ABSTRACT
Skeletal muscle wasting is a common feature of chronic kidney disease (CKD) and is clinically relevant due to associations with quality of life, physical functioning, mortality and a number of comorbidities. Satellite cells (SCs) are a population of skeletal muscle progenitor cells responsible for accrual and maintenance of muscle mass by providing new nuclei to myofibres. Recent evidence from animal models and human studies indicates CKD may negatively affect SC abundance and function in response to stimuli such as exercise and damage. The aim of this review is to collate recent literature on the effect of CKD on SCs, with a particular focus on the myogenic response to exercise in this population. Exercise is widely recognized as important for the maintenance of healthy skeletal muscle mass and is increasingly advocated in the care of a number of chronic conditions. Therefore a greater understanding of the impact of uraemia upon SCs and the possible altered myogenic response in CKD is required to inform strategies to prevent uraemic cachexia.
Keywords: exercise, intramuscular inflammation, sarcopenia, satellite cells, skeletal muscle
INTRODUCTION
Skeletal muscle wasting in CKD
Chronic kidney disease (CKD) is characterized by a progressive decline in renal function, often in conjunction with structural abnormalities. The prevalence of CKD Stages 3–5 is predicted to be 8.5% in the UK and 10.6% globally, with prevalence greatest at Stage 3, higher among women and increasing with age [1–3]. The number of patients receiving renal replacement therapy (RRT) has increased in the UK from 45 484 to 61 256 between 2007 and 2015 [4, 5].
Cachexia is highly prevalent in CKD (Table 1). It is associated with declining renal function [6–8] and therefore is prominent during the latter stages of the disease [9]. However, wasting is also reported in non-dialysis patients [7, 10, 11] and the rate of decline may be greater compared with patients receiving RRT [12]. Reduced muscle mass and strength is also common in renal transplant recipients [13, 14] and is associated with mortality and graft failure [15, 16].
Table 1.
Prevalence of sarcopenia in CKD and associations with mortality and physical function
| Reference | Population | Criteria | Prevalence (%) | Association |
|---|---|---|---|---|
| Souza et al. [17] | NDD |
|
|
ADL, gait speed, functional capacity, higher BMI |
| Zhou et al. [18] | NDD CKD (Stages 3–5) |
|
|
Measured GFR, functional reach, Berg balance score. |
| Pereira et al. [10] | NDD CKD (3–5) |
|
|
Mortality HR (association between mortality and sarcopenia according to BIA significant after multivariate adjustment) |
| Lamarca et al. [19] | HD CKD |
|
|
|
| Kittiskulnam et al. [20] | HD CKD |
|
|
Gait speed (Associations between data and mortality presented in another paper [21]. Significantly higher mortality rate in sarcopenic patients, according to low MM, but not in adjusted models) |
| Gracia-Iguacel et al. [22] | HD CKD |
|
|
No association between PEW and mortality but loss of MM associated with increased mortality |
| Carrero et al. [24] | HD | SGA | 39 | Mortality risk |
| Kittiskulnam et al. [21] | HD | HGS <26/16 kg men/women | 29.9 | Low HGS and slow gait speed associated with mortality risk |
| Chang et al. [25] | NDD CKD | HGS, SGA, BIA, MAMC, MAMA, MAC, SKF | N/A | Only HGS was significantly associated with composite endpoints of non-dialysis mortality and ESRD |
| Isoyama et al. [26] | Dialysis CKD |
|
|
|
| Wang et al. [27] | NDD CKD | LTI <10% reference value | 12.2% | Serum albumin, eGFR, age, IL-6, CVD |
ASMI, Appendicular Skeletal Muscle Index; BIA, bioelectrical impedance analysis; BSA, body surface area; CC, calf circumference; DEXA, dual-energy X-ray absorptiometry; SKF, skinfold thickness; HGS, handgrip strength; LTI, Lean Tissue Index.; MAC, mid-arm circumference; MAMA, mid-arm muscle area; MAMC, mid-arm muscle circumference; NDD, non-dialysis dependent; SGA, subjective global assessment; SM, skeletal muscle.
The prevalence of muscle wasting in CKD varies depending on the method of assessment, with different criteria referring to measures of body composition, functional outcomes or a combination (Table 1) [10, 17–27]. Even when employing the same measure, differing cut-off values are often used. For example, the European Working Group on Sarcopenia in Older People (EWGSOP) and Foundation for the National Institutes of Health (FNIH) determine sarcopenia according to hand-grip strength but with differing cut-offs (<30 kg/20 kg versus <26 kg/16 kg in men and women, respectively). This can significantly influence prevalence statistics. Zhou et al. [18] reported 29% of 148 non-dialysis patients fit the EWGSOP classification of sarcopenia, however, according to appendicular lean mass (<7.3 kg/m2 versus 5.5 kg/m2 for men and women, respectively) this increased to 36%, whereas 14% satisfied both criteria [18]. Every 1 mL/min/1.73 m2 decrement in measured GFR was associated with a 0.15 kg loss of lean mass, which was in turn positively associated with physical functioning [18]. Therefore, regardless of the criteria used, sarcopenia appears to worsen with disease progression and is associated with poorer performance of activities of daily living (ADL), slower gait speed, reduced physical functioning and inactivity [17]. Consensus on the definition and methods of assessment of sarcopenia in CKD are required and should employ multidimensional measures encompassing muscle size and functionality with cut-off points relevant to CKD [28].
The clinical relevance of reduced muscle mass and strength in CKD is exemplified through associations with mortality, depression, quality of life, diabetes and cardiovascular disease (CVD) [10,29–32]. For example, psoas cross-sectional area (CSA) independently predicts major adverse cardiovascular events in non-dialysis patients [33]. Reduced muscle mass is also associated with impaired exercise capacity and physical functioning [9, 11, 12, 34, 35], which likely contribute to reduced rates of physical activity in CKD [11, 36]. Roshanravan et al., [37] recently collated evidence from non-dialysis demonstrating every 0.1 m/s decrement in gait speed is associated with a 26% greater risk of death [37]. Reduced strength and physical capacity may account for the higher fall rates in CKD patients compared with the general population [38]. Falls are a major cause of acute injury and often initiate a decline in functional independence, culminating in greater reliance on health care services [39], which is predicted to cost the National Health Service in excess of £2.3 billion per years [39]. This exemplifies the importance of early detection of skeletal muscle impairment to allow for timely initiation of appropriate therapy in patients with CKD [40].
CKD adversely alters both protein synthesis and degradation, with proposed mechanisms including upregulation of the ubiquitin–proteasome system (UPS), caspase-3 and autophagy in response to factors that include metabolic acidosis, inflammation, mitochondrial dysfunction, oxidative stress and insulin resistance (IR) [40–43]. Satellite cells (SCs) are specialized stem-like cells that regulate skeletal muscle mass by initiating myogenesis, thereby facilitating growth and repair. Altered SC function is a feature of a number of conditions associated with loss of muscle mass, including unloading, denervation and atrophy induced by a number of chronic diseases [44]. Recent evidence indicates that CKD may also impair the functioning of these cells, presenting a novel mechanism contributing to uraemic cachexia [41]. The aim of this article is to review the available literature investigating the role of SCs in uraemic cachexia and to explore the potential for exercise to restore any lost or inhibited function of these cells.
Overview of myogenesis
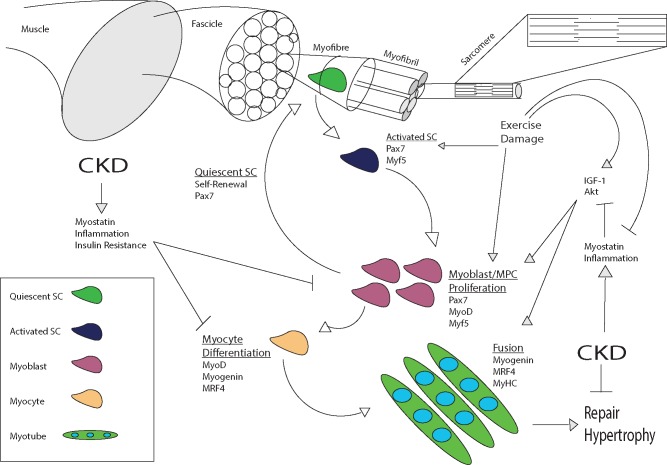
Skeletal muscle comprises long cylindrical multinucleated fibres that run the length of a tissue longitudinally and are grouped into bundles, referred to as fascicles (Figure 1). Myofibres are composed of numerous myofibrils organized in series, which are in turn composed of sarcomeres, the contractile unit of muscle tissue required for locomotion. Muscle fibres are highly specialized, with specific metabolic capacities to meet distinct contractile demands. As a result, mature muscle is post-mitotic and comprises terminally differentiated cells [45]. However, skeletal muscle is also highly plastic, displaying remarkable capacity to increase in size following hypertrophic stimuli or repair damaged myofibres. This is afforded by the population of SCs that supply new myonuclei to regenerating or expanding myotubes [46].
FIGURE 1.
Overview of myogenesis and regulatory processes and the possible effect of CKD. Arrows denote stimulatory/upregulatory impact and flat lines indicate negative/suppressive impact. SCs are activated by stimuli such as exercise and damage. Proliferating cells and myoblasts either return to quiescence or differentiate to become myocytes. Mature myocytes fuse to each other or existing myotubes, providing new myonuclei. CKD interferes with both proliferation and differentiation of SCs with factors including inflammation, myostatin signalling and IGF-1/Akt dysregulation proposed as primary mechanisms. See text for details.
Individual myofibres are enveloped by an elastic membrane, the sarcolemma, which comprises a plasma and basement membrane. Under homeostatic conditions, SCs reside in a niche between these membranes in a dormant state known as quiescence. Quiescent SCs are undifferentiated and non-proliferative, but upon activation by damage, exercise or growth factors, they are able to re-enter the cell cycle [47]. SC progeny, referred to as myoblasts or myogenic precursor cells (MPCs), proliferate and differentiate, committing to the myogenic lineage (myocytes). A small population of SCs will undergo asymmetric division, with one of their progeny progressing along the myogenic programme, whereas the other retains its stem cell–like capacity and returns to quiescence, maintaining the SC pool [46]. Myoblasts undergoing terminal differentiation will either fuse to each other or to existing myotubes, providing new myonuclei that are phenotypically and functionally indistinguishable from those surrounding them [48].
Myogenesis is regulated by the sequential expression of a number of transcription factors, namely paired-box protein-7 (Pax7) and a group of myogenic regulatory factors (MRFs). Quiescent SCs can be identified by the expression of Pax7 and the absence of myoblast determination protein (MyoD) and myogenin expression [46]. Upon activation, myogenic factor 5 (Myf5) is upregulated and subsequently proliferating MPCs express high levels of MyoD. Following several rounds of proliferation, terminal differentiation is initiated by myogenin and MRF4, whereas differentiated cells are also notable for their absence of Pax7 expression, due to downregulation by myogenin [46–49].
Numerous regulators participate in the orchestration of this complex process, acting via distinct signalling pathways to facilitate MRF expression and cellular progression through each stage of the myogenic program. It is beyond the scope of this review to discuss the many regulatory factors involved in myogenesis, however, interested readers are directed to the following comprehensive reviews [46, 49].
SC FUNCTION IN CKD
Research assessing the effect of CKD on myogenesis is relatively scarce. However, it has been suggested that mice subjected to subtotal nephrectomy exhibit reduced MPC abundance, with 18% fewer myonuclei located outside the sarcolemma of myofibres [50].
When assessed according to MRF expression, CKD mice showed reduced mRNA expression of Pax7, but in the presence of increased MyoD and myogenin, increased mRNA expression [51]. These mice were not subjected to injury and there was no evidence of ongoing regeneration, however, elevated muscle RING-finger protein-1 (MuRF1) and muscle atrophy F-box (MAFbx) mRNA expression supports the notion of elevated proteolysis, previously reported in CKD, potentially providing a stimulus for myogenesis [51].
In contrast, another study reported no difference in Pax7 gene expression or the abundance of Pax7+ cells between CKD and wild-type (WT) mice [52]. However, MyoD, Myf-5 and myogenin mRNA were supressed in CKD muscle [50, 52]. Reduced positive staining for MyoD was seen in SCs isolated from CKD mice, together with lower levels of ‘5-bromo-2’-deoxyuridine (BrdU) incorporation and embryonic myosin heavy chain (eMyHC) staining indicating downregulated SC proliferation and differentiation, respectively, compared with WT-derived SCs. This in vitro evidence suggests that CKD does not reduce the number of SCs but impairs their activation and differentiation [52].
The discrepancy of the influence of uraemia on MRF expression may be a product of differing methods, with a genetic model of slow progressive CKD being used by Avin et al. [51] compared with the more widely used 5/6 subtotal nephrectomy [52]. Regardless, both studies show CKD induced dysregulation of SCs in mice.
SCs are required to repair muscle after acute injury, indicated by the lack of regenerative myogenesis following ablation of Pax7+ cells [53, 54]. Nephrectomized mice subjected to cardiotoxin (CTX) injury show blunted MyoD and myogenin mRNA expression 72-h post-injury, persisting for 14 days [52]. While WT muscle achieved full repair after 14 days, at this point CKD mice presented expanded interstitial spaces, persistence of mononuclear cells and myofibres remained considerably smaller after a month [52]. Therefore the blunted myogenic gene expression seen in isolated SCs in vitro and whole muscle in CKD is replicated in an in vivo model of injury, culminating in impaired tissue regeneration. To our knowledge, no data are available assessing the myogenic response of humans with CKD to muscle damage. However, another scenario in which SCs are activated is in response to exercise.
Effects of exercise on SC function in CKD
Generally, exercise activates SCs, with numerous studies showing initiation of myogenesis following a single bout in healthy individuals [55–58]. This has been seen following protocols designed to induce muscle damage, however, SCs also respond to non-damaging and non-hypertrophic exercise [59]. Sedentary obese individuals showed an increase in Pax7+ and MyoD+ cells in type I fibres after a single bout of a resistance exercise protocol relevant to ‘real-world’ physical activity (8 × 8 leg extension repetitions at 70% 1 repetition maximum). Although no change in type II fibre–specific SCs was seen [60]. SC activation in the absence of myofibre damage suggests different mechanisms of action, with cytokines and growth factors proposed to play central roles [61].
Exercise has previously been shown to attenuate CKD-induced atrophy in mice [50]. This was due in part to the rescue of depressed protein synthesis and elevated proteolytic rates. Muscle overload, but not treadmill running, increased peripherally located myonuclei by 1.8-fold and increased the expression of MyoD, myogenin and embryonic MyHC [50]. This indicates that a model of resistance exercise can increase MPC abundance and activation compared with non-exercised uraemic mice, alleviating CKD-induced atrophy.
Exercise is increasingly being recognized as an important aspect in the treatment of CKD due to improvements in muscle size and function, physical capacity and CVD risk [40, 62]. However, the effect of CKD on SC function in patients and the myogenic response to exercise has received less attention.
Dialysis patients showed reduced SC content in type II compared with type I fibres [63]. However, when normalized for fibre area, this difference was no longer evident, suggesting this finding may be a product of disproportionate atrophy in type II fibres common in CKD patients [63]. The abundance of SCs per fibre increased by 15% in type I fibres following 16 weeks of high-intensity resistance training, with no increase in myonuclear content [63]. Interestingly, there was no change in the SC content, but an increase in myonuclear content was reported in type II fibres, indicating fibre-specific SC responses to training. [63].
We have previously reported the molecular response to resistance exercise in non-dialysis patients [64]. We found no change in MyoD or myogenin mRNA expression 24-h after an unaccustomed bout of exercise compared with baseline levels. Similarly, no acute myogenic response was seen following a period of resistance training [64]. This lack of exercise-induced myogenesis could diminish exercise adaptation, contributing to loss of muscle mass. However, these patients did show increases in muscle CSA (8%), volume (10%) and knee extensor strength (13%) after training [65]. SC activation during the early stages of training appears responsive to the degree of damage caused by unaccustomed exercise, which is attenuated with regular exercise [66]. Therefore it is possible the exercise protocol we employed did not cause sufficient tissue damage to stimulate SC activation. With regard to the training-induced hypertrophy, while we did not assess SC and myonuclear content, it is possible to achieve hypertrophy without the accrual of additional myonuclei if the existing pool is capable of supporting the transcriptional capacity of the expanded tissue [66].
Alternatively, the single sample point at 24-h post-exercise missed an effect. Comprehensive determination of the time course of gene expression over a 24-h period following resistance exercise reported upregulation of myogenic genes from 2- to 12-h, with MRF4, MyoD and myogenin peaking within 4–8 h in young healthy individuals [67]. Increases in Pax7+ cells have been reported 24-h post-exercise [56] and even peaking 72-h after and remaining above baseline values 120-h post-exercise [57]. However, these studies have generally employed more extreme protocols of exhaustive eccentric exercise, causing greater damage. The time course of a myogenic response to exercise, both damaging and non-damaging, requires more thorough investigation in CKD.
In summary, SCs are recognized to be essential regulators of skeletal muscle repair [68]. Their role in hypertrophy, however, has been the subject of debate, with some supporting an essential requirement [69], while others show hypertrophy can be achieved in their absence [68, 70]. These differences could be the product of the methods used to deplete SCs (irradiation versus genetic approaches) in animal models and hypertrophic stimuli (overload versus myostatin inhibition). In humans, a wealth of evidence shows SC activation following acute and chronic resistance exercise [57, 58, 60, 63, 66, 67, 71–73]. Indeed, positive correlations between acute SC response and chronic hypertrophic gains support a role for SC in skeletal muscle adaptation [74]. As previously mentioned, non-hypertrophic aerobic exercise also stimulates a myogenic activation, without increasing the SC pool, supporting a role in non-hypertrophic skeletal muscle remodelling [59]. While debate remains on the absolute requirement of SCs to hypertrophy in animal models, the human evidence showing myogenic activation after both resistance and endurance exercise overwhelmingly supports a central role of SCs in skeletal muscle adaptation to exercise [48].
Within the context of CKD, however, there is a shortage of research assessing the effect of CKD on SC function. The studies that have been performed indicate that uraemia impairs SC abundance and/or activation. This has been shown to correspond to blunted myogenic response to muscle injury and exercise, potentially contributing to sarcopenia.
MECHANISMS OF SC DYSFUNCTION IN CKD
Inflammation
SCs are receptive to and indeed reliant on signals from their local environment, mediated by factors such as disease, damage and exercise [46]. The involvement of cells of other lineages, namely haematopoietic, is now recognized as pivotal to SC function in response to exercise and tissue injury [75].
The early inflammatory response to acute injury is well characterized, with neutrophils entering muscle within 1–24 h [76–81] and producing large amounts of oxidative free radicals to remove cellular debris [82, 83]. Following neutrophil accumulation, macrophages become the dominant leucocyte population in regenerating skeletal muscle. An initial population of Ly6C+ monocytes/macrophages infiltrate regenerating muscle, phagocytizing necrotic debris and producing large amounts of pro-inflammatory cytokines before transitioning in situ to pro-regenerative Ly6C-macrophages [84–86]. Impaired regeneration following macrophage depletion illustrates their importance [84, 87, 88]. This is due to close interaction between myogenic cells and macrophages throughout myogenesis, with the former facilitating monocyte chemotaxis while macrophages in turn protect MPCs and myotubes against apoptosis [89, 90].
Pro-inflammatory macrophages associate with proliferating MPCs in human muscle [91] and produce soluble factors that stimulate MPC proliferation and cytokine secretion in vitro [91, 92]. Tumour necrosis factor α (TNFα) induces a dose-dependent increase in myoblast proliferation [93] but inhibits myogenic differentiation via nuclear factor κB (NF-κB) activation [94–96]. However, macrophages are also a source of insulin-like growth factor-1 (IGF-1) [97] and anti-inflammatory macrophages have been shown to co-localize with myogenin-positive MPCs in vivo, promoting differentiation and myotube fusion [91]. In sum, macrophage-derived growth factors and cytokines are involved in both MPC proliferation and differentiation, demonstrating the importance of the local inflammatory milieu in myogenesis.
CKD has a profound effect on this local inflammatory environment, characterized by low-grade inflammation in circulation. For example, systemic concentrations of C-reactive protein (CRP) were independently associated with elevated protein degradation and reduced protein synthesis and protein balance in maintenance haemodialysis (HD) patients [98], while interleukin-6 (IL-6) concentration was associated with reduced lean tissue mass in non-dialysis CKD patients [27].
However, CKD patients also show elevated mRNA expression of intramuscular IL-6, TNF-α, toll-like receptor-4 (TLR4) and myostatin, while NF-κB and p38 mitogen-activated protein kinase (MAPK) signalling is also upregulated [99–101]. Uraemic rodent models show greater cytokine production and macrophage infiltration in adipose tissue, while peritoneal macrophages isolated from partially nephrectomized mice show augmented M1 and blunted M2 polarization [102, 103]. Similar findings were recently reported in humans with end-stage renal disease (ESRD), as patients exhibited greater macrophage presence in adipose tissue [104]. With regard to skeletal muscle, excessive macrophage infiltration and prolonged pro-inflammatory cytokine expression were reported in CKD mice subjected to CTX-induced injury, culminating in delayed regeneration [52]. Therefore uraemia upregulates cytokine expression and inflammatory signalling pathways in peripheral tissues while also altering inflammatory cell infiltration and function.
This altered immune cell presence and function in peripheral tissues may be a product of circulatory factors, with CKD serum promoting M1 polarization and greater cytokine expression in macrophages derived from WT animals [102]. Serum also promotes a pro-inflammatory response in myogenic cell lines, evidenced by upregulation of TLR4 and TNF-α expression in C2C12 cells [100] and also impairing mitochondrial function [104]. Similarly, indoxyl sulfate, a uraemic toxin, recently reduced proliferation and differentiation of C2C12 while also downregulating both mRNA and protein expression of MyoD, myogenin and MyHC [105]. In sum, uraemia promotes a pro-inflammatory response from both immune and myogenic cells.
As in response to injury, inflammation is part of a normal exercise response and is an important regulator of SC activation [106]. However, recent micro-array analysis showed an overall pattern of blunted gene expression response to an acute bout of exercise in CKD patients before and after transplantation [107]. Enhancement of various gene pathways following exercise increased post-transplant, particularly those related to cytokine and chemokine activity [107]. In addition to investigating the myogenic response to resistance exercise described earlier [64], we have also assessed the intramuscular inflammatory response to an acute bout of resistance exercise in non-dialysis CKD patients before and after 8 weeks of progressive resistance training [65]. A considerable inflammatory response to exercise in the untrained state was reported, with IL-6 (53-fold), monocyte chemoattractant protein-1 (25-fold) and TNF-α (4-fold) all increasing significantly. This suggests a transient worsening of the inflammatory environment within muscle 24 h after a single bout of exercise. Whether this inflammatory cytokine upregulation is greater than that normally seen in healthy individuals is unclear, however, the response was dampened after a period of training [64]. In addition, IL-15 mRNA was suppressed significantly from baseline in the untrained state. IL-15 is another myokine with mitogenic properties [108] and has recently been shown to mitigate the negative influence of TNF-α on human myogenesis [109]. Training corrected this blunted IL-15 expression, which was also combined with the reversal of inflammatory cytokine expression [64]. There was no evidence of overt oxidative stress or protein catabolism following exercise in either the unaccustomed or trained state [64].
Collectively this indicates an altered intramuscular response to acute exercise in CKD patients. The apparent lack of a myogenic response in these patients following exercise has been discussed earlier. Whether this was due to elevated local inflammation is unclear, but evidence suggests that CKD patients were also unable to stimulate a myogenic response after a period of training, despite normalization of cytokine expression [64]. Considering the role of inflammation in myogenesis and the effect of cytokines and immune cells on SCs in culture, future research should address the effect of inflammatory factors present in uraemia on SC function.
IR and diabetes mellitus (DM)
The prevalence of IR in CKD has been reported to range from 10 to 100%, with variance likely due to differences in population and cause of disease, methods of measurement and criteria of IR [110]. Research assessing how IR interferes with myogenesis in CKD is lacking, however, much evidence has assessed its role in SC dysfunction in obesity and type 2 DM.
Large-scale cross-sectional evidence indicates patients with type 2 DM show decreased muscle mass, quality and strength and reduced physical function compared with non-diabetic counterparts [111, 112] and that losses of skeletal muscle mass, quality and strength with age are accelerated in the presence of type 2 DM [113, 114].
Myopathy is common in insulin-resistant states, with SC abundance and function central to this [115]. Lipotoxicity, caused either by high-fat diets or transgenic mouse models, prolongs muscle regeneration via impaired SC functioning [116, 117]. Animal models of type 2 DM show delayed regeneration following CTX injury, with impaired SC activation and proliferation in response to damage, as indicated by reduced BrdU incorporation [118]. Delayed regeneration coincided with impaired inflammatory response with attenuated macrophage accumulation in damaged areas, potentially contributing to persistent necrosis and collagen accumulation [118, 119].
SCs derived from insulin-resistant donors exhibit impaired glucose and lipid metabolism, indicating these cells retain donor characteristics in vitro [115, 120]. Interestingly, altered inflammatory signalling is also conserved in primary cell cultures derived from insulin-resistant individuals with evidence of increased NF-κB DNA binding and cytokine production [121] and dysfunctional IL-6-negative regulation [122]. The effect of inflammation on SC function has been discussed previously (see above) and may represent a common mechanism through which CKD and type 2 DM negatively influence myogenesis.
Exercise is routinely used in the management of diabetes. Reduced SC proliferation in obese Zucker rats was counteracted by loading along with protein expression of myogenin, MyoD and Akt [123]. However, no change in SC content was seen after 6 months of endurance exercise in obese male type 2 DM patients [124]. This may be due to the lack of hypertrophy, with patients showing no change in fibre composition, CSA or lean mass. Therefore exercise has the potential to ameliorate the negative influence of type 2 DM and IR on SC function, but further research is needed specifically in the context of CKD and to clarify the optimal exercise mode to use for maximum benefit.
IGF-1
IGF-1 signalling is central to maintaining a healthy muscle mass. Following IGF-1 receptor binding, a cascade of intracellular signalling is initiated that represents a crossroads in protein metabolism, stimulating protein synthesis via downstream targets, such as Protein Kinase B and mammalian target of rapamycin, while suppressing degradation through phosphorylation of Forkhead box O1 (FoxO1) and subsequent inhibition of the UPS [125, 126]. This is relevant to CKD, as rodent models show defective post-receptor insulin/IGF-1 signalling culminating in reduced Akt activation [127].
IGF-1 levels increase both locally and in circulation after exercise. Intramuscular IGF-1 also increases after tissue damage, primarily derived from macrophages [97, 128]. Most mitogens are generally believed to increase proliferation and inhibit differentiation, however, IGF-1 positively regulates both of these mutually exclusive processes through distinct signalling pathways. Early provision of IGF-1 by macrophages mediates MPC proliferation via MAPK signalling, whereas later IGF-1 secretion by macrophages and other cell types, including fibroblasts, supports differentiation via phosphatidylinositol-3-kinase-induced p70 S6 kinase activation [129]. Alternatively, the IGF-1 splice variant mechano-growth factor (MGF) is expressed early after mechanical stretch in rodents, promoting proliferation and blocking differentiation, whereas IGF-IEa is expressed later and promotes myoblast differentiation [130, 131]. A similar expression time course of these IGF-1 isoforms was replicated in humans after exercise [132].
Numerous studies using in vivo transgenic or knock-out models point to a major role of IGF-1 in the regulation of muscle mass [133]. Transgenic mice bearing heterozygous IGF-1 receptor (IGF-1R) mutation in MyoD+ cells show reduced muscle mass and myofibre CSA, while myogenic gene expression, proliferation and differentiation rates are also suppressed [52, 133]. IGF-1 signalling intersects with numerous members of other pathways in skeletal muscle [126], including transforming growth factor β1 (TGF-β1), which suppresses MyoD-dependent differentiation via Smad3 signalling [134]. However, IGF-1 prevents nuclear translocation of Smad3 and subsequent impact on gene expression due to cytoplasmic association with p-Akt [133]. Exercise-induced Akt activation has been seen to be blunted in mouse models of CKD and in human CKD patients, suggesting CKD induces an anabolic resistance [50, 64] that may impair SC function and also contribute to fibrosis by failing to regulate TGF-β1 signalling, as seen in IGF-1R+/− models [133]. This appears to be rescued by exercise training [50, 64], which was also associated with increased MRF expression, suggesting that this is one possible mechanism by which regular exercise training might be able to improve SC function.
Muscle overload designed to replicate resistance exercise increased IGF-1 mRNA in CKD mice and corrected atrophy [135]. Similarly, a cycling-based endurance training program increased IGF-1 expression in muscle of maintenance HD patients [136]. This highlights suppressed IGF-1 signalling as a therapeutic target to prevent muscle wasting, which appears can be positively modulated by exercise training. This is an attractive, safe and low-cost intervention that provides numerous other benefits to CKD patients.
Myostatin
Myostatin is a member of the TGF-β protein family and is a negative regulator of muscle mass. It is expressed in SCs and their progeny, where it inhibits G0-S phase progression and proliferation [137]. Myostatin knock-out myoblasts exhibit prolonged proliferation in differentiation medium due to extended expression of MyoD and myogenin [137].
In a recent publication, myostatin was shown to stimulate fibro/adipogenic precursor (FAP) cell proliferation. FAPs are bipotent progenitor cells capable of differentiating into adipocytes or fibrocytes. Myostatin increases fibrotic gene expression in FAPs, promoting fibrocyte differentiation and potentially accounting for greater FAP content and α-smooth muscle actin expression in CKD muscle following injury [138]. Blocking myostatin was effective in reducing FAP abundance and fibrotic gene expression in injured CKD mice compared with controls, while also decreasing fibrotic gene expression. Therefore it appears myostatin expression simultaneously inhibits myogenesis and promotes fibrosis by pushing FAPs towards fibrocyte differentiation [138].
Myostatin mRNA expression is elevated in CKD muscle, potentially due to TNF-α-induced NF-κB activation [51, 138, 139]. This appears to induce atrophy, as intramuscular antagonism of myostatin corrected rates of protein synthesis and degradation, preventing loss of muscle mass [139]. This was partially due to increased Akt phosphorylation, subsequently increasing phosphorylation of FoxO3a and FoxO1 [139]. In addition, myostatin inhibition also increased MyoD and myogenin expression at rest and in response to CTX injury in CKD mice [139].
The applicability of myostatin inhibition to CKD patients has not been investigated, but a number of clinical trials have been performed in healthy controls [140], elderly participants [141] and other patient populations [142]. These trials demonstrate anti-myostatin treatments to be generally well tolerated and to exert positive effects on muscle mass and functional outcomes [140, 143].
Another method of reducing myostatin expression is through exercise. Muscle overload attenuates declines in MyoD, myogenin and eMyHC expression in CKD muscle [50] and normalized elevated myostatin expression [135]. Regular endurance training also appears to improve myostatin mRNA expression levels in HD patients [136] and resistance exercise suppressed myostatin expression 24 h after a single bout in non-dialysis patients [64]. Therefore exercise may pose a viable therapeutic strategy of improving the effect of altered intramuscular myostatin signalling in CKD.
Nutrient availability
In addition to stimuli such as exercise, tissue damage and growth hormones, nutrient availability has emerged as an additional modulator of SC function. Protein provision, specifically of branched-chain amino acids (BCAAs) such as leucine, may have the potential to augment SC responses in vitro and in vivo following acute exercise, as comprehensively reviewed recently [144].
Dietary recommendations, especially those concerning protein, are complicated within a uraemic context. Failure to excrete non-volatile acids derived from dietary protein can result in metabolic acidosis, which is implicated in protein catabolism, systemic inflammation and CKD progression [145]. However, protein energy wasting is prevalent in CKD and frequently caused by inadequate dietary protein intake [146]. Low-protein diets are common in non-dialysis CKD patients, and hypertrophic benefits of exercise have been achieved consuming 0.6 g/kg/day [147]. However, considering the impact of protein availability on hypertrophic and specifically SC responses to exercise [144], further research is warranted to determine the optimal dosage and timing of protein intake post-exercise in order to maximize benefits without compromising other aspects of patient care.
Ageing
Another factor relevant to SC dysfunction within the context of CKD is ageing. The decline in skeletal muscle mass starts during the third decade of life, accelerating during the fifth [148] and SC dysfunction has been highlighted as a contributing factor [149]. Increasing age is associated with reduced fibre CSA and SC content [73], which disproportionately effects type II fibres [149, 150]. While 4 weeks of retraining in young participants restored the myofibre area and SC content lost during 2 weeks of immobilization, elderly individuals showed no such recovery [151]. Previous research also reports blunted acute SC responses after resistance exercise in type II–associated SCs, potentially due to delayed downregulation of myostatin [73, 152]. This indicates impaired or delayed SC response to exercise. Promisingly however, age-related declines in type II fibre size and SC content can be corrected following prolonged resistance exercise regimes lasting 12 weeks [149].
As with elderly individuals, dialysis patients reportedly show greater type II–specific fibre atrophy [153] and lower type II SC content [63]. Due to the chronic nature of renal disease, prevalence is high in the elderly. However, with neither study including age-matched controls, it is difficult to disentangle the contributions of aging and CKD to muscle wasting and SC dysfunction.
SUMMARY
Muscle wasting is prevalent in CKD patients and is associated with a number of negative outcomes. Impaired SC functioning has emerged as a novel mechanism of atrophy and muscle dysfunction in CKD. Research on this topic is in its infancy, but a greater understanding of the effect of uraemia on SCs and the mechanisms through which this occurs will allow for more targeted treatment strategies.
This is particularly pertinent as increasingly there are calls for exercise to be implemented into standard renal care due to its favourable impacts on a range of common comorbidities [154]. Despite the wealth of evidence in support of ‘exercise as medicine’ in CKD, routine prescription is uncommon [155]. More research is required to determine the necessary dose to prescribe to CKD patients, particularly in non-dialysis and transplant populations [156], to increase implementation to that seen in other chronic diseases.
FUNDING
This report is independent research supported by the National Institute for Health Research Leicester Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research Leicester BRC or the Department of Health.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Stevens PE, O’Donoghue DJ, de Lusignan S. et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007; 72: 92–99 [DOI] [PubMed] [Google Scholar]
- 2. Hill NR, Fatoba ST, Oke JL. et al. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Q-L,, Rothenbacher D.. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farrington K, Udayaraj U, Gilg J. et al. UK Renal Registry 11th Annual Report (December 2008): Chapter 4 ESRD prevalent rates in 2007 in the UK: national and centre-specific analyses. Nephron Clin Practice 2009; 111(Suppl 1): c43–c68 [DOI] [PubMed] [Google Scholar]
- 5. MacNeill SJ,, Ford D.. UK Renal Registry 19th Annual Report: Chapter 2 UK renal replacement therapy prevalence in 2015: national and centre-specific analyses. Nephron 2017; 137(Suppl 1): 45–72 [DOI] [PubMed] [Google Scholar]
- 6. Foley RN, Wang C, Ishani A. et al. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol 2007; 27: 279–286 [DOI] [PubMed] [Google Scholar]
- 7. Tynkevich E, Flamant M, Haymann J-P. et al. Decrease in urinary creatinine excretion in early stage chronic kidney disease. PloS One 2014; 9: e111949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leikis MJ. Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin J Am Soc Nephrol 2006; 1: 488–495 [DOI] [PubMed] [Google Scholar]
- 9. McIntyre CW, Selby NM, Sigrist M. et al. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant 2006; 21: 2210–2216 [DOI] [PubMed] [Google Scholar]
- 10. Pereira RA. et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrol Dial Transplant 2015; 30: 1718–1725 [DOI] [PubMed] [Google Scholar]
- 11. Segura-Ortí E. et al. Correlates of physical functioning and performance across the spectrum of kidney function. Clin Nurs Res 2017: 27: 579–596 [DOI] [PubMed] [Google Scholar]
- 12. John SG, Sigrist MK, Taal MW. et al. Natural history of skeletal muscle mass changes in chronic kidney disease stage 4 and 5 patients: an observational study. PLoS One 2013; 8: e65372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozkayar N, Altun B, Halil M. et al. Evaluation of sarcopenia in renal transplant recipients. Nephrourol Mon 2014; 6: e20055–e20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van den Ham ECH, Kooman JP, Schols AMWJ. et al. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am J Transplant 2005; 5: 1957–1965 [DOI] [PubMed] [Google Scholar]
- 15. Kasiske BL. Creatinine excretion after renal transplantation. Transplantation 1989; 48: 424–427 [DOI] [PubMed] [Google Scholar]
- 16. Oterdoom LH, van Ree RM, de Vries APJ. et al. Urinary creatinine excretion reflecting muscle mass is a predictor of mortality and graft loss in renal transplant recipients. Transplantation 2008; 86: 391–398 [DOI] [PubMed] [Google Scholar]
- 17. Souza VAd, Oliveira D, Barbosa SR. et al. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One 2017; 12: e0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou Y, Hellberg M, Svensson P, Höglund P, Clyne N. Sarcopenia and relationships between muscle mass, measured glomerular filtration rate and physical function in patients with chronic kidney disease stages 3–5. Nephrol Dial Transplant 2018; 33: 342–348 [DOI] [PubMed] [Google Scholar]
- 19. Lamarca F, Carrero JJ, Rodrigues JCD. et al. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging 2014; 18: 710–717 [DOI] [PubMed] [Google Scholar]
- 20. Kittiskulnam P, Carrero JJ, Chertow GM. et al. Sarcopenia among patients receiving hemodialysis: weighing the evidence. J Cachexia Sarcopenia Muscle 2017; 8: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kittiskulnam P, Chertow GM, Carrero JJ. et al. Sarcopenia and its individual criteria are associated, in part, with mortality among patients on hemodialysis. Kidney Int 2017; 92: 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gracia-Iguacel C. et al. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 2013; 33: 495–505 [DOI] [PubMed] [Google Scholar]
- 23. Kalantar-Zadeh K, Kopple JD, Block G. et al. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001; 38: 1251–1263 [DOI] [PubMed] [Google Scholar]
- 24. Carrero JJ, Chmielewski M, Axelsson J. et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008; 27: 557–564 [DOI] [PubMed] [Google Scholar]
- 25. Chang Y-T, Wu H-L, Guo H-R. et al. Handgrip strength is an independent predictor of renal outcomes in patients with chronic kidney diseases. Nephrol Dial Transplant 2011; 26: 3588–3595 [DOI] [PubMed] [Google Scholar]
- 26. Isoyama N. et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y-W, Lin T-Y, Peng C-H. et al. Factors associated with decreased lean tissue index in patients with chronic kidney disease. Nutrients 2017; 9: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carrero JJ, Johansen KL, Lindholm B. et al. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int 2016; 90: 53–66 [DOI] [PubMed] [Google Scholar]
- 29. Wang AY-M, Sea MM-M, Ho ZS-Y. et al. Evaluation of handgrip strength as a nutritional marker and prognostic indicator in peritoneal dialysis patients. Am J Clin Nutr 2005; 81: 79–86 [DOI] [PubMed] [Google Scholar]
- 30. Noori N, Kopple JD, Kovesdy CP. et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2010; 5: 2258–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beddhu S, Pappas LM, Ramkumar N. et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 2003; 14: 2366–2372 [DOI] [PubMed] [Google Scholar]
- 32. Martinson M, Ikizler TA, Morrell G. et al. Associations of body size and body composition with functional ability and quality of life in hemodialysis patients. Clin J Am Soc Nephrol 2014; 9: 1082–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harada K, Suzuki S, Ishii H. et al. Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol 2017; 119: 1275–1280 [DOI] [PubMed] [Google Scholar]
- 34. Johansen KL, Shubert T, Doyle J. et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int 2003; 63: 291–297 [DOI] [PubMed] [Google Scholar]
- 35. Kempeneers G, Noakes TD, van Zyl-Smit R. et al. Skeletal muscle limits the exercise tolerance of renal transplant recipients: effects of a graded exercise training program. Am J Kid Dis 1990; 16: 57–65 [DOI] [PubMed] [Google Scholar]
- 36. Beddhu S, Baird BC, Zitterkoph J. et al. Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 2009; 4: 1901–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roshanravan B. et al. Association between physical performance and all-cause mortality in CKD. J Am Soc Nephrol 2013; 24: 822–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cook WL, Tomlinson G, Donaldson M. et al. Falls and fall-related injuries in older dialysis patients. Clin J Am Soc Nephrol 2006; 1: 1197–1204 [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Health and Care Excellence Falls in older people: assessing risk and prevention. CG161. London: National Institute for Health and Care Excellence, 2013
- 40. Roshanravan B, Gamboa J, Wilund K.. Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am J Kidney Dis 2017; 69: 837–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang XH, Mitch WE.. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Workeneh BT, Mitch WE.. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr 2010; 91: 1128S–1132S [DOI] [PubMed] [Google Scholar]
- 43. Price S. Russ, Gooch Jennifer L., Donaldson Sue K.. et al. Muscle atrophy in chronic kidney disease results from abnormalities in insulin signaling. J Ren Nutr 2010; 20(5 Suppl): S24–S28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKenna CF, Fry CS.. Altered satellite cell dynamics accompany skeletal muscle atrophy during chronic illness, disuse, and aging. Curr Opin Clin Nutr Metab Care 2017; 20: 447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zammit PS. Function of the myogenic regulatory factors Myf5, MyoD, myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis Semin Cell Dev Biol 2017; 72: 19–32 [DOI] [PubMed] [Google Scholar]
- 46. Yin H, Price F, Rudnicki MA.. Satellite cells and the muscle stem cell niche. Physiol Rev 2013; 93: 23–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Le Grand F, Rudnicki MA.. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2007; 19: 628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snijders T, Nederveen JP, McKay BR. et al. Satellite cells in human skeletal muscle plasticity. Front Physiol 2015; 6: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. Satellite cells and skeletal muscle regeneration. Compr Physiol 2015; 5: 1027–1059 [DOI] [PubMed] [Google Scholar]
- 50. Wang XH, Du J, Klein JD. et al. Exercise ameliorates chronic kidney disease–induced defects in muscle protein metabolism and progenitor cell function. Kidney Int 2009; 76: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Avin KG, Chen NX, Organ JM. et al. Skeletal muscle regeneration and oxidative stress are altered in chronic kidney disease. PLoS One 2016; 11: e0159411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang L, Wang XH, Wang H. et al. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 2010; 21: 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lepper C, Partridge TA, Fan C-M.. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 2011; 138: 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patsalos A. et al. In situ macrophage phenotypic transition is affected by altered cellular composition prior to acute sterile muscle injury. J Physiol 2017; 595: 5815–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Caldow MK, Thomas EE, Dale MJ. et al. Early myogenic responses to acute exercise before and after resistance training in young men. Physiol Rep 2015; 3: e12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. McKay BR, Toth KG, Tarnopolsky MA. et al. Satellite cell number and cell cycle kinetics in response to acute myotrauma in humans: immunohistochemistry versus flow cytometry. J Physiol 2010; 588: 3307–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McKay BR, De Lisio M, Johnston APW. et al. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One 2009; 4: e6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nederveen JP, Joanisse S, Séguin CML. et al. The effect of exercise mode on the acute response of satellite cells in old men. Acta Physiol 2015; 215: 177–190 [DOI] [PubMed] [Google Scholar]
- 59. Joanisse S, McKay BR, Nederveen JP. et al. Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am J Physiol Regul Integr Comp Physiol 2015; 309: R1101–R1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pugh JK. et al. Satellite cell response to concurrent resistance exercise and high-intensity interval training in sedentary, overweight/obese, middle-aged individuals. Eur J Appl Physiol 2017; 118: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ambrosio F, Kadi F, Lexell J. et al. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology . Am J Phys Med Rehabil 2009; 88: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gould DW, Graham-Brown MPM, Watson EL. et al. Physiological benefits of exercise in pre‐dialysis chronic kidney disease. Nephrology 2014; 19: 519–527 [DOI] [PubMed] [Google Scholar]
- 63. Molsted S, Andersen JL, Harrison AP. et al. Fiber type‐specific response of skeletal muscle satellite cells to high‐intensity resistance training in dialysis patients. Muscle Nerve 2015; 52: 736–745 [DOI] [PubMed] [Google Scholar]
- 64. Watson EL, Viana JL, Wimbury D. et al. The effect of resistance exercise on inflammatory and myogenic markers in patients with chronic kidney disease. Front Physiol 2017; 8: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Watson EL, Greening NJ, Viana JL. et al. Progressive resistance exercise training in CKD: a feasibility study. Am J Kidney Dis 2015; 66: 249–257 [DOI] [PubMed] [Google Scholar]
- 66. Damas F, Libardi CA, Ugrinowitsch C. et al. Early-and later-phases satellite cell responses and myonuclear content with resistance training in young men. PLoS One 2018; 13: e0191039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang Y, Creer A, Jemiolo B. et al. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 2005; 98: 1745–1752 [DOI] [PubMed] [Google Scholar]
- 68. McCarthy JJ, Mula J, Miyazaki M. et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 2011; 138: 3657–3666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Adams GR, Caiozzo VJ, Haddad F. et al. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol 2002; 283: C1182–C1195 [DOI] [PubMed] [Google Scholar]
- 70. Lee S-J, Huynh TV, Lee Y-S. et al. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci USA 2012; 109: E2353–E2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKay BR, O’Reilly CE, Phillips SM. et al. Co‐expression of IGF‐1 family members with myogenic regulatory factors following acute damaging muscle‐lengthening contractions in humans. J Physiol 2008; 586: 5549–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Toth KG, McKay BR, De Lisio M. et al. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One 2011; 6: e17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKay BR, Ogborn DI, Bellamy LM. et al. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J 2012; 26: 2509–2521 [DOI] [PubMed] [Google Scholar]
- 74. Bellamy LM, Joanisse S, Grubb A. et al. The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 2014; 9: e109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Comp Physiol 2011; 1: 2029–2062 [DOI] [PubMed] [Google Scholar]
- 76. Fielding RA, Manfredi TJ, Ding W. et al. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol 1993; 265(1 Pt 2): R166–R172 [DOI] [PubMed] [Google Scholar]
- 77. Frenette J, Chbinou N, Godbout C. et al. Macrophages, not neutrophils, infiltrate skeletal muscle in mice deficient in P/E selectins after mechanical reloading. Am J Physiol Regul Integr Comp Physiol 2003; 285: R727–R732 [DOI] [PubMed] [Google Scholar]
- 78. Kawanishi N, Mizokami T, Niihara H. et al. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med Sci Sports Exerc 2016; 48: 1917–1924 [DOI] [PubMed] [Google Scholar]
- 79. Smith JK, Grisham MB, Granger DN. et al. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol Heart Circ Physiol 1989; 256: H789–H793 [DOI] [PubMed] [Google Scholar]
- 80. Tidball JG, Berchenko E, Frenette J.. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol 1999; 65: 492–498 [PubMed] [Google Scholar]
- 81. Brigitte M, Schilte C, Plonquet A. et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin‐induced myoinjury. Arthritis Rheum 2010; 62: 268–279 [DOI] [PubMed] [Google Scholar]
- 82. Nguyen HX, Lusis AJ, Tidball JG.. Null mutation of myeloperoxidase in mice prevents mechanical activation of neutrophil lysis of muscle cell membranes in vitro and in vivo. J Physiol 2005; 565: 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nguyen HX, Tidball JG.. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol 2003; 553: 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Arnold L, Henry A, Poron F. et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 2007; 204: 1057–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang H, Melton DW, Porter L. et al. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol 2014; 184: 1167–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V. et al. p38/MKP-1–regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol 2011; 195: 307–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu X, Liu Y, Zhao L. et al. Macrophage depletion impairs skeletal muscle regeneration: the roles of regulatory factors for muscle regeneration. Cell Biol Int 2017; 41: 228–238 [DOI] [PubMed] [Google Scholar]
- 88. Xiao W, Liu Y, Chen P.. Macrophage depletion impairs skeletal muscle regeneration: the roles of pro-fibrotic factors, inflammation, and oxidative stress. Inflammation 2016; 39: 2016–2028 [DOI] [PubMed] [Google Scholar]
- 89. Sonnet C. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci 2006; 119: 2497–2507 [DOI] [PubMed] [Google Scholar]
- 90. Chazaud B, Sonnet C, Lafuste P. et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 2003; 163: 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saclier M, Yacoub-Youssef H, Mackey AL. et al. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 2013; 31: 384–396 [DOI] [PubMed] [Google Scholar]
- 92. Cantini M, Massimino ML, Rapizzi E. et al. Human satellite cell-proliferation in vitro is regulated by autocrine secretion of IL-6 stimulated by a soluble factor(s) released by activated monocytes. Biochem Biophys Res Commun 1995; 216: 49–53 [DOI] [PubMed] [Google Scholar]
- 93. Li Y-P. TNF-α is a mitogen in skeletal muscle. Am J Physiol Cell Physiol 2003; 285: C370–C376 [DOI] [PubMed] [Google Scholar]
- 94. Langen RCJ, Schols AMWJ, Kelders MCJM. et al. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-κB. FASEB J 2001; 15: 1169–1180 [DOI] [PubMed] [Google Scholar]
- 95. Langen RCJ, van der Velden JLJ, Schols AMWJ. et al. Tumor necrosis factor-alpha inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 2004; 18: 227–237 [DOI] [PubMed] [Google Scholar]
- 96. Guttridge DC, Albanese C, Reuther JY. et al. NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cel Biol 1999; 19: 5785–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tonkin J, Temmerman L, Sampson RD. et al. Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther 2015; 23: 1189–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Deger SM, Hung AM, Gamboa JL. et al. Systemic inflammation is associated with exaggerated skeletal muscle protein catabolism in maintenance hemodialysis patients. JCI Insight 2017; 2: e95185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Garibotto G, Sofia A, Procopio V. et al. Peripheral tissue release of interleukin-6 in patients with chronic kidney diseases: effects of end-stage renal disease and microinflammatory state. Kidney Int 2006; 70: 384–390 [DOI] [PubMed] [Google Scholar]
- 100. Verzola D, Bonanni A, Sofia A. et al. Toll‐like receptor 4 signalling mediates inflammation in skeletal muscle of patients with chronic kidney disease. J Cachexia Sarcopenia Muscle 2017; 8: 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Verzola D, Procopio V, Sofia A. et al. Apoptosis and myostatin mRNA are upregulated in the skeletal muscle of patients with chronic kidney disease. Kidney Int 2011; 79: 773–782 [DOI] [PubMed] [Google Scholar]
- 102. Li C, Ding XY, Xiang DM. et al. Enhanced M1 and impaired M2 macrophage polarization and reduced mitochondrial biogenesis via inhibition of AMP kinase in chronic kidney disease. Cel Physiol Biochem 2015; 36: 358–372 [DOI] [PubMed] [Google Scholar]
- 103. Xiang DM, Song XZ, Zhou ZM. et al. Chronic kidney disease promotes chronic inflammation in visceral white adipose tissue. Am J Physiol Renal Physiol 2017; 312: F689–F701 [DOI] [PubMed] [Google Scholar]
- 104. Martinez Cantarin MP, Whitaker-Menezes D, Lin Z. et al. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol Dial Transplant 2017; 32: 943–951 [DOI] [PubMed] [Google Scholar]
- 105. Jheng JR, Chen Y-S, Ao UL. et al. The double‐edged sword of endoplasmic reticulum stress in uremic sarcopenia through myogenesis perturbation. J Cachexia Sarcopenia Muscle 2018; 9: 570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise. Exerc Immunol Rev 2012; 18: 42–97 [PubMed] [Google Scholar]
- 107. Coletta DK, Campbell LE, Weil J. et al. Changes in pre-and post-exercise gene expression among patients with chronic kidney disease and kidney transplant recipients. PLoS One 2016; 11: e0160327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pérez‐López A, McKendry J, Martin-Rincon M. et al. Skeletal muscle IL‐15/IL‐15Rα and myofibrillar protein synthesis after resistance exercise. Scand J Med Sci Sports 2018; 28: 116–125 [DOI] [PubMed] [Google Scholar]
- 109. O’Leary MF, Wallace GR, Bennett AJ. et al. IL-15 promotes human myogenesis and mitigates the detrimental effects of TNFα on myotube development. Sci Rep 2017; 7: 12997/1–12997/11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Spoto B, Pisano A, Zoccali C.. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol 2016; 311: F1087–F1108 [DOI] [PubMed] [Google Scholar]
- 111. Park SW, Goodpaster BH, Strotmeyer ES. et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006; 55: 1813–1818 [DOI] [PubMed] [Google Scholar]
- 112. Leenders M, Verdijk LB, van der Hoeven L. et al. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J Am Med Dir Assoc 2013; 14: 585–592 [DOI] [PubMed] [Google Scholar]
- 113. Park SW, Goodpaster BH, Strotmeyer ES. et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007; 30: 1507–1512 [DOI] [PubMed] [Google Scholar]
- 114. Park SW, Goodpaster BH, Lee JS. et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009; 32: 1993–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fujimaki S, Kuwabara T.. Diabetes-induced dysfunction of mitochondria and stem cells in skeletal muscle and the nervous system. Int J Mol Sci 2017; 18: 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fu X, Zhu M, Zhang S. et al. Obesity impairs skeletal muscle regeneration via inhibition of AMP-activated protein kinase. Diabetes 2015: db150647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tamilarasan KP, Temmel H, Das SK. et al. Skeletal muscle damage and impaired regeneration due to LPL-mediated lipotoxicity. Cell Death Dis 2012; 3: e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Nguyen M-H, Cheng M, Koh TJ.. Impaired muscle regeneration in ob/ob and db/db mice. Sci World J 2011; 11: 1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Krause MP, Al-Sajee D, D’Souza DM. et al. Impaired macrophage and satellite cell infiltration occurs in a muscle-specific fashion following injury in diabetic skeletal muscle. PLoS One 2013; 8: e70971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fujimaki S, Wakabayashi T, Takemasa T. et al. Diabetes and stem cell function. BioMed Res Int 2015; 2015: 592915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Green CJ, Pedersen M, Pedersen BK. et al. Elevated NF-κB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes 2011; 60: 2810–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Scheele C, Nielsen S, Kelly M. et al. Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6. PLoS One 2012; 7: e39657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Peterson JM, Bryner RW, Alway SE.. Satellite cell proliferation is reduced in muscles of obese Zucker rats but restored with loading. Am J Physiol Cell Physiol 2008; 295: C521–C528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Snijders T, Verdijk LB, Hansen D. et al. Continuous endurance‐type exercise training does not modulate satellite cell content in obese type 2 diabetes patients. Muscle Nerve 2011; 43: 393–401 [DOI] [PubMed] [Google Scholar]
- 125. Sandri M, Sandri C, Gilbert A. et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004; 117: 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schiaffino S, Mammucari C.. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet Muscle 2011; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bailey JL. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 2006; 17: 1388–1394 [DOI] [PubMed] [Google Scholar]
- 128. Lu H, Huang D, Saederup N. et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 2011; 25: 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Coolican SA, Samuel DS, Ewton DZ. et al. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 1997; 272: 6653–6662 [DOI] [PubMed] [Google Scholar]
- 130. Hill M, Goldspink G.. Expression and splicing of the insulin‐like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 2003; 549: 409–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yang SY, Goldspink G.. Different roles of the IGF‐I Ec peptide (MGF) and mature IGF‐I in myoblast proliferation and differentiation. FEBS Let 2002; 522: 156–160 [DOI] [PubMed] [Google Scholar]
- 132. Philippou A, Papageorgiou E, Bogdanis G. et al. Expression of IGF-1 isoforms after exercise-induced muscle damage in humans: characterization of the MGF E peptide actions in vitro. In Vivo 2009; 23: 567–575 [PubMed] [Google Scholar]
- 133. Dong Y, Lakhia R, Thomas SS. et al. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am J Physiol Endocrinol Metab 2013; 305: E367–E375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu D, Black BL, Derynck R.. TGF-β inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 2001; 15: 2950–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sun D, Chen Y, Rabkin R.. Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int 2006; 70: 453–459 [DOI] [PubMed] [Google Scholar]
- 136. Kopple JD, Storer T, Casburi R.. Impaired exercise capacity and exercise training in maintenance hemodialysis patients. J Renal Nutr 2005; 15: 44–48 [DOI] [PubMed] [Google Scholar]
- 137. McCroskery S, Thomas M, Maxwell L. et al. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 2003; 162: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dong J, Dong Y, Chen Z. et al. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int 2017; 91: 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang L, Rajan V, Lin E. et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 2011; 25: 1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Attie KM, Borgstein NG, Yang Y. et al. A single ascending‐dose study of muscle regulator ACE‐031 in healthy volunteers. Muscle Nerve 2013; 47: 416–423 [DOI] [PubMed] [Google Scholar]
- 141. Becker C, Lord SR, Studenski SA. et al. Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol 2015; 3: 948–957 [DOI] [PubMed] [Google Scholar]
- 142. Padhi D, Higano CS, Shore ND. et al. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrin Metab 2014; 99: E1967–E1975 [DOI] [PubMed] [Google Scholar]
- 143. Campbell C, McMillan HJ, Mah JK. et al. Myostatin inhibitor ACE‐031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo‐controlled clinical trial. Muscle Nerve 2017; 55: 458–464 [DOI] [PubMed] [Google Scholar]
- 144. Shamim B, Hawley JA, Camera DM.. Protein availability and satellite cell dynamics in skeletal muscle. Sports Med 2018; 48: 1329–1343 [DOI] [PubMed] [Google Scholar]
- 145. Zha Y, Qian Q.. Protein nutrition and malnutrition in CKD and ESRD. Nutrients 2017; 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Alp Ikizler T, Cano NJ, Franch H. et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013; 84: 1096–1107 [DOI] [PubMed] [Google Scholar]
- 147. Castaneda C, Gordon PL, Parker RC. et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis 2004; 43: 607–616 [DOI] [PubMed] [Google Scholar]
- 148. Janssen I, Heymsfield SB, Wang ZM. et al. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol 2000; 89: 81–88 [DOI] [PubMed] [Google Scholar]
- 149. Verdijk LB, Snijders T, Drost M. et al. Satellite cells in human skeletal muscle; from birth to old age. Age 2014; 36: 545–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Verdijk LB, Koopman R, Schaart G. et al. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 2007; 292: E151–E157 [DOI] [PubMed] [Google Scholar]
- 151. Suetta C, Frandsen U, Mackey AL. et al. Ageing is associated with diminished muscle re‐growth and myogenic precursor cell expansion early after immobility‐induced atrophy in human skeletal muscle. J Physiol 2013; 591: 3789–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Snijders T, Verdijk LB, Smeets JSJ. et al. The skeletal muscle satellite cell response to a single bout of resistance-type exercise is delayed with aging in men. Age 2014; 36: 9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kouidi E, Albani M, Natsis K. et al. The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol Dial Transplant 1998; 13: 685–699 [DOI] [PubMed] [Google Scholar]
- 154. Zelle DM, Klaassen G, van Adrichem E. et al. Physical inactivity: a risk factor and target for intervention in renal care. Nat Rev Nephrol 2017; 13: 152–168 [DOI] [PubMed] [Google Scholar]
- 155. Wilkinson TJ, Shur NF, Smith AC.. “Exercise as medicine” in chronic kidney disease. Scand J Med Sci Sports 2016; 26: 985–988 [DOI] [PubMed] [Google Scholar]
- 156. Cheema BS, Chan D, Fahey P. et al. Effect of progressive resistance training on measures of skeletal muscle hypertrophy, muscular strength and health-related quality of life in patients with chronic kidney disease: a systematic review and meta-analysis. Sports Med 2014; 44: 1125–1138 [DOI] [PubMed] [Google Scholar]