Abstract
Oncolytic viruses, such as oncolytic herpes simplex virus (oHSV), are a new class of cancer therapeutic, which selectively replicate and kill cancer cells, while inducing an inflammatory microenvironment, immunovirotherapy. Recently, an oHSV (talimogene laherparepvec) has been approved for the treatment of advanced melanoma. Glioblastoma (GBM) is an almost always lethal primary tumor in the brain that is highly immunosuppressive, and posited to contain GBM stem-like cells (GSCs). Immune checkpoint blockade has revolutionized therapy for some cancers, but not GBM. We have used a syngeneic GSC-derived orthotopic GBM model (005) to develop immunotherapeutic strategies. Curative therapy required oHSV expressing IL-12 in combination with two checkpoint inhibitors, anti-PD-1 and anti-CTLA-4. This response required CD4+ and CD8+ T cells, and macrophages in a complex interplay.
Keywords: : cancer immunotherapy, cancer stem cells, checkpoint inhibitors, glioma, HSV, immunovirotherapy, macrophage polarization, oncolytic virus
Glioblastoma, glioblastoma stem-like cells & immunosuppression
Glioblastoma (GBM) is the most common and aggressive form of primary malignant brain tumor with no effective treatments [1]. Despite significant advances in molecular understanding, diagnosis and the development of molecular targeted therapies, the current standard of care (surgical resection, radiation therapy and chemotherapy) only yields a median survival of approximately 15 months [2]. There are a number of factors that contribute to this: intratumoral heterogeneity, GBM stem-like cells (GSCs), tumor invasiveness, angiogenic and hypoxic microenvironment, blood–brain tumor-barrier and limited drug delivery, and immunodistinct location and immune suppression [2,3]. GBM is one of the most immunosuppressive tumors, due to a variety of mechanisms: impairment of MHC class I presentation and downregulation of co-stimulatory molecules (CD80, CD86); activation of STAT3 and upregulation of IDO; expression of a broad range of secreted immunosuppressive molecules, IL-1, TGFβ, IL-10, CSF-1, PGE-1; recruitment of immunosuppressive Tregs and T-cell exhaustion; influx of myeloid-derived suppressor cells and protumoral M2-like macrophages/microglia; and low mutational load, which contributes to poor tumor immunogenicity [3–5]. Tumor heterogeneity affects all treatment strategies, including immune-mediated, and is facilitated by tumor evolution and the presence of a subpopulation of cancer stem cells or GSCs, which contribute to tumor initiation, progression, maintenance, drug resistance and tumor relapse [6]. GSCs isolated from patient GBM specimens maintain the genetic/epigenetic features of the patient, and have characteristics of self-renewal, differentiation into more mature phenotypes of multiple lineages, and efficient formation of tumors in immune deficient mice that recapitulate the patients’ histopathology [6,7]. In addition, GSCs induce immunosuppression through recruitment or polarization of the macrophages/microglia toward immunosuppressive M2-like phenotypes [8,9]. Thus, GSCs are increasingly becoming a critical immunotherapeutic target, with GSC-derived tumor models representative of human GBM increasingly important.
Immune competent GBM stem cell model (005 GSC) recapitulates immune suppressive features of human disease
Immunotherapy is currently a revolutionary treatment modality in a subset of cancers and patients [10], unfortunately, GBM has so far not been successfully included in this revolution [11]. There are relatively few mouse syngeneic glioma models, many of which are immunogenic [12]. In addition, there are inducible genetically engineered mouse in situ arising brain tumor models, such as lentivirus transduced [13], which so far have not been used to evaluate oncolytic viruses. In order to test and develop new immunotherapeutic modalities for brain tumors, a new GSC-derived immunocompetent syngeneic orthotopic preclinical GBM model (005 GSCs) was recently described [14,15]. 005 GSCs expressing a GFP reporter gene were isolated from gliomas that arose after intracranial injection of lentivirus expressing activated oncogenic proteins H-Ras and Akt into GFAP-Cre tumor suppressor gene p53 heterozygous C57BL/6 mice [16]. 005 GSC-derived tumors recapitulate the afore-mentioned complex features of immunosuppressive GBM, such as: low levels of MHC class I and immune checkpoint PD-L1 expression, indicating this model is poorly immunogenic; however, both MHC class I and PD-L1 can be upregulated following treatment with IFN-γ [14,17]; lack of MHC class II expression, even after IFN-γ stimulation [17]; lack of cell surface expression of CD40, CD80 and CD86 [14]; low somatic mutation burden [5], with two known somatic mutations [16]; immunosuppressive tumor microenvironment (TME), including accumulation of Tregs and myeloid-derived suppressor cells [14,17–18]; and differentiate in vivo into mature phenotypes, such as expressing NeuN (neuronal marker) (Figure 1A), with heterogeneous histopathology [14]. This representative nonimmunogenic 005 GBM model has been used to test immunotherapeutic strategies for GBM.
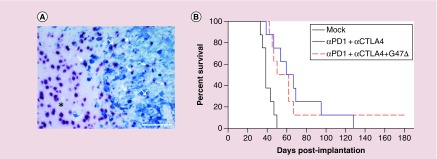
Figure 1. . In vivo differentiation of 005 GSCs and triple combination therapy in 005 GSC-derived GBM model.
(A) 005 glioblastoma stem-like cells (GSCs) differentiate in vivo into cells expressing neuronal marker (NeuN). Mice implanted with 005 GSCs (2 × 104) on day 0, sacrificed on day 24 and formalin-fixed paraffin-embedded brain tumor sections were double stained for GFP+ 005 tumor cells (blue; anti-GFP) and NeuN+ neuronal cells (red nuclear; anti-NeuN), as described [17]. A representative image obtained at 20× magnification is presented. Cells positive for both GFP and NeuN (GFP+NeuN+) are indicated by arrows. *nontumor area. Scale bar = 100 μm. (B) Triple combination therapy in 005 GSC-derived tumor model. Mice implanted with 2 × 104 005 GSCs on day 0 and treated with G47Δ (5 × 105 pfu) or phosphate-buffered saline (PBS) injected intratumorally on day 8 and anti-CTLA-4 (5 mg/kg) and anti-PD-1 (10 mg/kg) or isotype control IgG intraperitoneally on days 8, 11 and 14 (n = 8/group). Median survival of mock group (PBS+IgG) was significantly different from the dual combination (anti-PD-1+anti-CTLA-4; p = 0.001) or triple combination (anti-PD-1+anti-CTLA-4+G47Δ; p = 0.006). However, the triple combination treatment was no different compared with the dual combination treatment (anti-PD-1+anti-CTLA-4; p = 0.76) (log-rank analysis).
Oncolytic herpes simplex viruses for cancer therapy
Oncolytic viruses (OVs), a unique class of anticancer agent, are naturally occurring or genetically engineered to selectively replicate in and kill cancer cells while sparing normal tissue [19,20]. Immunovirotherapy takes advantage of the natural inflammatory responses to virus infection and immune responses to OV-induced cancer cell death to drive antitumor immunity [21–23]. This natural inflammatory response can be further enhanced through expression of various cytokines or other immunomodulatory molecules engineered into the virus [22,24]. Replication-competent oncolytic herpes simplex virus (oHSV) is designed for both oncolytic activity and safety [24,25]. OHSV replication in cancer cells leads to a cascade of antitumoral (antiviral) immunological events (in situ vaccine): activation of innate cytokine/chemokine signaling, oHSV propagation and cancer cell lysis (oncolysis), release of viral/tumor antigens and induction of innate and finally adaptive host immune responses [26]. Nine different oHSVs have been in or are in clinical trials, including 5 for GBM, with no serious adverse events attributed to the virus (NCT03152318) [19,24]. OHSV talimogene laherparepvec (T-Vec; expressing GM-CSF) was recently approved by the US FDA for patients with advanced melanoma, the first OV approved in the USA [25]. G47Δ, similar to T-Vec, but safe in the brain [27], is currently under clinical trial for recurrent GBM, as well as olfactory neuroblastoma and prostate cancer in Japan (UMIN000015995, UMIN000011636). G47Δ contains multiple genetic alterations: inactivation of ICP6, required for nucleotide metabolism, limits viral replication to cycling cells and attenuates pathogenicity; deletion of γ34.5, the major determinant of oHSV neurovirulence that blocks antiviral innate host responses in normal cells; deletion of ICP47, which inhibits MHC class I presentation and complements growth defects of γ34.5-deleted HSV; and insertion of a reporter gene LacZ, coding for β-galactosidase [24]. In the 005 GBM model, intratumoral G47Δ treatment significantly reduced the proportion of immunosuppressive Tregs (CD4+CD25+FoxP3+) within the tumor microenvironment; however, the extension of overall survival was limited [14]. GBM selective oHSV can also be constructed by retargeting to cell surface receptors like EGFRvIII or use of miRNA targets to miRNAs expressed in the nervous system but not GBM [28].
IL-12 armed oHSV enhances antitumor immune responses & efficacy
OHSV can easily accommodate insertions of large amounts of DNA (∼20 kb), and thus be ‘armed’ with therapeutic transgenes, including immunomodulatory (i.e., GM-CSF in T-Vec, IL-12 in G47Δ, NV1042 and M032), and antiangiogenic (i.e., angiostatin, endostatin, IL-12, PF4 and Vstat120) transgenes [24,29]. IL-12 is a master regulator of antitumor immunity that is particularly potent in oHSV, promoting proliferation of activated T- and NK cells, stimulating Th1 differentiation, and inducing IFN-γ production [30]. Systemic administration of IL-12 causes serious toxicity in patients [30]. No detrimental effects were reported when IL-12 was expressed locally from oHSV in 005 tumors [14,17] or in normal nonhuman primate brain [31]. In G47Δ-mIL12, the IL-12 fusion transgene is inserted in the ICP6 gene, with expression driven by the CMV immediate-early promoter [14]. In the 005 GBM model, local expression of IL-12 after intratumoral injection of G47Δ-mIL12 improved survival compared with virus without IL-12 transgene expression (G47Δ-empty) [14]. Two injections of G47Δ-mIL12 were better than a single injection [14,17]. G47Δ-mIL12 treatment was associated with a significant reduction of GFP+ 005 tumor cells (oncolysis), increased intratumoral infiltration of CD3+ T cells, reduction of Tregs (CD4+FoxP3+) with increased T-effector (CD8+)/Tregs (CD4+FoxP3+) ratio, increase in M1-like macrophages (pSTAT1+ and iNOS+), stimulation of Th1 differentiation (T-bet+) and increased IFN-γ production [14,17]. In the periphery, oHSV NV1042 expressing IL-12 significantly inhibited tumor growth in prostate and breast cancer transgenic tumor models [32]. The diverse antitumor immune properties of G47Δ-mIL12 indicate that combining G47Δ-mIL12 with other immunotherapeutic strategies might improve the therapeutic outcome.
Immune checkpoints
Immune checkpoint molecules, such as CTLA-4, PD-1 and its ligand PD-L1 play critical roles in regulating antitumor immune responses [33–35]. CTLA-4 is expressed on T cells and competes with co-stimulatory receptor CD28 for binding to their ligands CD80 (B7.1) and CD86 (B7.2), thereby inhibiting T-cell activation and stimulating Tregs [33,34]. PD-1 is expressed on a broad range of immune cells and is often upregulated in the TME, while PD-L1 is upregulated on activated leukocytes and myeloid cells and many cancer cells, often by IFN-γ [34,36]. PD-1 negatively regulates T effector cells to maintain homeostasis; however, chronic stimulation leads to exhaustion, as in tumors [36]. The role of PD-1 on Tregs, B cells, myeloid cells and NK cells is poorly understood. CTLA-4 and PD-1 regulate different T-cell inhibitory pathways; blocking co-stimulation, likely early in T-cell activation, and TCR signaling, likely blocking T-cell killing, respectively [37]. Although blocking CTLA-4 or PD-1 removes inhibitory signaling, antitumor activity is dependent on existing immune activation and T-cell recognition of tumor cells. Blocking either or both of these receptors by means of antibodies unleashes antitumor immunity mostly in cancers with an immunologically ‘hot’ TME (e.g., subsets of non-small-cell lung cancer and melanomas) [34,38–39]. In the TME, checkpoint blockade responses are associated with elevated levels of CD8+ TILs at the tumor periphery [40], expression of PD-L1 [41] and IFN-γ [42], high mutational and clonal neoantigen burden [43] and mismatch repair deficiency [44,45]. On the other hand, for cancers with an immunologically ‘cold’ TME (e.g., GBM, even a subset of melanomas), immune checkpoint blockade immunotherapy alone has not been successful so far [35,46], including a recently failed Phase III clinical trial of anti-PD-1 (nivolumab) in GBM (CheckMate-143) [11]. Similar to the results in the clinic with checkpoint inhibitors for GBM, treatment of mice bearing 005 GSC-derived tumors, representative of immunologically ‘cold’ tumors, with systemic immune checkpoint antibodies (anti-PD-1 or anti-PD-L1 or anti-CTLA-4) provided only modest prolongation of survival [17]. This contrasts with the results seen with mouse GL261 syngeneic tumors [47–49]. Even the combination of two antibodies (anti-PD-1+anti-CTLA-4) was not successful in the 005 GSC model, only delaying tumor progression without cures [17]. This limited antitumor efficacy of checkpoint blockade antibodies (either alone or in combination) was not due to the inability of the antibodies to cross the blood–brain or tumor barrier, since immune checkpoint antibodies (anti-PD-1 or anti-PD-L1) were detected in the tumor after intraperitoneal administration [17]. It is unclear whether this is true in patients, however, oHSV could be armed with immune checkpoint inhibitor transgenes.
Immune checkpoint inhibitor combinations with oHSV
Infection with oHSV induces antitumor responses [26]. T-Vec was shown to activate T-cell responses and induce regression of distant lesions in clinical trials for advanced melanoma [25]. With this complementary activity to that of immune checkpoint inhibitors, it was reasonable to combine T-Vec with immune checkpoint inhibitors [50]. The combination of T-Vec with anti-CTLA-4 antibody (ipilimumab) in advanced melanoma was more effective than ipilimumab alone, without additional toxicities [51]. A Phase IB clinical trial combining T-Vec with anti-PD-1 antibody (pembrolizumab) found that patients with ‘cold’ tumors (baseline lack of CD8+ T-cell infiltration or IFN-γ signature) had a high objective response rate (33% complete responses) compared with prior single agent pembrolizumab [52]. T-Vec induced an immunologically ‘hot’ TME, with increases in CD8+ T-cell infiltration and IFN-γ gene expression [52]. In the mouse 005 GBM model, dual combination treatment (G47Δ-mIL12+anti-CTLA-4) produced only a modest prolongation of survival, with increased tumor infiltration of CD3+ T cells and increased T effector (CD3+CD8+)/Tregs (CD4+FoxP3+) ratio [17]. Similar limited survival benefits were also observed when G47Δ-mIL12 was combined with anti-PD-1 or anti-PD-L1 antibodies [17]. This indicates that immune effects of the dual combination treatment were insufficient in the immunosuppressed 005 TME, likely reflective of the large immune differences between melanoma and GBM.
Triple combination therapy (systemic anti-PD-1, anti-CTLA-4 & intratumoral G47Δ-mIL12) cures mice from GBM
Since PD-1/PD-L1 and CTLA-4/B7 are two independent immune inhibitory pathways [37,53], we hypothesized that combination of systemic anti-CTLA-4 (which affects immune priming) and anti-PD-1 (which works on activated immune cells) would synergize with intratumoral G47Δ-mIL12 treatment. Indeed, triple combination therapy (systemic anti-PD-1, anti-CTLA-4 and intratumoral G47Δ-mIL12) cured 77% of treated mice with 005-derived tumors [17]. All cured mice from the triple combination therapy had immunological memory protection, since they remained protected following lethal tumor rechallenge even 6 months after initial treatment. These findings were reproduced in a second aggressive carcinogen-induced mouse glioma model, CT-2A [12]. This combination was safe, as no toxicity was observed in the treated mice [17]. Local IL-12 expression is required for triple combination efficacy, as the combination with G47Δ was not significantly better than dual checkpoint inhibitors (anti-PD-1+anti-CTLA-4) in extending survival, although one animal was cured (Figure 1B). The unprecedented curative antitumor efficacy of the triple combination therapy in 005 model was associated with a significant reduction of tumor cells (GFP+005), increased infiltration of T effector cells (CD3+CD8+) and proliferating T cells (CD3+Ki67+), reduction of Tregs (CD4+FoxP3+), increased T-effector/T-regulatory cell ratio, influx of macrophages (CD68+) with polarization to an antitumoral M1-type (CD68+pSTAT1+) and a significant reduction of PD-L1+ cells [17]. Immune cell depletion/inhibition studies demonstrated that CD4+, CD8+ T cells and macrophages are each required for triple combination therapeutic efficacy, with CD4+ T cells playing a critical role [17]. Interestingly, depletion of a single cell type leads to a large perturbations in the other cell types, indicating a complex interconnectedness between immune cell types.
Conclusion
In a representative mouse GCS-derived tumor model neither single (oHSV-IL12 or checkpoint blockade) nor dual combination (oHSV-IL12 plus checkpoint blockade) therapies were efficacious in overcoming GBM immunosuppression. However, the triple combination, oHSV expressing IL12 (G47Δ-mIL12) in combination with two systemic immune checkpoint inhibitors (anti-CTLA-4 and anti-PD-1), successfully cured GBM in the representative GSC-derived immune-competent 005 GBM model, that recapitulates human GBM. Multiple immune cell types, especially CD4+ T cells were involved in the therapeutic efficacy. This triple combination therapeutic strategy should be translatable to the clinic in GBM and other poorly immunogenic or nonimmunogenic cancers.
Future perspective
In recent clinical trials for advanced melanoma, often checkpoint responsive or immunologically ‘hot’ tumors, the combination of oHSV T-Vec and ipilimumab (anti-CTLA-4) had an objective response rate of 39%, much greater than ipilimumab alone (18%) [51]. Despite the promising results over half the patients did not respond, indicating that additional interventions are needed. For immunologically ‘cold’ tumors, like GBM, the prognosis is much worse, as illustrated by the failure of the Phase III CheckMate 143 trial with anti-PD-1 (nivolumab) [11]. We propose that for nonimmunogenic tumors like GBM, multiple immune modulations, such as the combination described here (oHSV-IL12 + anti-PD-1 + anti-CTLA-4), will be necessary to overcome the immunosuppressive TME and produce durable complete responses. It will be important to determine which specific immune cell subtypes, within the necessary CD4+ and CD8+ T cells and macrophages, and how they mediate this triple combination curative therapy. New techniques, such as mass cytometry [37] and single cell RNA-seq [54], will identify further complexicity to decipher. Understanding the immune changes occurring after this combination therapy should identify other targets/strategies to beneficially manipulate the immune response, such as other immune checkpoints, myeloid cell modifiers or the microbiome. Strategies developed for GBM should be applicable to other nonimmunogenic or checkpoint nonresponsive cancers. There are a variety of other OVs in clinical trial for GBM (poliovirus, retrovirus, adenovirus, measles virus, reovirus, parvovirus and Newcastle disease virus) [19], and it remains to be determined how well they will interact with immune checkpoint blockade. It will be interesting to examine whether arming oHSV with immune stimulatory molecules or co-stimulatory ligands will similarly improve efficacy and whether other cytokines/chemokines expressed from oHSV synergize with immune checkpoint blockade in GBM. Is this strategy translatable to other nonimmunogenic cancers? Although this is an exciting time for immunotherapy and immunovirotherapy, much additional research will be needed to translate its success to the majority of cancer patients.
Executive summary.
Glioblastoma, glioblastoma stem-like cells & immunosuppression
Glioblastoma (GBM) is a relatively nonimmunogenic/immunosuppressed tumor.
GBM stem-like cells (GSCs) contribute to immunosuppression and tumor recurrence, and thus they are critical targets for therapeutics.
Immune competent GBM stem cell model (005 GSC) recapitulates immune suppressive features of human disease
Mouse 005 GSC-derived brain tumors are immunologically ‘cold’ and provide a representative preclinical model to test immunotherapies.
Oncolytic herpes simplex viruses for cancer therapy
Oncolytic herpes simplex viruses (oHSV) talimogene laherparepvec has been approved for the use in advanced melanoma.
Despite reducing Tregs, G47Δ treatment did not significantly improve the survival in 005 GBM model.
IL-12 armed oHSV enhances antitumor immune responses & efficacy
OHSV can be ‘armed’ with therapeutic transgenes to improve efficacy.
Intratumoral viral expression of IL-12 (G47Δ-mIL12) enhances survival, but only modestly.
Immune checkpoint inhibitor combinations with oHSV
Intralesional injection of talimogene laherparepvec in melanoma patients turns immunologically ‘cold’ tumors into ‘hot’ tumors and promotes checkpoint inhibitor clinical responses.
Checkpoint inhibitor antibodies cross the blood–brain and tumor barrier.
Triple combination therapy (systemic anti-PD-1, anti-CTLA-4 & intratumoral G47Δ-mIL12) cures mice from GBM
Triple combination therapy cures most mice in two representative GBM models (005 and CT-2A) and protects them from lethal tumor rechallenge.
Curative therapy is associated with large increases in M1-like macrophages and T effector cells, and decreases in T regulatory cells.
CD4+ T cells are required for efficacy, while CD8+ T cells and macrophages contribute to therapeutic benefits.
Conclusion
Multiple immunotherapeutic interventions (e.g., oHSV, local IL-12 and two checkpoint inhibitors) are required to cure poorly immunogenic cancers like GBM.
Multiple immune cells contribute to therapeutic efficacy.
The triple combination approach may be effective in other minimally immunotherapy-responsive cancers.
Footnotes
Financial & competing interests disclosure
This work was funded in part by grants from NIH (R01NS032677 to R.L. Martuza and R01CA160762 to S.D. Rabkin) and The Thomas A. Pappas Chair in Neurosciences (to S.D. Rabkin). SD Rabkin and RL Martuza are inventors on patents relating to oHSV owned by Georgetown University and Massachusetts General Hospital that have been licensed to Amgen, for which they receive royalties. D Saha has no potential conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Alexander BM, Cloughesy TF. Adult glioblastoma. J. Clin. Oncol. 2017;35(21):2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014;6(11):1359–1370. doi: 10.15252/emmm.201302627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Razavi SM, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Front. Surg. 2016;3:11. doi: 10.3389/fsurg.2016.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017;19(8):1047–1057. doi: 10.1093/neuonc/nox026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29(12):1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14(2):132–144. doi: 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015;17(2):170–182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Audia A, Conroy S, Glass R, Bhat KPL. The impact of the tumor microenvironment on the properties of glioma stem-like cells. Front. Oncol. 2017;7:143. doi: 10.3389/fonc.2017.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reardon DA, Wucherpfennig K, Chiocca EA. Immunotherapy for glioblastoma: on the sidelines or in the game? Discov. Med. 2017;24(133):201–208. [PubMed] [Google Scholar]
- 12.Oh T, Fakurnejad S, Sayegh ET, et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J. Transl. Med. 2014;12:107. doi: 10.1186/1479-5876-12-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huszthy PC, Daphu I, Niclou SP, et al. In vivo models of primary brain tumors: pitfalls and perspectives. Neuro Oncol. 2012;14(8):979–993. doi: 10.1093/neuonc/nos135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheema TA, Wakimoto H, Fecci PE, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl Acad. Sci. USA. 2013;110(29):12006–12011. doi: 10.1073/pnas.1307935110. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Description of new immunocompetent glioblastoma (GBM) stem-like cells model in mice and importance of local IL-12 expression in the context of oncolytic herpes simplex virus (oHSV) for efficacy.
- 15.Lu L, Saha D, Martuza RL, Rabkin SD, Wakimoto H. Single agent efficacy of the VEGFR kinase inhibitor axitinib in preclinical models of glioblastoma. J. Neurooncol. 2015;121(1):91–100. doi: 10.1007/s11060-014-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marumoto T, Tashiro A, Friedmann-Morvinski D, et al. Development of a novel mouse glioma model using lentiviral vectors. Nat. Med. 2009;15(1):110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha D, Martuza RL, Rabkin SD. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32(2):253.e255–267.e255. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the multiple therapeutic interventions necessary to cure GBM in a representative syngeneic mouse model and the necessity for multiple immune cell types.
- 18.Saha D, Martuza RL, Rabkin SD. Curing glioblastoma: oncolytic HSV-IL12 and checkpoint blockade. Oncoscience. 2017;4(7–8):67–69. doi: 10.18632/oncoscience.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saha D, Ahmed SS, Rabkin SD. Exploring the antitumor effect of virus in malignant glioma. Drugs Future. 2015;40(11):739–749. doi: 10.1358/dof.2015.040.11.2383070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3(6):841–849. doi: 10.1001/jamaoncol.2016.2064. [DOI] [PubMed] [Google Scholar]
- 21.Ning J, Wakimoto H, Rabkin SD. Immunovirotherapy for glioblastoma. Cell Cycle. 2014;13(2):175–176. doi: 10.4161/cc.27039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14(9):642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters C, Rabkin SD. Designing herpes viruses as oncolytics. Mol. Ther. Oncolytics. 2015;2:15010. doi: 10.1038/mto.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bommareddy PK, Peters C, Saha D, Rabkin SD, Kaufman HL. Oncolytic herpes simplex viruses as a paradigm for the treatment of cancer. Annu. Rev. Cancer Biol. 2018;2:155–173. [Google Scholar]
- 26.Saha D, Wakimoto H, Rabkin SD. Oncolytic herpes simplex virus interactions with the host immune system. Curr. Opin. Virol. 2016;21:26–34. doi: 10.1016/j.coviro.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl Acad. Sci. USA. 2001;98(11):6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzacurati L, Marzulli M, Reinhart B, et al. Use of miRNA response sequences to block off-target replication and increase the safety of an unattenuated, glioblastoma-targeted oncolytic HSV. Mol. Ther. 2015;23(1):99–107. doi: 10.1038/mt.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning J, Wakimoto H. Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy. Front. Microbiol. 2014;5:303. doi: 10.3389/fmicb.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasek W, Zagozdzon R, Jakobisiak M. Interleukin 12: still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014;63(5):419–435. doi: 10.1007/s00262-014-1523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth JC, Cassady KA, Cody JJ, et al. Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum. Gene Ther. Clin. Dev. 2014;25(1):16–27. doi: 10.1089/humc.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passer BJ, Cheema T, Wu S, Wu CL, Rabkin SD, Martuza RL. Combination of vinblastine and oncolytic herpes simplex virus vector expressing IL-12 therapy increases antitumor and antiangiogenic effects in prostate cancer models. Cancer Gene Ther. 2013;20(1):17–24. doi: 10.1038/cgt.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 34.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat. Rev. Neurol. 2015;11(9):504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent review on immune checkpoint blockade for GBM.
- 36.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18(3):153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 37.Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120.e1117–1133.e1117. doi: 10.1016/j.cell.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstration that anti-CTLA-4 and anti-PD-1 checkpoint inhibitors work through distinct cellular mechanisms, providing strong rationale for combination useage.
- 38.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 40.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mcgranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351(6280):1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat. Rev. Clin. Oncol. 2017;14(11):655–668. doi: 10.1038/nrclinonc.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dejaegher J, Verschuere T, Vercalsteren E, et al. Characterization of PD-1 upregulation on tumor-infiltrating lymphocytes in human and murine gliomas and preclinical therapeutic blockade. Int. J. Cancer. 2017;141(9):1891–1900. doi: 10.1002/ijc.30877. [DOI] [PubMed] [Google Scholar]
- 48.Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 2016;4(2):124–135. doi: 10.1158/2326-6066.CIR-15-0151. [DOI] [PubMed] [Google Scholar]
- 49.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014;20(20):5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dummer R, Hoeller C, Gruter IP, Michielin O. Combining talimogene laherparepvec with immunotherapies in melanoma and other solid tumors. Cancer Immunol. Immunother. 2017;66(6):683–695. doi: 10.1007/s00262-017-1967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label Phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J. Clin. Oncol. 2017 doi: 10.1200/JCO.2017.73.7379. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial demonstrating improved efficacy without additional toxicity using oHSV talimogene laherparepvec in combination with anti-CTLA-4.
- 52.Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T Cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2017;170(6):1109.e1110–1119.e1110. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clinical trial showing that oHSV talimogene laherparepvec induces features of an immunologically ‘hot’ tumor that promote improved responses to checkpoint blockade.
- 53.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tirosh I, Izar B, Prakadan SM, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352(6282):189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]