ABSTRACT
Background
Renal patients with diabetes mellitus are at very high risk of death before and after chronic dialysis initiation. Risk factors for death in this population are not clearly identified.
Methods
We performed a retrospective survival analysis in 861 patients with diabetes mellitus consecutively followed up in the 2000–13 period in a nephrology setting.
Results
The mean age was 70 ± 10 years [men 65.2%; diabetes duration 13.7 ± 10.3 years; mean estimated glomerular filtration rate (eGFR) 42.4 ± 21.0 mL/min/1.73 m2). During follow-up (median 60 months; up 15 years), 263 patients died (184 before and 79 after dialysis initiation) and 183 started chronic dialysis. In multivariate analyses, age, elevated systolic and low diastolic arterial pressures, peripheral artery disease, cancer, loop diuretic use and atrial fibrillation at baseline and acute kidney injury (AKI), heart failure (HF) and amputation during follow-up were identified as risk factors for death. After adjustments on these parameters, eGFRs at the time of the first outpatient visit—eGFR <45 mL/min/1.73 m2 {hazard ratio [HR] 1.58 [95% confidence interval (CI) 1.15–2.17]}, P = 0.005 and eGFR <30 [HR 1.53 (1.05–2.05)], P = 0.004, but not eGFR <60—were powerful risk factors for death. When initiation of dialysis was entered into the multivariate models, it was not associated with a risk of premature death [HR 1.19 (95% CI 0.91–1.55), P = 0.2069], even in patients >80 years of age [HR 1.08 (95% CI 0.64–1.81), P = 0.7793].
Conclusions
In patients with diabetes mellitus, high systolic and low diastolic arterial pressure, peripheral artery disease and development of AKI and HF are significant risk factors for death. In addition to these parameters, eGFR <45 mL/min/1.73 m2 at the time of referral is also a powerful risk factor for death.
Keywords: diabetes, dialysis, epidemiology, renal function, risk of death
INTRODUCTION
In 2014, at least 387 million people worldwide were living with diabetes [1], and nearly 600 million people are expected to be diabetic in 2035 [2]. Diabetes increases the risk of premature death and reduces life expectancy by nearly 15 years [3]. The risk of death in patients with diabetes mellitus is markedly increased when renal function is impaired, and even more in end-stage renal disease (ESRD) and dialysis [4, 5].
Optimal management of these renal patients with diabetes mellitus poses numerous problems, including the optimal time to nephrology referral and the need for dialysis (especially when renal transplantation is not feasible). Some studies indicate that late referral to nephrologists is associated with reduced survival [6]. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines suggest referring patients with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, macrolbuminuria or rapid renal function decline (RRFD, i.e. eGFR decrease >5 mL/min/year) to nephrologists [7]. However, no specific guideline applies to the diabetic population. Whether earlier referral is justified in patients with diabetes mellitus and chronic kidney disease (CKD) is unknown, as the progression of renal disease may be slow. After referral, nephrologists face difficulties in the management of these elderly patients with diabetes mellitus, as survival of these patients may be reduced after initiation of dialysis [8]. Some studies advocate that a conservative approach should be discussed in elderly patients, especially those with diabetes [9]. With this in mind, it may be difficult for clinicians to refer these patients earlier to nephrologists as they feel that the patients may die before they need dialysis, that the duration of follow-up before reaching ESRD is uncertain and may be extremely long in some patients and that chronic dialysis may precipitate death [9]. However, referral to nephrologists is not only useful to delay ESRD, but it is important to optimize management of CKD patients and therefore improve patient survival before and after dialysis initiation. The late referral issue is important; however, at the time of referral, it is not usually possible to know precisely the duration of follow-up before dialysis or transplantation becomes necessary. From a practical point of view, the issue of the GFR cut-off value associated with a risk of deleterious outcome is also important. The two issues are of course interconnected. However, it is easier for clinicians to identify GFR values than a time to dialysis, which remains elusive in most cases. Earlier referral than the one indicated in the KDIGO guidelines may appear useful.
Finally, the right timing for referral is unclear despite the KDIGO guidelines and the benefit of dialysis is unproven in many instances in elderly CKD patients with diabetes mellitus. In this population, whether earlier referral is justified is unknown and whether dialysis initiation is detrimental is unclear.
In the present retrospective study, we estimated whether dialysis initiation and the eGFR value of patients at the time of referral are associated with risk factors for death in a large cohort of renal patients with diabetes mellitus followed up to 15 years.
MATERIALS AND METHODS
Study population
In the present study we retrospectively analysed 861 consecutive patients with type 2 diabetes who were referred as outpatients to nephrologists in our hospital during the 2000–13 period (Centre Hospitalier Universitaire, Hôpital Bretonneau, Tours, France). The study was approved by the Ethics Committee of Human Research of our hospital (approval number 2016-21).
Type 2 diabetes was defined according to the American Diabetes Association (ADA) criteria [10]. Data were retrieved from files at baseline and during follow-up. They included age, gender, comorbid conditions and diabetes duration.
At baseline, systolic and diastolic blood pressures and body mass index (BMI) were measured and information regarding the use of antihypertensive drugs [including renin–angiotensin system (RAS) blockers], glucose-lowering agents, lipid-lowering agents and antiplatelet medications were collected.
Baseline laboratory results (including serum creatinine and glycated haemoglobin) were obtained. Baseline albuminuria was defined based on urine dipstick or by 24-h urine protein; proteinuria was converted in albuminuria as described previously [11]. GFR was estimated using the Modification of Diet in Renal Disease (MDRD) equation [12].
Follow-up and outcomes
All files were individually (manually) reviewed. Acute kidney injury (AKI) in hospitalization during follow-up was diagnosed using the KDIGO criteria [7]. Only serum creatinine criteria were used to diagnose AKI, thus, urinary output criteria were omitted. Baseline serum creatinine was considered the lowest creatinine value (i.e. the reference creatinine value). We identified AKI by comparing the highest creatinine value found during hospitalization with the reference serum creatinine value. AKI was defined as a serum creatinine level of 150% or +0.3 mg/dL (+26.5 µmol/L) versus the reference serum creatinine level [13].
Stroke was defined in patients as focal neurological abnormalities associated with ischaemic or haemorrhagic tissular lesions found on computed tomography scan and/or magnetic resonance imaging. Amputation was defined as a lower limb amputation above the metatarsophalangeal joint. We also recorded revascularization of coronary arteries (angioplasty, coronary artery bypass grafting or coronary stent) and revascularization of peripheral arteries (angioplasty or bypass of aortic or lower limb arteries).
Information regarding major cardiovascular events such as acute coronary syndrome (ACS), atrial fibrillation (AF) or hospitalization for heart failure (HF) as well as infections requiring hospitalization and cancer during follow-up was recorded.
Patients were followed until death or the end of the study period (15 November 2015). The main outcome of the study was all-cause death (whether or not death occurred in patients who started chronic dialysis). Deaths were identified using the national death register and medical records from our centre or primary care physicians.
Statistical analyses
Results are presented as mean (standard deviation (SD)) for continuous variables [or median and interquartile range (IQR) if the distribution of the variable was skewed] and as percentages for categorical variables.
We performed univariate and multivariate Cox models to examine the association between baseline characteristics and risk of death. Analyses were performed in the whole population and sensitivity analyses were performed in subgroups of patients with advanced age (age ≥70, ≥75 and ≥80 years) because it was suggested in the literature that the risk of death associated with dialysis initiation was greater in older patients. We examined whether age at dialysis was a risk factor for death in patients who started dialysis. We also performed univariate and multivariate analyses to evaluate whether major events (cardiovascular events, stroke, hospitalization for infection, AKI) during follow-up were risk factors for death; these events were considered as time-dependent parameters. We only considered the first event even when it occurred several times during follow-up. A stepwise descending procedure was used to determine every final multivariate model (all conventional variables were included in the models: all univariate significant variables were included in a maximized multivariate model, then we determined an optimized model with a backward procedure). Kaplan–Meier curves were constructed and compared using a log-rank test. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). A P-value <0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
The mean age was 70.3 ± 10.0 years and most patients were men (65.2%). Overall, eGFR was 42.4 ± 21.0 mL/min/1.73 m2 (Table 1). Of note, 334 (44.3%) patients had a history of major cardiovascular disease. Albuminuria was present in 98.1% of patients and macroalbuminuria was present in 49% of patients. Among the 556 patients with eGFR <60 mL/min/1.73 m2, normoalbuminuria, microalbuminuria and macroalbuminuria were present in 22.1, 29.7 and 48.2% of patients, respectively.
Table 1.
Baseline characteristics
| Clinical characteristics (n = 861) | |
| Age (years) | 70.3 ± 10.0 |
| Sex (male) | 65.2 |
| BMI (kg/m2) | 30.7 ± 5.9 |
| Systolic/diastolic arterial pressure (mmHg) | 149 ± 23/78 ± 12 |
| Diabetes duration (years) | 13.7 ± 10.3 |
| Main reason for first outpatient visit in nephrology ward | |
| CKD | 54.3 |
| Albuminuria | 15.6 |
| Hypertension | 3.4 |
| Other | 26.7 |
| Comorbid conditions | |
| Hypertension | 93.5 |
| Coronary artery disease | 26.6 |
| HF | 20.4 |
| Peripheral artery disease | 19.2 |
| AF | 20.1 |
| Stroke | 7.0 |
| Renal artery stenosis | 3.4 |
| Smoking (active/former) | 8.2/33.1 |
| Diabetic retinopathy | 24.0 |
| Biological data | |
| Serum creatinine (µmol/L) | 176 ± 124 |
| eGFR (mL/min/1.73 m2) | 42.4 ± 21.0 |
| CKD stage | |
| 1 or 2 | 15.6 |
| 3a | 19.7 |
| 3b | 35.7 |
| 4 | 22.8 |
| 5 | 6.2 |
| Albuminuria (mg/day or mg/g of urine creatinine) | 955 ± 1794 |
| Normoalbuminuria | 21.4 |
| Microalbuminuria | 29.6 |
| Macroalbuminuria | 49.0 |
| Haemoglobin A1c | 7.25 ± 1.5 |
| Antihypertensive therapy | |
| ACE inhibitor/ARB/both | 33.5/42.5/3.7 |
| Calcium-channel blocker | 50.4 |
| Loop diuretic | 39.0 |
| Thiazide | 23.5 |
| Beta-blocker | 46.0 |
| Spironolactone | 3.5 |
| Others | 22.1 |
| Glucose-lowering therapy | |
| Insulin | 45.5 |
| Oral agents | 53.0 |
| Diet only | 7.5 |
| Other treatments | |
| Statin | 57.7 |
| Fibrate | 8.8 |
| Antiplatelet drug | 51.0 |
Results are presented as percentage or mean ± SD. Albuminuria: values after conversion of proteinuria in albuminuria when only proteinuria was available [11].
Albuminuria classes were defined as normoalbuminuria: albuminuria <30 mg/day, <30 mg/g or <20 mg/L; microalbuminuria: albuminuria ≥30 and <300 mg/day; ≥30 and <300 mg/g or ≥20 and <200 mg/L; macroalbuminuria: albuminuria ≥300 mg/day, ≥300 mg/g or ≥200 mg/L.
CKD Stages: 1–2: eGFR ≥60 mL/min/1.73 m2; 3a: 45–59.9; 3b: 30–44.9; 4: 15–29.9; 5: <15.
Despite a significant proportion of patients with CKD, only 207 (24%) had diabetic retinopathy. Angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker (ARB) were used in the most patients, and half of the patients received insulin (Table 1).
Baseline and follow-up parameters associated with the risk of death
Patients were followed up for a median duration of 60 months (IQR 39–78 months, range 4.5–193 months, total observation time 6113 patient-years). During this period, 263 patients died (including 79 after dialysis); the 5- and 10-year risks of death were 24.2 and 44.6%, respectively. Of note, 183 patients started chronic dialysis, whereas 13 patients had a conservative treatment [mostly due to comorbid conditions (61.5%) or the patient’s choice (31.0%)] during follow-up.
In univariate and multivariate analyses, baseline parameters associated with the risk of death included age, diastolic arterial pressure, peripheral artery disease, cancer, use of loop diuretics and AF (Table 2). Other parameters such as AKI were significantly associated with the risk of death in univariate and multivariate analyses (Table 2).
Table 2.
Baseline and follow-up parameters associated with the risk of death during follow-up
| Univariate analysis |
Stepwise multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Parameters | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Baseline | ||||||
| Gender (men versus women) | 1.09 | 0.84–1.42 | 0.5114 | – | ||
| Age (per 10 years) | 2.16 | 1.85–2.52 | <0.0001 | 1.94 | 1.64–2.29 | <0.0001 |
| Systolic arterial pressure (per +10 mmHg) | 0.97 | 0.92–1.03 | 0.2724 | – | ||
| Diastolic arterial pressure (per +10 mmHg) | 0.76 | 0.68–0.85 | <0.0001 | 0.88 | 0.79–0.99 | 0.0127 |
| BMI (kg/m2) | 1.00 | 0.99–1.00 | 0.4494 | – | ||
| Haemoglobin (per +1 g/L) | 0.99 | 0.98–0.99 | 0.0003 | – | ||
| Smoking (ever versus no smoking) | 1.14 | 0.89–1.47 | 0.2955 | – | ||
| Diabetic retinopathy | 1.05 | 0.81–1.38 | 0.7106 | – | ||
| Coronary artery disease | 1.63 | 1.27–2.10 | 0.0001 | – | ||
| Peripheral vascular disease | 1.99 | 1.53–2.58 | <0.0001 | 1.84 | 1.39–2.42 | <0.0001 |
| Lower limb amputation | 1.89 | 1.06–3.37 | 0.0319 | – | ||
| Hospitalization for HF | 2.43 | 1.88–3.15 | <0.0001 | – | ||
| Cancer | 1.74 | 1.26–2.40 | 0.0009 | 1.84 | 1.31–2.57 | 0.0004 |
| Loop diuretic use | 2.03 | 1.59–2.59 | <0.0001 | 1.75 | 1.34–2.27 | <0.0001 |
| AF | 1.83 | 1.83–3.14 | <0.0001 | 1.68 | 1.25–2.26 | 0.0006 |
| Glycosylated haemoglobin (per +1%) | 0.99 | 0.90–1.09 | 0.8040 | – | ||
| RAS blockers | 0.74 | 0.57–0.96 | 0.0245 | – | ||
| Follow–up | ||||||
| AKI | 3.25 | 2.53–4.18 | <0.0001 | 2.12 | 1.58–2.85 | <0.0001 |
| AF | 0.99 | 0.70–1.40 | 0.9513 | – | ||
| Acute coronary syndrome | 1.70 | 1.16–2.48 | 0.0062 | – | ||
| HF | 3.63 | 2.83–4.66 | <0.0001 | 2.44 | 1.83–3.27 | <0.0001 |
| Stroke | 1.53 | 1.01–2.31 | 0.0449 | – | ||
| Lower limb amputation | 2.35 | 1.31–4.20 | 0.0040 | 1.93 | 1.08–3.45 | 0.0275 |
| Hospitalization for serious infection | 3.64 | 0.51–26.1 | 0.1985 | – | ||
Initiation of chronic dialysis, age and risk of death
In this analysis we assessed whether initiation of dialysis could accelerate the risk of death.
In univariate analysis, dialysis was not associated with the risk of death (Table 3). The mean age at dialysis was 73.5 ± 9.2 years in the 183 patients who initiated chronic dialysis (Table 3). Similar results were observed when the analysis was restricted to older patients (≥70, ≥75 or ≥80 years at baseline); for these age groups, the mean age at dialysis was 77.1 ± 4.6, 79.8 ± 3.5 and 83.1 ± 2.8 years, respectively (Table 3).
Table 3.
Chronic dialysis initiation and risk of death during follow-up
| Information regarding patients who died during follow-up |
Information regarding patients who started chronic dialysis during follow-up |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | Number of patients who died (before/after dialysis initiation) | Age at baseline (years) | Age at death (years) | Number of patients who started dialysis | Age at baseline (years) | Age at dialysis initiation (years) | |
| Risk of death associated with chronic dialysis initiation (univariate analysis) | |||||||||
| All patients (n = 861) | 1.19 | 0.91–1.55 | 0.2069 | 263 (184/79) | 74.4 ± 8.8 | 79.4 ± 8.5 | 183 | 70.4 ± 8.9 | 73.5 ± 9.2 |
| Sensitivity analyses (subgroup analyses) | |||||||||
| Age ≥70 years (n = 474) | 1.13 | 0.82–1.56 | 0.4621 | 187 (135/52) | 78.7 ± 5.3 | 83.3 ± 5.6 | 101 | 77.1 ± 4.6 | 79.9 ± 5.0 |
| Age ≥75 years (n = 314) | 1.07 | 0.73–1.58 | 0.7304 | 135 (99/36) | 81.2 ± 4.1 | 85.7 ± 4.3 | 64 | 79.8 ± 3.5 | 82.6 ± 4.1 |
| Age ≥80 years (n = 139) | 1.08 | 0.64–1.81 | 0.7793 | 74 (54/20) | 84.1 ± 3.1 | 88.1 ± 3.3 | 27 | 83.1 ± 2.8 | 85.2 ± 3.3 |
| Risk of death associated with chronic dialysis initiation (multivariate analysis) | |||||||||
| Model 1 | 1.19 | 0.89–1.58 | 0.2443 | ||||||
| Model 2 | 1.40 | 0.42–4.71 | 0.5839 | ||||||
| Model 3 | 1.69 | 0.47–6.13 | 0.4228 | ||||||
Age is presented in mean ± SD.
Baseline parameters: age, diastolic arterial pressure, peripheral vascular disease, cancer, use of loop diuretics and AF.
Follow-up parameters: AKI, HF and amputation during follow-up.
Adjustments on baseline parameters (Model 1), follow-up parameters (Model 2) or both (Model 3).
In multivariate analyses, there was no significant association between initiation of dialysis and the risk of death after adjustments on baseline and/or follow-up risk factors for death (Table 3).
Among patients who started dialysis, age at dialysis was significantly associated with the risk of premature death in univariate and multivariate analyses [univariate: HR per +10 years 1.36 (95% CI 1.05–1.77), P = 0.0212; multivariate: 1.50 (1.10–2.05), P = 0.0104].
Risk of death according to renal function value or decline at the time of referral
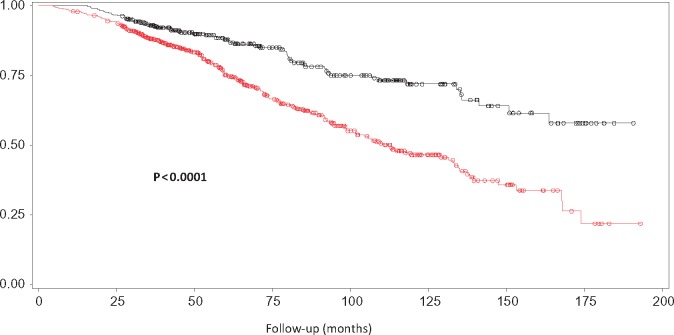
In univariate analyses, eGFR <30 mL/min/1.73 m2 (versus eGFR ≥30) at the time of referral was significantly associated with the risk of death (Table 4). However, eGFR <60 mL/min/1.73 m2 (versus eGFR ≥60) and <45 mL/min/1.73 m2 (versus eGFR ≥45) were also significant (Table 4 and Figure 1).
Table 4.
eGFR at the time of referral as a risk of death
| HR | 95% CI | P-value | |
|---|---|---|---|
| Univariate analysis | |||
| Baseline eGFR <60 (versus ≥60) | 2.88 | 1.78–4.64 | <0.0001 |
| Baseline eGFR <45 (versus ≥45) | 2.18 | 1.63–2.91 | <0.0001 |
| Baseline eGFR <30 (versus ≥30) | 1.84 | 1.44–2.36 | <0.0001 |
| Multivariate analysis | |||
| Model 1: adjustment on baseline parameters | |||
| Baseline eGFR <60 (versus ≥60) | 1.40 | 0.83–2.38 | 0.2114 |
| Baseline eGFR <45 (versus ≥45) | 1.63 | 1.20–2.23 | 0.0020 |
| Baseline eGFR <30 (versus ≥30) | 1.49 | 1.14–1.95 | 0.0034 |
| Model 2: adjustment on follow-up parameters | |||
| Baseline eGFR <60 (versus ≥60) | 2.25 | 1.39–3.65 | 0.0010 |
| Baseline eGFR <45 (versus ≥45) | 2.13 | 1.58–2.87 | <0.0001 |
| Baseline eGFR <30 (versus ≥30) | 2.03 | 1.54–2.68 | <0.0001 |
| Model 3: adjustment on baseline and follow-up parameters | |||
| Baseline eGFR <60 (versus ≥60) | 1.30 | 0.76–2.23 | 0.3425 |
| Baseline eGFR <45 (versus ≥45) | 1.58 | 1.15–2.17 | 0.0053 |
| Baseline eGFR <30 (versus ≥30) | 1.53 | 1.53–2.05 | 0.0040 |
eGFR denotes eGFR (determined using the MDRD equation (in mL/min/1.73 m2).
Baseline parameters: age, diastolic arterial pressure, peripheral vascular disease, cancer, use of loop diuretics and AF.
Follow-up parameters: AKI, HF and amputation during follow-up.
FIGURE 1:
Risk of death in patients with eGFR <45 mL/min/1.73 m2 (versus ≥45) at the time of referral. Symbols in red: patients with eGFR <45 mL/min/1.73 m2 at the time of referral to the nephrologist. Symbols in black: patients with eGFR ≥45 mL/min/1.73 m2 at the time of referral to the nephrologist.
In multivariate analyses, only eGFR <45 mL/min/1.73 m2 (versus eGFR ≥45) and eGFR <30 mL/min/1.73 m2 (versus eGFR ≥30) remained significant (Table 4), even when patients with baseline eGFR <15 mL/min/1.73 m2 were excluded from the analysis [HR 2.72 (95% CI 1.68–4.40), P <0.0001 and HR 2.05 (1.53–2.750), P <0.0001, respectively].
RRFD (eGFR change >5 mL/min/1.73 m2/year) [HR 1.45 (95% CI 0.88–2.39), P = 0.1479] and macroalbuminuria (versus normoalbuminuria or microalbuminuria) [HR 1.14 (95% CI 0.89–1.46), P = 0.3021] were not risk factors for death during follow-up.
As expected, eGFR <30 mL/min/1.73 m2 (versus eGFR ≥30), <45 (versus eGFR ≥45) and <60 (versus eGFR ≥60) were risk factors for dialysis initiation [HR 7.12 (95% CI 5.28–9.60) P <0.0001; HR 6.04 (3.76–9.70), P <0.0001; HR 20.7 (5.2–83.2), P <0.0001, respectively]; similar findings were observed for microalbuminuria versus normoalbuminuria [HR 2.03 (95% CI 1.07–3.84), P = 0.0297] and for macroalbuminuria versus microalbuminuria [HR 2.84 (1.97–4.11), P <0.0001].
DISCUSSION
In the present study conducted in elderly patients with diabetes mellitus who were followed up in a nephrology setting, almost half of the patients died after 10 years of follow-up. Major risk factors for death were age, diastolic arterial pressure, peripheral artery disease, cancer, use of loop diuretics and AF at baseline, and AKI, HF and lower limb amputation during follow-up. Initiation of chronic dialysis did not precipitate death, regardless of adjustments. An eGFR <45 mL/min/1.73 m2 (CKD Stage 3b or worse) at the time of nephrology referral was a powerful risk of death, after multiple adjustments, but not eGFR <60 mL/min/1.73 m2. These findings support the view that referral to nephrologists may be warranted not only in patients with CKD Stage 4 but also in patients with CKD Stage 3b.
The results of the present study indicate that dialysis initiation did not accelerate the risk of death, even in older patients (age ≥70– ≥80 years). In a recent single-centre observational study, the risk of death was not different in patients ≥80 years age choosing dialysis and in those receiving conservative treatment [14]. For this reason, it was proposed that dialysis initiation must be discussed on an individual basis [8, 9]. Our findings suggest that the risk of death is not markedly accelerated in our elderly patients with diabetes mellitus in whom the decision of dialysis was made and therefore this decision was probably adequate.
In the present study, eGFR <45 mL/min/1.73 m2 (CKD Stage ≥3b) at the time of the first nephrology visit was a powerful risk of death. This issue of early referral to nephrologists has been extensively studied in patients with advanced CKD (usually CKD Stage 5) [6]. Renal outcome and mortality were compared in patients with early versus later referral to nephrologists (in most studies early was defined as follow-up before dialysis of 1–6 months) [6]. Using this definition, early referral was associated with lower mortality as compared with later referral in the Cochrane’s systematic review where 63 887 patients were analysed [6]. However, these analyses were limited to patients with low eGFR (CKD Stage 5), were not stratified according to the presence of diabetes mellitus and not all patients were managed in a nephrology setting [6]. Moreover, these analyses were focused on the timing of referral but not on the value of eGFR at the time of referral. Our results thus provide new evidence regarding the right timing of referral using eGFR values. In effect, the expected duration of follow-up before dialysis is an important concept for nephrologists; however, the change of renal function over time is difficult to ascertain for non-nephrologists and may vary considerably, especially in subjects with diabetes mellitus [15, 16]. It was shown that the deterioration of renal function can be slow in many patients with diabetes mellitus, especially when low proteinuria is present [15, 17]. In contrast, some patients exhibit much more rapid deterioration of renal function [15, 16]. Our findings are thus important, as they focused on eGFR values and not on renal function changes over time.
The KDIGO guidelines suggest referral of patients to nephrologists in the following situations: eGFR <30 mL/min/1.73 m2, macroalbuminuria or RRFD (yearly eGFR change >5 ml/min/1.73 m2/year) [7] in order to optimize nephroprotection measures and to accurately prepare patients for dialysis (including the choice of techniques, the discussion regarding renal transplantation and the reduction of catheter use) [6, 7]. Our findings suggest that referral to nephrologists should be prompted much earlier (eGFR <45 mL/min/1.73 m2), not only to optimize nephroprotection measures but primarily to improve the survival of patients, regardless of whether dialysis will be necessary in these patients. Although macroalbuminuria and rapid deterioration of renal function were not associated with the risk of death during follow-up, these criteria remain useful to refer patients to nephrologists [18].
In our study, ∼80% of patients were receiving RAS blockers. The use of RAS blockers is advocated in patients with diabetes mellitus. Probably more patients should be using them in our population, but it should be noted that this figure is much greater than those reported in other French studies focused on renal risk in diabetes mellitus [19].
Of note, AKI remained significantly associated with the risk of death in multivariate analyses. Recently it was reported that AKI, in addition to GFR and albuminuria, was a strong predictor of cardiovascular and non-cardiovascular death in a large cohort of patients with diabetes mellitus. We noted that low diastolic blood pressure was a risk factor for death in our population. Other studies suggest that there is a J curve relationship between diastolic blood pressure and the risk of death [20].
Our study has several limitations. It is a monocentric study and therefore our findings need to be replicated; however, our cohort is large and the total observation period was important. Files were individually (manually) reviewed and there was no inclusion bias, as these patients were consecutively included.
Our study also has some strengths. Multivariate analyses were carefully designed to take into account potential confounders, including baseline parameters as well as parameters during follow-up associated with the risk of death. All patients were followed in the same centre and we could therefore retrieve all treatments, hospitalizations and biochemical evaluations. We believe that our cohort of patients is representative of the patients with diabetes mellitus followed up in nephrology settings in France. In effect, renal function of the 986 outpatients with diabetes mellitus who were referred to nephrologists in the ALICE multicentre French study was comparable to that found in our cohort [20]. The mortality rate of the 1341 patients with diabetes mellitus followed in French hospitals from the Survival, Type 2 Diabetes and Genetics study [21] was comparable to that found in our cohort. Our mortality rate is lower than that found in the patients included in the UK Prospective Diabetes Study [22]. However, this study was conducted almost 20 years ago and the mortality rate has greatly diminished in recent years in this population [23].
In conclusion, in the present study conducted in elderly patients with diabetes mellitus followed up in a nephrology setting, the risk of death was not modified by the initiation of dialysis and eGFR <45 mL/min/1.73 m2 (CKD Stage ≥3b) at the time of the first nephrology visit was a powerful risk of death, even in oldest patients. These findings support the view that earlier referral of patients with diabetes mellitus to nephrologists may be justified (at the CKD Stage 3b), even in the absence of macroalbuminuria or rapid degradation of renal function.
AUTHORS’ CONTRIBUTIONS
P.C. was involved in the collection of data and design and writing of the article. G.P., F.M., B.C., L.H., R.N., N.J. and B.E. were responsible for the analysis and discussion of the article. B.M. contributed towards the writing and discussion of the article. S.B. provided the statistical analysis for the work described. H.J-M. handled the design, writing and analysis of the article.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part.
REFERENCES
- 1. Zimmet PZ, Magliano DJ, Herman WH. et al. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol 2014; 2: 56–64 [DOI] [PubMed] [Google Scholar]
- 2. Guariguata L, Whiting DR, Hambleton L. et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014; 103: 137–149 [DOI] [PubMed] [Google Scholar]
- 3. McKinlay J, Marceau L.. US public health and the 21st century: diabetes mellitus. Lancet 2000; 356: 757–761 [DOI] [PubMed] [Google Scholar]
- 4. Ninomiya T, Perkovic V, de Galan BE. et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009; 20: 1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afkarian M, Sachs MC, Kestenbaum B. et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smart NA,, Dieberg G,, Ladhani M. et al. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev 2014; 6: CD007333. [DOI] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 8. Rosansky SJ, Cancarini G, Clark WF. et al. Dialysis initiation: what’s the rush? Semin Dial 2013; 26: 650–657 [DOI] [PubMed] [Google Scholar]
- 9. Rosansky SJ, Clark WF.. Has the yearly increase in the renal replacement therapy population ended? J Am Soc Nephrol 2013; 24: 1367–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015; 38: S8–S16 [DOI] [PubMed] [Google Scholar]
- 11. Inker LA, Levey AS, Pandya K. et al. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient meta-analysis. Am J Kidney Dis 2014; 64: 74–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Coresh J, Balk E. et al. National Kidney Foundation practice guidelines for chronic disease: evaluation, classification and stratification. Ann Intern Med 2003; 139: 137–147 [DOI] [PubMed] [Google Scholar]
- 13. Ronco C, House A, Haapio M.. Cardiorenal syndrome: refining the definition of a complex symbiosis gone wrong. Intensive Care Med 2008; 34: 957–962 [DOI] [PubMed] [Google Scholar]
- 14. Verberne WR, Geers AB, Jellema WT. et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol 2016; 11: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perkins BA, Ficociello LH, Ostrander BE. et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007; 18: 1353–1361 [DOI] [PubMed] [Google Scholar]
- 16. Krolewski AS, Skupien J, Rossing P. et al. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int 2017; 91: 1300–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab 2012; 38: 291–297 [DOI] [PubMed] [Google Scholar]
- 18. Kovesdy CP, Coresh J, Ballew SH. et al. Past decline versus current eGFR and subsequent ESRD risk. J Am Soc Nephrol 2016; 27: 2447–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monseu M, Gand E, Saulnier P-J. et al. Acute kidney injury predicts major adverse outcomes in diabetes: synergic impact with low glomerular filtration rate and albuminuria. Diabetes Care 2015; 38: 2333–2340 [DOI] [PubMed] [Google Scholar]
- 20. Cushman WC, Evans GW, Byington RP. et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362: 1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joly D, Choukroun G, Combe C. et al. Glycemic control according to glomerular filtration rate in patients with type 2 diabetes and overt nephropathy: a prospective observational study. Diabetes Res Clin Pract 2015; 108: 120–127 [DOI] [PubMed] [Google Scholar]
- 22. Adler AI, Stevens RJ, Manley SE. et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 23. Tancredi M, Rosengren A, Svensson AM. et al. Excess mortality among persons with type 2 diabetes. N Engl J Med 2015; 373: 1720–1732 [DOI] [PubMed] [Google Scholar]