ABSTRACT
Incremental haemodialysis has the potential to allow better preservation of renal function, is less invasive to the patient and has lower cost. Despite these advantages, it is not commonly applied. This may be due to uncertainty about how to account for renal function in the prescription of dialysis and measurement of dose.
In this issue, Vartia describes the practical basis for including the effect of renal function in the prescription and quantification of dialysis. He uses a well-known and validated urea kinetic model to calculate time average urea concentrations and the equivalent renal clearance (EKR) from dialysis. The effect of renal function is amplified by a weighting factor to account for the relatively greater effect of renal function compared with dialysis with the same urea clearance. In that way, patients on differing dialysis regimens can be dialysed with the same target dose.
A further step would be to use a downward adjusting factor for dialysis to convert the urea clearance by dialysis (as EKR) to a glomerular filtration rate (GFR) equivalent. A factor of 0.75 is suggested. In that way, dialysis dose can be reported as GFR equivalent in mL/min/1.73 m2, comparable between different types of dialysis and also to renal function without dialysis.
Keywords: GFR, incremental dialysis, renal function
Incremental dialysis: Despite increasing evidence to support its use, incremental haemodialysis is not universally applied [1]. If the potential benefits of incremental dialysis are as described in recent publications [2], then starting dialysis at a full dose will be subjecting patients to unnecessarily long or more frequent treatments at higher cost. In addition, full-dose haemodialysis may hasten the loss of residual renal function [3], putting the patient at further disadvantage [4]. Patients often start dialysis without a proven renal diagnosis [5] and it is not unreasonable to suppose that some of these patients may have an acute or reversible component to their kidney disease. If this is the case, then starting dialysis at a full dose and not monitoring residual renal function may be depriving the patient of the chance of renal recovery sufficient to stop dialysis [6], thereby unnecessarily trapping them on long-term dialysis.
Incremental dialysis has been the standard of care in peritoneal dialysis for many years [7]. Measurements of residual renal function are made regularly and are considered more important than dialysis dose [8]. Why has this not been adopted in haemodialysis?
One reason for the patchy adoption of incremental haemodialysis is that incremental dialysis requires a dynamic individualized dialysis prescription, taking renal function into account. A robust system for monitoring residual renal function is required. The quality of dialysis is usually assessed from measurements of dialysis dose [e.g. dialysis efficiency (Kt/V), urea reduction ratio (URR)], and there are no clear standards for including residual renal function in the dose assessment or prescription. Patients receiving incremental dialysis will have a lower dialysis dose (as measured by Kt/V, URR), frequency or treatment time than recommended in current guidelines and the dialysis provider may think that they will be penalized as a result.
CONVERTING CLEARANCE OF INTERMITTENT HAEMODIALYSIS TO A CONTINUOUS EQUIVALENT COMPARABLE WITH RENAL FUNCTION
URR and Kt/V only account for clearance during a single dialysis session. They ignore residual renal function, dialysis frequency and spacing. They are influenced by treatment duration, so these measures are only directly comparable between patients or universal targets when treatment frequency, spacing and duration are standardized (e.g. three times weekly for 4 h). The dialysis dose assessed by URR or Kt/V is not comparable to continuous clearance by the kidneys.
In contrast, clearance in peritoneal dialysis is almost continuous. It can be measured by dialysis effluent collection and added to that from urine collections. In that way, a combined renal + dialysis clearance can easily be calculated.
Three methods for calculating clearance in haemodialysis in a way that is comparable to continuous renal function (and to continuous dialysis such continuous ambulatory peritoneal dialysis) have been proposed. All three are clearance calculations in the form of the rate of the mass of urea removed (equal to the mass generated in steady state) divided by the blood urea concentration. They all have implicit units of volume/time. They differ only on the interpretation of concentration in blood and on the method of normalizing for body size. The first of these to be proposed was the solute removal index (SRI) [9], and this interpreted blood concentration as the highest (peak) urea level in the weekly cycle. Body water volume (V) was used to normalize for size, so the units were per week. The standard Kt/V (stdKt/V) is calculated in the same way as the SRI but uses the average predialysis urea concentration instead of the peak [10]. Equivalent renal clearance (EKR) uses time average urea concentration as the blood concentration and can be expressed in more familiar units of mL/min [11]. EKR can be normalized for body size using body surface area or V. There is now evidence that it would be better to normalize clearance to body surface area rather than V [12]. Toxicity is more likely to be proportional to the time average concentration than to the peak [13]. In that case, EKR normalized to surface area in units of mL/min/1.73 m2 would be more logical and has the additional benefit of being consistent with GFR reporting.
Each of these three methods can add the mass of urea removed by the kidneys to the mass removed by dialysis to calculate a combined clearance. They allow different renal replacement modalities and schedules to be compared directly. The article by Vartia [14] in this issue provides a rational basis for achieving this. Vartia’s solution has extended an online resource developed by Daugirdas [15].
ADJUSTING RENAL UREA CLEARANCE TO BETTER REFLECT ITS RELATIVE EFFECT ON THE PATIENT COMPARED WITH DIALYSIS
One problem with SRI, stdKt/V and EKR is that they count renal function quantified by urea clearance as equal to urea clearance by dialysis. This underestimates the relative contribution of renal function when compared with dialysis. Renal urea clearance underestimates GFR, and kidney function provides benefits to the patient other than clearance.
Casino and Basile [16] proposed a variable target to correct for this. According to this proposal, the target for the total (dialysis + renal) stdKt/V and EKR could be 1.7/week and 6 mL/min/1.73 m2 when residual renal urea clearance is 6 mL/min. As renal function decreases, target stdKt/V and EKR should rise progressively to 2.3/week and 12 mL/min/1.73 m2 when the patient is anuric.
Instead, Vartia [14] proposes an adjustment factor to give more weight to the renal function when compared with dialysis. Using Vartia’s method, the renal function component of stdKt/V or EKR is multiplied by an adjustment factor, so higher values are calculated when there is significant renal function. In this way, the target could be an stdKt/V of 1.7/week or EKR of 6 mL/min/1.73 m2 for all levels of renal function. Both Vartia’s and Casino and Basile’s [16] methods have the same result on solute levels and clearances.
An adjustment factor of 2 has been suggested for renal urea clearance [17], implying that, compared with haemodialysis with the same urea clearance, renal function is twice as effective in controlling uraemia. There is some rational basis for this. Urea is reabsorbed in the renal tubules, so urea clearance underestimates GFR. GFR is approximately urea clearance multiplied by 1.5. On the other hand, urea diffuses most rapidly of the dialysable uraemic toxins, so urea clearance by dialysis overestimates the clearance of other dialysable solutes. The average clearance by dialysis of a range of dialysable solutes would be around 0.75 times the urea clearance. Taking all this into account, the kidneys would be about twice as effective at clearing a range of dialysable solutes compared with dialysis with the same urea clearance. Of course, the kidneys provide other benefits to the patient besides clearing small and middle-sized solutes. But the loss of these cannot be compensated for by increasing the dialysis dose, so are not taken into account for incremental dialysis.
The minimum GFR required to avoid uraemic symptoms without dialysis is variable between patients, but in the region of 6–10 mL/min/1.73 m2. Renal urea clearance (and EKR) would be 4–7.5 mL/min/1.73 m2 at that point. There is evidence from a randomized controlled trial (RCT) that starting dialysis when symptoms develop is not harmful [18]. Current guidelines recommend the minimum dose of dialysis in anuric patients to be a single-pool Kt/V of 1.2. This is the minimum recommended dose required to control uraemic symptoms and avoid long-term complications. Perhaps, more importantly, an RCT demonstrated no improved outcome by providing a higher dose, despite increased treatment burden for the patient [19]. Thrice weekly haemodialysis in an anuric patient, delivered over 4 h as recommended, is equal to an EKR of 11.5 mL/min/1.73 m2, approximately double the urea clearance of failing kidneys at a point just before symptoms develop or dialysis starts.
DIALYSIS DOSE EXPRESSED AS THE GFR EQUIVALENT
Building on these proposals, and in full agreement with them, it seems that a logical next step would be to quantify dialysis as a GFR equivalent, adjusted to surface area. In this way, the different dialysis modalities and regimens could be compared not only to each other, but also to renal function without dialysis.
This would involve adjustment factors for both the urea clearance from dialysis and residual renal function. The dialysis adjustment would be <1 and the renal adjustment >1. In this way, the GFR equivalent should, ideally, be maintained at a level similar to the GFR before the patient started dialysis and before they developed uraemic symptoms (in the range of 6–10 mL/min/1.73 m2 for most patients). Values for the renal and dialysis adjustment factors of 1.5 and 0.75, respectively, are suggested by existing evidence, physics and guidelines. The renal adjustment factor is required only to convert renal urea clearance to GFR equivalent. If renal function is measured as GFR (e.g. mean of urea and creatinine clearance), then the renal adjustment is not needed.
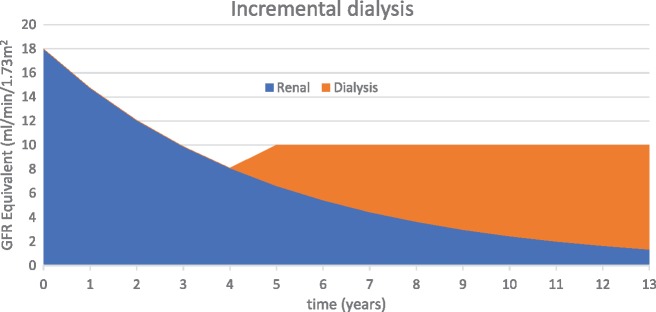
Figure 1 and Table 1 demonstrate how incremental dialysis could be prescribed and quantified using GFR equivalent. This approach is limited to solute clearance and prioritizes a reduction in treatment burden on the patient. Increasing treatment time and/or frequency may be preferred over other options as a way to control fluid overload or the accumulation of poorly dialysable solutes such as phosphate.
FIGURE 1.
Incremental dialysis to maintain GFR equivalent to 10 mL/min. The patient develops uraemic symptoms at year 4 when GFR decreases to <8 mL/min/1.73 m2. At that point, low-dose dialysis is started to boost GFR equivalent to 10 mL/min and the patient becomes asymptomatic. Thereafter, dialysis dose is regularly increased to maintain GFR equivalent at this level as renal function decreases.
Table 1.
Example of incremental dialysis to maintain GFR equivalent at 10 mL/min/1.73 m2
| Renal function (GFR; mL/min/1.73 m2) |
Dialysis prescription |
Total dose HD + renal |
||||
|---|---|---|---|---|---|---|
| Per session |
Per week | Per week | mL/min/1.73 m2 |
|||
| Session length | spKt/V | frequency | stdKt/V | EKR | GFR equivalent | |
| 10 | 00: 00 | 0 | 0 | 1.89 | 7.5 | 10 |
| 9 | 00: 00 | 0 | 0 | 1.7 | 6.7 | 9 |
| 8 | 02: 30 | 0.95 | 1 | 1.75 | 8.8 | 10 |
| 7 | 04: 00 | 1.52 | 1 | 1.67 | 9.2 | 10 |
| 6 | 02: 30 | 0.95 | 2 | 1.94 | 10.1 | 10 |
| 5 | 03: 00 | 1.14 | 2 | 1.9 | 10.4 | 10 |
| 4 | 04: 00 | 1.52 | 2 | 1.93 | 11.2 | 10 |
| 3 | 03: 00 | 1.14 | 3 | 2.17 | 11.8 | 10 |
| 2 | 03: 15 | 1.24 | 3 | 2.22 | 12.6 | 10 |
| 1 | 03: 30 | 1.33 | 3 | 2.21 | 12.9 | 10 |
| 0 | 04: 00 | 1.52 | 3 | 2.24 | 13.6 | 10 |
Due to the adjustment factors, anuric patients receive higher stdKt/V and EKR. HD, haemodialysis; spKt/V, single-pool Kt/V (per session).
Using a fixed target for GFR equivalent of 10 mL/min/1.73 m2 and renal and dialysis adjustment factors of 1.5 and 0.75, respectively, the same values for stdKt/V and EKR are calculated as proposed by Casino and Basile’s variable target model [16] (Table 1).
In peritoneal dialysis, residual renal function is usually quantified as the mean of urea and creatinine clearance, in effect already adjusting it to a GFR equivalent. Current guidelines suggest a minimum Kt/V of 1.7/week. Without dialysis, this would require renal function at a GFR of 9.7 mL/min/1.73 m2 [20]. For anuric patients, guidelines recommend a minimum Kt/V of 1.7/week but suggest that higher doses may be required. In common practice, V is calculated using the Watson equations in peritoneal dialysis. This method is known to overestimate V by about 20% in dialysis patients [21]. Taking this into account, a Kt/V of 1.7/week equals an EKR of 8.5 mL/min/1.73 m2. Assuming the same downward adjustment for peritoneal dialysis as with haemodialysis, the minimum recommended dose for peritoneal dialysis would be equivalent to a GFR of 6 mL/min/1.73 m2. This seems rather low, but just within the range where dialysis would normally be started and at the lower end of that recommended for haemodialysis. The majority of anuric patients treated by peritoneal dialysis have a higher dose of dialysis than this [22]. Thus the same adjustment factors could be used to calculate a GFR equivalent for both peritoneal dialysis and haemodialysis. Using GFR equivalent to prescribe and quantify peritoneal dialysis would be consistent with the current practice and guidelines.
GFR equivalent could quantify all forms of dialysis in the same way as we already do for quantifying kidney function in patients who are not on dialysis. This would simplify reporting, as the units and ranges are already familiar to patients and clinicians. This method of quantifying dialysis can be standardized with reference to the online calculators. GFR equivalent may be particularly useful in dialysis for acute kidney injury (or chronic kidney disease with an acute or reversible component), as the point where renal function has recovered sufficiently to stop dialysis would be more easily identified.
CONFLICT OF INTEREST STATEMENT
The author was not funded for the preparation of this manuscript. This manuscript has not been published previously or submitted to any other journal. The author has no conflicts of interest related to this manuscript.
REFERENCES
- 1. Mathew AT, Obi Y, Rhee CM. et al. Incremental dialysis for preserving residual kidney function–does one size fit all when initiating dialysis? Semin Dial 2018;31:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong J, Vilar E, Davenport A. et al. Incremental haemodialysis. Nephrol Dial Transplant 2015;30:1639–1648 [DOI] [PubMed] [Google Scholar]
- 3. Liu Y, Zou W, Wu J. et al. Comparison between incremental and thrice-weekly hemodialysis: a systematic review and meta-analysis. Nephrology (Carlton) 2018. Mar 13. 10.1111/nep.13252 [DOI] [PubMed] [Google Scholar]
- 4. Termorshuizen F, Dekker FW, van Manen JG. et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004;15:1061–1070 [DOI] [PubMed] [Google Scholar]
- 5. Gilg J, Methven S, Casula A. et al. UK Renal Registry 19th Annual Report: chapter 1 UK RRT adult incidence in 2015: national and centre-specific analyses. Nephron 2017;13(Suppl 1):11–44 [DOI] [PubMed] [Google Scholar]
- 6. Fernández-Lucas M, Teruel-Briones JL, Gomis A. et al. Recovery of renal function in patients receiving haemodialysis treatment. Nefrologia 2012;32:166–171 [DOI] [PubMed] [Google Scholar]
- 7. Woodrow G, Fan SL, Reid C. et al. Renal Association clinical practice guideline on peritoneal dialysis in adults and children. BMC Nephrol 2017;18:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perl J, Bargman JM.. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis 2009;53:1068–1081 [DOI] [PubMed] [Google Scholar]
- 9. Keshaviah P. The solute removal index–a unified basis for comparing disparate therapies. Perit Dial Int 1995;15:101–104 [PubMed] [Google Scholar]
- 10. Gotch FA. Modeling the dose of home dialysis. Home Hemodial Int 1999;3:37–40 [DOI] [PubMed] [Google Scholar]
- 11. Casino FG, Lopez T.. The equivalent renal urea clearance: a new parameter to assess dialysis dose. Nephrol Dial Transplant 1996;11:1574–1581 [PubMed] [Google Scholar]
- 12. Ramirez SP, Kapke A, Port FK. et al. Dialysis dose scaled to body surface area and size-adjusted, sex-specific patient mortality. Clin J Am Soc Nephrol 2012;7:1977–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tennekes HA, Sánchez-Bayo F.. The molecular basis of simple relationships between exposure concentration and toxic effects with time. Toxicology 2013;309:39–51 [DOI] [PubMed] [Google Scholar]
- 14. Vartia A. Residual renal function in incremental haemodialysis. Nephrol Dial Transplant 2018;11:857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daugirdas JT. Solute solver ‘what if’ module for modeling urea kinetics. Nephrol Dial Transplant 2016;31:1934–1937 [DOI] [PubMed] [Google Scholar]
- 16. Casino FG, Basile C.. The variable target model: a paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant 2017;32:182–190 [DOI] [PubMed] [Google Scholar]
- 17. Basile C, Casino FG, Kalantar-Zadeh K.. Is incremental hemodialysis ready to return on the scene? From empiricism to kinetic modelling. J Nephrol 2017;30:521–529 [DOI] [PubMed] [Google Scholar]
- 18. Cooper BA, Branley P, Bulfone L. et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609–619 [DOI] [PubMed] [Google Scholar]
- 19. Eknoyan G, Beck GJ, Cheung AK. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002;347:2010–2019 [DOI] [PubMed] [Google Scholar]
- 20. Guest S, Akonur A, Ghaffari A. et al. Intermittent peritoneal dialysis: urea kinetic modeling and implications of residual kidney function. Perit Dial Int 2012; 32:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindley EJ, Chamney PW, Wuepper A. et al. A comparison of methods for determining urea distribution volume for routine use in on-line monitoring of haemodialysis adequacy. Nephrol Dial Transplant 2009;24:211–216 [DOI] [PubMed] [Google Scholar]
- 22. Jansen MA, Termorshuizen F, Korevaar JC. et al. Predictors of survival in anuric peritoneal dialysis patients. Kidney Int 2005; 68:1199–1205 [DOI] [PubMed] [Google Scholar]