Significance
The brain uses temporal regularities to anticipate the timing of future events, and adjust attention and action accordingly. We investigated whether subsecond temporal predictions formed in two distinct predictive contexts, when the stream of events is rhythmic or when the specific interval between two events is known, are functionally and neurally distinct. We show that individuals with cerebellar dysfunction were impaired in forming temporal predictions based on single intervals, but not in rhythmic contexts. In contrast, individuals with basal ganglia dysfunction resulting from Parkinson’s disease showed the reverse pattern. This double dissociation constitutes causal evidence in favor of distinct computational and neural mechanisms for interval- and rhythm-based temporal prediction, and highlights the contribution of these subcortical structures to attentional orienting.
Keywords: temporal predictions, attention, cerebellum, basal ganglia
Abstract
Predicting the timing of upcoming events is critical for successful interaction in a dynamic world, and is recognized as a key computation for attentional orienting. Temporal predictions can be formed when recent events define a rhythmic structure, as well as in aperiodic streams or even in isolation, when a specified interval is known from previous exposure. However, whether predictions in these two contexts are mediated by a common mechanism, or by distinct, context-dependent mechanisms, is highly controversial. Moreover, although the basal ganglia and cerebellum have been linked to temporal processing, the role of these subcortical structures in temporal orienting of attention is unclear. To address these issues, we tested individuals with cerebellar degeneration or Parkinson’s disease, with the latter serving as a model of basal ganglia dysfunction, on temporal prediction tasks in the subsecond range. The participants performed a visual detection task in which the onset of the target was predictable, based on either a rhythmic stream of stimuli, or a single interval, specified by two events that occurred within an aperiodic stream. Patients with cerebellar degeneration showed no benefit from single-interval cuing but preserved benefit from rhythm cuing, whereas patients with Parkinson’s disease showed no benefit from rhythm cuing but preserved benefit from single-interval cuing. This double dissociation provides causal evidence for functionally nonoverlapping mechanisms of rhythm- and interval-based temporal prediction for attentional orienting, and establishes the separable contributions of the cerebellum and basal ganglia to these functions, suggesting a mechanistic specialization across timing domains.
Humans use environmental regularities to predict not only the content of future events, but also their timing. In baseball, the hitter must anticipate where and when the ball will cross the plate; in symphonies, the gestures of the conductor allow the musicians to collectively anticipate when to play the first note. The importance of temporal predictions extends beyond movement control, as they are used to guide attention in time to optimize the perception of upcoming events (1–4). Whether predicting the moment of hitting the pitched baseball or anticipating the final note of a musical piece, this ability is critical for efficiently interacting with a dynamic world, and is considered a pivotal component of attentional control (5, 6).
A fundamental question is whether temporal predictions are mediated by context-specific mechanisms, or by a common mechanism. Temporal predictions can be formed when the stimulus stream is (quasi)-periodic, such as in speech, music, or biological motion; for example, behavioral performance is facilitated for events falling on-, relative to off-beat, of either visual (7) or auditory (8) rhythmic streams. This has been attributed to the entrainment (i.e., synchronization) of endogenous oscillations with the external periodic signal (6, 9), supported by findings of increased phase concentration of neural activity in rhythmic streams (2, 3, 10). However, temporal predictions can also be formed in aperiodic streams or in isolation if the interval between two events is known (1, 11–13). For example, based on previous exposures, a driver can anticipate when the traffic light will turn green from when the pedestrian light turns red. It has been suggested that perceiving one event (e.g., the pedestrian light turning red) triggers implicit tracking of ongoing time, with attentional preparation increasing such that it peaks when the memorized interval has elapsed (14). In support of this, EEG studies in humans (15, 16) and neurophysiological recordings in nonhuman primates (17, 18) have shown that the rate of ramping activity is flexibly adjusted according to the expected interval, peaking when it elapses. While recent behavioral and EEG evidence suggests that both mechanisms may coexist (4, 19–21), others argued that temporal predictions in both contexts can be explained using a single mechanism; for example, rhythmic prediction may entail the repeated application of a single-interval predictive mechanism. To date, the issue remains highly contentious (4, 22–24).
A solution to this conundrum is inspired by findings on the role of subcortical structures in temporal processing. Research with human participants using explicit timing tasks, in which temporal quantities must be explicitly detected, compared, or reproduced, has repeatedly shown a central role for the cerebellum and basal ganglia (25–29). Recently it was shown that the cerebellum is crucial for explicit timing of single intervals, such as determining which of two isolated intervals is longer, but is not essential when the task requires judging which of two streams is more periodic (30, 31). In contrast, the basal ganglia have been implicated in rhythmic judgments, as well as sensitivity to the beat structure in streams (28, 29), with inconsistent evidence regarding explicit timing of single intervals (25, 26, 32–34). However, it is not clear whether the organizational principles for temporal prediction mirror those identified in studies of explicit perceptual timing. First, based on distinct cortical activation patterns observed in neuroimaging studies, it has been proposed that explicit perceptual timing and temporal prediction are functionally dissociable (35–37). Second, temporal prediction has been associated with cortical regions, such as the left inferior parietal and premotor cortices, with inconsistent findings regarding the role of subcortical structures (1, 38–40). Thus, whether the cerebellum and basal ganglia have a role in temporal predictions for attentional orienting remains unclear.
Here, we took a neuropsychological approach to address these questions, testing individuals with cerebellar degeneration (CD), Parkinson’s disease (PD, used as a model of basal ganglia dysfunction), and healthy control participants on temporal orienting tasks. We focused on subsecond intervals, given that the benefits of attentional orienting in time are robustly observed in this range, as well as evidence highlighting the critical role of the cerebellum in subsecond timing (15, 25, 30, 41). In three conditions, participants detected a target embedded in a visual stream (Fig. 1). In the “rhythmic” condition, the target coincided with an isochronous stream. In the “single-interval” condition, the target timing was predictable based on aperiodic repeated presentation of pairs of stimuli that defined the target interval. In the “random” condition, the stream was aperiodic and the target onset time was unpredictable. To increase the generalizability of the results, we tested two subsecond target intervals, 600 and 900 ms, in each condition. We observed a striking double dissociation: individuals with CD exhibited a selective impairment in forming interval-based temporal predictions, whereas individuals with basal ganglia degeneration were selectively impaired in forming rhythm-based temporal predictions.
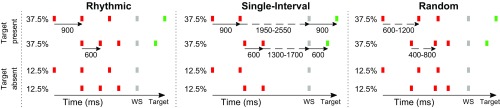
Fig. 1.
Experimental paradigm. Participants viewed a stream of flickering colored squares and detected a target (green square) by providing a speeded response (75% of the trials, Upper half). When there was no target, they were to withhold response (25% of the trials, Lower half). (Left) Rhythmic condition. Identical time interval between all stimuli. (Center) Single-interval condition. The interval between the white square (warning signal, WS) and target was the same as that between the two red squares, but the interval between the two pairs was random. (Right) Random: all intervals are randomly jittered. In all three conditions, the stimulus rate could be slow (Upper row) or fast (Lower row).
Results
Formation of temporal predictions in the rhythmic and single-interval conditions should manifest as faster reaction times (RT) relative to the random condition (referred to as “RT benefit”). Impairment in forming temporal predictions should be expressed in smaller RT benefit for a patient group relative to controls. Given age differences between the two patient groups (as expected from the typical differences in age of pathology onset), two control groups were tested, one age-matched to each patient group.
We first performed four repeated-measures ANOVA on the RT data, one for each of the four groups (CD, n = 11; CD-matched, n = 11; PD, n = 12; PD-matched, n = 12), with the factors “condition” and “target interval.” In all groups, RTs differed significantly between the experimental conditions (condition factor: all Fs > 5, all Ps < 0.05, all > 0.34) (see SI Appendix, Table S1 for the comprehensive results of the ANOVAs, and SI Appendix, Fig. S1 for the results of each participant). Because there were no interactions with target interval (all Fs < 2.05, all Ps > 0.15, all < 0.16), we averaged across the two target intervals when analyzing the differences between conditions.
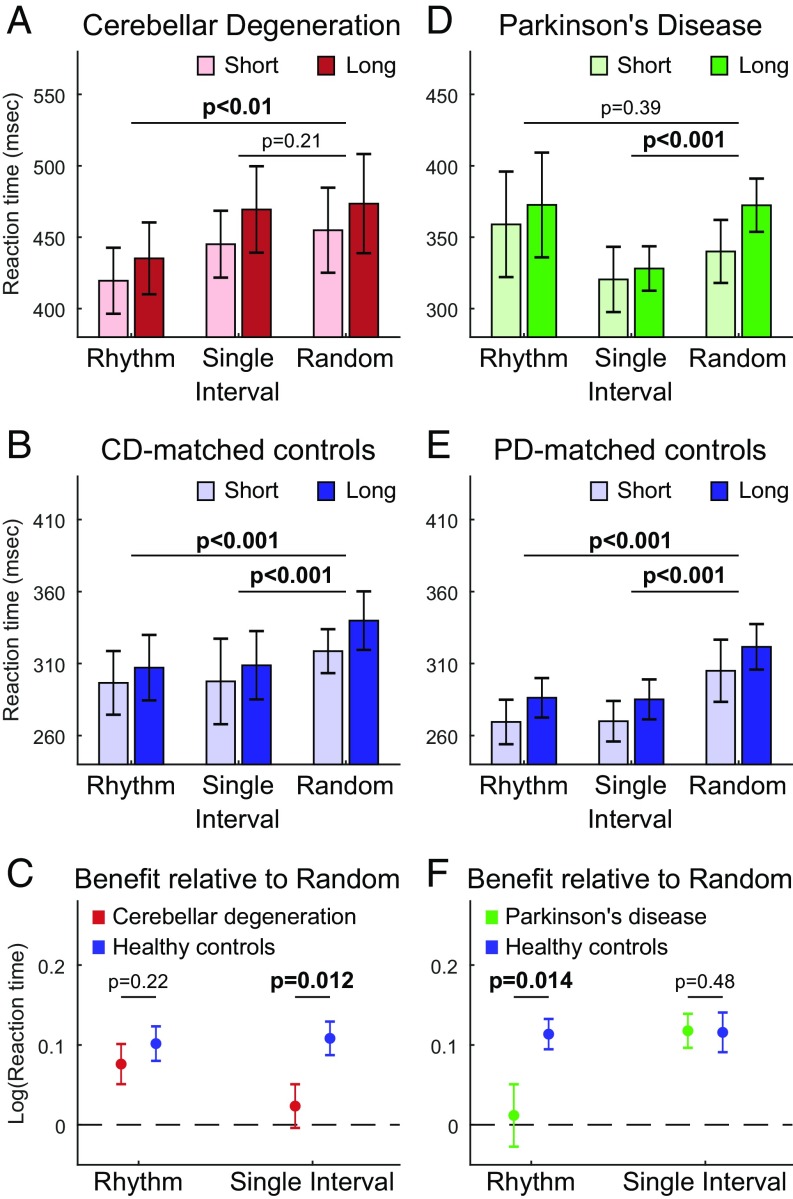
Next, we used planned contrasts to elucidate the how RTs differed between conditions in each group. In the CD group (Fig. 2A), RTs were significantly faster compared with the random condition in the rhythmic condition [t(10) = 3.02, P = 0.006, Cohen’s d = 0.92], but not the single-interval condition [t(10) = 0.86, P = 0.21]. In contrast, in the CD-matched group (Fig. 2B), RTs were significantly faster than the random condition in both the rhythmic [t(10) = 4.72, P = 0.0004, Cohen’s d = 1.49] and the single-interval conditions [t(10) = 5.15, P = 0.0002, Cohen’s d = 1.63].
Fig. 2.
(A) Mean RTs for CD patients to the visual target, demonstrating RT benefit in the rhythmic condition but not in the single-interval condition. (B) Mean RTs for CD-matched healthy controls, demonstrating RT benefits in both predictive conditions. (C) Comparison of RT benefits in the two predictive conditions relative to the random condition between the CD and CD-matched groups, presented as the mean difference in log-transformed RTs. Positive values indicate larger benefit. CD leads to significant impairment in the single-interval condition only. (D) Mean RTs for PD patients, demonstrating RT benefit in the single-interval condition but not in the rhythmic condition. (E) Mean RTs for PD-matched healthy controls, demonstrating RT benefits in both predictive conditions. (F) Same as C for the PD vs. PD-matched groups. PD leads to significant impairment in the rhythm condition only. Bold font indicates significant difference (P < 0.05). All statistical analyses were performed after RT data had been log-transformed (Materials and Methods). The y axes in the panels of A, B, D, and E have different baseline values but span an identical range. In all panels, error bars represent SEM.
We then directly compared the RT differences between conditions between the CD and CD-matched groups using a mixed-effect ANOVA with factors “group” and “condition.” Most importantly, this analysis revealed a significant interaction between group and condition [F(2, 40) = 3.88, P = 0.029, = 0.16] (Fig. 2C and SI Appendix, Table S1). To elucidate this interaction, we used planned interaction contrasts, directly comparing the RT benefit scores [e.g., RT(random) – RT(rhythmic)] between groups, separately for each predictive condition. For the single-interval condition, the RT benefit was significantly smaller in the CD group [t(20) = 2.46, P = 0.012, uncorrected, Cohen’s d = 1.05]. However, the RT benefit for the rhythmic condition did not differ significantly between the two groups [t(20) = 0.6, P = 0.22, uncorrected]. Thus, cerebellar dysfunction leads to impairment in forming interval-based temporal predictions, supported by both the absence of an RT benefit for this condition relative to baseline, and by the group difference. However, the ability to form rhythm-based temporal prediction is preserved.
In the PD group, a different pattern was observed (Fig. 2D). Planned contrasts conducted on the condition factor revealed that RTs were significantly faster compared with the random condition in the single-interval condition [t(11) = 5.53, P = 0.00009, Cohen’s d = 1.58], but not in the rhythmic condition [t(11) = 0.3, P = 0.39], a pattern opposite than obtained for the CD group. For the PD-matched group (Fig. 2E), RTs were significantly faster relative to the random condition in both the rhythmic [t(11) = 5.99, P = 0.00005, Cohen’s d = 1.81] and the single-interval conditions [t(11) = 4.67, P = 0.0003, Cohen’s d = 1.41], a pattern similar to that observed with the CD-matched group.
When directly comparing the PD and PD-matched groups using a mixed-effects ANOVA, we again observed a significant interaction between group and condition [F(2, 44) = 3.94, P = 0.029, = 0.15] (Fig. 2F and SI Appendix, Table S1). Planned interaction contrasts revealed that the RT benefit for the single-interval condition did not differ significantly between the two groups [t(22) = 0.06, P = 0.48, uncorrected]. However, the RT benefit for the rhythmic condition was significantly smaller in the PD group [t(22) = 2.34, P = 0.014, uncorrected, d = 1]. Thus, basal ganglia dysfunction leads to impairment in forming rhythm-based temporal predictions, supported by both the absence of an RT benefit for this condition relative to baseline, and by the group difference. However, the ability to form interval-based temporal prediction is preserved.
Finally, we directly compared the RT benefits from the two predictive conditions between the two patient groups. For each patient, we took the RT benefit scores calculated for the planned contrasts (the red and green data points in Fig. 2 C and F, respectively; see above), and analyzed them using a two-way mixed-effects ANOVA. Neither the main effect of condition [F(1, 21) = 1.03, P = 0.32] nor of group [F(1, 21) = 0.22, P = 0.64] was significant. Crucially, however, there was a significant interaction [F(1, 21) = 9.12, P = 0.007, 0.3]. Simple effect contrasts revealed that CD patients had significantly larger benefit in the rhythmic condition than in the single-interval condition [t(10) = 2.55, P = 0.029, Cohen’s d = 0.81], whereas PD patients had significantly larger benefit in the single-interval condition than in the rhythmic condition [t(11) = 2.28, P = 0.044, Cohen’s d = 0.69]. These results confirm the opposite pattern of RT benefits displayed by the two patient groups compared with their respective control groups.
Discussion
We investigated the role of subcortical structures in forming temporal predictions to allocate attention in time based on a rhythmic series of events, or on the memory of a single isolated interval. Converging evidence from the within-group and between-group analyses highlighted a double dissociation between individuals with cerebellar and basal ganglia dysfunction. These results indicate that interval- and rhythm-based temporal predictions are functionally nonoverlapping, and provide causal evidence for the crucial but context-specific roles of the cerebellum and basal ganglia in the neural circuitry of temporal prediction.
Nonoverlapping Mechanisms for Rhythm and Isolated Interval Temporal Prediction.
Anticipating the timing of future events is essential for adjusting attentional and motor resources in time (5). This ability has been repeatedly demonstrated in continuous rhythmic contexts (2, 3, 7, 8, 10), as well as in aperiodic contexts when the duration of an isolated interval is known from previous exposures (1, 11–13, 15–18). Three models have been proposed to account for temporal prediction in these two contexts. One is that isolated interval prediction entails a comparison of elapsed time to a memory representation of the interval (11, 14), with rhythmic prediction reflecting repeated application of this interval-based process (4, 42–44). A different model is that temporal prediction relies on synchronization of endogenous neural oscillations to (quasi-) rhythmic structure in the stimulus stream (6, 9), with single-interval prediction reflecting alignment of a single oscillatory cycle (22, 45, 46). A third possibility is that rhythm-based and interval-based mechanisms coexist, and are engaged in a context-dependent manner (4, 19–21).
Our findings of a double dissociation between the single-interval and rhythmic conditions stand against the idea of a single unified mechanism for temporal predictions. If rhythmic predictions depended on the iteration of an interval-based process, the CD group should have been impaired in both contexts. Conversely, if single-interval predictions depended on alignment of a single cycle of a rhythmic process, the PD group should have been impaired in both contexts. Instead, our findings suggest that distinct, nonoverlapping mechanisms support interval- and rhythm-based temporal predictions. We speculate that this dualism enables the organism to efficiently respond to different computational problems. For example, timing isolated intervals relies on a memory representation of the target interval, whereas, for rhythmic contexts, temporal information is embedded in the stimulus stream, minimizing the demands on memory. In line with this, a concurrent working memory load interferes with isolated interval, but not rhythmic temporal prediction (47, 48). In contrast, an interval-based mechanism might be necessary to generate temporal predictions in the absence of external input.
Causal Role of the Cerebellum and Basal Ganglia in Temporal Prediction.
Neuroimaging studies of temporal predictions have, in general, highlighted the prominent role of cortical regions, in particular the left inferior parietal lobe, supplementary motor area (SMA), and right inferior frontal cortex (1, 35, 38–40). The engagement of subcortical structures has been less consistent. This might partially reflect the difficulty in neuroimaging studies to distinguish between activations related to temporal prediction and other task requirements. Our neuropsychological results emphasize that the cerebellum and basal ganglia are necessary for temporal predictions in a context-dependent manner.
The cerebellum has been repeatedly implicated in subsecond timing (25, 30, 41, 49), but its role in temporal predictions has only been examined in a few studies, using tasks that posed additional demands, such as forming spatiotemporal predictions (50), learning temporal regularities across exposures (51), or switching between different temporal groupings of rhythmic elements (52). Our task minimized such demands, enabling us to isolate the role of the cerebellum in temporal prediction per se. More important, prior work has not assessed whether cerebellar involvement in temporal orienting is context-specific, as observed in other timing domains (27, 30). Our results challenge the idea that the cerebellum is necessary for any form of prediction or timing, instead identifying its unique role in temporal predictions that are based on representing the interval between events.
The basal ganglia are central to current models of timing, especially those in which temporal representations rely on endogenous periodic processes (53, 54). In line with this, the basal ganglia have been associated with timing in rhythmic contexts, both when reported explicitly or when forming temporal predictions (28, 29, 55–58), consistent with our findings from the PD group. However, the role of the basal ganglia in single-interval temporal predictions in humans has been unclear, with some reports of preserved performance (59, 60) and others of impairment, with the results complicated by variation due to medication state (61). Moreover, to date there has been no direct comparison between single-interval and rhythmic temporal predictions. Our finding of preserved single-interval predictions in the PD group suggest that the contribution of the basal ganglia to temporal predictions is restricted to rhythmic contexts.
Studying patients with neurological disorders has been foundational to understanding brain function, providing an important tool for identifying functional specialization. Nevertheless, this approach has well-known limitations, such as that the pathology is heterogeneous across individuals, and could extend beyond or spares critical tissue within the region of interest. In addition, preserved function could reflect compensatory processes. Furthermore, our PD patients were tested while taking medication, possibly masking impairments in interval-based timing (however, see ref. 62). However, double dissociations, as observed here, mitigate these concerns, as well as more general issues regarding task-difficulty differences (63). While it is possible that with more extensive lesions the impairments would not have remained selective, the current results point to asymmetric specialization of the cerebellum and basal ganglia in interval- and rhythm-based temporal predictions. Moreover, the persistence of the selective impairments in individuals with degenerative disorders that, in many cases, have been symptomatic for years, argues against a compensatory account.
Finally, the present results are not at odds with hypotheses concerning interactions between the cerebellum and basal ganglia in representing temporal relations (64), nor do they undermine the necessity of cortical regions, such as the SMA and inferior parietal lobe, which have been associated with temporal prediction (1, 65). Indeed, given the vast evidence for dynamic communication between cortical and subcortical areas in timing (50, 53), it is reasonable to assume that subcortical and cortical circuits interact to support temporal predictions. Understanding the roles of cortical and subcortical nodes in networks for temporal prediction is an important challenge for future research.
Shared Principles of Subcortical Computation Across Timing Domains.
Previous research in other timing domains, such as explicit perceptual and motor timing, has also examined the importance of the cerebellum and basal ganglia for single intervals and rhythmic timing, although few studies have directly compared them. Cerebellar dysfunction impairs perceptual timing of isolated intervals but not judgments involving rhythmic sequences (30, 31). This asymmetry is also seen in motor timing, where CD impairs producing precisely timed movements defined by intervals, but not periodic movements when these can emerge from a different control parameter, such as maintaining a constant angular velocity in circle drawing (27, 66). For the basal ganglia, this structure has been repeatedly implicated in perceptual sensitivity to rhythmic structure and rhythmic movements (28, 29, 67), with a less clear role in motor and perceptual timing of isolated intervals. An impressive literature, mostly involving studies with rodents, has implicated the basal ganglia in interval timing. However, this work has mainly focused on suprasecond intervals (68–70). For subsecond intervals, human neuropsychology studies are inconsistent (71), with some reporting impairments (32, 72) but others performance within normal range (25, 34). Thus, the basal ganglia appear to show an opposite asymmetry to the cerebellum, being essential to motor and perceptual timing of rhythms but not of isolated intervals. Furthermore, although only a few fMRI studies have contrasted explicit timing in interval and rhythmic contexts, the results point to dissociated activation patterns within cerebellar and striatal networks, respectively (73, 74).
Our findings indicate that temporal predictions follow a similar organization to that of explicit perceptual and motor timing. This parallelism across timing domains suggests that, at least at the subcortical level, common computations may be enlisted across a range of timing tasks (75), with distinct computations associated with the cerebellum and basal ganglia. One hypothesis is that the cerebellum and basal ganglia are involved in a core representation of time that is required regardless of whether timing is done explicitly for movement coordination or for prediction. In support of this, temporal predictions and explicit timing show similar scalar properties (76).
In contrast to the similar constraints noted above for explicit timing and temporal prediction, previous work has pointed to nonoverlapping cortical activations for these two domains (35–37). This suggests that different organizational principles may hold for the cortex. For example, cortical activations could reflect the utilization of subcortical core temporal representations according to the task goals, such as providing an explicit report of a temporal property or coordinating shifts of attention. Importantly, cortical temporal processing may also be sensitive to the mode of temporal representation, as evident by findings of distinct cortical engagement during rhythmic and interval-explicit perceptual timing (73). Future work should examine whether this cortical dissociation is specific to explicit timing or also occur for temporal predictions, as well as explore if they arise from differences in the distribution of subcortical inputs.
To conclude, our results indicate that predictive adjustment of attention in time is mediated by distinct functional and neural mechanisms, depending on whether predictions are based on a periodic stream or derived from aperiodic isolated intervals. This dissociation requires modifying current models of temporal prediction to recognize context-dependent representations of temporal information. Furthermore, our results highlight the contribution of the cerebellum and basal ganglia for the temporal orienting of attention, expanding a perspective that has generally focused on the cerebral cortex.
Materials and Methods
Participants.
Thirteen patients with CD, 12 patients with PD, and 23 healthy individuals were recruited for the study. Participants were prescreened to have normal or corrected-to-normal vision, intact color vision, and no professional musical training or engagement in amateur-level musical activity in the 5 y before testing (e.g., playing an instrument or singing in a choir). Two participants in the CD group were not tested in the main experiment due to inability to perform the task (see below), leading to a final sample size of 11 CD patients. All participants provided written informed consent before their participation. The study and all its procedures were approved by the Institutional Review Board of the University of California, Berkeley.
The patients in the CD group (seven females, nine right-handed, mean age 51.6 y, SD 14.2) had been diagnosed with spinocerebellar ataxia, either linked to a specific genetic subtype (six participants) or unknown/idiopathic etiology (five participants). At the time of testing, all were evaluated with the International Cooperative Ataxia Rating Scale (ICARS) (77). The mean ICARS score was 34.4 (SD 11.6). We did not test patients who presented symptoms of multisystem atrophy. Patients in the PD group (three females, seven right-handed, mean age 68.4 y, SD 8.1) were tested on their standard dopaminergic medication schedule and evaluated at the time of testing with the Unified Parkinson’s Disease Rating Scale, (UPDRS) (78). The mean score on the motor section of the UPDRS was 14.2 (SD 4.5). A medical history was obtained for the patients in the CD and PD group to verify that none of the participants had other neurological disorders.
There was a substantial difference in age between the two CD and PD groups, typical to the different onset time of symptoms in these two conditions. Given this, we recruited two control groups, one from the same age range as that of the CD group (CD-matched group, n = 11, six females, eight right-handed, mean age 52.8 y, SD 12.3) and the other as that of the PD group (PD-matched group, n = 12, seven females, nine right-handed, mean age 67.8 y, SD 7.7). Individuals in both control groups reported not having any neurological disorders or significant history of neurological incidents. The patient groups did not differ significantly from the respective control groups in age (CD vs. CD-matched controls, P = 0.84; PD vs. PD-matched controls, P = 0.86). All of the participants completed the Montreal Cognitive Assessment scale (MoCA) as a simple assessment of overall cognitive competence. Although we did not select participants to provide a match on this measure, there were no differences between each patient group and their matched control (CD: mean = 27.5; CD-matched controls: mean = 28.1, P = 0.39; PD: mean = 28.2; PD-matched controls: mean = 28.5, P = 0.66) or between the two patient groups (P = 0.29).
Stimuli and Task.
Stimuli were filled color squares (∼3.5° visual angle per side) presented for 100 ms. Each trial consisted of two or three red squares, followed by a white square acting as a warning signal (WS), and then a green square defined as the target. The WS-target interval was either 600 ms (“short” trial) or 900 ms (“long” trial). Participants were instructed to make a speeded button press using a computer keyboard as soon as they detected the target.
Three experimental conditions were presented in separate blocks, differing in the temporal structure of the stream of red squares (Fig. 1). In the rhythmic condition, there were three red squares. The interstimulus intervals (ISIs) between all stimuli were identical to the target interval of that trial. This made the target timing fully predictable as it occurred on-beat within the induced rhythm. In the single-interval condition there were two red squares. The ISI between them was identical to the target interval. However, the interval between the second red square and the WS was randomly jittered, with a mean that was 2.5 times the WS signal-target interval (−13.3%, −6.6%, 0%, +6.6%, +13.3 of 1,500 or 2,250 ms for short and long trials, respectively, uniform distribution). This strongly reduced the periodicity in the stimulus train relative to the rhythmic condition, as the WS signal occurred, on average, at 180° phase, relative to a “beat” that, in theory, could have been created by the two red squares (see ref. 4). However, target timing was fully predictable due to the repetition of the interval between the two red squares. In the random condition, there were three red squares. The stream ISIs were randomly jittered around 600 or 900 ms (−33.3%, −16.6%, 0%, +16.6%, +33.3%, uniform distribution). This strongly reduced rhythmicity in the stimulus train, and also made the onset time of the target unpredictable. For all three conditions, 25% of the trials were “catch trials” in which no target was presented, to minimize the incentive to conduct anticipatory responses (13).
Procedure.
The experiment was conducted in a quiet, dimly lit room. Stimuli were presented on a gray background on a computer monitor (viewing distance ∼50 cm). Stimulus presentation and response acquisition were controlled using the Psychophysics Toolbox (79, 80) for MATLAB (Mathworks). Upon arrival, participants provided consent, demographic information, and completed the MoCA. In addition, the patients provided a clinical history and were evaluated with the relevant neurological examination (ICARS and UPDRS for the CD and PD groups, respectively). Participants then performed three practice blocks, one for each condition, starting with the Random condition, then the two predictive conditions (order counterbalanced across participants). Each practice block started with condition-specific instructions and included 16 trials, 8 with the short- and 8 with the long-target interval, in random order, with four of these being catch trials (i.e., no target). The practice blocks included several pauses during which the experimenter verified that the participant understood the task, could differentiate fast and slow trials, and could describe the mode of temporal predictability (e.g., rhythm vs. single interval). Participants then completed two triplets of test blocks, with each triplet composed of one block of each of the three conditions (random, rhythmic, single interval). Each test block consisted of 32 trials, 16 with the short interval and 16 with the long interval, presented in random order. Within each of the two intervals, four randomly selected trials (25%) were catch trials. The block order was randomized within each triplet. Participants received feedback (error message of the monitor) if the responded prematurely, responded on a catch trial, or if they did not respond within 3 s from target onset.
Statistical Analysis.
Trials were discarded if a response was detected before the onset of the target stimulus, or if the RT was shorter than 100 ms or longer than 3,000 ms (3% of the trials, no difference between groups or conditions). The RTs from the remaining trials were log-transformed to reduce the skewedness inherent in RT distributions. The log-transformed data were analyzed using standard parametric methods (see below). Trials were then discarded if the transformed RT was more than three SDs above or below the mean transformed RT, separately for each condition and target interval (0.5% of the trials, no difference between conditions).
To assess the benefit of temporally predictive context in each group, log-transformed RTs were subjected to a two-way repeated-measures ANOVA with factors condition (random/rhythmic/single interval) and target interval (600 ms/900 ms). Formation of temporal predictions in each of the predictive conditions should be expressed in faster RTs relative to the random condition. Therefore, we conducted one-tailed planned contrasts to compare each predictive condition (rhythmic or single interval) to the random condition.
To directly compare each patient group to its respective control group, we conducted a two-way mixed-effects ANOVA, with group (patients/controls) as a between-subject factor, and condition (random/rRhythmic/single interval) as a within-subject factors. This analysis was conducted across the target-interval factor, as the within-group analyses revealed no interaction of this factor with condition, the factor of primary interest. Impaired ability to form temporal predictions should be expressed in smaller RT benefit for the predictive conditions relative to the random condition in the patient versus the control group. Therefore, we conducted one-tailed planned interaction contrasts to compare each predictive condition with the random condition between the patient group and the respective control group. This entailed calculating, for each participant, the RT benefit score of the predictive condition (e.g., the rhythmic condition) relative to the random condition [e.g., RT(random) – RT(rhythmic)], and comparing these RT benefit scores between groups using a two-sample uncorrected t test.
Finally, to directly compare the ability to benefit from the rhythmic and single-interval conditions between the two patient groups, we used the RT benefit scores that were calculated for each patient for the rhythmic and single-interval conditions (see above). These scores were submitted to a two-way mixed ANOVA with group (CD/PD) as a between-subject factor and condition (rhythmic/single interval) as a within-subject factor. In all analyses, effect sizes were estimated using Cohen’s d or partial . All t tests compared the differences in log-transformed RTs between the conditions of interest. We also note that the RT benefits scores were not correlated with age for either group.
Supplementary Material
Acknowledgments
We thank Claudia Tischler and Kate Duberg for their assistance data collection. This work was supported by National Institute of Health Grants NS092079 and NS105839.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1810596115/-/DCSupplemental.
References
- 1.Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 3.Henry MJ, Obleser J. Frequency modulation entrains slow neural oscillations and optimizes human listening behavior. Proc Natl Acad Sci USA. 2012;109:20095–20100. doi: 10.1073/pnas.1213390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breska A, Deouell LY. Neural mechanisms of rhythm-based temporal prediction: Delta phase-locking reflects temporal predictability but not rhythmic entrainment. PLoS Biol. 2017;15:e2001665. doi: 10.1371/journal.pbio.2001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nobre AC, van Ede F. Anticipated moments: Temporal structure in attention. Nat Rev Neurosci. 2018;19:34–48. doi: 10.1038/nrn.2017.141. [DOI] [PubMed] [Google Scholar]
- 6.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanabria D, Capizzi M, Correa A. Rhythms that speed you up. J Exp Psychol Hum Percept Perform. 2011;37:236–244. doi: 10.1037/a0019956. [DOI] [PubMed] [Google Scholar]
- 8.Jones MR, Moynihan H, MacKenzie N, Puente J. Temporal aspects of stimulus-driven attending in dynamic arrays. Psychol Sci. 2002;13:313–319. doi: 10.1111/1467-9280.00458. [DOI] [PubMed] [Google Scholar]
- 9.Large EW, Jones MR. The dynamics of attending: How people track time-varying events. Psychol Rev. 1999;106:119–159. [Google Scholar]
- 10.Lakatos P, et al. The spectrotemporal filter mechanism of auditory selective attention. Neuron. 2013;77:750–761. doi: 10.1016/j.neuron.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Los SA, Heslenfeld DJ. Intentional and unintentional contributions to nonspecific preparation: Electrophysiological evidence. J Exp Psychol Gen. 2005;134:52–72. doi: 10.1037/0096-3445.134.1.52. [DOI] [PubMed] [Google Scholar]
- 12.Lange K, Rösler F, Röder B. Early processing stages are modulated when auditory stimuli are presented at an attended moment in time: An event-related potential study. Psychophysiology. 2003;40:806–817. doi: 10.1111/1469-8986.00081. [DOI] [PubMed] [Google Scholar]
- 13.Correa A, Lupiáñez J, Tudela P. The attentional mechanism of temporal orienting: Determinants and attributes. Exp Brain Res. 2006;169:58–68. doi: 10.1007/s00221-005-0131-x. [DOI] [PubMed] [Google Scholar]
- 14.Durstewitz D. Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci. 2003;23:5342–5353. doi: 10.1523/JNEUROSCI.23-12-05342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miniussi C, Wilding EL, Coull JT, Nobre AC. Orienting attention in time. Modulation of brain potentials. Brain. 1999;122:1507–1518. doi: 10.1093/brain/122.8.1507. [DOI] [PubMed] [Google Scholar]
- 16.Trillenberg P, Verleger R, Wascher E, Wauschkuhn B, Wessel K. CNV and temporal uncertainty with ‘ageing’ and ‘non-ageing’ S1-S2 intervals. Clin Neurophysiol. 2000;111:1216–1226. doi: 10.1016/s1388-2457(00)00274-1. [DOI] [PubMed] [Google Scholar]
- 17.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 18.Mendoza G, Méndez JC, Pérez O, Prado L, Merchant H. Neural basis for categorical boundaries in the primate pre-SMA during relative categorization of time intervals. Nat Commun. 2018;9:1098. doi: 10.1038/s41467-018-03482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breska A, Deouell LY. Automatic bias of temporal expectations following temporally regular input independently of high-level temporal expectation. J Cogn Neurosci. 2014;26:1555–1571. doi: 10.1162/jocn_a_00564. [DOI] [PubMed] [Google Scholar]
- 20.Breska A, Deouell LY. When synchronizing to rhythms is not a good thing: Modulations of preparatory and post-target neural activity when shifting attention away from on-beat times of a distracting rhythm. J Neurosci. 2016;36:7154–7166. doi: 10.1523/JNEUROSCI.4619-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohenkohl G, Coull JT, Nobre AC. Behavioural dissociation between exogenous and endogenous temporal orienting of attention. PLoS One. 2011;6:e14620. doi: 10.1371/journal.pone.0014620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obleser J, Henry MJ, Lakatos P. What do we talk about when we talk about rhythm? PLoS Biol. 2017;15:e2002794. doi: 10.1371/journal.pbio.2002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breska A, Deouell LY. Dance to the rhythm, cautiously: Isolating unique indicators of oscillatory entrainment. PLoS Biol. 2017;15:e2003534. doi: 10.1371/journal.pbio.2003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoefel B, Ten Oever S, Sack AT. The involvement of endogenous neural oscillations in the processing of rhythmic input: More than a regular repetition of evoked neural responses. Front Neurosci. 2018;12:95. doi: 10.3389/fnins.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 26.Rao SM, et al. Distributed neural systems underlying the timing of movements. J Neurosci. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 28.Grahn JA, Rowe JB. Finding and feeling the musical beat: Striatal dissociations between detection and prediction of regularity. Cereb Cortex. 2013;23:913–921. doi: 10.1093/cercor/bhs083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Grube M, Cooper FE, Chinnery PF, Griffiths TD. Dissociation of duration-based and beat-based auditory timing in cerebellar degeneration. Proc Natl Acad Sci USA. 2010;107:11597–11601. doi: 10.1073/pnas.0910473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grube M, Lee KH, Griffiths TD, Barker AT, Woodruff PW. Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Front Psychol. 2010;1:171. doi: 10.3389/fpsyg.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Teki S, Grube M, Griffiths TD. A unified model of time perception accounts for duration-based and beat-based timing mechanisms. Front Integr Nuerosci. 2012;5:90. doi: 10.3389/fnint.2011.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch G, et al. Impaired reproduction of second but not millisecond time intervals in Parkinson’s disease. Neuropsychologia. 2008;46:1305–1313. doi: 10.1016/j.neuropsychologia.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Coull JT, Davranche K, Nazarian B, Vidal F. Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia. 2013;51:309–319. doi: 10.1016/j.neuropsychologia.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Bueti D, Walsh V, Frith C, Rees G. Different brain circuits underlie motor and perceptual representations of temporal intervals. J Cogn Neurosci. 2008;20:204–214. doi: 10.1162/jocn.2008.20017. [DOI] [PubMed] [Google Scholar]
- 38.Coull JT, Frith CD, Büchel C, Nobre AC. Orienting attention in time: Behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38:808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- 39.Bolger D, Coull JT, Schön D. Metrical rhythm implicitly orients attention in time as indexed by improved target detection and left inferior parietal activation. J Cogn Neurosci. 2014;26:593–605. doi: 10.1162/jocn_a_00511. [DOI] [PubMed] [Google Scholar]
- 40.Davranche K, Nazarian B, Vidal F, Coull J. Orienting attention in time activates left intraparietal sulcus for both perceptual and motor task goals. J Cogn Neurosci. 2011;23:3318–3330. doi: 10.1162/jocn_a_00030. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi MJ, Kantele M, Walsh V, Carlson S, Kanai R. Dissociable neuroanatomical correlates of subsecond and suprasecond time perception. J Cogn Neurosci. 2014;26:1685–1693. doi: 10.1162/jocn_a_00580. [DOI] [PubMed] [Google Scholar]
- 42.Drake C, Botte M-CC. Tempo sensitivity in auditory sequences: Evidence for a multiple-look model. Percept Psychophys. 1993;54:277–286. doi: 10.3758/bf03205262. [DOI] [PubMed] [Google Scholar]
- 43.Keele SW, Nicoletti R, Ivry RI, Pokorny RA. Mechanisms of perceptual timing: Beat-based or interval-based judgements? Psychol Res. 1989;50:251–256. [Google Scholar]
- 44.Merchant H, Zarco W, Pérez O, Prado L, Bartolo R. Measuring time with different neural chronometers during a synchronization-continuation task. Proc Natl Acad Sci USA. 2011;108:19784–19789. doi: 10.1073/pnas.1112933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Besle J, et al. Tuning of the human neocortex to the temporal dynamics of attended events. J Neurosci. 2011;31:3176–3185. doi: 10.1523/JNEUROSCI.4518-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanics G, et al. Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci. 2010;30:13578–13585. doi: 10.1523/JNEUROSCI.0703-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capizzi M, Sanabria D, Correa Á. Dissociating controlled from automatic processing in temporal preparation. Cognition. 2012;123:293–302. doi: 10.1016/j.cognition.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 48.de la Rosa MD, Sanabria D, Capizzi M, Correa A. Temporal preparation driven by rhythms is resistant to working memory interference. Front Psychol. 2012;3:308. doi: 10.3389/fpsyg.2012.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson F, Hesslow G. Theoretical considerations for understanding a Purkinje cell timing mechanism. Commun Integr Biol. 2014;7:e994376. doi: 10.4161/19420889.2014.994376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trillenberg P, Verleger R, Teetzmann A, Wascher E, Wessel K. On the role of the cerebellum in exploiting temporal contingencies: Evidence from response times and preparatory EEG potentials in patients with cerebellar atrophy. Neuropsychologia. 2004;42:754–763. doi: 10.1016/j.neuropsychologia.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Nozaradan S, Schwartze M, Obermeier C, Kotz SA. Specific contributions of basal ganglia and cerebellum to the neural tracking of rhythm. Cortex. 2017;95:156–168. doi: 10.1016/j.cortex.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 53.Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- 55.Grahn JA, Henry MJ, McAuley JD. FMRI investigation of cross-modal interactions in beat perception: Audition primes vision, but not vice versa. Neuroimage. 2011;54:1231–1243. doi: 10.1016/j.neuroimage.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Praamstra P, Pope P. Slow brain potential and oscillatory EEG manifestations of impaired temporal preparation in Parkinson’s disease. J Neurophysiol. 2007;98:2848–2857. doi: 10.1152/jn.00224.2007. [DOI] [PubMed] [Google Scholar]
- 57.Te Woerd ES, Oostenveld R, de Lange FP, Praamstra P. Impaired auditory-to-motor entrainment in Parkinson’s disease. J Neurophysiol. 2017;117:1853–1864. doi: 10.1152/jn.00547.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotz SA, Schmidt-Kassow M. Basal ganglia contribution to rule expectancy and temporal predictability in speech. Cortex. 2015;68:48–60. doi: 10.1016/j.cortex.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 59.Bareš M, Lungu OV, Husárová I, Gescheidt T. Predictive motor timing performance dissociates between early diseases of the cerebellum and Parkinson’s disease. Cerebellum. 2010;9:124–135. doi: 10.1007/s12311-009-0133-5. [DOI] [PubMed] [Google Scholar]
- 60.Jurkowski AJ, Stepp E, Hackley SA. Variable foreperiod deficits in Parkinson’s disease: Dissociation across reflexive and voluntary behaviors. Brain Cogn. 2005;58:49–61. doi: 10.1016/j.bandc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson’s disease: Heterogeneity in temporal performance. Exp Brain Res. 2008;184:233–248. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- 62.Jones CRG, Malone TJL, Dirnberger G, Edwards M, Jahanshahi M. Basal ganglia, dopamine and temporal processing: Performance on three timing tasks on and off medication in Parkinson’s disease. Brain Cogn. 2008;68:30–41. doi: 10.1016/j.bandc.2008.02.121. [DOI] [PubMed] [Google Scholar]
- 63.Coltheart M. Assumptions and methods in cognitive neuropsychology. In: Rapp B, editor. The Handbook of Cognitive Neuropsychology: What Deficits Reveal about the Human Mind. Psychology Press; New York: 2001. pp. 3–21. [Google Scholar]
- 64.Schwartze M, Kotz SA. A dual-pathway neural architecture for specific temporal prediction. Neurosci Biobehav Rev. 2013;37:2587–2596. doi: 10.1016/j.neubiorev.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Praamstra P, Kourtis D, Kwok HF, Oostenveld R. Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci. 2006;26:5448–5455. doi: 10.1523/JNEUROSCI.0440-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlerf JE, Spencer RMC, Zelaznik HN, Ivry RB. Timing of rhythmic movements in patients with cerebellar degeneration. Cerebellum. 2007;6:221–231. doi: 10.1080/14734220701370643. [DOI] [PubMed] [Google Scholar]
- 67.O’Boyle DJ, Freeman JS, Cody FWJ. The accuracy and precision of timing of self-paced, repetitive movements in subjects with Parkinson’s disease. Brain. 1996;119:51–70. doi: 10.1093/brain/119.1.51. [DOI] [PubMed] [Google Scholar]
- 68.Mello GBM, Soares S, Paton JJ. A scalable population code for time in the striatum. Curr Biol. 2015;25:1113–1122. doi: 10.1016/j.cub.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 69.Soares S, Atallah B, Paton J. Midbrain dopamine neurons control judgment of time. Science. 2016;354:1273–1127. doi: 10.1126/science.aah5234. [DOI] [PubMed] [Google Scholar]
- 70.Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- 71.Jones CRG, Jahanshahi M. Motor and perceptual timing in Parkinson’s disease. In: Merchant H, de Lafuente V, editors. Neurobiology of Interval Timing. Springer; New York: 2014. pp. 265–290. [Google Scholar]
- 72.Cope TE, Grube M, Singh B, Burn DJ, Griffiths TD. The basal ganglia in perceptual timing: Timing performance in multiple system Atrophy and Huntington’s disease. Neuropsychologia. 2014;52:73–81. doi: 10.1016/j.neuropsychologia.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teki S, Grube M, Kumar S, Griffiths TD. Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci. 2011;31:3805–3812. doi: 10.1523/JNEUROSCI.5561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teki S, Griffiths TD. Brain bases of working memory for time intervals in rhythmic sequences. Front Neurosci. 2016;10:239. doi: 10.3389/fnins.2016.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Breska A, Ivry RB. Taxonomies of timing: Where does the cerebellum fit in? Curr Opin Behav Sci. 2016;8:282–288. doi: 10.1016/j.cobeha.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piras F, Coull JT. Implicit, predictive timing draws upon the same scalar representation of time as explicit timing. PLoS One. 2011;6:e18203. doi: 10.1371/journal.pone.0018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trouillas P, et al. The Ataxia Neuropharmacology Committee of the World Federation of Neurology International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 78.Fahn S, Elton RL. The unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson’s Disease. McMellam Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 79.Brainard DH. The psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 80.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.