ABSTRACT
Backgound
Fungal peritonitis (FP) is one of the most important causes of peritoneal dialysis (PD) failure, often burdened by increased morbility and mortality. This study evaluates the clinical course of FP cases that arose between 1983 and 2016 in a single PD unit.
Methods
We conducted a retrospective observational analysis of FP episodes recorded in the Baxter POET (Peritonitis Organism Exit sites Tunnel infections) registry and clinical records. FP incidence rate, PD and patients’ survival and clinical characteristics of the study population were analysed, taking into account the evolution of clinical practice during the study period as a result of technical innovation, scientific evidence and guideline history.
Results
Fourteen FP cases (2.8%) were detected. The overall incidence of PD peritonitis was one episode/27 patient-months. Candida parapsilosis was the most frequently (50%) detected yeast. Seventy-five per cent of cases were considered secondary FP. This group experienced 2.6±1.7 bacterial peritonitis before FP, most frequently due to Staphylococcus and Enterococcus species. Most patients were treated with fluconazole for ≥8 days. All subjects were hospitalized for a median time of 25 days. Tenckhoff catheter removal occurred in all cases of FP and all patients were transferred to haemodialysis. Two patients died. From December 2010 to December 2016, no FP episodes were recorded.
Conclusions
FP is confirmed as a significant cause of PD drop out and increases patients’ mortality risk. Prompt diagnosis of FP, targeted antifugal therapy and rapid PD catheter removal are essential strategies for improved patient and PD survival.
Keywords: fungal peritonitis, haemodialysis, management protocol, peritoneal dialysis, survival analysis
INTRODUCTION
Fungal peritonitis (FP) represents a critical complication of peritoneal dialysis (PD), being often associated with treatment failure, and increased morbidity and mortality [1, 2]. The incidence of FP is heterogeneous worldwide, ranging from 2 to 23.8% in industrialized and developing countries, respectively [3–15]. Differences in clinical management and socioeconomic environment, such as low educational status and unhygienic conditions, probably account for this discrepancy [16]. Prolonged antibiotic treatment, previous bacterial peritonitis, and gynaecological and bowel-source infections are accepted as prominent risk factors for FP [1, 2]. The clinical relevance of FP has fuelled heated debates concerning the best prophylactic and therapeutic interventions capable of counteracting the risks and consequences of the disease. The efficacies of prophylactic schedules with antifungal drugs, such as fluconazole or nystatin, have received a considerable amount of interest although with inconclusive results. [17–24]. Both early PD catheter removal and prompt antifungal treatment are considered the best strategies to improve patients’ survival in FP [1, 2, 25, 26]. Permanent transfer to haemodialysis remains frequent among survivors, although PD resumption has been described, especially in less fragile patients [9, 12].
The present retrospective observational study evaluates the clinical course and outcomes of 14 FP cases that arose over the last 34 years (from 1983 to 2016) at the Renal Unit of Desio Hospital (Italy), with a particular focus on patients’ demography, dialysis features, peritonitis incidence, fungal agents, antimycotic treatment and PD survival.
MATERIALS AND METHODS
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Patients and statistical analysis
Between January 1983 and December 2016, 589 cases of peritonitis occurred in 542 PD patients followed at the Renal Unit of Desio Hospital, of which 14 episodes involved FP.
Patient characteristics were retrospectively collected from the Baxter POET (Peritonitis Organism Exit sites Tunnel infections) registry and clinical records (outpatient and inpatient medical records and laboratory database). The following clinical data were reported: gender, age, cause of end-stage renal disease (ESRD), residual renal function, Charlson Comorbidity Index (CCI) timing of treatment, PD modality [continuous ambulatory peritoneal dialysis (CAPD) or automatic peritoneal dialysis (APD)] and risk factors for FP.
Peritonitis events were defined as suggested by current guidelines [27]. FP was confirmed with a positive culture for fungi and a negative Gram stain. Clinical presentation, as well as empirical or targeted antibiotic treatment (intraperitoneal or systemic), were analysed in all patients with a confirmed FP event. The rate of any type of peritonitis was computed and reported as events/number of patients/month, which were stratified on five 7-year periods.
All patients who had at least one episode of FP were considered. FP events not preceded by any bacterial peritonitis were classified as primary FP. Previous bacterial peritonites were described for every subject with secondary FP. The peritoneal effluent white cell count (EWCC), performed at the moment of culture testing, was recorded. The time from PD start to the first FP was assessed. Risk factors for FP were analysed.
All causative fungal agents and their mycotic susceptibility were collected. Patients and PD outcomes (patient mortality, PD survival and shift to haemodialysis) were analysed. Data are expressed as episodes/patient-month, percentage change and mean ± SD.
PD: technique, surveillance and peritonitis management
All patients performed PD (CAPD/APD) through a double-cuffed straight Tenckhoff catheter, inserted by surgical technique. Generally, PD was started after a standard break-in period of 2–4 weeks.
Incident patients, after the implantation of the catheter, and prevalent patients, after repeated infective episodes, underwent a training period before home delivery.
PD (CAPD/APD) was performed by means of disconnect systems (Baxter Healthcare, Deerfield, IL, USA, and Fresenius Medical Care, Deutschland GmbH, Germany), with lactate-buffered glucose-containing dialysate solutions. Between 1983 and 2016, empirical and targeted antibiotic treatment of bacterial peritonitis was ensured according to international guidelines [27–31]. Furthermore, three historical insights resulted in a considerable step forward regarding the evolution of PD over the last 34 years. First, in 1989, the Y set was introduced as a connection system. Secondly, in 2005, as suggested by the International Society of Peritoneal Dialysis guidelines [31], step-by-step protocols for infection prevention were introduced with particular focus on the following issues: (i) exit-site care, using detersion with chlorhexidine solution, and local mupirocin application for exit-site/tunnel infections; (ii) antibiotic prophylaxis in the case of invasive diagnostic procedures; (iii) prevention of FP according to our centre’s protocol: prophylaxis against FP by administration of 400 mg fluconazole on Day 1 followed by 200 mg daily for at least 10 days, for all PD patients exposed to an antibiotic course longer than 14 days due to peritonitis or other non-peritoneal infections, such as pulmonitis or enteritis; and (iv) a structured retraining programme. Thirdly, starting from 2006, biocompatible solutions were systematically adopted for both CAPD and APD dwells.
Microbiological investigations
Identification of isolates
In our diagnostic laboratory, Sabouraud’s Dextrose Agar (SDA) was used for the isolation of all yeast from PD effluents. SDA is a ground powder used to cultivate, isolate, identify and maintain saprophytes and pathogenic fungi, especially Candida albicans, in non-sterile specimens.
The soil selectivity is due to its acidic pH (5.6), which inhibits bacterial growth, except for Lactobacillu acidophilus.
After 3 days of incubation at 25°C, C. albicans grew with colourless or tenuous pink-coloured colonies, while other fungal colonies were coloured from intense pink to red. Identification was performed using commercial isolation media, which can differentiate yeast species based on colony colour (bioMerieux), and, more recently, VITEK® 2 YST ID card (bioMerieux) and Matrix Assisted Laser Desorption Ionization Time-of-Flight VITEK® MS, which offer rapid and accurate recognition of a broad range of pathogenic yeasts.
The test result is considered to be negative if there is no presence of colonies on the plate with the described characteristics or if the identification tests are negative.
Susceptibility testing
The antifungal susceptibility of each strain was performed using the VITEK® two yeast susceptibility card (AST-YS, bioMerieux) and the interpretation of the results complied with the European Committee on Antimicrobial Susceptibility Testing breakpoint tables.
RESULTS
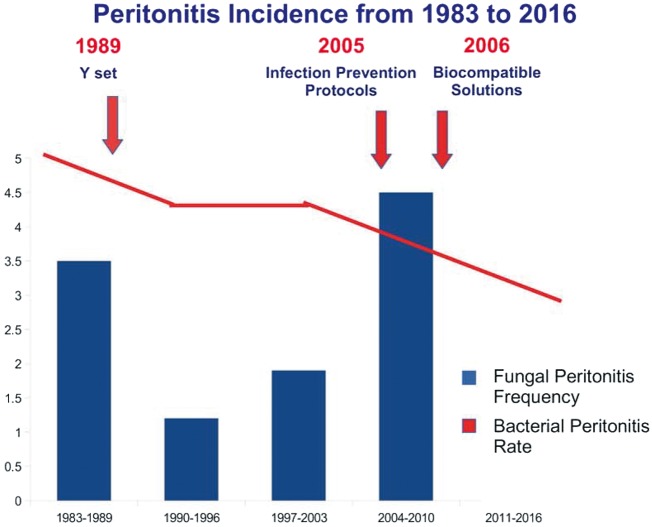
A total of 589 cases of peritonitis occurring among 539 PD patients were observed between January 1983 and December 2016, with an overall peritonitis rate of one episode/27 patient-months. Of those, 14 (2.8%) episodes were defined as FP. The peritonitis incidence decreased from one episode/20 patient-months to one episode/45 patient-months between the first (1983–89) and last (2011–16) 7-year periods. FP incidence declined from 1983 to 1996, subsequently progressively increased to the highest peak in the 2004–10 period and then fell to zero in the last 6 years (2011–16).
In four FP cases, occurring between 1984 and 1989, data were limited to the Baxter POET registry without additional information from medical and laboratory records.
Patients’ demographic and clinical characteristics are summarized in Table 1. Most of the patients were male and over 50 years old; 14% were diabetics, 78% had already experienced at least one bacterial peritonitis event, 20% did not present any risk factors and only one patient was a colonic diverticulosis carrier. One-half of the included population was undergoing CAPD at the time of the FP event and had a median residual renal function of 1000 mL/day. The mean time from PD start to FP was 3.8 ± 4.2 years.
Table 1.
Patients’ demographic and clinical characteristics
| Male/female (%) | 57.1/42.9 |
| Age (years), mean ± SD | 67 ± 10 |
| Cause of ESRD (%) | |
| Diabetes | 14 |
| Ischaemic nephropathy | 14 |
| ADPKD | 14 |
| Glomerulonephritis | 22 |
| Other | 36 |
| Residual renal function (mL), mean ± SD | 978 ± 696 |
| Time on PD (years), mean ± SD | 3.8 ± 4.2 |
| CAPD:APD (%) | 50:50 |
ADPKD, autosomal dominant polycystic kidney disease.
No fungal tunnel or exit-site infections were reported among PD population over the years.
Candida parapsilosis was the most commonly represented fungus (50%). Among the FP cases, only 3 (21.4%) were defined as primary FP, while 11 (78.6%) were considered secondary FP. The patients in the secondary FP group experienced 2.6 ± 1.7 bacterial peritonitis episodes before mycotic peritoneal infection, most frequently due to Staphylococcus species and Enterococcus species, but also due to Enterobacter species and Pseudomonas species to a lesser extent (Table 2). The peritoneal EWCC, performed at the moment of culture testing, was higher in the secondary FP group (2985 ± 5914 N/MMC versus 1700 ± 1673 N/MMC). All FP cases received multiple antibiotic courses before FP diagnosis was performed due to the systematic diagnostic delay of laboratory methods. Vancomycin, gentamicin, ciprofloxacin and third-generation cephalosporins were the most represented medications.
Table 2.
Clinical presentation and outcomes
| Pathogenic yeast (%) | |
| C. parapsilosis | 50 |
| Candida species | 28.6 |
| C. albicans | 21.4 |
| Peritonitis risk factors [number of patients (%)] | |
| Previous bacterial peritonitis | 11 (78.6) |
| Previous antibiotic treatment | 14 (100) |
| Bowel-source infection (e.g. diverticulitis) | 1 (7) |
| Gynaecological-source infection | 0 |
| None | 3 (21.4) |
| Peritoneal effluent cell count (N/MMC), mean ± SD | 2688 ± 5197 |
| Primary FP | 3 patients (35 %) |
| Secondary FP | 11 patients (75 %) |
| Treatment [number of patients (%)] | |
| Fluconazole | 13 (92.9) |
| Voriconazole | 1 (7.1) |
| Treatment duration (days), mean ± SD | 22 ± 14 |
| Fluconazole | 200 mg/48 h |
| Voriconazole | 400 mg/24 h |
| Hospitalization (days), mean ± SD | 27 ± 19 |
| Outcome [number of patients (%)] | |
| Death | 2 (14) |
| Tenckhoff removal | 12 (86) |
| Shift to haemodialysis | 11 (79) |
| PD resumption | 1 (7) |
N/MMC, number per cubic millimeter.
Determination of sensitivity to antifungal agents was possible in all patients. Eleven isolated fungi were susceptible to fluconazole, amphotericin B, imidazole, nystatin and voriconazole. Three cases were resistant to amphotericin B, imidazole and fluconazole (respectively, C. parapsilosis, Candida guilliermondii and Candida krusei). All patients but one were treated with fluconazole for at least 8 days (average time of treatment 22 ± 14 days) at a dose of 200 mg every 48 h, after a loading dose of 400 mg. The exception underwent therapy with voriconazole (400 mg/24 h) for 21 days, according to sensitivity test.
All FP cases were hospitalized with a median hospitalization time of 25 days.
Tenckhoff catheter was removed in all FP cases after a median period of 4 days (ranging from 1 day to 8 days) from FP diagnosis without short-term complications. All patients were transferred to haemodialysis therapy after implantation of a central venous catheter without complications. PD was resumed in only one woman (1.7%) 2 months after FP resolution because of her haemodynamic instability due to a severe chronic ischaemic heart disease; others continued haemodialysis without complications. Two patients died from FP (mortality rate 14.3%).
From December 2010 to December 2016, no FP episodes were recorded.
DISCUSSION
FP is a relatively rare but serious complication in PD patients, leading to significant patient morbidity and mortality, and technique failure [1, 2]. Our data are consistent with the current literature.
In our population, FP was due mostly to C. parapsilosis and, to a lesser extent, to C. albicans.
In PD patients, C. albicans has been historically reported to be more common than non-albicans Candida species but, in recent reports, a shift in the prevalence of pathogenic yeast has been observed [3–15]. Candida albicans or C. parapsilosis are the most represented species [1, 2]. Their relative frequency varies depending on the population involved, geographical region, previous antifungal exposure and patient age [32].
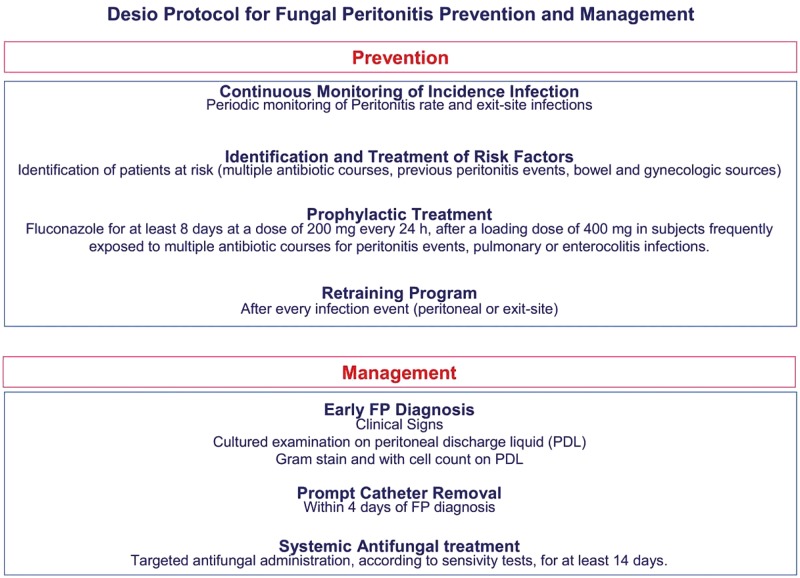
Worldwide FP incidence accounts for 2–23.8% of all peritonitis episodes [3–15]. We registered a very low FP frequency (2.8%) in a PD centre burdened by restrained load of peritoneal infections with a peritonitis rate that was constantly reducing. Over the study period, the incidence of FP had a swinging and variable course until no incidence in the final time period (2011–16) (Figure 1). This favourable trend was probably due to our specific intervention protocol and prophylactic strategy (Figure 2). Current guidelines strongly suggest that every dialysis centre has its own FP management protocol [30, 31].
FIGURE 1:
Peritonitis incidence from 1983 to 2016. Over this time period, PD treatment evolution, characterized by Y set introduction, the application of infection prevention protocols and the use of biocompatible solutions, allowed a significant reduction of the bacterial peritonitis rate. FP incidence had a swinging and variable course until its total prevention during the final time period (2011–16).
FIGURE 2:
Desio protocol for FP prevention and management. A proper protocol for FP prevention and management yielded a drastic reduction of the peritonitis rate.
As suggested [16], the extreme variability in FP incidence is probably due to differing levels of industrialization and differing welfare states and resources at the country level. Proper local strategies are recommended by international guidelines to reduce FP incidence [30, 31, 33].
If the peritonitis rate in a dialysis centre is not acceptable, an effective root cause analysis can identify the problem and the appropriate measures needed to rectify it [33, 34]. The process for the reduction of peritonitis rates includes the identification of the need for reducing peritonitis, the identification of peritonitis causes and intervention. Peritonitis surveillance programmes, infection prevention protocols, patient training and staff education are well-known approaches to reduce PD-related fungal infection [34–36]. In our centre, we have implemented a master plan to maintain the attention of medical and nursing staff at a high level. Peritonitis monitoring, proper interventions, periodic retraining of patients (every year and after every peritonitis event), and a continuing medical and nursing team education programme are our main ways to reduce preventable peritonitis and FP (Figure 2).
Different FP risk factors [1, 2] have been identified and various action strategies have been suggested to reduce FP incidence [26]. The identifiable risk factors include preceding bacterial peritonitis and exposure to an antimicrobial agent [2]. In our PD population, these are precisely the most represented risk factors. Only a small amount (20%) of PD patients in the study did not present any risk factors.
It has been postulated that exposure to antibiotics results in intestinal overgrowth of fungi, particularly Candida species. The selective suppression of bacteria during the use of antibiotics would further allow the proliferation of fungi if there was contamination of the peritoneal fluid, leading to an increased risk of FP [25].
The main factors associated with the development of FP include two important operative mechanisms: fungal overgrowth in the gastrointestinal tract and declining peritoneal defence because of peritonitis. It has been suggested that antibiotic therapy destroys the normal bacterial flora of the colon and promotes the colonization and overgrowth of yeast in the digestive tract, with future migration into the peritoneal cavity by routes that are currently not well defined [25].
Our patients with primary FP had no particular risk factors, nor were they more severely compromised than other patients. They may not have observed asepsis during PD exchanges, but did not present exit-site infections, previous antibiotic therapy or extraperitoneal fungal infections in the days before peritonitis development.
Conversely, secondary FP occurred in patients who had previously undergone multiple courses of antibiotics. Although Gram-negative and polymicrobial peritonitis encourages further formation of FP [35, 36], our PD population particularly experienced Gram-positive peritonitis events before FP.
These considerations introduce a potential target group for prophylaxis. A large number of studies [17–24] have examined the use of prophylaxis against FP with either oral nystatin or an antimicotic drug, given during antibiotic therapy, with conflicting results, without providing clear evidence or opinions. Current guidelines recommend washing hands, mupirocin use, exit-site care and cleansing as better tools to reduce the risk of PD-related peritonitis [30, 31]. Adequate training programmes, taught by qualified and dedicated staff have proved crucial for the monitoring and control of PD peritonitis trends [36, 37].
Thanks to our PD programme (Figure 2), we were able to identify an increase in FP incidence from 2005–10 and to improve the quality of our infection prevention protocol. From September 2009, we administered fluconazole (400 mg on Day 1, then 200 mg/day for ≥10 days), associated with targeted antibiotic treatment, to every patient who experienced more than one peritonitis or other infective event, such as pulmonitis or enteritis, lasting for >14 days.
Amphotericin, although it is commonly used to treat fungal infections, has a high toxic potential and a low therapeutic index. Even at low doses, it is frequently associated with severe adverse effects, most often renal impairment and gastrointestinal disorders. Moreover, intraperitoneal injection involves severe abdominal pain that causes strong discomfort to patients [37, 38].
Taking into account all these considerations, at the same effectiveness, our centre adopted fluconazole as the first-choice treatment for all cases of FP.
Even if some side effects from azole use, such as hepatotoxicity or QT interval (time between the start of the Q wave and the end of the T wave in the heart's electrical cycle) prolongation [39, 40], were reported, our PD population did not experienced any kinds of complications related to fluconazole administration.
The extensive use of fluconazole, accompanied by a careful retraining programme, has allowed us to dramatically reduce the incidence of FP. From December 2010 no FP episode was reported. No exit-site or tunnel fungal infections were reported at all. As is already known, prophylactic exit-site treatment has not yet been demonstrated to yield a real advantage regarding exit-site or peritoneal infection incidence [41], so we do not suggest prophylactic use of topical mupirocin or gentamicin for exit-site care, only for curative treatment.
Prevention of FP should involve a strategy for the detection and management of potential risk factors in both the host and the environment. Reducing the overall peritonitis rate is still the best way to reduce absolute FP incidence. Programmes with higher baseline rates of FP will likely find prophylaxis to be more beneficial, while those with low baseline rates may not detect a benefit and, in these settings, its use may not be justified in view of the possibility of the emergence of resistant strains. Each PD programme must examine its own history of FP and decide whether a prophylaxis protocol might be beneficial.
Early administration of antifungal drugs and early removal of PD catheters are the cornerstones of FP treatment [25, 26].
FP management poses a difficult challenge: early commencement of therapy is critical for successful treatment, but the absence of a typical clinical picture may delay the FP diagnosis. Infecting organisms can be difficult to isolate and microbiological cultures, nowadays available, have a minimum technical delay of 2 days before the correct response can be obtained [42]. EWCC is an important prognostic factor of peritonitis outcome [43] and it could be used to stratify PD patients who are more at risk of a negative outcome. Our data suggest that, with respect to primary FP, patients with secondary FP have a more intensive inflammatory response with a higher EWCC level at the onset of peritonitis . The Gram stain is another test that could be useful for early FP diagnosis as it provides immediate recognition of fungal hyphae. Its routine use is strongly recommended.
The approach to PD catheter management has changed considerably over the years. From 2005, guidelines [30, 31] have defined FP as a strong indication for immediate catheter removal with a shift to haemodialysis.
The practice of rapid PD catheter removal, combined with antifungal therapy, on confirming FP diagnosis is very common in many dialysis unit [3–15], including ours (Figure 2). Other groups recommend drug treatment of FP and delaying catheter removal until the dialysate effluent has become clear [43]. The current literature suggests that this approach is recommended only for elderly or frail patients with little capacity to support a shift to haemodialysis [44]. The PD catheter was removed in all our patients after a median time of 4 days from the onset of peritonitis.
Catheter removal in combination with antifungal therapy resulted in the best overall outcome, with the lowest rates of FP episodes and death compared with either therapeutic intervention on its own [2].
In our centre, FP was confirmed as an important cause of definitive PD drop out (100%), burdened by a considerable risk of morbidity and mortality (14%). The worldwide mortality rate is variable, ranging between 5 and 40% [3–15], being at the highest level in patients with loss of residual kidney function [45] and in those whose peritoneal catheter was not removed quickly once diagnosed [1, 2]. In our cohort, patients who died because of FP had a higher burden of comorbidity, less residual urine output (on average 800 mL/day) [46] and a longer hospitalization time (50–60 day). Only one had a long PD vintage (14 years). These factors could have contributed to FP-related deaths. Our recorded mortality is not among the lowest reported in the literature. Precisely for this reason, our peritonitis surveillance and prevention protocols have been strengthened, resulting a complete collapse of FP cases from 2011 onwards.
CONCLUSION
FP is a rare complication of PD that is associated with significant morbidity, mortality and technique failure. In our centre, during a 34-year period, only 2.8% of all peritonitis events were FP cases, and all cases were caused by Candida species, with the majority due to C. parapsilosis. For us, the best approach to improve patients’ survival is prompt diagnosis of FP, targeted antifungal therapy and rapid PD catheter removal. Prophylactic use of fluconazole and continuing patient and medical staff training are powerful tools to reduce the incidence of PD-related peritonitis and improve PD patients’ survival.
AUTHORS’ CONTRIBUTIONS
S.A., M.E.G., M.P., G.S and B.D. contributed to collection of data. S.A., A.G. and R.S. contributed to paper drafting and review.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Wang AY, Yu AW, Li PK. et al. Factors predicting outcome of fungal peritonitis in peritoneal dialysis: analysis of a 9-year experience of fungal peritonitis in a single center. Am J Kidney Dis 2000; 36: 1183–1192 [DOI] [PubMed] [Google Scholar]
- 2. Miles R, Hawley CM, McDonald SP. et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 2009; 76: 622–628 [DOI] [PubMed] [Google Scholar]
- 3. Nagappan R, Collins JF, Lee WT.. Fungal peritonitis in continuous ambulatory peritoneal dialysis--the Auckland experience. Am J Kidney Dis 1992; 20: 492–496 [DOI] [PubMed] [Google Scholar]
- 4. Warady BA, Bashir M, Donaldson LA.. Fungal peritonitis in children receiving peritoneal dialysis: a report of the NAPRTCS. Kidney Int 2000; 58: 384–389 [DOI] [PubMed] [Google Scholar]
- 5. The Turkish Multicenter Peritoneal Dialysis Study Group (TULIP). The rate, risk factors, and outcome of fungal peritonitis in CAPD patients: experience in Turkey. Perit Dial Int 2000; 20: 338–341 [PubMed] [Google Scholar]
- 6. Bibashi E, Memmos D, Kokolina E. et al. Fungal peritonitis complicating peritoneal dialysis during an 11-year period: report of 46 cases. Clin Infect Dis 2003; 36: 927–931 [DOI] [PubMed] [Google Scholar]
- 7. Ram R, Swarnalatha G, Neela P. et al. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: a single centre experience in India. Nephron Clin Pract 2008; 110: c207–c212 [DOI] [PubMed] [Google Scholar]
- 8. Khan FY, Elsayed M, Anand D. et al. Fungal peritonitis in patients undergoing continuous ambulatory peritoneal dialysis in Qatar. J Infect Dev Ctires 2011; 5: 646–651 [DOI] [PubMed] [Google Scholar]
- 9. Chavada R, Kok J, van Hal S. et al. Seeking clarity within cloudy effluents: differentiating fungal from bacterial peritonitis in peritoneal dialysis patients. PLoS One 2011; 6: e28247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prasad NK, Singh K, Rizwan A. et al. Microbiology and outcomes of peritonitis in Northern India. Perit Dial Int 2012; 34: 188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levallois J, Nadeau-Fredette A-C, Labbé A-C. et al. Ten-year experience with fungal peritonitis in peritoneal dialysis patients: antifungal susceptibility patterns in a North-American center International. Int J Infect Dis 2012; 16: e41–e43 [DOI] [PubMed] [Google Scholar]
- 12. Nadeau-Fredette AC, Bargman JM.. Characteristics and outcomes of fungal peritonitis in a modern North American cohort. Perit Dial Int 2015; 35: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumar KV, Mallikarjuna HM, Gokulnath Jayanthi S.. Fungal peritonitis in continuous ambulatory peritoneal dialysis: the impact of antifungal prophylaxis on patient and technique outcomes. Indian J Nephrol 2014; 24: 297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakao M, Yamamoto I, Maruyama Y. et al. 33 years of peritoneal dialysis-associated peritonitis: a single-center study in Japan. Ther Apher Dial 2016; 20: 60–65 [DOI] [PubMed] [Google Scholar]
- 15. Giacobino J, Montelli AC, Barretti P. et al. Fungal peritonitis in patients undergoing peritoneal dialysis (PD) in Brazil: molecular identification, biofilm production and antifungal susceptibility of the agents. Med Mycol 2016; 54: 725–732 [DOI] [PubMed] [Google Scholar]
- 16. Stinghen AE,, Barretti P,, Pecoits-Filho R.. Factors contributing to the differences in peritonitis rates between centers and regions. Perit Dial Int 2007; 27 (Suppl 2): S281–S285 [PubMed] [Google Scholar]
- 17. Záruba K, Peters J, Jungbluth H.. Successful prophylaxis for fungal peritonitis in patients on continuous ambulatory peritoneal dialysis: six years’ experience. Am J Kidney Dis 1991; 17: 43–46 [Erratum in: Am J Kidney Dis 1991; 17: 726] [DOI] [PubMed] [Google Scholar]
- 18. Thodis E, Vas SI, Bargman JM. et al. Nystatin prophylaxis: its inability to prevent fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 1998; 18: 583–589 [PubMed] [Google Scholar]
- 19. Wong PN, Lo KY, Tong GM. et al. Prevention of fungal peritonitis with nystatin prophylaxis in patients receiving CAPD. Perit Dial Int 2007; 27: 531–536 [PubMed] [Google Scholar]
- 20. Lye WC. Nystatin prophylaxis for fungal peritonitis: to be or not to be? Perit Dial Int 2007; 27: 511–513 [PubMed] [Google Scholar]
- 21. Restrepo C, Chacon J, Manjarres G.. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int 2010; 30: 619–625 [DOI] [PubMed] [Google Scholar]
- 22. Prabhu MV, Subhramanyam SV, Gandhe S. et al. Prophylaxis against fungal peritonitis in CAPD—a single center experience with low-dose fluconazole. Ren Fail 2010; 32: 802–805 [DOI] [PubMed] [Google Scholar]
- 23. Wong P-N, Tong GMW, Wong Y-Y. et al. Alternating mupirocin/gentamicin is associated with increased risk of fungal peritonitis as compared with gentamicin alone - results of a randomized open-label controlled trial. Perit Dial Int 2016; 36: 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen CM, Ho MW, Wang JH.. Fungal peritonitis in peritoneal dialysis patients: effect of fluconazole treatment and use of the twin-bag disconnect system. J Microbiol Immunol Infect 2004; 37: 115–120 [PubMed] [Google Scholar]
- 25. Prasad N, Gupta A.. Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 2005; 25: 207–222 [PubMed] [Google Scholar]
- 26. Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int 2009; 29 (Suppl 2): S161–S165 [PubMed] [Google Scholar]
- 27. Keane WF, Bailie GR, Boeschoten E. et al. International Society for Peritoneal Dialysis. Adult Peritoneal dialysi-related peritonitis treatment recommendations: 2000 update. Perit Dial Int 2000; 20: 396–411 [PubMed] [Google Scholar]
- 28. The Ad Hoc Advisory Committee on Peritonitis Management. Continuous ambulatory peritoneal dialysis (CAPD) peritonitis treatment recommendations: 1989 update. Perit Dial Int 1989; 9: 247–256 [PubMed] [Google Scholar]
- 29. Keane WF, Everett ED, Golper TA. et al. Peritoneal dialysis-related peritonitis treatment recommendations. 1993 update. The Ad Hoc Advisory Committee on Peritonitis Management. International Society for Peritoneal Dialysis. Perit Dial Int 1993; 13: 14–28 [PubMed] [Google Scholar]
- 30. Piraino B, Bailie GR, Bernardini J. et al. ISPD Guidelines/recommendations peritoneal dialysis-related infections recommendations: 2005 update Perit Dial Int 2005; 25: 107–131 [PubMed] [Google Scholar]
- 31. Kam-Tao Li P, Szeto CC, Piraino B. et al. DG ISPD Guidelines/recommendations peritoneal dialysis-related infections recommendations: 2010 update Perit Dial Int 2010; 30: 393–423 . [DOI] [PubMed] [Google Scholar]
- 32. Antinori S, Milazzo L, Sollima S. et al. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med 2016; 34: 21–28 [DOI] [PubMed] [Google Scholar]
- 33. Piraino B, Bernardini J, Brown E. et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31: 614–630 [DOI] [PubMed] [Google Scholar]
- 34. Anupkumar S. Reducing peritoneal dialysis-related peritonitis rate. Ochsner J 2014; 14: 386–391 [PMC free article] [PubMed] [Google Scholar]
- 35. Chou CY, Kao MT, Kuo HL. et al. Gram negative and polymicrobial peritonitis are associated with subsequent fungal peritonitis in CAPD patients. Perit Dial Int 2006; 26: 607–608 [PubMed] [Google Scholar]
- 36. Bernardini J. Training and retraining: impact on peritonitis. Perit Dial Int 2010; 30: 434–436 [DOI] [PubMed] [Google Scholar]
- 37. Patel GP, Crank CW, Leikin JB.. An evaluation of hepatotoxicity and nephrotoxicity of liposomal amphotericin B (L-AMB). J Med Toxicol 2011; 7: 12–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johansen HK, Gøtzsche PC.. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database Syst Rev 2014; 9: CD000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kyriakidis I, Tragiannidis A, Munchen S. et al. Clinical hepatotoxicity associated with antifungal agents. Expert Opin Drug Saf 2017; 16: 149–165 [DOI] [PubMed] [Google Scholar]
- 40. Pham CP, de Feiter PW, van der Kuy PH. et al. Long QTc interval and torsade de pointes caused by fluconazole. Ann Pharmacother 2006; 40: 1456–1461 [DOI] [PubMed] [Google Scholar]
- 41. Chen SS, Sheth H, Piraino B. et al. Long-term exit-site gentamicin prophylaxis and gentamicin resistance in a peritoneal dialysis program. Perit Dial Int 2016; 36: 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Posteraro B, Efremov L, Leoncini E. et al. Are the conventional commercial yeast identification methods still helpful in the era of new clinical microbiology diagnostics? A meta-analysis of their accuracy. J Clin Microbiol 2015; 53: 2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu R, Chen Y, Luo S. et al. Clinical characteristics and outcomes of peritoneal dialysis-related peritonitis with different trends of change in effluent white cell count: a longitudinal study. Perit Dial Int 2013; 33: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wong P-N, Lo K-Y, Tong GMW. et al. Treatment of fungal peritonitis with a combination of intravenous amphotericin B and oral flucytosine, and delayed catheter replacement in continous amulatory peritoneal dialysis. Perit Dial Int 2008; 28: 155–162 [PubMed] [Google Scholar]
- 45. Lye WC. Catheter removal for fungal peritonitis: sooner or later. Perit Dial Int 2008; 28: 130–133 [PubMed] [Google Scholar]
- 46. Liu YL, Huang CC, Kao MT.. Residual renal function predicts outcome of fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 2006; 26: 407–409 [PubMed] [Google Scholar]