ABSTRACT
Background
Aortic stiffness is one of the earliest signs of cardiovascular disease (CVD) in patients with chronic kidney disease and an independent predictor of mortality. It is thought to drive left ventricular (LV) remodelling, an established biomarker for mortality. The relationship between direct and indirect measures of aortic stiffness and LV remodelling is not defined in dialysis patients, nor are the reproducibility of methods used to assess aortic stiffness using cardiac magnetic resonance (CMR) imaging.
Methods
Using 3T CMR, we report the results of (i) the interstudy, interobserver and intra-observer reproducibility of ascending aortic distensibility (AAD), descending aortic distensibility (DAD) and aortic pulse wave velocity (aPWV) in 10 haemodialysis (HD) patients and (ii) the relationship between AAD, DAD and aPWV and LV mass index (LVMi) and LV remodelling in 70 HD patients.
Results
Inter- and intra-observer variability of AAD, DAD and aPWV were excellent [intraclass correlation (ICC) > 0.9 for all]. Interstudy reproducibility of AAD was excellent {ICC 0.94 [95% confidence interval (CI) 0.78–0.99]}, but poor for DAD and aPWV [ICC 0.51 (−0.13–0.85) and 0.51 (−0.31–0.89)]. AAD, DAD and aPWV associated with LVMi on univariate analysis (β = −0.244, P = 0.04; β =−0.315, P < 0.001 and β = 0.242, P = 0.04, respectively). Only systolic blood pressure, serum phosphate and a history of CVD remained independent determinants of LVMi on multivariable linear regression.
Conclusions
AAD is the most reproducible CMR-derived measure of aortic stiffness in HD patients. CMR-derived measures of aortic stiffness were not independent determinants of LVMi in HD patients. Whether one should target blood pressure over aortic stiffness to mitigate cardiovascular risk still needs determination.
Keywords: aortic stiffness, chronic kidney disease, end-stage renal disease, haemodialysis, left ventricular remodelling
INTRODUCTION
The pathophysiological processes that drive cardiovascular disease (CVD) in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) are complex and multifactorial [1, 2]. They lead to changes in cardiac structure and function, including left ventricular (LV) hypertrophy (LVH), LV dilatation and myocardial fibrosis, typically termed uraemic cardiomyopathy [3]. The development of aortic stiffness is fundamental to the development of LVH and uraemic cardiomyopathy, offsetting the finely tuned coupling of the heart and arterial system (the ‘arterial–ventricular interaction’) [4]. Aortic stiffening in patients with CKD and ESRD occurs at an accelerated rate compared with the normal ageing process and arteriosclerosis is the predominant pathogenic process [4]; indeed, studies in patients with ESRD suggest that accelerated vascular ageing directly affects cardiovascular outcomes [5]. The importance of aortic stiffness as a marker of cardiovascular dysfunction in early-stage CKD is clear [6] and is detectable before overt cardiac dysfunction [7]. Large studies in dialysis patients show that increased aortic stiffness predicts outcomes [5, 8–10] and suggest associations with increased LV mass (LVM) and LV remodelling [11, 12].
The techniques used to measure aortic stiffness, however, have been varied and not standardized. Aortic stiffness can be measured non-invasively, directly with aortic distensibility (AD) or indirectly with aortic pulse wave velocity (aPWV). Each of these can be measured by a number of modalities, including cardiac magnetic resonance (CMR) imaging, tissue Doppler or mechano-transducer, and the relative pros and cons of these have been discussed, including the reproducibility of these measures in healthy volunteers and in CKD disease populations [13]. None of these techniques have been validated against invasive measures in patients with CKD. Although CMR-derived aPWV measurement has been validated against invasive catheterization in patients with coronary artery disease, CMR-derived measures of AD have not [14]. CMR is the established gold standard for the assessment of cardiac structure and function [15]. Its proven reproducibility in patients on dialysis [16] and multiparametric capabilities make it an ideal platform to comprehensively phenotype cardiovascular function and disease in patients with renal disease.
No study has assessed the reproducibility of CMR-derived direct and indirect measures of aortic stiffness in prevalent dialysis patients or assessed the relationship between LVH, LV remodelling and measures of aortic stiffness using CMR. If these measures were shown to be reproducible in this population, because they can be acquired as part of a comprehensive cardiovascular assessment using CMR, it would obviate the need for these measures to be repeated using alternative non-invasive methods for patients undergoing CMR either in a clinical trial or as part of their clinical care.
The aims of this study were to assess the relationship between aortic stiffness and LV remodelling and to characterize the determinants of aPWV, ascending AD (AAD) and descending AD (DAD) in patients on haemodialysis (HD). As aortic stiffness is a potential therapeutic target in ESRD as a surrogate marker of outcome, demonstrating the reproducibility of CMR-derived aPWV, AAD and DAD in this population is of great importance. These analyses were also undertaken and are presented in this study.
MATERIALS AND METHODS
The baseline CMR scans of 70 HD patients from the CYCLE-HD trial (ISRCTN 11299707), assessing the effects of intradialytic exercise on cardiac structure, were analysed. Inclusion and exclusion criteria are as previously described [17]. Demographic data, medical comorbidity, dialysis vintage, haematological and biochemical data were collected prospectively. Blood pressure (BP) was measured non-invasively using a brachial cuff (Datex Ohmeda GE S/5) 24 h after dialysis (on an interdialytic day) after the patient had been resting for at least 30 min. The study was given local research and ethics committee approval and written informed consent was obtained prior to recruitment.
CMR image protocol
Complete LV and aortic functional analysis was undertaken on a 3T CMR platform (Skyra, Siemens Medical Imaging, Erlangen, Germany) with an 18-channel phased-array receiver coil. Dialysis patients were scanned on non-dialysis days, not after the ‘long-break’, with all scans conducted between 18 and 24 h of the most recent dialysis to standardize fluid status. The CMR protocols for acquiring cine imaging were as previously described [18], conforming to internationally recognized standards [19]. Briefly, electrographic gated breath-hold steady-state free precession long-axis cine images in two-, three- and four-chamber views were acquired. Short-axis cine images covering the left ventricle were taken at 8-mm slice thickness, no gap, field of view 28 × 30 cm, matrix 208 × 256, repetition time 2.9 ms, echo time 1.2 ms, flip angle 64°−79°, temporal resolution <50 ms, 80% phase, with 30 phases per cardiac cycle, in-plane image resolution 1.1 × 1.5 mm to 1.3 × 1.7 mm.
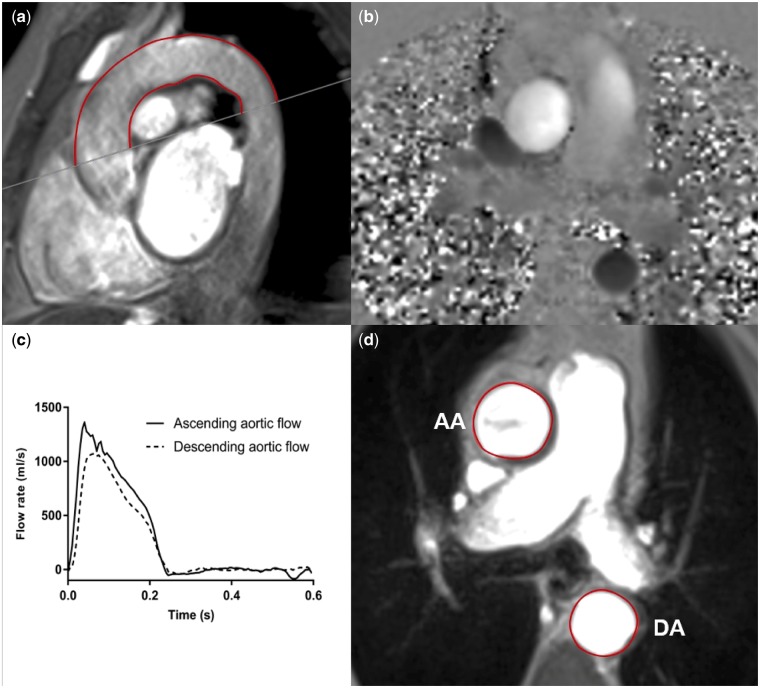
High temporal resolution, through-plane, phase-contrast cines were acquired perpendicular to the ascending and descending aorta at the level of the pulmonary artery bifurcation, permitting calculation of transit time for aPWV (velocity encoding 150 cm/s, slice thickness 5 mm, acquired temporal resolution 10 ms, typical field of view 400 mm, acquired spatial resolution 1.6 × 1.6 × 5 mm, repetition time 10.88 ms, echo time 3.13 ms). Sagittal-oblique cines of the ascending and descending aorta were captured for distance measurement (Figure 1). Steady-state free procession cines were taken at the pulmonary artery bifurcation, perpendicular to the aorta to facilitate calculation of distensibility (Figure 1). Brachial BP was measured concomitantly using an automated device for derivation of pulse pressure (PP) (Datex Ohmeda GE S/5) and AD was calculated using the following formula, as previously described [13]:
FIGURE 1:
Assessment of aPWV and AD using two-dimensional phase-contrast CMR. For the aPWV calculation, axial aortic contours were mapped onto phase–contrast cines (a), allowing the waveform transit time to be calculated from flow curves of the ascending and descending aorta (b). Distance was measured using a sagittal–oblique cine. Outer and inner borders of the aortic arch were manually drawn and the mean distance of these two borders was calculated (mm) (c). AD was calculated from axial cine images by contouring the change in ascending and descending aortic area (d) and the PP measured simultaneously. AA, ascending aorta; DA, descending aorta.
CMR image analysis
Scans were analysed offline by blinded observers. A single reader completed all LV structural and functional analysis (M.P.M.G.-B.) and a separate independent reader completed analysis of all aortic stiffness analysis (S.F.A.). Image quality was assessed as being excellent, good, acceptable or poor.
LV analysis
LV analysis was completed using the software package CMR42 (Circle Cardiovascular Imaging, Calgary, AB, Canada). LV volume and LVM were quantified excluding papillary muscles and trabeculations as previously described [20], with epicardial and endocardial manually contoured on a contiguous stack of multiphase ventricular short-axis cines (10–12 slices) at end diastole and end systole. Volumetric and LVM data were indexed to body surface area.
Aortic stiffness
Measures of aortic stiffness were analysed using the semi-automated software package JIM version 6 (Xinapse Systems, Essex, UK) as previously described [21].
aPWV
Every ninth slice of the ascending and descending aorta was manually contoured and propagated using the gradient echo cine. Contouring every ninth slice was based on local experience that has yielded the most reliable propagation of contours. Contours were mapped onto the phase-contrast cine (Figure 1), allowing the temporal shift to be determined. Contours that did not propagate well were manually adjusted. The cut made to define the axial slice for the phase-contrast sequences was superimposed on the sagittal–oblique cine and the average distance was measured around the aortic arch (Figure 1). aPWV was calculated as previously described [13, 21].
Aortic distensibility
The ascending and descending aortic borders were manually contoured for every seventh slice and propagated through the cardiac cycle (Figure 1). Contouring every seventh slice was based on local experience that has yielded the most reliable propagation of contours. PP, derived from brachial BP taken during CMR, was imputed. AAD and DAD were calculated as previously described [13].
Reproducibility of aPWV, AAD and DAD
Interstudy repeatability was undertaken for the 10 HD patients who underwent repeat CMR scans. These patients were approached after their initial scan and gave separate consent to undertake a further identical CMR scan within 14 days as per study protocol. Interobserver variability was assessed by analysis of 10 scans independently by two operators. Intra-observer variability was assessed by reanalysis of 10 scans by the same reader, 2 weeks apart. Despite the measures taken to ensure consistency of volume status (scanning on a non-dialysis day and not after the long-break) concerns remained that haemodynamic status would be significantly different between scans, which would affect cardiac loading. Changes in heart rate and BP on interstudy scan visits were recorded to identify any significant changes in haemodynamics. In addition, a change in LV end-diastolic volume (ΔLVEDV) between scans was taken as a surrogate marker of change in cardiac loading and hydration status in HD patients. To identify the potential influence of changes in volume status on CMR-derived measures of aortic stiffness, relationships between changes in cardiac loading and changes in measures of aortic stiffness between scans were assessed. This is as in our previous work describing the reproducibility of native T1 mapping in HD patients [16].
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences, version 24.0 (IBM, Armonk, NY, USA). The normality of data was determined by the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and non-normally distributed data as median (25th and 75th percentile). Paired t-tests were used to compare differences between means of repeated measures. A two-tailed P-value < 0.05 was considered statistically significant.
Determinants of aortic stiffness, LVM and LV remodelling
For the purposes of regression, non-normally distributed data was log transformed to normalize the data. Univariate and multivariable linear regression analyses were conducted to assess the independent determinants of aortic stiffness and the ability of aortic stiffness measures to determine LV structure and concentric remodelling [LVM index (LVMi) and LVM/volume ratio].
For the determinants of aortic stiffness, multivariable linear regression was performed, with each stiffness parameter (aPWV, AAD, DAD) as the dependent variable. Predetermined variables known to influence aortic stiffness were included in each model: age [22], systolic BP (SBP) [23], HD vintage [24], diabetes [25], CVD [26] and body mass index [27]. For testing the associations of aortic stiffness with LV structure, multivariable linear regression models were created for each parameter (aPWV, AAD and DAD), due to high collinearity between measures of arterial stiffness. Associations with LVMi and the LVM:volume ratio were assessed using the coefficient of determination (R2) to compare the strength of each regression model. Covariates known to influence LV structure were included in each model: age [28], SBP [29], serum phosphate [30], haemoglobin [31] and CVD [32].
Variance inflation factors were calculated to test for multi-collinearity between covariates.
Reproducibility study
Agreement between the techniques, operators and within-operator techniques was quantified by intraclass correlation (ICC) and appraised by Bland–Altman analysis. Reproducibility was considered ‘excellent’ for ICC >0.90, ‘good’ for ICC between 0.80 and 0.89, ‘moderate’ for ICC between 0.60 and 0.79 and ‘poor’ for ICC <0.60. Pearson’s or Spearman’s correlations were used to assess potential relationships between ΔLVEDV and changes in aPWV, AAD and DAD (ΔaPWV, ΔAAD and ΔDAD) between scans to assess the possible effects on clinical changes in cardiac loading and fluid status on CMR-derived measures of aortic stiffness.
RESULTS
Baseline demographic details are shown in Table 1. aPWV, AAD and DAD were analysable in 69 of 70 scans. Image quality for sagittal–oblique aortic cine was excellent in 10, good in 32 and acceptable in 27, and for phase-contrast images it was excellent in 18, good in 30 and acceptable in 21. Contours propagated for phase-contrast images (requiring no adjustment) in 62 of 69 scans, with a small amount of manual adjustment required in seven scans. Image quality of aortic cines was excellent in 11, good in 25 and acceptable in 33. Contours propagated for aortic cine images in 64 of 69 scans, with a small amount of manual adjustment required in 5 scans. LV structural and functional analysis was possible in all 70 scans. Image quality for LV analysis was excellent in 24, good in 39 and acceptable in 7.
Table 1.
Characteristics of the total ESRD population (N = 70)
| Variable | Values |
|---|---|
| Age (years) | 59 (46, 69) |
| Male, n (%) | 51 (73) |
| Ethnicity, n (%) | |
| White | 29 (41) |
| BAME | 39 (56) |
| Other | 2 (3) |
| HD vintage (months) | 19.5 (8.8, 48.5) |
| Previous transplant, n (%) | 17 (24) |
| Active on transplant list, n (%) | 18 (26) |
| BMI (kg/m2) | 27 (24, 31) |
| SBP (mmHg) | 144 ± 37 |
| DBP (mmHg) | 66 ± 17 |
| PP (mmHg) | 78 ± 31 |
| Heart rate (bpm) | 73 (66, 80) |
| Ever smoked, n (%) | 37 (57) (n=65) |
| Pack-years | 1 (0, 20) (n=65) |
| Co-morbidity, n (%) | |
| Type 2 diabetes | 26 (37) |
| Hypertension | 46 (66) |
| CVD | 22 (31) |
| Heart failure | 5 (7) |
| Coronary artery disease | 14 (20) |
| Previous MI | 4 (6) |
| Previous stroke | 2 (3) |
| Haematology and biochemistry | |
| Haemoglobin (g/L) | 111.8 ± 15.8 |
| CRP (mg/L) (n=69) | 5 (5, 13) |
| Phosphate (mmol/L) | 1.50 (1.3, 1.9) |
| Sodium (mmol/L) | 136.2 ± 2.7 |
| URR (%) | 76.1 ± 6.6 |
| PTH (pmol/L) | 32.1 (12.1, 79.9) (n=69) |
| HbA1c (%) | 5.3 (4.9, 6.4) (n=67) |
| Total cholesterol (mmol/L) | 3.9 ± 1.1 |
| Medications, n (%) | |
| Erythropoietin | 55 (79) |
| Vitamin D supplement | 52 (74) |
| Phosphate binder | 55 (79) |
| Beta-blocker | 29 (41) |
| ACEi/ARB | 13 (19) |
| Diuretic | 15 (21) |
| Alpha-blocker | 14 (20) |
| Calcium blocker | 27 (39) |
| Statins | 39 (56) |
| NSAIDs | 34 (49) |
| Insulin | 14 (20) |
| Oral hypoglycaemia | 8 (11) |
Normally distributed data presented as mean ± SD, non-normally distributed data presented as median (25th, 75th percentile).
BAME, Black, Asian and minority ethnic; BMI, body mass index; MI, myocardial infarction; CRP, C-reactive protein; URR, urea reduction ratio; PTH, parathyroid hormone; HbA1c, haemoglobin A1c; ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; NSAIDs, non-steroidal anti-inflammatory drugs.
Description of CMR-derived LV structure and function and all measures of aortic stiffness are shown in Table 2. There was a low level of collinearity (≤2) and no significant non-linear effects in all regression analyses.
Table 2.
LV and aortic characteristics of the total population
| Cardiac and arterial parameters | Patients (n = 70) |
|---|---|
| aPWV (m/s) | 7.72 (5.6, 10.9) (n =69) |
| AAD (mmHg−1×10−3) | 2.19 (1.2, 3.8) (n=69) |
| DAD (mmHg−1×10−3) | 2.58 (1.8, 3.9) (n=69) |
| LVEDV index (mL/m2) | 81.9 (66.2, 105.9) |
| LV end-systolic volume index (mL/m2) | 35.4 (26.8, 48.1) |
| LV stroke volume index (mL/m2) | 45.1 (37.4, 55.6) |
| LV ejection fraction (%) | 56.4 (50.7, 60.9) |
| LVMi (g/m2) | 58.5 (48.2, 72.6) |
| LVM/volume ratio (g/mL) | 0.71 ± 0.14 |
Normally distributed data presented as mean ± SD, non-normally distributed data presented as median (25th, 75th percentile). LV indices indexed to body surface area.
Determinants of aortic stiffness
AAD {1.59 mmHg−1×10−3 [95% confidence interval (CI) 1.0–2.8] versus 2.62 mmHg−1×10−3 [1.6–4.5], P = 0.023} and DAD [1.95 mmHg−1×10−3 (95% CI 1.0–2.9) versus 3.03 mmHg−1×10−3 (2.3–4.3), P = 0.005] were significantly reduced in diabetic patients. AAD was reduced in patients with CVD [1.63 mmHg−1×10−3 (95% CI 1.1–2.5) versus 2.62 mmHg−1×10−3 (1.5–4.4), P = 0.034]. There was no difference in any measures of aortic stiffness between gender, ethnicity, any other medical comorbidity and medication use. Univariate associations with measures of aortic stiffness are shown in Supplementary data, Appendix 1. Age and SBP were associated with all measures of aortic stiffness, although diastolic BP (DBP) or heart rate had no association. Multivariable determinants of aortic stiffness are included in Supplementary data, Appendix 1. Only age and SBP were significant determinants of aortic stiffness, independent of other, pre-specified, recognized influences.
Relationship between measures of aortic stiffness, LV structure and remodelling
All measures of aortic stiffness were associated with LVMi on univariate analysis (Table 3). SBP, DBP, PP, CVD, haemoglobin and phosphate also associated with LVMi. There were no differences in LVMi between gender, ethnicity, those with diabetes or hypertension or in the use of medications such as angiotensin-converting enzyme inhibitors or statins. Overall, there were no significant associations between any measure of aortic stiffness and LV concentric remodelling (LVM:volume ratio). However, patients with a mass:volume ratio >0.65 (n = 48) had significantly reduced AAD and DAD and significantly increased aPWV when compared with patients with an LVM:volume ratio <0.65 (n = 22) [1.81 mmHg−1×10−3 (95% CI 1.0–3.3) versus 3.02 (1.6–4.8), P = 0.021; 2.49 mmHg−1×10−3 (95% CI 1.6–3.4) versus 3.37 mmHg−1×10−3 (2.3–5.1), P = 0.37; 8.19 mmHg−1×10−3 (95% CI 6.1–11.6) versus 6.12 mmHg−1×10−3 (5.2–10.1), P = 0.043, respectively].
Table 3.
Univariate associations between LVMi and remodelling and aortic and clinical characteristics
| Variable | LVEF (%) |
LVMi (g/m2)a |
LVM:volume ratio (g/mL) |
|||
|---|---|---|---|---|---|---|
| β (SE) | P-value | β (SE) | P-value | β (SE) | P-value | |
| aPWV (m/s)a | −0.125 (0.05) | 0.30 | 0.242 (0.08) | 0.04 | 0.095 (0.09) | 0.44 |
| AAD (mmHg−1×10−3)a | 0.020 (0.02) | 0.87 | −0.244 (0.04) | 0.04 | −0.077 (0.05) | 0.53 |
| DAD (mmHg−1×10−3)a | 0.063 (0.03) | 0.61 | −0.315 (0.05) | <0.01 | −0.005 (0.06) | 0.97 |
| Age (years)a | 0.038 (0.06) | 0.76 | −0.032 (0.12) | 0.79 | −0.008 (0.12) | 0.95 |
| HD vintage (months)a | −0.061 (0.02) | 0.61 | −0.040 (0.03) | 0.74 | −0.041 (0.04) | 0.74 |
| CVD | 0.225 (0.02) | 0.06 | −0.250 (0.03) | 0.04 | −0.129 (0.04) | 0.29 |
| Haemoglobin (g/dL) | −0.051 (<0.01) | 0.68 | −0.260 (<0.01) | 0.03 | 0.072 (<0.01) | 0.55 |
| Phosphate (mmol/L)a | −0.027 (0.06) | 0.82 | 0.332 (0.12) | <0.01 | −0.013 (0.12) | 0.92 |
| SBP (mmHg) | −0.090 (<0.01) | 0.46 | 0.524 (<0.01) | <0.01 | 0.117 (<0.01) | 0.34 |
| DBP (mmHg) | −0.172 (<0.01) | 0.15 | 0.387 (<0.01) | 0.01 | 0.198 (<0.01) | 0.10 |
| PP (mmHg) | −0.013 (<0.01) | 0.91 | 0.442 (<0.01) | <0.01 | 0.058 (<0.01) | 0.63 |
β , standardized beta coefficient; SE, standard error; LVEF, LV ejection fraction.
Log transformed data.
Multivariable linear regression for each measure of aortic stiffness against LVMi was performed (Table 4). After adjusting for the variables in Model 1, aPWV and DAD remained independent determinants of LVMi. Once SBP was included in Model 2, none of the measures of aortic stiffness were significant determinants. Phosphate and SBP remained significant independent determinants of LVMi in all regression models; additionally CVD remained significant in the model including AAD (Supplementary data, Appendix 2).
Table 4.
Multivariable linear regression models to assess the ability of aPWV, AAD and DAD to determine LVMia
| Model | R2 | β (SE) | P-value |
|---|---|---|---|
| Model 1b | |||
| aPWV (m/s)a | 0.334 | 0.279 (0.04) | 0.02 |
| AAD (mmHg−1×10−3)a | 0.314 | −0.226 (0.05) | 0.12 |
| DAD (mmHg−1×10−3)a | 0.360 | −0.307 (0.05) | <0.01 |
| Model 2c | |||
| aPWV (m/s)a | 0.440 | 0.155 (0.089) | 0.23 |
| AAD (mmHg−1×10−3)a | 0.432 | 0.124 (0.058) | 0.45 |
| DAD (mmHg−1×10−3)a | 0.428 | −0.047 (0.063) | 0.75 |
β, standardized beta coefficient; SE, standard error. aLog transformed data.
Adjusted for age, phosphate, haemoglobin and CVD.
Adjusted for Model 1 + SBP.
Reproducibility
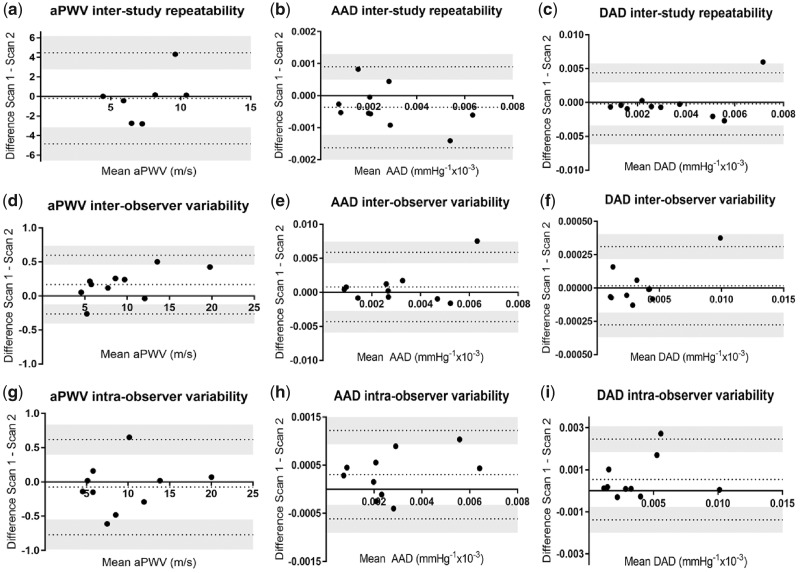
The median time between interstudy scans was 7 days (95% CI 5.2–9.5). Interstudy repeatability and inter- and intra-observer variability for all measures of aortic stiffness are shown in Table 5. For interstudy scans, all 10 subjects had analysable data for AD, but only 7 patients had analysable scans for aPWV. Analysis of Bland–Altman plots demonstrated no evidence of systematic bias, with most data points within 95% CIs (Figure 2).
Table 5.
Interstudy repeatability and inter and intra-observer variability of aPWV, AAD and DAD
| Parameter (n = 10) | Study 1 | Study 2 | ICC (95% CI) | Bias ± SD difference | BA limits of agreement |
|---|---|---|---|---|---|
| Interstudy | |||||
| aPWV (m/s) (n=7) | 8.19 ± 3.5 | 8.91 ± 4.5 | 0.51 (−0.31, 0.89) | −0.19 ± 2.37 | −4.8, 4.5 |
| AAD (mmHg−1×10−3) | 2.49 ± 1.72 | 2.85 ± 2.0 | 0.94 (0.78, 0.99) | −3 × 10−4 ± −6 × 10−4 | −2 × 10−3, 9 × 10−4 |
| DAD (mmHg−1× 10−3) | 3.20 ± 2.7 | 3.41 ± 1.9 | 0.51 (−0.13, 0.85) | −2 × 10−4 ± 2 × 10−3 | −0.005, 4 × 10−3 |
| Interobserver | |||||
| aPWV (m/s) | 9.35 ± 4.8 | 9.18 ± 4.7 | 0.99 (0.99, 1.00) | 0.17 ± 0.22 | −0.3, 0.6 |
| AAD (mmHg−1×10−3) | 2.63 ± 1.8 | 2.68 ± 1.8 | 0.99 (0.99, 0.99) | 7.9 × 10−4 ± 2.6 × 10−3 | −4 × 10−3, 6 × 10−3 |
| DAD (mmHg−1×10−3) | 3.47 ± 2.6 | 3.45 ± 2.6 | 0.99 (0.99, 1.00) | 1.73e−005 ± 2×−4 | −2.7 × 10−4, 3 × 10−4 |
| Intra-observer | |||||
| aPWV (m/s) | 9.35 ± 4.8 | 9.27 ± 4.9 | 0.99 (0.98, 0.99) | −0.08 ± 0.35 | −0.8, 0.6 |
| AAD (mmHg−1×10−3) | 2.63 ± 1.8 | 2.93 ± 1.9 | 0.97 (0.88, 0.99) | 3 × 10−4 ± 4 × 10−4 | −6 × 10−4, 1.2 × 10−3 |
| DAD (mmHg−1×10−3) | 3.47 ± 2.6 | 4.01 ± 2.9 | 0.94 (0.77, 0.98) | 5 × 10−4 ± 9 × 10−4 | −1.4 × 10−3, 2.5 × 10−3 |
Data are presented as mean ± SD. BA, Bland–Altman.
FIGURE 2:
Bland–Altman graphs for interstudy repeatability of (a) aPWV, (b) AAD and (c) DAD; interobserver variability of (d) aPWV, (e) AAD and (f) DAD; and intra-observer variability of (g) aPWV, (h) AAD and (i) DAD.
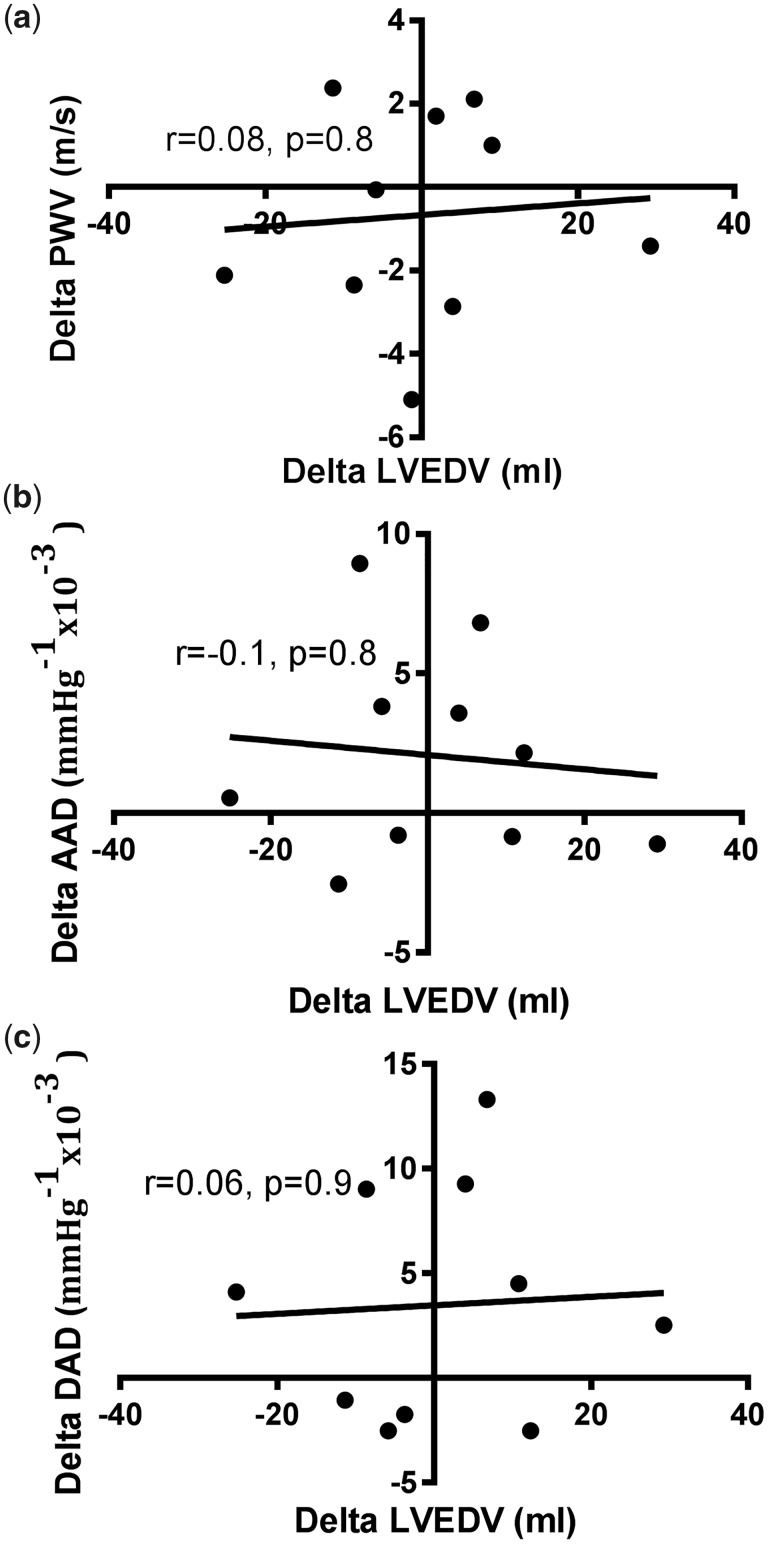
There were no significant changes in LVEDV between scans for interstudy scans (139.3 ± 21 versus 138.4 ± 27.8 mL, P = 0.9). There was no significant relationship between ΔLVEDV and ΔaPWV, ΔAAD or ΔDAD between scans (Figure 3). There were no changes in SBP (143.1 ± 42.5 versus 145.2 ± 42.4 mmHg, P = 0.9), DBP (66.3 ± 21.6 versus 66 ± 13.3 mmHg, P = 0.9), PP (76.8 ± 27.2 versus 79.2 ± 41.2 mmHg, P = 0.8) or heart rate (72.8 ± 11.9 versus 73.1 ± 10.8 bpm, P = 0.9) between interstudy scans.
FIGURE 3:
Correlations between interstudy change in (A) ΔLVEDV and ΔaPWV, (b) ΔLVEDV and ΔAAD and (c) ΔLVEDV and ΔDAD.
DISCUSSION
This is the first study to describe and compare the reproducibility of AAD, DAD and aPWV in the same patients and the only study to assess the reproducibility of these parameters in HD patients. We have shown the reproducibility of AAD to be significantly better than DAD and aPWV when assessed by CMR, although only seven patients had paired analysable interstudy scans for aPWV. While this does suggest there may be important limitations and difficulties in acquiring aPWV compared with AD in HD patients, overall the analysability of aPWV scans and AD scans was similar. It is reassuring that we were unable to demonstrate any significant correlations between interstudy changes in markers of cardiac loading and hydration status (ΔLVEDV) and interstudy changes in aPWV, AAD or DAD (ΔaPWV, ΔAAD or ΔDAD). This suggests the small clinical changes that typically occur day to day in HD patients do not significantly influence CMR-derived measures of aortic stiffness, provided patients are scanned under the controlled conditions we describe, that is, on a day between their dialysis sessions and not after their long break.
Age and SBP were the only independent determinants of all measures of aortic stiffness and the model was strongest for AAD. This relationship between age, SBP and aortic stiffness is supported by numerous studies in patients with ESRD, including studies using CMR [33–36]. With ageing, progressive fragmentation of elastin fibres and cross-linking of collagen in the tunica media leads to arteriosclerosis [37], and age-related stiffening has been found to be marked most in the ascending aorta, possibly as it has the highest concentration of elastin and collagen [38]. We have shown that AAD and DAD were significantly reduced in diabetic patients compared with non-diabetics, but aPWV was not significantly different between the groups. This is contrary to a previous study where aPWW, AAD and DAD were all shown to be different between type 2 diabetic patients and controls [26]. It is likely that this study and our study are underpowered to detect a between-group difference in aPWV in diabetic patients and this should be assessed in future, larger studies. We have also shown that AAD was significantly increased in patients with CVD compared with those without CVD, but no difference was seen between DAD and aPWV. Although one might expect the measures of aortic stiffness to be different in patients with and without a history of CVD, this finding may be because the vascular remodelling that occurs in ESRD is distinct from the processes of hypertension, normal ageing and drivers of CVD in the general population. For example, dilatation of arterial lumens, intima–media hypertrophy and alterations to intrinsic elastic properties of the walls are significantly altered in patients with ESRD compared with non-uraemic controls [39]. This is attributed to the unique combination of traditional and non-traditional risk factors to which renal patients are subject, which may override or alter the effects of CVD driven by traditional risk factors.
AAD, DAD and aPWV were all determinants of LVMi on univariate analysis, but only SBP, CVD and phosphate remained independent determinants of LVMi on multivariable regression. The ascending aorta has been described as the most clinically relevant aortic area to predict age-related LVM in healthy subjects [40], so it is somewhat surprising that AAD was not an independent determinant of LVMi in our participants. Stiffening in the ascending aorta, versus more distal areas, should theoretically have a more profound effect on LV structure due to its higher elastin content and the buffering action that this affords (the windkessel effect). The reasons we were unable to demonstrate the independent effects of AAD on LVMi are unclear, but may be because in prevalent HD patients, arteriopathy has plateaued and other pathological mechanisms (including SBP) become more influential. Further data are required to decide whether strategies to reduce BP or strategies to directly target aortic stiffness play a more important role in mitigating cardiovascular risk and these results should be viewed as hypothesis generating. Indeed, it is unclear whether improvement of aortic stiffness in patients with CKD is due to improvement in BP or aortic remodelling [41], as hypertension and aortic disease are intrinsically related. Certainly from these cross-sectional data it is unclear whether SBP is high due to aortic stiffness or whether aortic stiffness is driven by high SBP. The observation that measures of aortic stiffness are not independent determinants of LVMi might appear to be at odds with the results from previous studies [11, 42]. However, our results are in line with larger studies of HD patients, which found no independent relationships between carotid–femoral PWV (cfPWV) and LVMi [42, 43]. Importantly, these were all non-CMR studies and echocardiography is known to overestimate LVM in HD patients, which may account for the discrepancy between our results and smaller studies that have used echocardiography [44].
It has been demonstrated in both CMR and non-CMR studies that measures of aortic stiffness predict cardiovascular mortality in patients with advanced CKD and ESRD [5, 9]. There is also a graded relationship between increased LVM and cardiovascular death [45]. However, there are no studies that directly report the relationships between aortic stiffness, LVH, mortality and cardiovascular events using CMR. Mark et al. [5] described a weak but significant correlation between AAD and LVMi (r =−0.21, P = 0.021) and AAD was an independent predictor of survival. The relationship between LVM and outcome was not reported, nor was it reported for any independent association between AAD and LVH. It remains unclear whether LVMi or measures of aortic stiffness are better predictors of outcome in HD patients and future studies should seek to clarify this.
The explanation for AAD losing significance in the multivariable model before DAD and aPWV is not entirely clear. Redheuil et al. [23] showed that the relationship between AD (assessed at 3T) and age is non-linear. In healthy patients, AD markedly decreases before the age of 50 years and tended towards a plateau while aPWV continued to increase. Our patients were mostly >50 years of age and would be expected to have accelerated vascular aging. You would therefore expect the effect of AD on LVMi at this stage to be diminished compared with aPWV. Another explanation might be that while we are considering AAD, DAD and aPWV as surrogates of aortic stiffness, they may have discrete and differing roles in the pathogenesis of CVD-related outcomes. Although the study does not include patients with CKD, the Dallas Heart Study showed that aPWV predicted extracardiac vascular events, such as stroke, but AAD predicted non-fatal cardiac events [46]. It is possible that this is true in patients with CKD and the assessment of both measures may yield complementary rather than duplicating information.
Our data did not show any independent relationship between concentric remodelling and measures of aortic stiffness. However, the interaction between aortic stiffening and LV remodelling is supported by biological plausibility and the observation that AAD and DAD were significantly reduced and aPWV significantly increased in patients with LVM/LVEDV >0.65 compared with those with LVM/LVEDV <0.65. This finding was not a pre-specified analysis, however, and should be viewed as explorative. Serum phosphate is known to closely regulate medial vascular smooth muscle growth and calcification and has long been proposed as a mediator of increased arterial stiffness. The finding that phosphate was an independent determinant of LVMi above the ability of aPWV is as previously reported in a study of CKD patients using CMR [47].
Limitations
This study was cross-sectional in design and causality cannot be inferred from the results we present. We did not directly compare CMR measures of aortic stiffness with more traditional non-invasive methods such as cfPWV. The sample size we have reported is relatively small, but we limited covariates for regression models to try to avoid overfitting. While this study offers important information about the reproducibility of and the relationship between measures of aortic stiffness and LVMi, we have not assessed any relationships with mortality or cardiovascular events. This study was conducted at 3T and the results are not necessarily generalizable to studies at 1.5T. To determine the PP for the calculation to derive AD, we used brachial PP taken at the time of aortic cine during the CMR scan as a proxy for central PP. Some studies have used estimated carotid artery PP as a closer approximation of central PP, derived from brachial BP using calibrated tonometric devices such as SphygmoCor. However, while both brachial and calibrated central pressures underestimate invasively measured central BP, this deviation has been shown to be consistently greater in the SpygmoCor. Nevertheless, absolute differences in brachial BP and carotid artery BP derived from devices such as SphgmoCor are likely to be small [48]. Although BP was measured on an interdialytic day, after 30 min of rest, the gold standard of ambulatory BP monitoring was not used to assess BP. While the reproducibility of aPWV was worse than AAD, interstudy scans for aPWV were only possible in 7 of 10 scans. While this does, potentially, give important information about the technique reliability, it would have been ideal to have completed 10 interstudy reproducibility scans for all patients.
CONCLUSION
Our study shows that AAD is the most reproducible CMR-derived measure of aortic stiffness in HD patients. All measures of CMR-derived aortic stiffness were determinants of LVMi, but not independent of BP. Future studies should compare the abilities of AAD, DAD and aPWV to predict hard outcomes in patients with CKD and ESRD and assess the relationship between modifications of these parameters and changes in outcomes in interventional studies.
Ethics approval and consent to participate
The study was given local research and ethics committee approval and written informed consent was obtained prior to recruitment.
Consent for publication
All authors have read the final version of this manuscript and have agreed to its submission and consent to publication if accepted.
Availability of data and materials
All data are available for analysis should statistical review be required.
Supplementary Material
ACKNOWLEDGEMENTS
This study is part of the research portfolio supported by the NIHR Leicester Biomedical Research Centre and the Leicester Clinical Research Facility based at the University Hospitals of Leicester and the University of Leicester.
FUNDING
This study is independent research arising from a Clinician Scientist Award (to J.B., CS2013-13-014) supported by the NIHR. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
AUTHORS’ CONTRIBUTIONS
M.P.M.G.-B. contributed to study conception, data collection, analysis and manuscript preparation. S.F.A. contributed to data collection, analysis and manuscript revision. F.Y.L. contributed to statistical analysis and manuscript review. W.H.H. contributed to data analysis and manuscript review. J.O.B. contributed to study conception and manuscript revision. G.P.M. contributed to study conception, oversight of data collection and analysis and final draft manuscript. G.G. contributed to data analysis and manuscript revision and K.P. contributed to image acquisition and manuscript revision.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Schiffrin EL, Lipman ML, Mann JFE.. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007; 116: 85–97 [DOI] [PubMed] [Google Scholar]
- 2. Breidthardt T, McIntyre CW.. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med 2010; 12: 13–20 [DOI] [PubMed] [Google Scholar]
- 3. Gross M, Ritz E.. Hypertrophy and fibrosis in the cardiomyopathy of uremia–beyond coronary heart disease. Semin Dial 2008; 21: 308–318 [DOI] [PubMed] [Google Scholar]
- 4. Moody WE, Edwards NC, Chue CD. et al. Arterial disease in chronic kidney disease. Heart 2013; 99: 365–372 [DOI] [PubMed] [Google Scholar]
- 5. Mark PB, Doyle A, Blyth KG. et al. Vascular function assessed with cardiovascular magnetic resonance predicts survival in patients with advanced chronic kidney disease. J Cardiovasc Magn Reson 2008; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moody WE, Ferro CJ, Edwards NC. et al. Cardiovascular effects of unilateral nephrectomy in living kidney donors. Hypertension 2016; 67: 368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards NC, Ferro CJ, Townend JN. et al. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart 2008; 94: 1038–1043 [DOI] [PubMed] [Google Scholar]
- 8. Blacher J, Guerin AP, Pannier B. et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99: 2434–2439 [DOI] [PubMed] [Google Scholar]
- 9. Verbeke F, Van Biesen W, Honkanen E. et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the Calcification Outcome in Renal Disease (CORD) study. Clin J Am Soc Nephrol 2011; 6: 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shoji T, Emoto M, Shinohara K. et al. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 2001; 12: 2117–2124 [DOI] [PubMed] [Google Scholar]
- 11. London GM, Marchais SJ, Safar ME. et al. Aortic and large artery compliance in end-stage renal failure. Kidney Int 1990; 37: 137–142 [DOI] [PubMed] [Google Scholar]
- 12. Odudu A, Eldehni M, McCann G. et al. Characterisation of cardiomyopathy by cardiac and aortic magnetic resonance in patients new to hemodialysis. Eur Radiol 2016; 26: 2749–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adenwalla SF, Graham-Brown MP, Leone FM. et al. The importance of accurate measurement of aortic stiffness in patients with chronic kidney disease and end-stage renal disease. Clin Kidney J 2017; 10: 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grotenhuis HB, Westenberg JJM, Steendijk P. et al. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity‐encoded MRI. J Magn Reson Imaging 2009; 30: 521–526 [DOI] [PubMed] [Google Scholar]
- 15. Grothues F, Smith GC, Moon JCC. et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002; 90: 29–34 [DOI] [PubMed] [Google Scholar]
- 16. Graham-Brown MPM, Rutherford E, Levelt E. et al. Native T1 mapping: inter-study, inter-observer and inter-center reproducibility in hemodialysis patients. J Cardiovasc Magn Reson 2017; 19: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham-Brown MPM, March DS, Churchward DR. et al. Design and methods of CYCLE-HD: improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: a randomised control trial. BMC Nephrol 2016; 17: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh A, Ford I, Greenwood JP. et al. Rationale and design of the PRognostic Importance of MIcrovascular Dysfunction in asymptomatic patients with Aortic Stenosis (PRIMID-AS): a multicentre observational study with blinded investigations. BMJ Open 2013; 3: 004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kramer CM, Barkhausen J, Flamm SD. et al. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson 2013; 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh A, Steadman CD, Khan JN. et al. Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 tesla: a comparison of feature‐tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015; 41: 1129–1137 [DOI] [PubMed] [Google Scholar]
- 21. Khan JN, Wilmot EG, Leggate M. et al. Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging 2014; 15: 1263–1269 [DOI] [PubMed] [Google Scholar]
- 22. Redheuil A, Yu W-C, Wu CO. et al. Reduced ascending aortic strain and distensibility earliest manifestations of vascular aging in humans. Hypertension 2010; 55: 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niiranen TJ, Kalesan B, Hamburg NM. et al. Relative contributions of arterial stiffness and hypertension to cardiovascular disease: the Framingham Heart Study. J Am Heart Assoc 2016; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang M, Tsai W, Chen J. et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology 2007; 12: 591–597 [DOI] [PubMed] [Google Scholar]
- 25. van der Meer RW, Diamant M, Westenberg JJM. et al. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson 2007; 9: 645–651 [DOI] [PubMed] [Google Scholar]
- 26. van Popele NM, Grobbee DE, Bots ML. et al. Association between arterial stiffness and atherosclerosis the Rotterdam Study. Stroke 2001; 32: 454–460 [DOI] [PubMed] [Google Scholar]
- 27. Ferreira I, Henry RMA, Twisk JWR. et al. The metabolic syndrome, cardiopulmonary fitness, and subcutaneous trunk fat as independent determinants of arterial stiffness: the Amsterdam Growth and Health Longitudinal Study. Arch Intern Med 2005; 165: 875–882 [DOI] [PubMed] [Google Scholar]
- 28. Foley RN, Parfrey PS, Harnett JD. et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 1995; 47: 186–192 [DOI] [PubMed] [Google Scholar]
- 29. Foley RN, Parfrey PS, Harnett JD. et al. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 1996; 49: 1379–1385 [DOI] [PubMed] [Google Scholar]
- 30. Stróżecki P, Adamowicz A, Nartowicz E. et al. Parathormon, calcium, phosphorus, and left ventricular structure and function in normotensive hemodialysis patients. Ren Fail 2001; 23: 115–126 [DOI] [PubMed] [Google Scholar]
- 31. Foley RN, Parfrey PS, Harnett JD. et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kid Dis 1996; 28: 53–61 [DOI] [PubMed] [Google Scholar]
- 32. Nitta K, Iimuro S, Imai E. et al. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin Exp Nephrol 2013; 17: 730–742 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Utescu MS, Couture V, Mac-Way F. et al. Determinants of progression of aortic stiffness in hemodialysis patients a prospective longitudinal study. Hypertension 2013; 62: 154–160 [DOI] [PubMed] [Google Scholar]
- 34. London GM, Pannier B, Guerin AP. et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 2001; 12: 2759–2767 [DOI] [PubMed] [Google Scholar]
- 35. Zimmerli LU, Mark PB, Steedman T. et al. Vascular function in patients with end-stage renal disease and/or coronary artery disease: a cardiac magnetic resonance imaging study. Kidney Int 2007; 71: 68–73 [DOI] [PubMed] [Google Scholar]
- 36. Temmar M, Liabeuf S, Renard C. et al. Pulse wave velocity and vascular calcification at different stages of chronic kidney disease. J Hypertens 2010; 28: 163–169 [DOI] [PubMed] [Google Scholar]
- 37. O'Rourke MF, Hashimoto J.. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007; 50: 1–13 [DOI] [PubMed] [Google Scholar]
- 38. Rogers WJ, Hu YL, Coast D. et al. Age-associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol 2001; 38: 1123–1129 [DOI] [PubMed] [Google Scholar]
- 39. Guerin AP, Pannier B, Marchais SJ. et al. Arterial structure and function in end-stage renal disease. Curr Hypertens Rep 2008; 10: 107–111 [DOI] [PubMed] [Google Scholar]
- 40. Redheuil A, Yu W-C, Mousseaux E. et al Age-related changes in aortic arch geometry: relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol 2011; 58: 1262–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edwards NC, Steeds RP, Stewart PM. et al. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol 2009; 54: 505–512 [DOI] [PubMed] [Google Scholar]
- 42. Nitta K, Akiba T, Uchida K. et al. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertens Res 2004; 27: 47–52 [DOI] [PubMed] [Google Scholar]
- 43. Kim ED, Sozio SM, Estrella MM. et al. Cross-sectional association of volume, blood pressures, and aortic stiffness with left ventricular mass in incident hemodialysis patients: the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease (PACE) study. BMC Nephrol 2015; 16: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stewart GA, Foster J, Cowan M. et al. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int 1999; 56: 2248–2253 [DOI] [PubMed] [Google Scholar]
- 45. Zoccali C, Benedetto FA, Mallamaci F. et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int 2004; 65: 1492–1498 [DOI] [PubMed] [Google Scholar]
- 46. Maroules CD, Khera A, Ayers C. et al. Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas Heart Study. J Cardiovasc Magn Reson 2014; 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chue CD, Edwards NC, Moody WE. et al. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart 2012; 98: 219–224. [DOI] [PubMed] [Google Scholar]
- 48. Ding F, Fan W, Zhang R. et al. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens 2011; 24: 1306–1311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available for analysis should statistical review be required.