapheresis (PLEX) or high cut-off (HCO) dialysis]. There were also some efforts to characterize FLCs’ structure and physico-chemical properties in order to investigate potential inhibitors of FLCs’ intratubular aggregation.
Herein, our purpose is to review all the relatively new therapeutic strategies from a nephrologist’s perspective, focusing on the use of extracorporeal techniques to reduce the levels of FLCs in serum, especially with HCO dialysis membranes. We also summarize the knowledge acquired over the last two decades regarding the implication of FLCs in the renal pathology of MM.
PATHOGENESIS: THE ROLE OF FLCs
The first documentation of the existence of FLCs dates from 1845 [5], when a 38-year-old greengrocer from London developed bone pains and generalized swelling. His physician noticed a unique protein in his urine and sent a sample to Henry Bence Jones, the chemical pathologist from whom these proteins would take their name. More than a century later, in 1963, Edelman would characterize these molecules as FLCs [6].
Under normal conditions, plasma cells synthesize 40% more light than heavy chains [7], and this excess of FLCs is thought to favour accurate assembly of intact immunoglobulins. As a result, ∼500 mg of polyclonal FLCs are given to blood circulation every day [7, 8]. In humans, light chains are encoded by two different gene loci, resulting in the serologically distinguishable light chain types, lambda and kappa. While the former is a 22.5 kDa monomer, the latter tend to be a dimeric protein of 45 kDa. The small molecular weight confers FLCs a high distribution volume, so intravascular space contains only 15–20% of them.
FLCs filtrate through glomerular endothelium and basal membrane, reaching the proximal tubule, where they are endocyted by the megalin–cubilin complex and then degraded into their constituent amino acids inside tubular cells.
In the setting of a plasma cell dyscrasia, FLC production increases regardless of what type of immunoglobulin is produced. In fact, ∼80% of all MM patients produce monoclonal intact immunoglobulins, with 95% of these also producing monoclonal serum FLCs [9]. Although heavy chains and intact immunoglobulins remain in blood circulation, FLCs go through the glomerular filtration barrier to proximal tubule lumen and exceed the capacity of absorption-catabolism of tubular epithelial cells. As a consequence, these molecules are easily detected in urine.
RENAL LESIONS IN MM: SOME CONSIDERATIONS
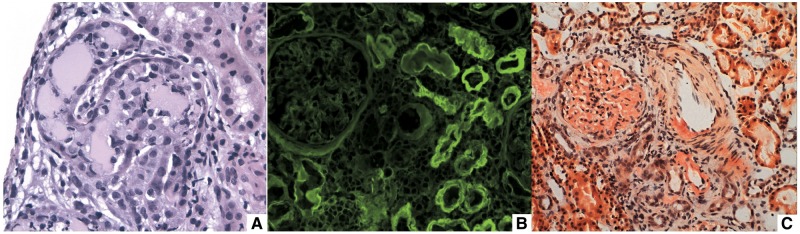
When FLC production is increased, FLCs can be isolated from urine (Bence Jones protein) but they can also remain in distal tubular structures, forming crystalline aggregates with the Tamm–Horsfall glycoprotein (THP) or uromodulin, appearing as eosinophilic casts under light microscopy examination; the so-called myeloma cast nephropathy (MCN) (Figure 1A). In MCN, there is proteinuria without albuminuria, since the glomerular basement membrane remains undamaged.
FIGURE 1.
Clinicopathologic renal lesions in MM. (A) Eosinophilic intratubular casts in MCN (haematoxylin and eosin stain); (B) tubular and glomerular basement memebranes stained by anti-lambda antibodies in LCDD (immunofluorescence microscopy); (C) orange stain of mesangium and arteriole corresponding to AL fibril deposits (Congo red stain).
On the contrary, FLCs can deposit on the glomerular basement membrane and mesangium as well, producing disruption of the filtration barrier (Figure 1B and C). If FLCs are disposed as beta-shaped arranged fibrils interacting with serum amyloid P, they will cause light chain amyloidosis (AL), whereas if FLCs are deposited themselves without any organization they will produce light chain deposit disease (LCDD). In both pathologic entities, there is albuminuria because there is glomerular filtration barrier damage. AL is usually presented as nephrotic syndrome, often without renal dysfunction, whereas LCDD displays renal dysfunction, microhaematuria and albuminuria [10].
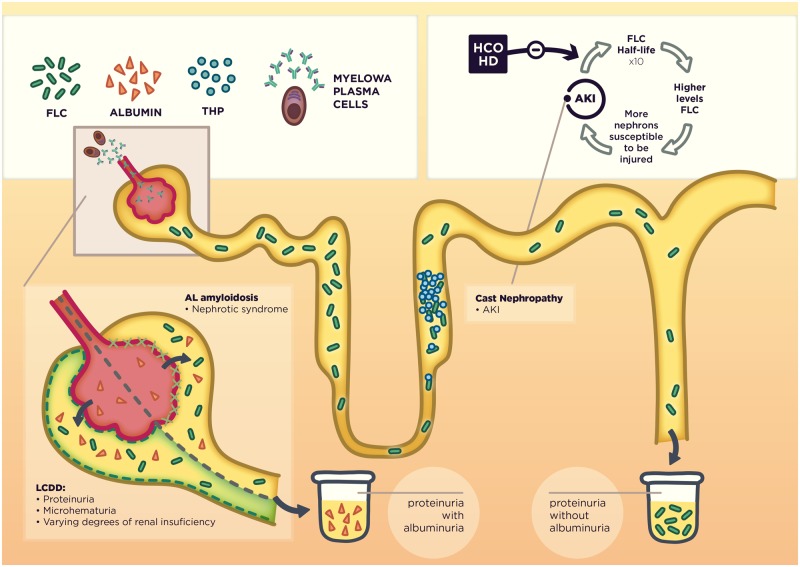
Therefore, FLCs cause different types of kidney injury in MM depending on which histological compartment is affected. The presence and type of proteinuria (Bence Jones proteinuria or albuminuria) remains the cornerstone for the clinical approach to characterize the kidney injury in MM (Figure 2).
FIGURE 2.
Mechanisms of FLC-induced nephropathy and its clinical correlation. In the upper right corner, we show the theoretical advantage of using extracorporeal techniques for FLC removal.
Nasr et al. [10] retrospectively reported renal pathology findings of 190 MM patients. Kidney biopsies were obtained as part of the standard clinical practice, and main indications were acute kidney injury (AKI) (55%), progressive renal failure with proteinuria (27%), proteinuria without decrease in glomerular filtration rate (9%) and nephrotic syndrome (14%). To be included in the study, the diagnosis of MM must have been made before kidney biopsy or shortly thereafter. They found that the most common renal lesion (33%) was MCN, followed by LCDD (22%) and AL (21%). Remarkably, 23% of the pathologic diagnostics were not clearly caused by the paraprotein.
They reported a 9% incidence of acute tubular necrosis, identifying at least one precipitating factor in 60% of cases: non-steroidal anti-inflammatory drugs use (40%), dehydration (30%), hypercalcaemia (30%), zoledronate (30%) and intravenous iodinated contrast (10%). Nephroangiosclerosis (6%) or diabetic nephropathy (5%) followed as the most common non-paraprotein-associated lesions.
This observation highlights the importance of the renal biopsy to perform a correct diagnosis and makes clear that kidney dysfunction in MM is not always related to it. This concept is crucial to the interpretation of the results raised by interventional studies, as theoretically the extracorporeal clearance of FLCs is only effective when there is paraprotein-mediated kidney injury. In the Nasr et al. study [10], MCN was the lesion with the highest dialysis requirements (37%). Moreover, in other studies focusing on patients with AKI, the prevalence of MCN rose to 66–87% [11, 12].
KIDNEY INJURY AS A PROGNOSTIC FACTOR IN MM
Focusing on AKI and dialysis needs, Haynes et al. [12] studied the clinical outcomes of 107 MM patients with AKI. They found three main protective factors: use of chemotherapy, serum albumin >35 g/L and dialysis independence. There were 88 patients requiring haemodialysis (HD), and only 15 of them (17%) reached dialysis independence during the follow-up, although the so-called ‘novel therapies’ (bortezomib and lenalidomide) were not used in this study. Dialysis independence is higher in more recent studies using these new chemotherapy agents, ranging from 21% to 50% [13–16]. Although these studies are small, it seems that renal injury reversibility could be increased by using novel therapies. However, even with these new therapies, kidney function recovery rates still remain low (<50%).
EXTRACORPOREAL TECHNIQUES: PLEX
The half-life of circulating FLCs is 3–6 h in normal renal function [17] but it shows an inverse correlation with glomerular filtration rate, as the kidney is the main FLC catabolism site. Thus, FLC half-life increases to 10-fold in patients with CKD stage 5 [18]. As such, in AKI secondary to MCN, the functioning nephrons would be overexposed to FLCs for a significant period of time, even after successful chemotherapy. Removing FLCs in this situation will cease the ‘vicious circle’, accelerating the FLC decrement by an artificial ‘catabolism’, and therefore reducing the FLC burden in the non-injured nephrons that would otherwise collapse (Figure 2).
Plasma exchange or PLEX was the only extracorporeal technique to remove circulating FLCs until 2005, when the HCO-HD membranes appeared. Since then, several studies have been conducted to evaluate its efficacy. Nevertheless, there are only three published randomized and controlled clinical trials [19–21] (Table 1), and all of them failed to demonstrate a therapeutic effect of PLEX on MM renal dysfunction. However, all studies show limitations in terms of drawing robust conclusions. Although there is a lack of statistical power in the first two studies [19, 20], the most recent, conducted by Clark et al. [21], does not report the renal histology and FLC monitoring, making it very difficult to ascertain whether the study groups were comparable regarding the type and severity of renal disease. The inconclusive results led to PLEX being downgraded from Category II to a Category III indication in MM with AKI by the American Society for Apheresis [22] in 2007.
Table 1.
Randomized and controlled trials evaluating the efficacy of plasma exchange in MM-associated AKI
| Zucchelli et al. [19] | Johnson et al. [20] | Clark et al. [21] | |
|---|---|---|---|
| Year | 1988 | 1990 | 2005 |
| Number of patients | 29 | 21 | 97 |
| Study population |
|
|
|
| Intervention | Corticosteroids + cyclophosphamide in both groups:
|
Prednisone + melphalan in both groups:
|
Melphalan + prednisone or vincristine-adriamycin-dexamethasone:
|
| Dialysis need | 24/ 29 (13 HD; 11 peritoneal dialysis) | 12/21 (PLEX:5 ; No PLEX:7) | 29/97 (PLEX 15; control 14) |
| Outcomes |
|
|
|
| Biopsies | 17 biopsies performed (16 MCN; 1 proximal tubular affection) | 16 biopsies (13 MCN; 3 tubulointerstitial injury without casts) | No biopsies performed |
| Serum FLC determination | Not available | Not available | Not performed |
| Commentaries |
|
|
|
GFR, glomerular filtration rate; NS, non-significant.
In 2008, Leung et al. [23] published a retrospective study on PLEX considering renal biopsy findings and FLC reduction. Forty patients were treated with PLEX (an average of six sessions) and 85% received chemotherapy as well. In MCN, the renal response greatly depended on the attained FLC reduction, being significantly higher in those patients achieving circulating FLC reduction >50%. Remarkably, these results were not observed in patients without MCN. This study clearly illustrates the importance of the histological diagnosis and the relationship between FLC reduction and clinical outcomes in MCN, endorsing therapeutic schemes based on FLC levels instead of pre-fixed or arbitrary protocols. Further and well-designed clinical trials are needed to definitively refuse or accept the utility of PLEX as an adjuvant therapy.
EXTRACORPOREAL TECHNIQUES: HCO MEMBRANES
In 2005, a new generation of HD membranes known as ‘protein leaking membranes’ were designed as an alternative way to provide greater clearances of high molecular weight substances involved in uraemia, which are not removed by high flux membranes [24]. The so-called ‘high cut-off membranes’ owed their name to their wide pore size, which enhanced the molecular weight cut-off to ∼50–60 kDa [25]. By using these HCO membranes, the extracorporeal treatments could be more intensive and longer-lasting than those by PLEX, thus reducing the FLC rebound and preventing PLEX complications such as clotting or severe hypoproteinaemia. However, it has to be noted that albumin (68 kDa) leaks across those HCO membranes, which is their main limitation.
The most relevant studies on FLC removal by using HCO filters were published in 2007 [26] and 2008 [27], both led by Dr Hutchison. These articles established some core concepts in terms of dialysis characteristics (clearance, time, convection use, albumin leakage and membrane surface) to establish rational therapeutic schemes to evaluate the effects of FLC reduction on clinical outcomes. One of the most relevant conclusions reached in these studies [26, 27] is that HCO-HD could be useful in accelerating the decrease of serum FLCs provided by effective chemotherapy. Therefore, without a concomitant decrease in the FLC production rate, extracorporeal depuration will only generate a transient decrease of FLCs followed by a ‘rebound’ thereafter.
Accordingly, 19 patients with MM, AKI HD-dependent and diagnosis of MCN by renal histology were initially included in a study to receive standard chemotherapy regimen and HCO-HD [28]. Six patients had their chemotherapy interrupted because of complications and they displayed a moderate FLC reduction, <40%. In contrast, patients combining chemotherapy and HCO-HD showed an 85% decline in the FLC levels and, more importantly, all achieved the renal recovery end-point, defined as dialysis independence 2 weeks after last dialysis session. Although cautious, the observed 100% recovery rate (13/13) is promising, particularly when compared with recovery rates (∼20%) reported in previous studies [12, 13]. Nonetheless, it seems reasonable to conclude that, independently of whether HCO-HD is useful or not, its use should be limited to patients eligible for receiving an effective chemotherapeutic treatment.
Two successive studies [29, 30] examined if early reductions in serum FLCs were associated with better outcomes and if there was a specific FLC reduction threshold to achieve the renal improvement. In both series of patients (39 and 67, respectively) renal recovery rate was 60%. The authors found that FLC reduction on Day 21 was associated with renal recovery. Also, although they did not find a clear threshold, there was a linear relationship between the FLC reduction and the probability of renal recovery, as 60% FLC reduction was associated with 80% dialysis independence. This correlation was presumed in previous works [23], suggesting that there could be some biochemical targets in order to improve renal outcomes and, consequently, survival rates in MM patients.
Finally, two randomized clinical trials were conducted to prove the efficacy of HCO-HD as an adjuvant therapy for HD-dependent AKI in MM patients receiving concomitant chemotherapy (Table 2). One of them, the European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE) trial [31], has not yet been published, but preliminary results were given in abstract form. Meanwhile, the MYRE trial [32], has recently been published. There are some considerations to be taken into account when interpreting the results reported in said trials. In the EuLITE trial, the proportion of patients in which chemotherapy was discontinued was not reported. On the other hand, there was a higher proportion of patients having severe infectious complications in the experimental group, raising some concerns on group differences regarding immunoparesis, MM severity or antibiotic-dose adjustment. Finally, the use of two filters in series could provide higher FLC clearance, but implied higher risk of hypoalbuminaemia and its related complications. When published, the definitive results may enable us to undertake a thorough analysis of this work. In the MYRE trial [32] the primary end point consisting of HD independence at 3 months was not achieved, although renal recovery at 6 and 12 months was higher in the HCO-HD arm. The study was underpowered to identify early clinically relevant differences while there was no clear hypothesis for the observed delayed therapeutic effect. In fact, as previously described [29], FLC decrease at Day 21 should be a predictor of renal recovery with a median time of dialysis independence of 27 days (ranging from 7 to 107 days).
Table 2.
Randomized and controlled trials evaluating the efficacy of HCO-HD in MCN
| EuLITE [31] (2008–15; not published) | MYRE [32] (2011–16) | |
|---|---|---|
| Patient number | 90 |
|
| Study population |
|
|
| Calculation sample size |
|
|
| Analysis | Intention to treat | Intention to treat |
| Treatment |
|
|
| HD protocol |
|
|
| End points; results |
|
|
| Other relevant data |
|
|
Haematologic response overall: it includes partial (decrease of >50% FLC), very good partial (>90%) or complete response (normal level or ratio).
In conclusion, we currently know that HCO membranes enhance FLC clearance in HD-dependent AKI secondary to MCN. The advantage of HCO-HD use upon standard of care is still controversial; definitive results of some studies and more Randomized Controlled trial are needed. However, it must be noted that the International Myeloma Working Group (IMWG), in their 2016 recommendations on myeloma-related renal impairment, has already stated that the current data supports the use of HCO-HD in AKI HD-dependent secondary to MCN with an evidence grade B [33].
LOOKING FOR PROGNOSIS FACTORS: TIME, BIOPSY OR BOTH?
As the pathogenic role of FLCs on intratubular cast formation is accepted, we can hypothesize that there is some relationship between the duration of FLC exposure and the irreversibility of renal injury. Hutchinson et al. [30], found a more favourable prognosis in patients who initiated the HCO-HD technique within 7 days of presentation compared with those who initiated it later. This is the only available information we already have in terms of HCO-HD therapy delay, but it seems reasonable considering that immediate decrease of FLCs could entail less renal injury.
However, in real life, usually it is not possible to determine for how long the kidneys have been injured by circulating FLCs. There is no data about the natural history of MCN or subclinical effects of FLCs on renal structures, because histological diagnosis is only considered when there is AKI, so the outcomes might be different depending on the acute/chronic lesion burden in the kidney (as it is described in other glomerular or tubular entities). Therefore, the renal biopsy could help to clarify which patients are more likely to respond when chronologic diagnosis is unknown.
Although the information regarding the prognostic value of kidney biopsy is scarce, some studies attempted to relate histological lesions with clinical prognosis. In Johnson’s study [20], for example, low cast burden in biopsy (<5/specimen) was significantly related to better renal prognosis. Summarized in Table 3 are the most relevant studies in terms of clinical and pathologic relationships [4, 34, 35]. Overall, cast burden and tubular atrophy seem the best predictors for worse renal outcomes in MCN. However, as previously pointed out, renal injury in MM could be caused by other entities (MM-related and non-related), so the histological confirmation of MCN is recommended before initiating HCO-HD. Beyond identifying MCN, the kidney biopsy could become a tool to predict which patients are more likely to respond with intensive HCO-HD treatment. Even a ‘control’ biopsy could guide us in maintaining the therapy in patients likely to achieve delayed recovery of renal function. All of these concepts must be proven and properly explored in further studies, with the aim of stratifying which patients are more likely to respond. Perhaps the question is not whether HCO-HD works or not, but in which kind of patients it could have the most positive impact.
Table 3.
Main studies evaluating histological prognosis predictors in MCN biopsies
| Rota et al. [4] | Ecotière et al. [34] | Basnayake et al. [35] | |
|---|---|---|---|
| Number of patients | 34 (26 MCN) | 70 | 4 |
| Population |
|
|
|
| Clinical outcomes |
|
|
|
| Follow-up | No specified | Median 17.5 months (1–146) | No specified |
| Biopsy time | <3 weeks | Not specified | At 6 weeks after full treatment and no response |
| Biopsy items evaluated |
|
|
|
| Results | Tubular atrophy (P=0.05) between recovered and non-recovered renal function | Whole study population:
|
|
| Commentaries/ other information |
|
|
|
eGFR, estimated glomerular filtration rate.
MOLECULAR THERAPIES: COMPLEMENT DETERMINING REGION INHIBITION
While kappa chains tend to be more common in MCN and LCDD, lambda chains are more amyloidogenic [10]. However, as previously anticipated, each light chain type shows a different ‘nephritogenicity’, beyond kappa or lambda classification. In fact, the severity of renal injury is not always directly related to FLC levels in plasma or urine [36, 37]. One of the major reasons for such inconsistency, beyond some well-known precipitating factors such as dehydration, is the affinity of the FLC variable domain with the THP, which is a constitutive protein synthetized and secreted into the tubular lumen by tubular cells. This protein was isolated from the cast of MCN kidneys for the first time in 1990 [38] by Paul Sanders and represented a milestone in terms of knowledge on this illness. Over the following two decades, Sanders studied the interaction between FLC and THP and introduced the basis for new therapy strategies [39, 40, 41].
Each light chain contains two globular subunits, termed variable (VL) and constant (CL) domains. Three hypervariable segments of VL [complement determining regions (CDRs): CDR1, CDR2 and CDR3] produce loop structures conforming to a hydrophobic interior that interact with antigens [42]. In 2001, Ying and Sanders [39] identified the CDR3 region as a single binding site of light chains for THP, and the different affinity of several FLCs for THP was also proved. In 2012 [40] they published a relevant study that was focused on synthesizing a competitive inhibitor peptide. Firstly, they used information from previous affinity data of different CDR3 domains and synthesized a peptide based on CDR3 peptide sequences known to interact strongly with THP. The final inhibitor peptide was examined by a competitive Enzyme-Linked Immunosorbent Assay and was proved to show intensive inhibitory activity of human FLC-THP binding. In the second part of the study, this inhibitor was tested in vivo in a rodent model of AKI MCN, induced by intraperitoneal administration of FLCs. The authors compared the efficacy of injecting the competitor peptide intraperitoneally or vehicle alone 2 h before FLC injection. Interestingly, there was less cast burden and lower serum creatinine (Cr) in the treated rats. Despite these promising results, further studies are warranted.
CONCLUSIONS
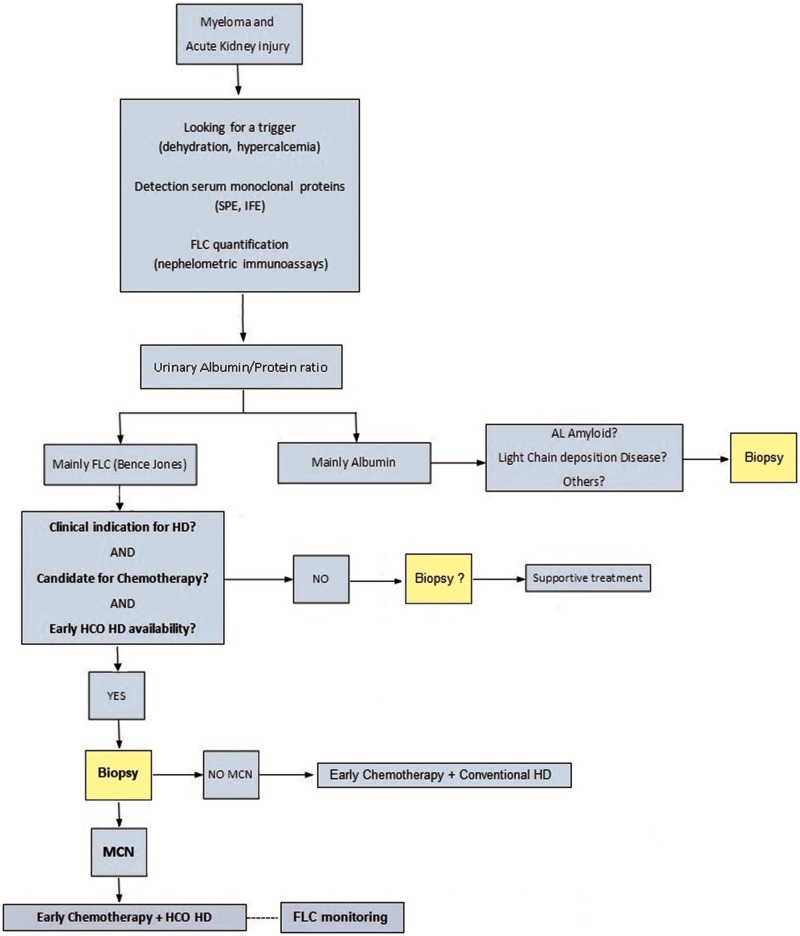
Efficacy on FLC reduction by HCO-HD filters has been demonstrated, but its impact on renal clinical outcomes remains unclear. According to the evidence, HCO-HD use should be restricted to MM patients on chemotherapy with histological diagnosis of MCN and HD requirements (Figure 3). The next step should be to identify which patients could benefit the most. It seems that some factors could influence the outcomes of these therapies (time of initiation, type and chronicity of renal lesions, lambda or kappa FLCs, drug adjustments and duration of treatment). Certainly, the information provided by the kidney biopsy is relevant for guiding treatment in cases with severe renal involvement. From our perspective, more efforts are needed to improve kidney outcomes in patients with MM and renal injury.
FIGURE 3.
Proposed algorithm for AKI management and use of HCO-HD in MM patients. Clinical criteria for using HCO in bold, according to available evidence; all of them should be met simultaneously before initiating HCO-HD. FLC monitoring is recommended when using HCO-HD to assess the technique efficacy and be aware of biochemical outcomes related with favourable prognosis. IFE, immunofixation electrophoresis; SPE, serum protein electrophoresis.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of Lluís Chavarria in the illustration of this review. This research was supported by The Red de Investigación Renal (European Regional Development Funds ISCIII Red Temática de Investigación Cooperativa en Salud Red de Investigación Renal; RD16/0009/0003).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Greenlee RT, Murray T, Bolden S. et al. Cancer statistics, 2000. CA Cancer J Clin 2000; 50: 7–33 [DOI] [PubMed] [Google Scholar]
- 2. Bladé J, Fernández-Llama P, Bosch F. et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 1998; 158: 1889–1893 [DOI] [PubMed] [Google Scholar]
- 3. Rayner HC, Haynes AP, Thompson JR. et al. Perspectives in multiple myeloma: survival, prognostic factors and disease complications in a single centre between 1975 and 1988 . Q J Med 1991; 79: 517–525 [PubMed] [Google Scholar]
- 4. Rota S, Mougenot B, Baudouin B. et al. Multiple myeloma and severe renal failure: a clinicopathologic study of outcome and prognosis in 34 patients. Medicine (Baltimore) 1987; 66: 126–137 [DOI] [PubMed] [Google Scholar]
- 5. Rathore R, Coward RA, Woywodt A.. What’s in a name? Bence Jones protein. Clin Kidney J 2012; 5: 478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Edelman GM, Gally JA.. The nature of Bence-Jones proteins : chemical similarities to polypeptide chains of myeloma globulins and normal γ-globulins. J Exp Med 1962; 116: 207–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waldmann TA, Strober W, Mogielnicki RP.. The renal handling of low molecular weight proteins: II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J Clin Invest 1972; 51: 2162–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon A. Light chains of human immunoglobulins. Methods Enzymol 1985; 116: 101–121 [DOI] [PubMed] [Google Scholar]
- 9. Kyle RA, Gertz MA, Witzig TE. et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003; 78: 21–33 [DOI] [PubMed] [Google Scholar]
- 10. Nasr SH, Valeri AM, Sethi S. et al. Clinicopathologic correlations in multiple myeloma: A case series of 190 patients with kidney biopsies. Am J Kidney Dis 2012; 59: 786–794 [DOI] [PubMed] [Google Scholar]
- 11. Pozzi C, Pasquali S, Domini U. et al. Prognostic factors and effectiveness of treatment in acute renal failure due to multiple myeloma: a review of 50 cases. Clin Nephrol 1987; 28: 1–9 [PubMed] [Google Scholar]
- 12. Haynes RJ, Read S, Collins GP. et al. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant 2010; 25: 419–426 [DOI] [PubMed] [Google Scholar]
- 13. Dimopoulos MA, Roussou M, Gavriatopoulou M. et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomibbased regimens: identification of predictive factors. Clin Lymphoma Myeloma 2009; 9: 302–306 [DOI] [PubMed] [Google Scholar]
- 14. Ludwig H, Adam Z, Hajek R. et al. Light chaininduced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol 2010; 28: 4635–4641 [DOI] [PubMed] [Google Scholar]
- 15. Morabito F, Gentile M, Ciolli S. et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol 2010; 84: 223–228 [DOI] [PubMed] [Google Scholar]
- 16. Dimopoulos MA, Roussou M, Gkotzamanidou M. et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 2013; 27: 423–429 [DOI] [PubMed] [Google Scholar]
- 17. Miettinen TA, Kekki M.. Effect of impaired hepatic and renal function on Bence Jones protein catabolism in human subjects. Clin Chim Acta 1967; 18: 395–407 [Google Scholar]
- 18. Hutchison CA, Harding S, Hewins P. et al. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008; 3: 1684–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zucchelli P, Pasquali S, Cagnoli L. et al. Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 1988; 33: 1175–1180 [DOI] [PubMed] [Google Scholar]
- 20. Johnson WJ, Kyle RA, Pineda AA. et al. Treatment of renal failure associated with multiple myeloma plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 1990; 150: 863–869 [PubMed] [Google Scholar]
- 21. Clark WF, Stewart AK, Rock GA. et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med 2005; 143: 777–784 [DOI] [PubMed] [Google Scholar]
- 22. Szczepiorkowski ZM, Bandarenko N, Kim HC. et al. Guidelines on the use of therapeutic apheresis in clinical practice—Evidence-based approach from the Clinical Applications Committee of the American Society for Apheresis. J Clin Apher 2007; 22: 106–175 [DOI] [PubMed] [Google Scholar]
- 23. Leung N, Gertz MA, Zeldenrust SR. et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int 2008; 73: 1282–1288 [DOI] [PubMed] [Google Scholar]
- 24. Ward RA. Protein-leaking membranes for hemodialysis: a new class of membranes in search of an application? J Am Soc Nephrol 2005; 16: 2421–2430 [DOI] [PubMed] [Google Scholar]
- 25. Gondouin B, Hutchison C.. High cut-off dialysis membranes: current uses and future potential. Adv Chronic Kidney Dis 2011; 18: 180–187 [DOI] [PubMed] [Google Scholar]
- 26. Hutchison CA, Cockwell P, Reid S. et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. Am Soc Nephrol 2007; 18: 886–895 [DOI] [PubMed] [Google Scholar]
- 27. Hutchison C, Harding S, Mead G. et al. Serum free-light chain removal by high cutoff hemodialysis: optimizing removal and supportive care. Artif Organs 2008; 32: 910–917 [DOI] [PubMed] [Google Scholar]
- 28. Hutchison CA, Bradwell AR, Cook M. et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol 2009; 4: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutchison CA, Cockwell P, Stringer S. et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol 2011; 22: 1129–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutchison CA, Heyne N, Airia P. et al. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 2012; 27: 3823–3828 [DOI] [PubMed] [Google Scholar]
- 31. Hutchison CA, Cook M, Heyne N. et al. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomized contol trial. Trials 2008; 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bridoux F, Carron PL, Pegourie B. et al. Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy. A randomized clinical trial. JAMA 2017; 318: 2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dimopoulos MA, Sonneveld P, Leung N. et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol 2016; 34: 1544–1557 [DOI] [PubMed] [Google Scholar]
- 34. Ecotière L, Thierry A, Debiais-Delpech C. et al. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant 2016; 31: 64–72 [DOI] [PubMed] [Google Scholar]
- 35. Basnayake K, Cheung CK, Sheaff M. et al. Differential progression of renal scarring and determinants of late renal recovery in sustained dialysis dependent acute kidney injury secondary to myeloma kidney J Clin Pathol 2010; 63: 884–887 [DOI] [PubMed] [Google Scholar]
- 36. Woodruff R, Sweet B.. Multiple myeloma with massive Bence Jones proteinuria and preservation of renal function. Aust NZ J Med 1977; 7: 60–62 [DOI] [PubMed] [Google Scholar]
- 37. Chauveau D, Choukroun G.. Bence Jones proteinuria and myeloma kidney. Nephrol Dial Transplant 1996; 11: 413–415 [PubMed] [Google Scholar]
- 38. Sanders PW, Booker BB, Bishop JB. et al. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J Clin Invest 1990; 85: 570–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ying W-Z, Sanders PW.. Mapping the binding domain of immunoglobulin light chains for Tamm-Horsfall protein. Am J Pathol 2001; 158: 1859–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ying W-Z, Allen CE, Curtis LM. et al. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest 2012; 122: 1777–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leung N. Treating myeloma cast nephropathy without treating myeloma. J Clin Invest 2012; 122: 1605–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanders PW. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol 2013; 23: 1777–1781 [DOI] [PubMed] [Google Scholar]