Abstract
Tyrosine phosphorylation is a common means of regulating protein functions and signal transduction in multiple cells. Protein tyrosine phosphatases (PTPs) are a large family of signaling enzymes that remove phosphate groups from tyrosine residues of target proteins and change their functions. Among them, receptor-type PTPs (RPTPs) exhibit a distinct spatial pattern of expression and play essential roles in regulating neurite outgrowth, axon guidance, and synaptic organization in developmental nervous system. Some RPTPs function as essential receptors for chondroitin sulfate proteoglycans that inhibit axon regeneration following CNS injury. Interestingly, certain RPTPs are also important to regulate functions of immune cells and development of autoimmune diseases. PTPσ, a RPTP in the LAR subfamily, is expressed in various immune cells and regulates their differentiation, production of various cytokines and immune responses. In this review, we highlight the physiological and pathological significance of PTPσ and related molecules in both nervous and immune systems.
Keywords: Protein tyrosine phosphatase σ (PTPσ), Neuroplasticity, Chondroitin sulfate proteoglycan (CSPG), receptor, Axon regeneration, Immune cell, Dendritic cell, T lymphocyte, Multiple sclerosis
1. Introduction
Protein tyrosine kinases and protein tyrosine phosphatases (PTPs) play essential roles in regulating signal transduction in multiple cell types by tyrosine phosphorylation and dephosphorylation, respectively. PTPs include non-receptor-type PTPs and receptor-type PTPs (RPTPs), which are vital cell surface proteins that have intracellular tyrosine phosphatase activity and extracellular domains with sequence homology to cell adhesion molecules. In contrast to the well-studied protein tyrosine kinases, the properties of most RPTPs remain less clear, including their specific substrates, regulation, biological functions and possible roles in human disorders (Han et al., 2016; Takahashi and Craig, 2013). The RPTP family includes eight sub-families based on their extracellular domain structures. Among them, type IIa RPTPs include three members in vertebrates, the leukocyte common antigen-related (LAR), PTPσ, and PTPδ. These RPTPs contain typical cell adhesion Ig and fibronectin III domains, which contribute to their interactions with the extracellular matrix (ECM) molecules. Structurally, RPTPs have two cytoplasmic tandem regions, the membrane-proximal PTP domain (D1, catalytically active) and the membrane-distal PTP domain (D2, inactive) (Takahashi and Craig, 2013). Additional splicing of small exon segments contributes to the structural diversity of the ectodomain (Pulido et al., 1995).
During development, LAR and PTPσ are widely expressed by multiple tissues including the nervous system, lung, kidney and thymus, while PTPδ is more restricted in the nervous system (Schaapveld et al., 1998; Shishikura et al., 2016; Sommer et al., 1997; Wang et al., 1995; Zhang et al., 1998). In mammalian nervous system, type IIa RPTPs (LAR, PTPσ, and PTPδ) are important for regulating synaptogenesis (Takahashi and Craig, 2013; Um and Ko, 2013), especially formation of excitatory synapses by interacting with postsynaptic netrin-G ligand-3 (NGL-3) (Kwon et al., 2010). The type IIa RPTPs are also expressed in adult nervous system, but their functional significance is not known completely. Deleting each of these RPTPs shows distinctive morphological and functional phenotypes in the nervous system of adult mice (Kolkman et al., 2004; Meathrel et al., 2002; Uetani et al., 2006; Uetani et al., 2000; Yeo et al., 1997). Among these three RPTPs, LAR and PTPσ have been shown to be important functional receptors that bind chondroitin sulfate proteoglycans (CSPGs) with high affinity and mediate their inhibitory effects through the convergent and divergent signaling pathway (Fisher et al., 2011; Ohtake and Li, 2015; Ohtake et al., 2016; Shen et al., 2009). Following CNS injury, severed axons fail to regenerate in adult mammalians partly due to upregulation of various inhibitory ECM molecules around the lesion, especially the potent axon growth inhibitors of CSPGs. Whether PTPδ functions as a receptor of CSPGs remains to be determined.
Multiple PTPs are expressed preferentially in the haematopoietic system and some of them have been implicated in regulating development and functions in the immune system (Pike and Tremblay, 2013). Altered PTP activities may induce inappropriate proliferation, survival and activation of immune cells, and contribute to pathophysiology of inflammation and autoimmunity diseases. Thus, PTPs have the complex and diverse functions in the immune system and may become molecular targets for treating immune related disorders. Out of the three type-IIa RPTPs, LAR is expressed on immature thymocytes although its phosphatase activity appears unnecessary for T cell development and function (Kondo et al., 2010; Terszowski et al., 2001). LAR and PTPσ are specifically co-expressed in murine plasmacytoid dendritic cells (pDCs) and deletion of both simultaneously increases interferon production by pDCs (Bunin et al., 2015). Recently, we demonstrate that PTPσ is expressed in multiple immune cells, including DCs, and is critical for regulating their activation, cytokine production and immune-mediated inflammation (Ohtake et al., 2017). Because PTPσ is highly expressed in both nervous and immune systems and has crucial functions in both systems, this review will focus on the diverse functions of PTPσ and its related genes, and their significance in mediating neurological and autoimmune disorders.
2. RPTPs regulates neuronal growth and synapse formation during development
Three type-IIa RPTPs show divergent distribution patterns in the developmental nervous system. PTPσ is widely expressed throughout the central and peripheral nervous system during embryonic development. The high levels of its mRNA are especially detected in several structures, including ventricular and subventricular zones, cortex, dorsal root ganglia (DRG), cranial nerve ganglia, olfactory epithelium, and retina (Wang et al., 1995). During postnatal development, its expression levels decrease in most brain regions, although certain areas still exhibit high levels, such as hippocampus. In contrast, LAR mRNA is expressed in neural and non-neural tissues with different patterns from PTPσ. High levels of LAR mRNA are detected in epithelial layer of various embryonic tissues and in neuroepithelium and DRGs during embryogenesis. LAR expression shows developmental regulation during neural cell growth and differentiation (Longo et al., 1993). During embryonic development, PTPδ expression is relatively low and limited to differentiated cells of several structures, including olfactory bulb, cortical plate, thalamic nuclei, and olivary nucleus (Schaapveld et al., 1998; Sommer et al., 1997).
RPTPs are localized to the growth cone at axonal tip of immature neurons, while they are largely localized to the dendritic spines and excitatory synapse of mature neurons (Wyszynski et al., 2002). RPTPs are localized to distinct types of synapses. In culture hippocampal neurons, PTPσ-immunostained puncta overlapped with the axonal vesicular glutamate transport protein 1, indicating its localization to CNS axons (Takahashi et al., 2011). LAR is concentrated in mature synapses in hippocampal neuronal cultures, and is vital for development and maintenance of excitatory synapses. LAR knockdown causes loss of excitatory synapses and dendritic spines, decrease of surface AMPA receptors, impaired dendritic targeting of cadherin-beta-catenin complex and dysfunction in excitatory synapses (Dunah et al., 2005; Woo et al., 2009). In contrast, in addition to expression in excitatory synapses, PTPδ accumulates in inhibitory presynapses and regulates their genesis by interacting with postsynaptic adhesion molecule Slit and Trk-like family proteins 3 (Slitrk3) (Takahashi et al., 2012). Thus, individual RPTPs appear to target different synapse types probably by binding to diverse ligands in distinct types of synapses.
RPTPs, including PTPσ and LAR, mediate various cell-ECM adhesions by binding some extracellular ligands, such as heparan sulfate proteoglycans (HSPGs) and CSPGs (Aricescu et al., 2002; Coles et al., 2011; Johnson et al., 2006). HSPGs are the cell-surface and ECM glycoproteins usually containing one or more covalently attached heparan sulfate (HS) chains, a type of glycosaminoglycan (GAG). CSPGs are a diverse family of ECM molecules characterized by containing chondroitin sulfate (CS) chains. Each of the HSPGs and CSPGs comprises of a core protein and a variable number of highly sulfated GAG side chains frequently with negative charge. GAGs are long unbranched polysaccharides with repeating disaccharides, which comprise an amino sugar (N-acetylglucosamine or N-acetylgalactosamine) along with a uronic sugar (glucuronic acid or iduronic acid) or galactose. GAGs are classified into CS, HS, hyaluronic acid, dermatan sulfate, and keratan sulfate based on their component sugars. CS chains contain N-acetylgalactosamine and glucuronic acid in contrast to N-acetylglucosamine and glucuronic acid in HS chains. RPTPs interact with ECM molecules by binding the GAG chains of proteoglycans (Dickendesher et al., 2012; Fisher et al., 2011; Shen et al., 2009). PTPσ binds to CS-D, CS-E, and DS, but not to CS-A or CS-C (Dickendesher et al., 2012). Recently, Geller’s group demonstrated that the main binding sites for PTPσ are located to neurons in adult mouse brain, but are not to GAG chains of proteoglycans (Yi et al., 2014). Further studies are required to clarify these discrepancies.
The highly-controlled sulfation patterns on CS chains are important for mediating CSPG functions (Miller and Hsieh-Wilson, 2015; Yu et al., 2018) although various studies revealed inconsistent results on the roles of different CS chains. CS-E, not CS-A, could suppress outgrowth of both cerebral cortical and DRG neurons (Brown et al., 2012; Butterfield et al., 2010; Karumbaiah et al., 2011; Verna et al., 1989), but CS-E was also reported to promote neurite growth of hippocampal and cortical neurons (Pulsipher et al., 2014; Tully et al., 2004). CS-C was either inhibitory or non-inhibitory to neurite growth reported by different groups (Brown et al., 2012; Butterfield et al., 2010). In contrast, CS-A, not CS-C, was found inhibitory to neurite growth of cerebellar granule cells (Wang et al., 2008). Therefore, it will be important to further elucidate the spatiotemporal regulation and precise functions of various CS chains, their specific molecular interactions and downstream signaling pathways.
CS and HS chains share common uronic sugar residues (glucuronic acid and iduronic acid) and a linkage region of xylose–galactose, but they consist of different amino sugar disaccharides, the N-acetylgalactosamine for CS and the N-acetylglucosamine for HS added by distinct enzymes (Lindahl et al., 2015; Yu et al., 2018). CSPGs and HSPGs regulate growth, guidance, and connections of CNS neurons, but these two subclasses of sulfated proteoglycans have distinct functions on neuronal growth, typically with suppression by the former and promotion by the latter on axonal elongation (Bandtlow and Zimmermann, 2000; Coles et al., 2011; Silver and Miller, 2004). Presence of CSPGs remarkably attenuated neurite outgrowth of cultured DRG neurons (Dill et al., 2008; Snow et al., 1990; Snow and Letourneau, 1992). In contrast, treatment with HSPGs, including glypican-2, strongly promoted elongation of cultured neurons in vitro (Coles et al., 2011; Hantaz-Ambroise et al., 1987; Lander et al., 1982).
HSPGs and CSPGs regulate axon growth during development through their HS or CS chains. HS treatment induced axon defasciculation and growth in incorrect directions in cultured cockroach embryos, while CS treatment had no effect. Enzymatic degradation of HS by heparitinase resulted in axon perturbation, whereas degradation of CS had no effect (Wang and Denburg, 1992). Similarly, heparin, a glycosaminoglycan containing heparan sulfate, resulted in abnormal axon behavior in retinal ganglion cells from Xenopus laevis embryos (Walz et al., 1997). CSPGs also play a remarkable role in guiding axons by forming inhibitory areas during development. CSPG expression shows precise spatial and temporal pattern in developing retina. CSPG was detected during retinal development of rat at embryonic day 12.5 (E12.5), gradually decreased from the central area to the periphery, and no longer detectable at E17 (Abbott et al., 2007). Treatment of cultured embryonic retina with chondroitinase ABC (ChABC) to remove CS GAG chains induced abnormal retinal axon projections into the region where normal axons could not enter (Snow et al., 1991). Therefore, proteoglycans regulate axon guidance and extension during development probably by interacting with RPTPs, including PTPσ.
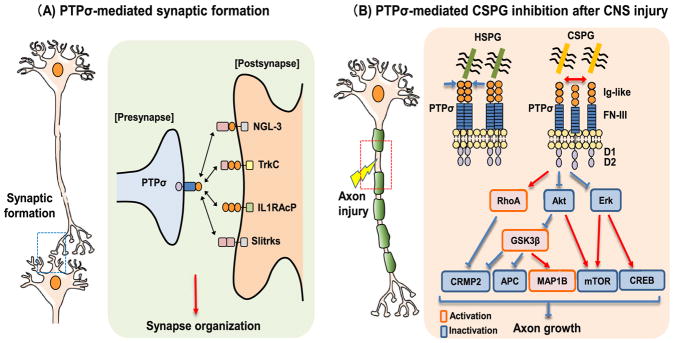
RPTPs play an important role in mediating synapse formation during development. Based on the expression patterns and phenotypes in transgenic animals, PTPσ and PTPδ appear to play more important roles than LAR for synapse organization during development. Functional interactions of RPTPs with a number of postsynaptic binding partners generate a complicated synaptic network and control synapse formation. Presynaptic RPTPs form trans-synaptic adhesion complexes with multiple postsynaptic adhesion molecules (Fig. 1A), including NGL-3 (Kwon et al., 2010; Woo et al., 2009), neurotrophin receptor TrkC (Takahashi et al., 2011), interleukin-1 (IL1) receptor accessory protein-like 1 (IL1RAPL1) (Valnegri et al., 2011; Yoshida et al., 2011), its paralog IL1RAPL2 (Pavlowsky et al., 2010; Valnegri et al., 2011; Yoshida et al., 2011), IL-1 receptor accessory protein (IL-1RAcP) (Yoshida et al., 2012), and Slitrks (Takahashi et al., 2012; Yim et al., 2013). Though all three type-IIa RPTPs can bind to NGL-3 through their first two FNIII domains and also to IL-1RAcP, Slitrks bind to PTPσ and PTPδ selectively through the Ig domains. In contrast, TrkC binds to PTPσ and IL1RAPL1 binds to PTPδ only.
Fig. 1.
PTPσ is important to regulate synapse formation by interacting with several postsynaptic proteins (A) and to mediate CSPG inhibition of neuronal growth as a receptor through multiple intracellular pathways (B). (A) PTPσ is localized to the growth cone at axonal tip in immature neurons during development and serves as a presynaptic hub by interacting with diverse postsynaptic partners, including NGL-3, TrkC, IL1RAcP and Slitrks, thus regulating the synapse formation in developmental neuron. (B) PTPσ can bind both HSPGs and CSPGs. Binding with the former induces PTPσ clustering and inactivation, promoting axon growth. In contrast, binding with CSPGs prevents PTPσ dimerization and activates this receptor and its downstream signaling pathways, inhibiting axon growth after CNS injury. Intracellularly, activation of PTPσ by CSPGs activate RhoA-Rock signaling and inactivate Akt and Erk pathways. Activation and/or inactivation of these signaling pathways mediate CSPG inhibition by other downstream signaling molecules, including GSK3β, CRMP2, APC, MAP1B, mTOR and CREB. Ig-like: immunoglobulin-like domains; FN-III: fibronectin type III domain; D1: D1 catalytic domain; D2: D2 non-catalytic domain.
Axonal RPTPs accumulate NGL-3 on dendrites and appear to promote excitatory postsynaptic development by interactions with NGL-3 and postsynaptic density protein (PSD-95). NGL-3 mainly induced excitatory presynaptic differentiation of contacting axons in co-cultured system and this effect is suppressed by treatment with soluble LAR ectodomain (Woo et al., 2009). Induced aggregation of NGL-3 on dendrites resulted in dendritic clustering of excitatory, but not inhibitory, postsynaptic proteins, including the scaffold protein PSD-95. Accordingly, the C-terminus of NGL-3 has been shown to bind the PDZ domains of PSD-95 (Kim et al., 2006). Although all three type IIa RPTPs can bind and interact with NGL-3, PTPσ appears to be the main pre-synaptic component based on their exclusive excitatory synaptic localization, affinity for NGL-3 and expression pattern in the brain.
Presynaptic PTPσ interacts with postsynaptic TrkC and seems to regulate development of excitatory synapses transduced by the intracellular domain of PTPσ. Trks, a family of tyrosine kinases, are well characterized as the receptors of neurotrophic factors that control neural survival, differentiation, proliferation, the fate of neural precursors, axon and dendrite growth and patterning. With unbiased expression screen in co-culture system, PTPσ, but not PTPδ and LAR, was identified as a TrkC binding partner (Takahashi et al., 2011). Moreover, TrkC induced excitatory, but not inhibitory, presynaptic differentiation and this effect is abolished by axonal expression of mutated PTPσ lacking the intracellular region.
RPTPs promote excitatory synapse development also by interacting with members in the IL-1/Toll receptor family (IL1RAPL1, IL1RAPL2 and IL1RAcP) and activating PSD-95 and Jun-N-terminal kinase (JNK) signaling pathways. IL1-RAcP interacts with all three type IIa RPTPs, although IL1RAPL1 binds PTPδ, but not PTPσ and LAR. IL1RAPL1-PTPδ interactions recruited GTPase-activating protein to regulate excitatory synapse formation, as well as dendritic synapse number and morphogenesis (Valnegri et al., 2011; Yoshida et al., 2011). IL-1RAcP includes two isoforms, the ubiquitously-expressed IL-1RAcP and the CNS-specific IL-1RAcPb (Smith et al., 2009). Both isoforms regulate differentiation of excitatory presynapses, while IL-1RAcPb also contributes to formation of dendritic spines. Because IL-1RAcP interacted with PTPδ through the extracellular domain and the IL-1RAcP-mediated formation of presynapses is partly abolished in cortical neurons derived from PTPδ-deficient mice (Yoshida et al., 2012), the interactions of IL-1RAcP with other RPTPs, such as PTPσ and/or LAR, may also play a significant role in this action. Given that IL1RAPL1-dificient neurons have reduced levels of phosphorylated PSD-95 and JNK proteins, these signaling pathways appear to mediate the RPTP-IL1RPL1 interactions (Pavlowsky et al., 2010).
Slitrks play important roles in regulating synapse development by forming protein complex with RPTPs (Yim et al., 2013). Slitrks are a family of transmembrane receptors that contain six leucine-rich repeat domains. All six members (Slitrk1–6) are able to induce presynaptic differentiation in neuronal co-cultures. Five Slitrks, except for Slitrk3, could interact with PTPσ and contribute to excitatory synaptogenic activities. Only Slitrk3 can induce inhibitory, but not excitatory, pre-synaptic differentiation in vitro and in vivo by interact with presynaptic PTPδ (Takahashi et al., 2012). Of note, because CSPG plays an important role in restricting neuronal plasticity following injury, interactions of RPTPs with postsynaptic adhesion molecules during development may also be regulated by competing with CSPGs in a spatiotemporal manner.
3. PTPσ mediates neuroplasticity in mature neurons
Although a number of brain regions show obvious mRNA distribution of PTPσ, LAR and PTPδ in the adult mice, their expression patterns are very diverse (Schaapveld et al., 1998; Shishikura et al., 2016; Wang et al., 1995). During postnatal development, the expression level of PTPσ decrease dramatically in most brain regions, but high levels continue to be detected in hippocampus, especially the pyramidal cell layer (Kwon et al., 2010). Strong signals for PTPσ are also detected in several other areas of adult mammals, including the internal granule layer of olfactory bulb, piriform cortex, tenia tecta, septal area and cerebellum. Strong LAR signals are found in the internal granule layer of olfactory bulb, subventricular zone of caudate putamen and cerebellum, but are less abundantly in layer IV of cortex and septal area. In contrast, PTPδ protein or mRNA is expressed in the hippocampus (particularly the CA2 and CA3 regions), layer IV of cortex, granule cell layer of cerebellum, thalamic nuclei, and mitral and granular cell layers of the olfactory bulb, but it is undetectable in septal area. Therefore, three type IIa RPTPs exhibit overlapping, but differential distribution patterns in the developed brain.
PTPσ modulates the neuronal plasticity in mature neurons. PTPσ-deficient mice exhibit severe growth retardation, high neonatal mortality and neurological deficiencies, including thinning of the corpus callosum and cerebral cortex, hippocampal dysgenesis, abnormal pituitary development, motor dysfunction and defective proprioception (Meathrel et al., 2002; Uetani et al., 2006). PTPσ null mice also have increased hippocampal mossy fibers and axon sprouting during aging, consistent with PTPσ limiting synapse formation. PTPσ deficiency increases the frequency of miniature post-synaptic currents mediated by α-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid (AMPA) receptor, accompanied by an increase in synapse density, but decreases synaptic efficiency. Moreover, the role of PTPσ in CNS plasticity has been demonstrated in learning and memory. PTPσ null mice show reduced long-term potentiation (LTP, an activity-dependent synaptic plasticity) in hippocampal Schaffer collateral-CA1 synapses and enhanced performance in a novel object recognition memory test (Horn et al., 2012).
LAR deficient mice have smaller basal forebrain cholinergic neurons and reduced cholinergic innervation of their target neurons in the dentate gyrus (Yeo et al., 1997). Mice lacking the phosphatase domains of LAR have spatial learning deficiency and hyperactivity (Kolkman et al., 2004). PTPδ knockout mice also exhibit marked motor dysfunction, impaired visuospatial processing with low survival rates (Uetani et al., 2006; Uetani et al., 2000), and impaired learning and memory tasks and enhanced LTP (Uetani et al., 2000). The phenotype differences in deficiency of three RPTPs may result from diverse ligands, subcellular distribution or downstream signaling pathways.
PTPσ regulates synaptic plasticity, learning and memory in adult CNS, but it is unclear whether its function is associated with the interactions with perineuronal nets (PNN) molecules, especially CSPGs. PNNs are composed of ECM macromolecules (including hyaluronan, CSPGs, tenascin-R, link proteins and other components) and organize these molecules into specific structures (Busch and Silver, 2007; Hagihara et al., 1999; Kwok et al., 2011; Massey et al., 2006). In extracellular space, CSPGs interact with HA polymer on cell surface and induce a stable connections with HA polymer chains and other linked proteins. The C-terminal domains of CSPG core proteins bind tenascin-R to form highly organized constructions of PNNs. PNNs wrap the neuronal cell body, proximal dendrites and synapses of various neuronal populations. CNS starts to form PNNs in the late stage of development, especially during critical periods of synaptic maturation (Kwok et al., 2011). PTPσ is one of the two identified RPTP receptors for CSPGs, the major components of PNN (Shen et al., 2009), but Geller’s group did not detect PTPσ binding to PNN in the mouse brain (Yi et al., 2014). Further studies are required to illustrate the molecular mechanisms for PTPσ-mediated neuronal plasticity.
PNNs may play critical roles in the mature CNS, including synapse stabilization, neuroprotection, ionic buffering and neuronal plasticity. PNNs have been implicated to maintain and modify cognition, learning and memory functions. PNN formation in amygdala of mice affects developmental shift in the ability to erase fear memories by extinction (Gogolla et al., 2009). Organization of CSPGs in PNNs mediates formation of erasure-resistant fear memories because local treatment of amygdala with ChABC to digest CSPGs in adult mice re-enabled the subsequent erasure of fear memories by extinction. PNNs also play critical roles in controlling neuronal plasticity in hippocampus. Deficiency of PNN structures in transgenic mice shows reduced LTP by theta-burst stimulation in CA1 region of hippocampus. Pretreatment of cultured hippocampal slices with ChABC induces similar reduction of LTP in vitro (Bukalo et al., 2001). Deletion of brevican, one of brain-specific CSPGs, in mutant mice, or treatment of wild-type mice with anti-brevican antibody resulted in impaired LTP, although they exhibited normal behavior and spatial memory (Brakebusch et al., 2002).
4. PTPσ functions as a CSPG receptor to mediate axon regeneration in the CNS
After CNS injuries, reactive astrocytes generate extremely high levels of CSPGs, which potently suppress axon growth into and beyond the lesion area (Bradbury et al., 2002; Busch and Silver, 2007; Jones et al., 2003). The major CSPGs expressed in the CNS include lecticans (neurocan, versican, aggrecan, and brevican), phosphacan and NG2. Lecticans share many similar domains at both N- and C-termini of the core proteins and their core proteins are linked by a central CS-GAG anchoring backbone bound one or numerous long chain CS-GAG polysaccharide (Yamaguchi, 2000). Lecticans function as linkers of the ECM molecules. Phosphacan is the extracellular domain of transmembrane RPTPβ. NG2 is a transmembrane CSPG and has no significant homologies to other CSPGs. Because removing GAGs or preventing their sulfation neutralizes most suppression of axon growth by CSPGs in vitro (Gilbert et al., 2005; Sherman and Back, 2008; Wang et al., 2008), the sulfated GAG chains play crucial roles for CSPG function. CSPGs has been known to impede axon elongation for almost three decades, but the detailed mechanisms are not well known. The general mechanisms identified include binding functional CSPG receptors on neuronal membrane, formation of non-permissive PNNs that causes steric hindrance of growth-promoting adhesion molecules such as laminin and integrins, and facilitating functions of chemo-repulsive molecules, such as Sema 3A and 5A (Condic et al., 1999; Kantor et al., 2004; Tan et al., 2011). Transmembrane receptors appear important in conveying CSPG inhibition, including PTPσ, LAR, and Nogo receptors 1 and 3 (Dickendesher et al., 2012; Fisher et al., 2011; Lang et al., 2015; Shen et al., 2009; Xu et al., 2015). Especially, PTPσ and LAR bind CSPGs with high affinity and convey their inhibitions of axon extension after CNS injury.
CSPG neurocan can bind and interact with PTPσ through the CSPG GAG chains and a few positively-charged residues in the first Ig-like domain of PTPσ (Shen et al., 2009). DRGs derived from PTPσ deficient mice increased neurite growth on CSPG and this effect was specific to CSPG because PTPσ deletion does not overcome suppression of myelin-associated glycoprotein. PTPσ knockout mice showed regeneration of lesioned ascending sensory neurons into the CSPG-rich lesion area although regenerating axons failed to pass injured area (Shen et al., 2009). Corticospinal tract (CST) axons regrew into and beyond the lesion area in adult PTPσ knockout mice after spinal cord injury (SCI) (Fry et al., 2010). Consistently, PTPσ knockout mice exhibited enhanced regeneration of optic nerve and peripheral nerve after injury (McLean et al., 2002; Sapieha et al., 2005; Thompson et al., 2003). Moreover, PTPσ plays a crucial role in converting growth cones into a dystrophic state by tightly stabilizing them within CSPG substrates. Systemic treatment with a membrane permeable peptide mimetic of the PTPσ wedge domain, which regulates a variety of downstream signaling, restored extensive serotonergic innervation to the spinal cord caudal to lesion and promoted recovery of locomotor and urinary functions in adult rats with contusive SCI (Lang et al., 2015).
Similarly, CSPGs bind LAR dose-dependently also through CSPG GAG chains and the first Ig-like domain of LAR. LAR inhibition by transgenic deletion or pharmacological blockade with sequence-targeting blocking small peptides stimulated regrowth of serotonergic and CST axons, and functional recovery after transection SCI (Fisher et al., 2011; Xu et al., 2015). As another member of type IIa RPTPs, whether PTPδ also mediates CSPG suppression as a transmembrane receptor is unknown. However, PTPδ has recently been reported to contribute to Semaphorin-3A mediated growth cone collapse response in neuronal cultures probably by dephosphorylating tyrosine residues at the C-terminus of Fyn and activing this kinase (Nakamura et al., 2017). Notably, Semaphorin-3A is a component of PNN together with CSPGs and is able to bind the sugar chains of CSPGs with high affinity (Carulli et al., 2013).
Both CSPGs and HSPGs are the ligands of PTPσ in nervous system with a similar binding affinity. Crystallographic analysis suggests that CSPGs can prevent PTPσ dimerization (Fig. 1B), while HSPGs induce PTPσ clustering (Coles et al., 2014; Coles et al., 2011). CSPGs and HSPGs share the same binding site of PTPσ in the first Ig-like domain and they compete for PTPσ binding, resulting in opposite effects on axon elongation. CSPGs inhibit axon outgrowth in neurons derived from wild-type mice, but this effect is diminished in PTPσ-deficient neurons (Fry et al., 2010; Sapieha et al., 2005; Shen et al., 2009; Thompson et al., 2003). In contrast, HSPGs dramatically promote axon outgrowth in wild-type, but not PTPσ-deficient neurons. Therefore, the CSPGs/HSPGs ratio is critical for determining PTPσ dimerization status in regulating axon growth. However, some issues remain regarding the interactions between proteoglycans and RPTPs. The CS-C GAG chain used in previous study (Coles et al., 2011) did not bind to PTPσ (Dickendesher et al., 2012). Recently, Geller’s group found that the fourth FNIII-containing domain of PTPσ alone could bind to HSPG heparin independently of three Ig domains, but not to CS-E or dermatan sulfate (Katagiri et al., 2017). It will be interesting to further study detailed mechanisms for interactions between CSPGs/HSPGs and PTPσ and potential role of the newly-identified binding domain of PTPσ for heparin.
Several other studies further support that PTPσ and LAR are important functional receptors for CSPGs. Newly-formed neurons from neuronal restricted precursors are intrinsically insensitive to CSPGs because low level expression of PTPσ and LAR. Several factors secreted by cultured neuronal and glial restricted precursors decrease CSPG inhibition and stimulate axon growth in vitro (Ketschek et al., 2012). Both PTPσ and LAR are expressed selectively in bad-regenerating neurons and have overlapping cellular distributions in Lamprey, a type of jawless fish (G. Zhang et al., 2014), indicating that PTPσ and LAR mediate poor intrinsic regenerative capacity of bad-regenerating neurons and also serve as functional receptors for CSPGs in non-mammals.
PTPσ and LAR mediate CSPG inhibitions through both convergent and divergent downstream pathways in neurons. Recently, we compared effects of PTPσ and LAR on the activities of multiple signaling molecules using cultures of a neuronal cell line (Neuro-2A) to over-express these receptors and primary cultures of postnatal cerebellar neurons derived from knockout mice (Fisher et al., 2011; Ohtake et al., 2016). PTPσ and LAR employ a few signaling pathways commonly, including RhoA, Akt, extracellular signal-regulated kinase (Erk) and microtubule- associated protein 1B (MAP1B). Their actions in transmitting CSPG effects to inhibit axon growth also involve distinct signal molecules, including the use by PTPσ of collapsing response mediator protein 2 (CRMP2, Fig. 1B), adenomatous polyposis coli (APC), S6 ribosomal protein (S6) and cAMP response element-binding protein (CREB), whereas the use by LAR of cofilin, protein kinase Cζ (PKCζ) and liver kinase B1 (LKB1). Consistent with these results, deletion of both PTPσ and LAR showed additive effects in enhancement of axon growth in adult neurons in vitro. Thus, several intracellular signaling pathways are known to mediate CSPG actions through the RPTP receptors.
Previous reports also suggest that CSPGs use diverse pathways to suppress neuronal growth, including glycogen synthase kinase 3β (GSK3β) and PKC (Dill et al., 2008; Powell et al., 2001). Cultured axons contain transcripts encoding RhoA and applying CSPGs to axon compartment enhanced axonal RhoA synthesis (Walker et al., 2012). Inhibition of RhoA transcripts in axons increased their growth in the presence of CSPGs. Treatment of neurons with LAR inhibitory peptides or deletion of PTPσ elevated both Erk and Akt activities (Sapieha et al., 2005; Xie et al., 2001). In spite of recent progress in understanding intracellular signals of CSPGs, further studies are required to clarify some issues remained. For examples, Rho/Rock pathways inhibit both cofilin and CRMP2, but only PTPσ inhibits CRMP2 whereas only LAR inhibits cofilin (Ohtake et al., 2016). Likewise, mTOR/S6 and CREB are activated by Akt and Erk, but only PTPσ inhibits S6 and CREB signaling pathways.
5. PTPσ regulates functions of immune cells and development of autoimmune diseases
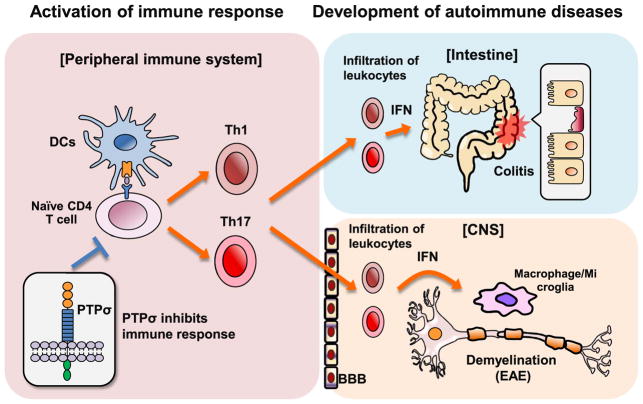
PTPσ is expressed by immune cells and has important functions in the immune system (Fig. 2). PTPσ, but not LAR and PTPδ, is expressed in human pDCs controlled by transcription factor E2–2 (Bunin et al., 2015). PTPσ is rapidly downregulated by pDC activation and its deficiency induces production of interferon α (IFNα) in pDCs. Antibody-mediated forced PTPσ activation inhibited Toll-like receptor 9-mediated pDC activation. pDCs can secrete type I IFN (IFNα or β) rapidly and abundantly in response to various stimuli and regulate viral infections through virus-specific T-cell responses. Accordingly, hyper-activation of pDCs is implicated in several autoimmune diseases, including inflammatory bowel disease (Cervantes-Barragan et al., 2012; Ganguly et al., 2013; Swiecki et al., 2010). DC-specific deletion of PTPσ in mice with a LAR null background leads to IFN production by pDCs, leukocyte infiltration and mild spontaneous colitis (Bunin et al., 2015). Consistently, three single nucleotide polymorphisms (SNPs) in human PTPRS (PTPσ) gene have been linked to ulcerative colitis, an inflammatory bowel disease characterized by dysregulation of auto-immune responses (Muise et al., 2007). PTPσ normally functions as a positive regulator of intestinal epithelial barrier by orchestrating epithelial cell adhesion through targeting of adherens junction proteins, including E-cadherin, β-catenin and ezrin (Murchie et al., 2014). Together, PTPσ is essential to balance DC tyrosine phosphorylation mediated by various kinases (Fujita et al., 2013) and to inhibit tyrosine phosphorylation-dependent pDC activation, spontaneous IFN production and immune-mediated intestinal inflammation.
Fig. 2.
PTPσ inhibits activation of immune cells and development of autoimmune diseases, including colitis and EAE. PTPσ deficiency induces activation of DCs (cDC and pDC), differentiation of CD4+ T cells into specific Th1 and Th17 cells, infiltration of leukocytes and generation of pro-inflammatory related cytokines including IFN. Thus, PTPσ inhibition facilitates development of autoimmune diseases, such as colitis and CNS autoimmune demyelinating disease EAE.
We recently identified critical role of PTPσ in regulating auto-immune disorders in the nervous system and extended the function of PTPσ in immune system (Ohtake et al., 2017). We demonstrate that transgenic deletion or pharmacological inhibition of PTPσ significantly exacerbates clinical symptoms and axon/myelin damages in the spinal cord of mice with experimental autoimmune encephalomyelitis (EAE), a widely-used model for multiple sclerosis (MS). Following immunization with myelin oligodendrocyte glycoprotein (MOG) peptide, PTPσ knockout mice showed pro-inflammatory responses in the spinal cord and lymphoid organs by changing expression levels of various inflammatory-related genes, including specific cytokines (Ifng and Il17a) and chemokines (Ccl2, Ccl5 and Cxcl10) (Ohtake et al., 2017). Interestingly, PTPσ deficiency stimulated activation of conventional DCs (cDCs), affected differentiation of CD4+ T lymphocytes directly, reduced number of regulatory T (Treg) cells, and facilitated infiltration of T cells and activation of macrophages and microglia in the CNS (Fig. 2). Moreover, adoptive transfer of PTPσ-deficient cDCs stimulated with lipopolysaccharide to wild-type mice augmented EAE clinical symptoms following pulsed MOG35–55 peptide immunization. PTPσ deletion also enhanced expression of genes mediating immune responses, including Tbx21 in Th1 and Rorc in Th17 cells (Ohtake et al., 2017). Consistently, PTPσ deficiency has been reported to reduce expression level of Foxp3, a lineage-specific marker for Treg (Fontenot et al., 2005; Ziegler, 2006), which delay the onset of spontaneous EAE (Lowther et al., 2013). Therefore, PTPσ is an important negative regulator for activation of pro-inflammatory responses of cDCs, differentiation of CD4+ T cells into Th1 and Th17 cells, and development of a CNS autoimmune disease. Notable, it is interesting to determine whether PTPσ directly regulates function of myelinating glia. PTPζ (PTPRZ), a member in the RPTP family, plays a negative role in oligodendrocyte differentiation during CNS development and remyelination in demyelinating CNS diseases by increasing apoptosis of mature oligodendrocytes (Harroch et al., 2002; Kuboyama et al., 2012).
PTPσ appears to regulate immune responses as a receptor of various immune cells by mediating constitutive signaling derived from the ECM ligands. The upstream signaling and physiological ligands that modulate PTPσ activity in immune cells or other cell types remain to be defined. HSPGs, the known ligands of RPTPs, have been reported to inhibit IFN production by macrophages in atherosclerosis mouse model (Gordts et al., 2014). Syndecan-1, a HSPG, could suppress various inflammatory responses and EAE development in mice and its deficiency exacerbated disease symptoms and impaired recovery in EAE mice (X. Zhang et al., 2013). In contrast, inhibition of CSPGs (also recognized ligands for RPTPs, but distinct type of proteoglycans from HSPGs) may become a therapeutic approach for autoimmune disease of MS. CSPGs are upregulated around the edge of MS lesions, whereas reduced in the center of active and chronic inactive plaques (Sobel and Ahmed, 2001). Treatment with disaccharidic degradation product of CSPGs significantly alleviated clinical symptoms in EAE mice through reducing IFNγ and TNFα production by splenocytes (Rolls et al., 2006; Zhou et al., 2010). Moreover, some cytokines, such as IFNγ and TGFβ1, could alter the expression level of CSPGs (Fujiyoshi et al., 2010; Smith and Strunz, 2005).
Also as a member of type IIa RPTPs, LAR is expressed during a certain stage of thymocyte development, but not in B cells. The physiological function of LAR in immune system remains controversial. One study indicates that the phosphatase activity of LAR is not required for T-cell development, repertoire selection and function (Terszowski et al., 2001), but the other group reported that LAR deficiency affected differentiation and expansion of immature thymocytes as well as negative and positive selection (Kondo et al., 2010). Moreover, T-cell receptor stimulation in response to calcium was significantly lower in thymocytes from LAR knockout mice, suggesting that LAR modulates T-cell receptor signaling for thymocyte differentiation.
6. PTPσ is a therapeutic molecular target for CNS injury and autoimmune disorders
Surmounting potent inhibition by CSPG-rich scar and its receptor-mediated downstream signaling is an important therapeutic target for achieving successful axon regeneration and functional recovery after CNS injuries. So far, the main in vivo therapeutic approach to overcome inhibition of CSPGs is enzymatic digestion with local application of ChABC. However, several disadvantages may prevent using this enzyme as a therapeutic choice for patients. ChABC cannot completely digest GAG chains from core glycoproteins and may leave undigested carbohydrate side chains on the molecules, which though less potent are still inhibitory (Lemons et al., 2003). ChABC loses its enzymatic activity rapidly at body temperature and cannot cross the blood brain barrier. A group attempted to generate the thermostabilized ChABC (Lee et al., 2010). A single local application could not be sufficient to overcome the inhibition due to continuous generation of CSPGs after injury. Bacterial ChABC may also induce autoimmune reactions after pulsed injections. Therefore, new strategies to overcome inhibitory effects by CSPGs are required to facilitate CNS axon regeneration and functional recovery. An alternative approach to overcome scar-sourced inhibition is to design novel compounds that block functions of CSPGs or their receptors, including PTPσ.
In contrast to invasive approach of applying ChABC locally, systemic administration of peptides, antibodies and chemicals could block CSPG inhibition efficiently. A peptide (called intracellular PTPσ peptide, ISP) mimetic of the catalytic domain of PTPσ could overcome CSPG inhibition efficiently. Systemic treatment with ISP enhanced serotonergic axon regrowth and facilitated functional recovery of locomotor and urinary systems in rat with contusive SCI (Lang et al., 2015). Previously, we demonstrated that peptide antagonists for LAR reduced CSPG inhibition in vitro and increased descending serotonergic axon growth and promoted sustained locomotor recovery in SCI mice after systemic administration (Fisher et al., 2011). Consistently, silencing PTPσ with lentivirus-mediated RNA interference promoted axon regeneration, synapse formation, and recovery of motor and sensory functions without affecting scar formation in rats with contusion SCI (Zhou et al., 2014). Because PTPσ dimerization suppresses its phosphatase activity and CSPGs activate PTPσ by preventing it from dimerizing after binding as ligands, the approach to induce and stabilize PTPσ dimerization could be effective for blocking its function. Indeed, a new PTPσ IgG monoclonal antibody could induce PTPσ dimerization and promote neurite outgrowth in SH-SY5Y cells, a neuroblastoma cell line (Wu et al., 2017). Recently, a novel CSPG inhibitor, fluorosamine (peracetylated 4-fluorinated analog of glucosamine) has been shown to reduce CSPG contents and to promote process outgrowth and differentiation in oligodendrocyte precursor cells, thus accelerating re-myelination after focal CNS demyelination in mice (Keough et al., 2016). Therefore, blocking CSPGs, their receptors, or CSPG-receptor interactions could overcome scar-mediated growth inhibitions and promote functional recovery after CNS injury. Given that multiple factors contribute to the repair failure after CNS injury, combing CSPG signaling blockade with other strategies, such as cell transplantation, is likely to be more effective.
Suppressing DC activation mediated by PTPσ may become an attractive immunotherapy for several immune diseases. PTPσ act as an inhibitory receptor on DCs that prevents DC hyperactivation, subsequent IFN production, and autoimmune related inflammation. pDC hyperactivation occurred in inflammatory bowel disease (Baumgart et al., 2011) and in colitis associated with Wiskott-Aldrich syndrome (Prete et al., 2013). Increased numbers of cDCs and pDCs accumulated in the cerebrospinal fluid and white matter of MS patients (Lande et al., 2008; Prete et al., 2013) and abnormalities in DCs have been associated with several stages of MS related disorders. Because PTPσ is important for suppressing immune and autoimmune responses mediated by pDCs, cDCs, CD4+ T lymphocytes and other cells, and its suppression contributes to various immune-related diseases, such as EAE and spontaneous mild colitis (Bunin et al., 2015; Ohtake et al., 2017), selective activation of this signaling protein may become an effective strategy for treating autoimmune related disorders.
Both PTPσ and its ligands, such as CSPGs, may become molecular targets for treating MS related disorders and other immune diseases. Any approaches that block PTPσ dimerization might activate this phosphatase and reduce overactivation of immune cells. Treatment with the disaccharide degradation product of CSPG (CSPG-DS), a CSPG derivative, significantly decreases production of IFNγ by brain-in-filtrating lymphocytes in EAE mice (Rolls et al., 2006; Zhou et al., 2010). CSPG-DS treatment also markedly alleviated the clinical symptoms of EAE by reducing infiltrating T cells and activated microglia. In contrast, treatment with CS-A, a complete CS GAG chain unattached to proteoglycans, exacerbated EAE symptoms and increased infiltrated T cells in the CNS and their differentiation into pathogenic types of Th1 and Th17 (Zhou et al., 2010). It is interesting to dissect the precise mechanisms for CSPG-DS and CS-A to regulate EAE development and whether PTPσ mediates these effects although CS-A does not bind PTPσ (Dickendesher et al., 2012).
7. Prospective
PTPσ plays important physiological and pathological roles in the nervous system of mammalians during development and in adults. PTPσ and other type IIa RPTPs are expressed in presynaptic terminals and make trans-synaptic adhesion complexes with post-synaptic adhesion molecules to organize synapse development and to modulate placidity of mature neurons, including learning and memory. Importantly, PTPσ, as well as LAR, functions as important receptors for CSPG inhibitors generated by reactive scar tissues. On the other hand, PTPσ is expressed by various immune cells, especially pDCs, cDCs and CD4+ T cells, and plays crucial roles for regulating immune responses and development of autoimmune diseases, including EAE. The tremendous advances in our understanding of PTPσ functions provide opportunity for developing effective therapeutic strategies for the disorders associated with dysfunctions of PTPσ and its related genes. Because of the diverse regulatory roles of PTPσ in different tissue systems, this phosphatase may become an important target for treating a number of human diseases, such as CNS injuries, MS and other autoimmune diseases. However, it seems important to develop effective approaches to target PTPσ and its signaling pathway selectively. For example, PTPσ suppression may have beneficial effects in the nervous system by promoting axon regeneration and neural repair, but it may also result in damaging roles in the other systems, such as inducing DC hyper-activation and autoimmune responses. Moreover, it is very important to further characterize functions of PTPσ and related molecules, and their detailed cellular and molecular mechanisms in various cell types. Better understanding physiology and pathophysiology of PTPσ-associated genes is likely to felicitate development of highly effective therapies for various neurological and autoimmune diseases.
Acknowledgments
This work was supported by research grants to SL from NIH (1R01NS079432 and 1R01EY024575) and Shriners Research Foundation (SHC-86300-PHI, SHC-86200-PHI-16 and SHC-85100).
References
- Abbott AP, Harris RC, Ryder KS. Application of hole theory to define ionic liquids by their transport properties. J Phys Chem B. 2007;111:4910–4913. doi: 10.1021/jp0671998. [DOI] [PubMed] [Google Scholar]
- Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol. 2002;22:1881–1892. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Metzke D, Guckelberger O, Pascher A, Grotzinger C, Przesdzing I, Dorffel Y, Schmitz J, Thomas S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn’s disease and ulcerative colitis. Clin Exp Immunol. 2011;166:46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H, Krug M, Bockers TM, Zhou X, Kreutz MR, Montag D, Gundelfinger ED, Fassler R. Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol. 2002;22:7417–7427. doi: 10.1128/MCB.22.21.7417-7427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci U S A. 2012;109:4768–4773. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, Schachner M, Dityatev A. Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience. 2001;104:359–369. doi: 10.1016/s0306-4522(01)00082-3. [DOI] [PubMed] [Google Scholar]
- Bunin A, Sisirak V, Ghosh HS, Grajkowska LT, Hou ZE, Miron M, Yang C, Ceribelli M, Uetani N, Chaperot L, Plumas J, Hendriks W, Tremblay ML, Hacker H, Staudt LM, Green PH, Bhagat G, Reizis B. Protein tyrosine phosphatase PTPRS is an inhibitory receptor on human and murine Plasmacytoid dendritic cells. Immunity. 2015;43:277–288. doi: 10.1016/j.immuni.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Butterfield KC, Conovaloff A, Caplan M, Panitch A. Chondroitin sulfate-binding peptides block chondroitin 6-sulfate inhibition of cortical neurite growth. Neurosci Lett. 2010;478:82–87. doi: 10.1016/j.neulet.2010.04.070. [DOI] [PubMed] [Google Scholar]
- Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F. Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci. 2013;57:10–22. doi: 10.1016/j.mcn.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science (New York, NY) 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles CH, Mitakidis N, Zhang P, Elegheert J, Lu W, Stoker AW, Nakagawa T, Craig AM, Jones EY, Aricescu AR. Structural basis for extracellular cis and trans RPTPsigma signal competition in synaptogenesis. Nat Commun. 2014;5:5209. doi: 10.1038/ncomms6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condic ML, Snow DM, Letourneau PC. Embryonic neurons adapt to the inhibitory proteoglycan aggrecan by increasing integrin expression. J Neurosci. 1999;19:10036–10043. doi: 10.1523/JNEUROSCI.19-22-10036.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–712. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill J, Wang H, Zhou FQ, Li S. Inactivation of glycogen synthase kinase-3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci. 2011;31:14051–14066. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia. 2010;58:423–433. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- Fujita H, Kitawaki T, Sato T, Maeda T, Kamihira S, Takaori-Kondo A, Kadowaki N. The tyrosine kinase inhibitor dasatinib suppresses cytokine production by plasmacytoid dendritic cells by targeting endosomal transport of CpG DNA. Eur J Immunol. 2013;43:93–103. doi: 10.1002/eji.201242699. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi T, Kubo T, Chan CC, Koda M, Okawa A, Takahashi K, Yamazaki M. Interferon-gamma decreases chondroitin sulfate proteoglycan expression and enhances hindlimb function after spinal cord injury in mice. J Neurotrauma. 2010;27:2283–2294. doi: 10.1089/neu.2009.1144. [DOI] [PubMed] [Google Scholar]
- Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in auto-immunity. Nat Rev. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science (New York, NY) 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Gordts PL, Foley EM, Lawrence R, Sinha R, Lameda-Diaz C, Deng L, Nock R, Glass CK, Erbilgin A, Lusis AJ, Witztum JL, Esko JD. Reducing macrophage proteoglycan sulfation increases atherosclerosis and obesity through enhanced type I interferon signaling. Cell Metab. 2014;20:813–826. doi: 10.1016/j.cmet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol. 1999;410:256–264. [PubMed] [Google Scholar]
- Han KA, Jeon S, Um JW, Ko J. Emergent synapse organizers: LAR-RPTPs and their companions. Int Rev Cell Mol Biol. 2016;324:39–65. doi: 10.1016/bs.ircmb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Hantaz-Ambroise D, Vigny M, Koenig J. Heparan sulfate proteoglycan and laminin mediate two different types of neurite outgrowth. J Neurosci. 1987;7:2293–2304. [PMC free article] [PubMed] [Google Scholar]
- Harroch S, Furtado GC, Brueck W, Rosenbluth J, Lafaille J, Chao M, Buxbaum JD, Schlessinger J. A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions. Nat Genet. 2002;32:411–414. doi: 10.1038/ng1004. [DOI] [PubMed] [Google Scholar]
- Horn KE, Xu B, Gobert D, Hamam BN, Thompson KM, Wu CL, Bouchard JF, Uetani N, Racine RJ, Tremblay ML, Ruthazer ES, Chapman CA, Kennedy TE. Receptor protein tyrosine phosphatase sigma regulates synapse structure, function and plasticity. J Neurochem. 2012;122:147–161. doi: 10.1111/j.1471-4159.2012.07762.x. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron. 2006;49:517–531. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by Heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Karumbaiah L, Anand S, Thazhath R, Zhong Y, McKeon RJ, Bellamkonda RV. Targeted downregulation of N-acetylgalactosamine 4-sulfate 6-O-sulfo-transferase significantly mitigates chondroitin sulfate proteoglycan-mediated inhibition. Glia. 2011;59:981–996. doi: 10.1002/glia.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Higashi S, Morgan AA, Bangayan NJ, Junka R, Yu P, Geller HM. Identification of a novel binding domain for heparin in RPTPσ, but not LAR or RPTPδ: implications for proteoglycan signaling. Neuroscience Meeting Planner: Society for Neuroscience Program No. 308.17/BB6 2017 [Google Scholar]
- Keough MB, Rogers JA, Zhang P, Jensen SK, Stephenson EL, Chen T, Hurlbert MG, Lau LW, Rawji KS, Plemel JR, Koch M, Ling CC, Yong VW. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun. 2016;7:11312. doi: 10.1038/ncomms11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketschek AR, Haas C, Gallo G, Fischer I. The roles of neuronal and glial precursors in overcoming chondroitin sulfate proteoglycan inhibition. Exp Neurol. 2012;235:627–637. doi: 10.1016/j.expneurol.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Burette A, Chung HS, Kwon SK, Woo J, Lee HW, Kim K, Kim H, Weinberg RJ, Kim E. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- Kolkman MJ, Streijger F, Linkels M, Bloemen M, Heeren DJ, Hendriks WJ, Van der Zee CE. Mice lacking leukocyte common antigen-related (LAR) protein tyrosine phosphatase domains demonstrate spatial learning impairment in the two-trial water maze and hyperactivity in multiple behavioural tests. Behav Brain Res. 2004;154:171–182. doi: 10.1016/j.bbr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Kondo S, Kishi H, Muraguchi A. Regulatory role of leukocyte-common-antigen-related molecule (LAR) in thymocyte differentiation. Eur J Immunol. 2010;40:1296–1302. doi: 10.1002/eji.200939743. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Fujikawa A, Masumura M, Suzuki R, Matsumoto M, Noda M. Protein tyrosine phosphatase receptor type z negatively regulates oligodendrocyte differentiation and myelination. PLoS One. 2012;7:e48797. doi: 10.1371/journal.pone.0048797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Kwon SK, Woo J, Kim SY, Kim H, Kim E. Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation. J Biol Chem. 2010;285:13966–13978. doi: 10.1074/jbc.M109.061127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gafa V, Serafini B, Giacomini E, Visconti A, Remoli ME, Severa M, Parmentier M, Ristori G, Salvetti M, Aloisi F, Coccia EM. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-beta. J Neuropathol Exp Neurol. 2008;67:388–401. doi: 10.1097/NEN.0b013e31816fc975. [DOI] [PubMed] [Google Scholar]
- Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982;94:574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Sandy JD, Anderson DK, Howland DR. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp Neurol. 2003;184:981–990. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- Lindahl U, Couchman J, Kimata K, Esko JD. Proteoglycans and Sulfated Glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2015. pp. 207–221. [Google Scholar]
- Longo FM, Martignetti JA, Le Beau JM, Zhang JS, Barnes JP, Brosius J. Leukocyte common antigen-related receptor-linked tyrosine phosphatase. Regulation of mRNA expression. J Biol Chem. 1993;268:26503–26511. [PubMed] [Google Scholar]
- Lowther DE, Chong DL, Ascough S, Ettorre A, Ingram RJ, Boyton RJ, Altmann DM. Th1 not Th17 cells drive spontaneous MS-like disease despite a functional regulatory T cell response. Acta Neuropathol. 2013;126:501–515. doi: 10.1007/s00401-013-1159-9. [DOI] [PubMed] [Google Scholar]
- Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knockout mice. J Neurosci. 2002;22:5481–5491. doi: 10.1523/JNEUROSCI.22-13-05481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meathrel K, Adamek T, Batt J, Rotin D, Doering LC. Protein tyrosine phosphatase sigma-deficient mice show aberrant cytoarchitecture and structural abnormalities in the central nervous system. J Neurosci Res. 2002;70:24–35. doi: 10.1002/jnr.10382. [DOI] [PubMed] [Google Scholar]
- Miller GM, Hsieh-Wilson LC. Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans. Exp Neurol. 2015;274:115–125. doi: 10.1016/j.expneurol.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muise AM, Walters T, Wine E, Griffiths AM, Turner D, Duerr RH, Regueiro MD, Ngan BY, Xu W, Sherman PM, Silverberg MS, Rotin D. Protein-tyrosine phosphatase sigma is associated with ulcerative colitis. Curr Biol. 2007;17:1212–1218. doi: 10.1016/j.cub.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Murchie R, Guo CH, Persaud A, Muise A, Rotin D. Protein tyrosine phosphatase sigma targets apical junction complex proteins in the intestine and regulates epithelial permeability. Proc Natl Acad Sci U S A. 2014;111:693–698. doi: 10.1073/pnas.1315017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura F, Okada T, Shishikura M, Uetani N, Taniguchi M, Yagi T, Iwakura Y, Ohshima T, Goshima Y, Strittmatter SM. Protein tyrosine phosphatase delta mediates the Sema3A-induced cortical basal dendritic Arborization through the activation of Fyn tyrosine kinase. J Neurosci. 2017;37:7125–7139. doi: 10.1523/JNEUROSCI.2519-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y, Li S. Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res. 2015;1619:22–35. doi: 10.1016/j.brainres.2014.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y, Wong D, Abdul-Muneer PM, Selzer ME, Li S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci Rep. 2016;6:37152. doi: 10.1038/srep37152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake Y, Kong W, Hussain R, Horiuchi M, Tremblay ML, Ganea D, Li S. Protein tyrosine phosphatase sigma regulates autoimmune encephalomyelitis development. Brain Behav Immun. 2017;65:111–124. doi: 10.1016/j.bbi.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlowsky A, Gianfelice A, Pallotto M, Zanchi A, Vara H, Khelfaoui M, Valnegri P, Rezai X, Bassani S, Brambilla D, Kumpost J, Blahos J, Roux MJ, Humeau Y, Chelly J, Passafaro M, Giustetto M, Billuart P, Sala C. A postsynaptic signaling pathway that may account for the cognitive defect due to IL1RAPL1 mutation. Curr Biol. 2010;20:103–115. doi: 10.1016/j.cub.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Pike KA, Tremblay ML. Regulating naive and memory CD8 T cell homeostasis—a role for protein tyrosine phosphatases. FEBS J. 2013;280:432–444. doi: 10.1111/j.1742-4658.2012.08587.x. [DOI] [PubMed] [Google Scholar]
- Powell EM, Mercado ML, Calle-Patino Y, Geller HM. Protein kinase C mediates neurite guidance at an astrocyte boundary. Glia. 2001;33:288–297. doi: 10.1002/1098-1136(20010315)33:4<288::aid-glia1027>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, Aiuti A, Vermi W, Cancrini C, Metin A, Hambleton S, Bredius R, Notarangelo LD, van der Burg M, Kalinke U, Villa A, Benvenuti F. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med. 2013;210:355–374. doi: 10.1084/jem.20120363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Serra-Pages C, Tang M, Streuli M. The LAR/PTP delta/PTP sigma subfamily of transmembrane protein-tyrosine-phosphatases: multiple human LAR, PTP delta, and PTP sigma isoforms are expressed in a tissue-specific manner and associate with the LAR-interacting protein LIP. 1. Proc Natl Acad Sci U S A. 1995;92:11686–11690. doi: 10.1073/pnas.92.25.11686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. Directing neuronal signaling through cell-surface glycan engineering. J Am Chem Soc. 2014;136:6794–6797. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Cahalon L, Bakalash S, Avidan H, Lider O, Schwartz M. A sulfated disaccharide derived from chondroitin sulfate proteoglycan protects against inflammation-associated neurodegeneration. FASEB J. 2006;20:547–549. doi: 10.1096/fj.05-4540fje. [DOI] [PubMed] [Google Scholar]
- Sapieha PS, Duplan L, Uetani N, Joly S, Tremblay ML, Kennedy TE, Di Polo A. Receptor protein tyrosine phosphatase sigma inhibits axon regrowth in the adult injured CNS. Mol Cell Neurosci. 2005;28:625–635. doi: 10.1016/j.mcn.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Schaapveld RQ, Schepens JT, Bachner D, Attema J, Wieringa B, Jap PH, Hendriks WJ. Developmental expression of the cell adhesion molecule-like protein tyrosine phosphatases LAR, RPTPdelta and RPTPsigma in the mouse. Mech Dev. 1998;77:59–62. doi: 10.1016/s0925-4773(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science (New York, NY) 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LS, Back SA. A ‘GAG’ reflex prevents repair of the damaged CNS. Trends Neurosci. 2008;31:44–52. doi: 10.1016/j.tins.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Shishikura M, Nakamura F, Yamashita N, Uetani N, Iwakura Y, Goshima Y. Expression of receptor protein tyrosine phosphatase delta, PTPdelta, in mouse central nervous system. Brain Res. 2016;1642:244–254. doi: 10.1016/j.brainres.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Smith DE, Lipsky BP, Russell C, Ketchem RR, Kirchner J, Hensley K, Huang Y, Friedman WJ, Boissonneault V, Plante MM, Rivest S, Sims JE. A central nervous system-restricted isoform of the interleukin-1 receptor accessory protein modulates neuronal responses to interleukin-1. Immunity. 2009;30:817–831. doi: 10.1016/j.immuni.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Letourneau PC. Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan (CS-PG) J Neurobiol. 1992;23:322–336. doi: 10.1002/neu.480230311. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Snow DM, Watanabe M, Letourneau PC, Silver J. A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development (Cambridge, England) 1991;113:1473–1485. doi: 10.1242/dev.113.4.1473. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- Sommer L, Rao M, Anderson DJ. RPTP delta and the novel protein tyrosine phosphatase RPTP psi are expressed in restricted regions of the developing central nervous system. Dev Dyn. 1997;208:48–61. doi: 10.1002/(SICI)1097-0177(199701)208:1<48::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Craig AM. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Arstikaitis P, Prasad T, Bartlett TE, Wang YT, Murphy TH, Craig AM. Postsynaptic TrkC and presynaptic PTPsigma function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, Ota M, Yasuda H, Tsumoto T, Aruga J, Craig AM. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat Neurosci. 2012;15(389–398):S381–382. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terszowski G, Jankowski A, Hendriks WJ, Rolink AG, Kisielow P. Within the hemopoietic system, LAR phosphatase is a T cell lineage-specific adhesion receptor-like protein whose phosphatase activity appears dispensable for T cell development, repertoire selection and function. Eur J Immunol. 2001;31:832–840. doi: 10.1002/1521-4141(200103)31:3<832::aid-immu832>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Thompson KM, Uetani N, Manitt C, Elchebly M, Tremblay ML, Kennedy TE. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol Cell Neurosci. 2003;23:681–692. doi: 10.1016/s1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Tully SE, Mabon R, Gama CI, Tsai SM, Liu X, Hsieh-Wilson LC. A chondroitin sulfate small molecule that stimulates neuronal growth. J Am Chem Soc. 2004;126:7736–7737. doi: 10.1021/ja0484045. [DOI] [PubMed] [Google Scholar]
- Uetani N, Kato K, Ogura H, Mizuno K, Kawano K, Mikoshiba K, Yakura H, Asano M, Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N, Chagnon MJ, Kennedy TE, Iwakura Y, Tremblay ML. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um JW, Ko J. LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 2013;23:465–475. doi: 10.1016/j.tcb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Valnegri P, Montrasio C, Brambilla D, Ko J, Passafaro M, Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPdelta and RhoGAP2. Hum Mol Genet. 2011;20:4797–4809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verna JM, Fichard A, Saxod R. Influence of glycosaminoglycans on neurite morphology and outgrowth patterns in vitro. Int J Dev Neurosci. 1989;7:389–399. doi: 10.1016/0736-5748(89)90060-9. [DOI] [PubMed] [Google Scholar]
- Walker BA, Ji SJ, Jaffrey SR. Intra-axonal translation of RhoA promotes axon growth inhibition by CSPG. J Neurosci. 2012;32:14442–14447. doi: 10.1523/JNEUROSCI.0176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, McFarlane S, Brickman YG, Nurcombe V, Bartlett PF, Holt CE. Essential role of heparan sulfates in axon navigation and targeting in the developing visual system. Development (Cambridge, England) 1997;124:2421–2430. doi: 10.1242/dev.124.12.2421. [DOI] [PubMed] [Google Scholar]
- Wang L, Denburg JL. A role for proteoglycans in the guidance of a subset of pioneer axons in cultured embryos of the cockroach. Neuron. 1992;8:701–714. doi: 10.1016/0896-6273(92)90091-q. [DOI] [PubMed] [Google Scholar]
- Wang H, Yan H, Canoll PD, Silvennoinen O, Schlessinger J, Musacchio JM. Expression of receptor protein tyrosine phosphatase-sigma (RPTP-sigma) in the nervous system of the developing and adult rat. J Neurosci Res. 1995;41:297–310. doi: 10.1002/jnr.490410303. [DOI] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Kwon SK, Choi S, Kim S, Lee JR, Dunah AW, Sheng M, Kim E. Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses. Nat Neurosci. 2009;12:428–437. doi: 10.1038/nn.2279. [DOI] [PubMed] [Google Scholar]
- Wu CL, Hardy S, Aubry I, Landry M, Haggarty A, Saragovi HU, Tremblay ML. Identification of function-regulating antibodies targeting the receptor protein tyrosine phosphatase sigma ectodomain. PLoS One. 2017;12:e0178489. doi: 10.1371/journal.pone.0178489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyszynski M, Kim E, Dunah AW, Passafaro M, Valtschanoff JG, Serra-Pages C, Streuli M, Weinberg RJ, Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Xie Y, Yeo TT, Zhang C, Yang T, Tisi MA, Massa SM, Longo FM. The leukocyte common antigen-related protein tyrosine phosphatase receptor regulates regenerative neurite outgrowth in vivo. J Neurosci. 2001;21:5130–5138. doi: 10.1523/JNEUROSCI.21-14-05130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Park D, Ohtake Y, Li H, Hayat U, Liu J, Selzer ME, Longo FM, Li S. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol Dis. 2015;73:36–48. doi: 10.1016/j.nbd.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo TT, Yang T, Massa SM, Zhang JS, Honkaniemi J, Butcher LL, Longo FM. Deficient LAR expression decreases basal forebrain cholinergic neuronal size and hippocampal cholinergic innervation. J Neurosci Res. 1997;47:348–360. doi: 10.1002/(sici)1097-4547(19970201)47:3<348::aid-jnr13>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Yi JH, Katagiri Y, Yu P, Lourie J, Bangayan NJ, Symes AJ, Geller HM. Receptor protein tyrosine phosphatase sigma binds to neurons in the adult mouse brain. Exp Neurol. 2014;255:12–18. doi: 10.1016/j.expneurol.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Yasumura M, Uemura T, Lee SJ, Ra M, Taguchi R, Iwakura Y, Mishina M. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. J Neurosci. 2011;31:13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Shiroshima T, Lee SJ, Yasumura M, Uemura T, Chen X, Iwakura Y, Mishina M. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J Neurosci. 2012;32:2588–2600. doi: 10.1523/JNEUROSCI.4637-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Pearson CS, Geller HM. Flexible roles for proteoglycan sulfation and receptor signaling. Trends Neurosci. 2018;41:47–61. doi: 10.1016/j.tins.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Honkaniemi J, Yang T, Yeo TT, Longo FM. LAR tyrosine phosphatase receptor: a developmental isoform is present in neurites and growth cones and its expression is regional- and cell-specific. Mol Cell Neurosci. 1998;10:271–286. doi: 10.1006/mcne.1998.0663. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wu C, Song J, Gotte M, Sorokin L. Syndecan-1, a cell surface proteoglycan, negatively regulates initial leukocyte recruitment to the brain across the choroid plexus in murine experimental autoimmune encephalomyelitis. J Immunol. 2013;191:4551–4561. doi: 10.4049/jimmunol.1300931. [DOI] [PubMed] [Google Scholar]
- Zhang G, Hu J, Li S, Huang L, Selzer ME. Selective expression of CSPG receptors PTPsigma and LAR in poorly regenerating reticulospinal neurons of lamprey. J Comp Neurol. 2014;522:2209–2229. doi: 10.1002/cne.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Nagarkatti P, Zhong Y, Nagarkatti M. Immune modulation by chondroitin sulfate and its degraded disaccharide product in the development of an experimental model of multiple sclerosis. J Neuroimmunol. 2010;223:55–64. doi: 10.1016/j.jneuroim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HX, Li XY, Li FY, Liu C, Liang ZP, Liu S, Zhang B, Wang TY, Chu TC, Lu L, Ning GZ, Kong XH, Feng SQ. Targeting RPTPsigma with lentiviral shRNA promotes neurites outgrowth of cortical neurons and improves functional recovery in a rat spinal cord contusion model. Brain Res. 2014;1586:46–63. doi: 10.1016/j.brainres.2014.08.048. [DOI] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]