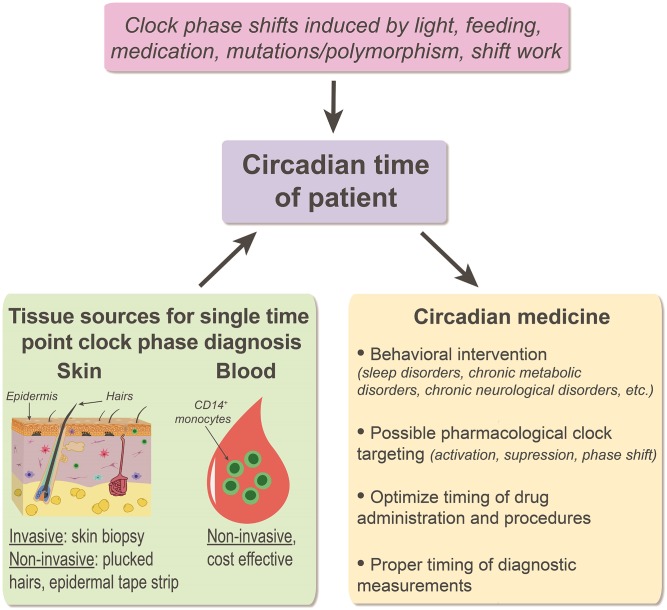
The nascent field of circadian medicine posits that timing of medical interventions, including the administration of drugs and surgical procedures, can be important for maximum therapeutic efficacy and minimum side effects. However, with today’s chaotic work schedules and light pollution, the phase of the body clock—the circadian time—varies between individuals within the same geographical time zone. Therefore, to fully deliver on its promise, the field needs practical and accurate diagnostic methods to determine the phase of an individual’s internal clock. In PNAS, Wu et al. (1) make two advances toward this goal by suggesting that (i) skin, an easily accessed tissue, is an ideal sample source for determining the phase of the body clock, and (ii) a single sample measuring the expression of 29 circadian biomarker genes may be sufficient to determine an individual’s body-clock phase. In an independent study in Journal of Clinical Investigation, Wittenbrink et al. (2) suggest that the same goal can be achieved with a single measurement of circadian biomarker genes in monocytes isolated from blood.
Earth’s rotation around its axis creates the diverse environments of day and night. In response, organisms, from simple bacteria to humans, have evolved an intrinsic, autonomous timing system: the circadian (Latin for “around the day”) clock—a system thought to provide a survival advantage. In humans, the circadian clock facilitates the anticipation of, and adaptation to, the daytime environment of activity and feeding and the nighttime environment of sleep and fasting. Therefore, it is unsurprising that at an organismal level, the circadian clock is an important regulator of activity, sleep, and metabolism (3).
Initially, it was thought that only the central clock (neurons comprising the suprachiasmatic nucleus of the hypothalamus) contained the timing mechanisms and that the clock’s effect on different organs was mediated indirectly by hormones and neurons. However, research since the 1990s revealed that nearly all cells of the body contain the same timing mechanism (4) and that in each organ, up to 20% of the transcriptome exhibits diurnal expression patterns (5). Outside the core clock genes, the diurnally regulated genes of an organ are relatively specific and often confer the central functions of that organ, explaining the broad effects of the circadian clock on organismal physiology. The timing of the central clock is set by light signals traveling from the retina via a neuronal pathway to the suprachiasmatic nucleus. In turn, the central clock coordinates the phase of the peripheral clocks so that there is generally a fixed relationship between the phases of peripheral clocks and the central clock. However, while the central clock seems to be primarily sensitive to light, the timing of peripheral clocks can be set by multiple additional signals, including the timing of food intake (6, 7); in such cases, peripheral clocks may not accurately report the phase of the systemic clock.
Individuals with genetic alterations in core circadian clock genes have an internal clock that is out of phase with wall time (8, 9). Also, in today’s society, many have schedules that do not match the day–night cycle, either through work or social activities, a situation that also creates circadian misalignment. Furthermore, peripheral clocks can be misaligned to the central clock (e.g., through unusual timing of food intake). Epidemiological studies suggest that circadian misalignment increases the propensity to diseases, including diabetes, obesity, cardiovascular disease, and cancer (reviewed in ref. 3). Other research has suggested that timing of medical interventions improves outcomes. Manifestations of disease (e.g., blood pressure) often vary over the day. The metabolism of many drugs is diurnal. Cell proliferation in rapidly dividing epithelia, such as skin epidermis (10), is also diurnal, and chemotherapy can be timed to minimize toxic effects in normal, fast-renewing tissues. In addition, because the concentrations of many hormones vary over the day, in clinical practice, their measurements need to be performed at certain times.
Circadian medicine is an emerging field of medicine that aims to use knowledge about the circadian clock to improve diagnoses and treatment outcomes (Fig. 1). However, the circadian time of different individuals varies, which may make it difficult to determine the proper timing of tests or interventions. For example, early-morning cortisol and testosterone measurements may not give the correct information in someone working night shifts. Consequently, both research and clinical applications would benefit from inexpensive, practical methods to determine an individual’s circadian time.
Fig. 1.
Diagnostic assays in circadian medicine. Individual internal clock can differ from wall time due to the variety of genetic and nongenetic factors (pink box). Prediction of patient’s individual circadian phase based on assaying gene expression in a single tissue sample, such as skin or blood (green box), can help optimize a variety of clinical decisions (yellow box).
Currently, the dim-light melatonin onset assay is thought to be the most accurate method to establish circadian time (11). Because this method requires standardized conditions and multiple measurements of melatonin in blood or saliva over several hours, it is not suitable in routine clinical practice or large epidemiological studies. Wu et al. (1) sampled human epidermis every 6 h for 24 h from 20 individuals and obtained single epidermal samples from 219 individuals. After measuring global mRNA expression, the authors used computational approaches to define 188 diurnal epidermal genes and a set of 29 biomarker genes whose combinatorial expression could accurately determine circadian phase in 30 of 36 single-time point skin samples. The ability to predict circadian phase based on a single tissue sample is a major advance.
Wu et al. (1) sampled epidermis from full-thickness 2-mm skin biopsy tissues. Although collecting skin biopsy tissue for determining circadian phase might be applied in some research studies and occasional clinical diagnostic cases of circadian disruption, this method is too invasive and impractical for wide use in circadian medicine. There are, however, less invasive ways to sample the epidermis, including repeated tape stripping. Interestingly, this method, which has been shown to be suitable for determining gene expression patterns by global microarrays, samples cells from all layers of human epidermis (12). Lastly, plucked scalp or beard hair follicles are a source of epithelial cells that can be used to determine gene expression (13). Further studies are required, but if samples removed by either tape stripping or hair plucking perform as well as epidermis from skin biopsies, then that would be a practical assay for routine clinical use and epidemiological studies.
Previously, investigators have analyzed gene expression or metabolites in blood cells to determine circadian time (14, 15). While a blood drawing is less invasive than a skin biopsy, Wu et al. (1) argue that the more robust circadian oscillations in the epidermis make the epidermal assay more accurate. A new approach based on isolating monocytes from blood (2) yields results that are more in line with those obtained using epidermis—also predicting circadian time from a single sample. Further comparative work will be needed to determine the relative merit of the two approaches. Both approaches are likely to be sensitive to selective modulations of the peripheral clock such as by unusual feeding times, which has been shown to affect the skin circadian clock (6) and may affect blood cell clocks as well. Furthermore, local or systemic infections and inflammatory diseases may have strong effects on the clock-controlled immune genes in sampled tissues, potentially reducing the diagnostic power of at least some diurnal biomarker genes in individuals with underlying conditions.
In addition to suggesting a suitable tissue source for determining the phase of the circadian clock in circadian medicine and research studies, Wu et al. (1) provide the most comprehensive analysis of the diurnal transcriptome in the human epidermis to date, suggesting circadian-modulated biology in human skin. Among the highly diurnal epidermal genes with currently unknown functions in skin are FUS and TSC22D3. FUS is a multifunctional RNA-binding protein, broadly implicated in maintenance of genomic integrity and mRNA and microRNA processing, while TSC22D3 is a glucocorticoid-induced leucine zipper protein implicated in mediating the antiinflammatory and immunosuppressive effects of steroids. In its predicted function, the circadian transcriptome of the human epidermis is similar to that of the mouse epidermis (10), indicating that the role of the circadian clock in skin is highly conserved between mice and humans, pointing to the utility of mice as a model for the role of the circadian clock in skin.
Studies on mouse skin have shown that the circadian clock modulates cell cycle in hair follicle epithelial cells and, consequently, aspects of hair growth (16, 17). In epidermal cells, the circadian clock coordinates daily gene oscillations that couple metabolism with the cell cycle (18). Also, epidermal cell sensitivity to DNA damage from solar radiation is modulated by the circadian clock (10, 19), suggesting important roles for the clock in skin states induced by DNA damage, such as premature aging and cancer. Furthermore, the clock modulates the skin immune response (reviewed in ref. 20). The gene set defined by Wu et al. (1) can now be used to study how the clock modulates these phenomena in human skin. Given that a single time point suffices to accurately determine circadian phase, the gene set can be immediately applied to existing gene expression datasets from skin diseases, including skin cancer. Especially interesting will be studies that compare the clock in simultaneously sampled normal and disease-affected skin. Such datasets exist, for psoriasis for example, an immune dysfunction disease with a possible circadian clock link (reviewed in ref. 20).
In conclusion, two recent papers (1, 2) point to epidermis and blood monocytes as sources for single-sample gene expression assays that can determine the phase of the body clock. The monocyte assay has already been adapted to a clinically compatible platform and is ready for evaluation in larger studies. The biomarker assay in epidermis currently requires a skin biopsy and, to be widely used, would benefit from adaptation to a more noninvasive sampling method and transfer to a platform suitable for routine clinical use.
Acknowledgments
M.V.P. is supported by a grant from the Pew Charitable Trust and NIH Grants AR073159, AR067273, and AR069653. B.A. is supported by the Irving Weinstein Foundation and NIH Grants AR056439, AR044882, and AR069962.
Footnotes
The authors declare no conflict of interest.
See companion article on page 12313.
References
- 1.Wu G, et al. Population-level rhythms in human skin with implications for circadian medicine. Proc Natl Acad Sci USA. 2018;115:12313–12318. doi: 10.1073/pnas.1809442115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittenbrink N, et al. High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest. 2018;128:3826–3839. doi: 10.1172/JCI120874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol. 2016;26:R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci USA. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, et al. Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell Rep. 2017;20:1061–1072. doi: 10.1016/j.celrep.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damiola F, et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patke A, et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell. 2017;169:203–215.e13. doi: 10.1016/j.cell.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 10.Geyfman M, et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc Natl Acad Sci USA. 2012;109:11758–11763. doi: 10.1073/pnas.1209592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- 12.Wong R, et al. Use of RT-PCR and DNA microarrays to characterize RNA recovered by non-invasive tape harvesting of normal and inflamed skin. J Invest Dermatol. 2004;123:159–167. doi: 10.1111/j.0022-202X.2004.22729.x. [DOI] [PubMed] [Google Scholar]
- 13.Akashi M, et al. Noninvasive method for assessing the human circadian clock using hair follicle cells. Proc Natl Acad Sci USA. 2010;107:15643–15648. doi: 10.1073/pnas.1003878107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughey JJ. Machine learning identifies a compact gene set for monitoring the circadian clock in human blood. Genome Med. 2017;9:19. doi: 10.1186/s13073-017-0406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laing EE, et al. Blood transcriptome based biomarkers for human circadian phase. eLife. 2017;6:e20214. doi: 10.7554/eLife.20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plikus MV, et al. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci USA. 2013;110:E2106–E2115. doi: 10.1073/pnas.1215935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin KK, et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stringari C, et al. In vivo single-cell detection of metabolic oscillations in stem cells. Cell Rep. 2015;10:1–7. doi: 10.1016/j.celrep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaddameedhi S, Selby CP, Kaufmann WK, Smart RC, Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci USA. 2011;108:18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plikus MV, et al. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms. 2015;30:163–182. doi: 10.1177/0748730414563537. [DOI] [PMC free article] [PubMed] [Google Scholar]