Abstract
Aim:
Investigate associations of leisure time physical activity (LTPA) with DNA methylation and miRNAs during pregnancy.
Patients & methods:
LTPA, candidate DNA methylation and circulating miRNAs were measured (average 15 weeks gestation) in pregnant women (n = 92).
Results:
Each additional hour of prepregnancy LTPA duration was associated with hypermethylation in C1orf212 (β = 0.137, 95% CI: 0.004–0.270) and higher circulating miR-146b-5p (β = 0.084, 95% CI: 0.017–0.151). Each additional metabolic equivalent hour of early-pregnancy LTPA energy expenditure was associated with higher circulating miR-21-3p (β = 0.431, 95% CI: 0.089–0.772) in women carrying female offspring, and lower circulating miR-146b-5p (β = -0.285, 95% CI: -0.528 to -0.043) and miR-517-5p (β = -0.406, 95% CI: -0.736 to -0.076) in women carrying male offspring.
Conclusion:
Our findings suggest that LTPA may influence maternal epigenetic biomarkers, possibly in an offspring sex-specific manner.
Keywords: : DNA methylation, miRNA, peripheral blood, physical activity, pregnancy, sex-specific
Physical activity is recommended across the lifespan for its cardiovascular and metabolic benefits [1]. Physical activity before and during pregnancy has been associated with lower risk of pregnancy complications, such as gestational diabetes mellitus [2] and preeclampsia [3], and improved fetal growth [4,5]. Effects of physical activity on inflammation, blood pressure control, lipid metabolism and glucose metabolism contribute to observed cardiovascular and metabolic benefits in the general population [6] and among pregnant women [7,8]; however, specific mechanisms are not well understood.
Previous studies have reported changes in epigenetic regulation of gene expression by DNA methylation and miRNAs after physical activity [9,10]. Regions in genes involved in systemic or local inflammation [11] and glucose metabolism [11,12] were differentially methylated in blood and skeletal muscle in response to an acute bout of aerobic physical activity (one cycling session) [11] and long-term (6 months) aerobic physical activity interventions (spinning, aerobics) [12] in adults. Changes in levels of circulating miRNAs involved in inflammatory pathways and angiogenesis have also been observed after long-term physical activity interventions (cycling and rowing) among healthy, physically active men [13,14]. Physical activity-related epigenetic changes during the perinatal period, a period of major physiologic changes in the mother, have not been well studied. Recently, McCullough et al. published the first, and to our knowledge the only, study of maternal physical activity during pregnancy and epigenetic regulation of imprinted genes in newborn cord blood at birth [15]. In their study, physical activity during pregnancy was associated with lower methylation in an imprinted region of PLAGL1. To our knowledge, previous studies have not examined associations of maternal physical activity before or during pregnancy with peripheral blood DNA methylation or circulating miRNAs in maternal blood.
Epigenetic regulatory mechanisms such as DNA methylation and miRNA levels may play important roles in systemic physiological function and placental development and function [16,17]. Circulating epigenetic biomarkers have previously been used to characterize pathophysiology of pregnancy complications [18,19] and adverse outcomes (e.g., macrosomia [20]) in early pregnancy. Physical activity-associated epigenetic changes may also be useful biomarkers for identifying high-risk women during pregnancy.
Placental and fetal growth responses to maternal behaviors and in utero exposures may be offspring sex-specific [21]. We have previously reported offspring sex-specific associations of maternal physical activity before and during pregnancy with offspring birth size [5]. Therefore, effects of maternal physical activity on circulating epigenetic biomarkers during pregnancy may also be differentially dependent on offspring sex.
The objective of this study was to examine associations of candidate epigenetic biomarkers (DNA methylation and miRNA levels) in maternal peripheral blood with prepregnancy and early-pregnancy physical activity and to determine if these associations differ by offspring sex. MiRNAs that have previously been associated with pregnancy complications and mechanisms related to pregnancy complications were identified from the literature. DNA methylation sites in genes that have been previously associated with environmental exposures and pregnancy outcomes were also identified from the literature.
Methods
Study setting & study population
Data used in this study were collected as part of the Omega study, a prospective pregnancy cohort designed to assess risk factors of pregnancy complications. Details about the study design have been published previously [22]. Briefly, pregnant women initiating prenatal care at clinics associated with Swedish Medical Center and Tacoma General Hospital in Washington State were recruited from 1996 to 2008. Women were eligible to participate in the Omega study if they were at least 18 years old, were able to speak and read English, initiated prenatal care prior to 20 weeks gestation, and planned to carry the pregnancy to term and deliver at one of the two study hospitals.
The current study sampled from among Omega study participants included in a case–cohort study investigating traffic-related air pollution and pregnancy complications. We included 100 randomly selected controls with healthy pregnancies (without gestational diabetes, preeclampsia, preterm birth or low birthweight) in the current analysis. Participants with missing prepregnancy or early-pregnancy leisure time physical activity (LTPA; n = 6), implausible values for prepregnancy or early-pregnancy LTPA duration (>35 h per week; n = 1 for prepregnancy, n = 3 for early pregnancy), or missing covariates (n = 1 missing gestational age at blood draw) were excluded. The Omega study was approved by the institutional review boards of Swedish Medical Center and Tacoma General Hospital. All participants gave written informed consent.
Data collection
Participants completed an in-person interview in early pregnancy (average 15 weeks gestation). Trained study interviewers collected sociodemographic characteristics, reproductive and medical history, and physical activity before and during early pregnancy. Maternal peripheral blood was collected shortly after enrollment in the study. Participants were followed until delivery, and trained study personnel abstracted maternal medical records for information on pregnancy outcomes. Prepregnancy BMI was calculated using reported height and prepregnancy weight.
Leisure time physical activity
Prepregnancy physical activity was assessed in all participants using the following questions: ‘Which activities did you participate in on a regular basis during the year before you became pregnant?’, and for each activity reported: ‘How many times per week?’, ‘How many months did you regularly participate in this activity?’, and ‘How much time did you spend at the activity per episode?’. Participants were provided examples of LTPA including walking, swimming, jogging, weightlifting, dance/aerobics, bicycling, hiking and yoga. Each activity reported was matched to its metabolic equivalent (MET) value [23], a measure of energy expenditure of a physical activity defined as the ratio of metabolic rate during a specific activity to a reference resting metabolic rate of 3.5 ml O2·kg-1·min-1. Total duration of each LTPA reported was calculated and summed to obtain total LTPA duration per week. Energy expenditure of each LTPA was calculated by multiplying each individual activity duration by its corresponding MET value. Individual MET hours were summed to obtain average prepregnancy LTPA energy expenditure per week.
Early-pregnancy physical activity was similarly assessed. Total duration of each LTPA reported in the week prior to the study interview was calculated and summed to obtain total early-pregnancy LTPA duration. Energy expenditure of each LTPA was calculated, and individual MET hours were summed to obtain early-pregnancy LTPA energy expenditure per week. Participants were categorized according to recommendations for moderate/vigorous physical activity before and during pregnancy (not active, active <150 min per week, active ≥150 min per week).
Sample collection, preprocessing, DNA & RNA extraction
Maternal peripheral blood samples were collected at 16 weeks gestation, on average (interquartile range: 15–17 weeks). After collection, samples were stored at 4°C until processing. Maternal peripheral blood buffy coat specimens were prepared from whole blood collected in early pregnancy. DNA, for methylation profiling, was extracted from maternal buffy coat samples using the Gentra PureGene Cell kit for DNA preparations (Qiagen, CA, USA). Approximately 200 μl of plasma was used for extracting small RNAs using the Exiqon miRCURY™ RNA Biofluids Isolation Kit (Exiqon, MA, USA). Integrity, purity and quantity of purified miRNA was assessed using spectrophotometry and an Agilent 2100 Bioanalyzer capillary electrophoresis system (Agilent Technologies, Inc., CA, USA).
Candidate DNA methylation site & miRNA selection & profiling, data processing & normalization
DNA methylation sites in genes that have been previously associated with environmental exposures and pregnancy outcomes were identified from the literature: NSAF, NXN, MSGN1, C1orf212, NOS2A, H19, HSD11B2 and F2. References for selected candidate DNA methylation sites are presented in Supplementary Table 1. Selected candidate DNA methylation sites are presented in Supplementary Table 2. Genomic DNA (250–300 ng) was bisulfide treated, using EpiTect Fast DNA Bisulfite Kit (Qiagen). Following cleanup of the converted product, DNA was brought up to a concentration of 10 ng/μl and 20 ng of converted DNA was run in the Pyromark PCR reaction as per manufacturer's protocol (Qiagen). Each 25 μl PCR reaction consisted of 12.5 μl 2× PyroMark PCR Master Mix (Qiagen), 5 pmol forward primer, 5 pmol reverse primer, 20 ng of bisulfite-treated DNA and water. Thermocycling conditions were 15 min at 95°C followed by 45 cycles of 30 s at 94°C, 30 s at 56°C and 30 s at 72°C, with a final extension of 10 min at 72°C. After visual determination of a single band on an agarose gel, 8 μl of the PCR product was used in a Qiagen Q24 Pyrosequencing assay using the manufacturer's protocol. Briefly, the biotin-labeled amplicon was bound to streptavidin-coated sepharose beads. The product was then denatured to remove unbound DNA and washed via a vacuum workstation. The resulting bound ssDNA was mixed with sequencing primer, heated at 80°C for 2 min and allowed to slowly cool. DNA methylation status was determined on this final product using a Pyromark Q24 instrument. By detecting the intensity of light generated during each nucleotide dispensation, the sequence information was collected. Final results were analyzed using Pyromark Q24 software.
MiRNAs that have previously been associated with pregnancy complications and mechanisms related to pregnancy complications were identified from the literature: miR-126-3p, -155-5p, -21-3p, -146b-5p, -210-3p, -222-3p, -223-3p, -517-5p, -518a-3p and -29a-3p. References for selected candidate miRNAs are presented in Supplementary Table 1. We constructed a custom targeted panel of the candidate miRNAs and two control miRNA assays using ExiqonLNA™ primers. Quantitative polymerase chain reaction (qPCR) was conducted in duplicate using 96-well qPCR plates. Reactions were run on an ABI PRISM 7000 real-time PCR machine (Applied Biosystems, CA, USA), using default cycling conditions. An exogenous miRNA cel-miR-39 was added as a positive control for technical factors including RNA extraction, complementary DNA synthesis and PCR amplification [24], and an endogenous ‘housekeeping’ miRNA, miR-423-3p, was chosen for normalization, based on previous recommendations [25]. We recorded threshold cycle (CT) values on two measurements per sample. Original plasma samples were split, completely independent RNA preps were done, an independent RT reaction was performed for each replicate, and each replicate was run on a different qPCR 96-well plate. CT values of duplicates differing by greater than 0.2-times the standard deviation were retested, and replicates were averaged for analyses. Lab personnel were blinded to physical activity. Data from miRNA qPCR arrays were imported into SDS Enterprise Software (V2.2.2, Applied Biosystems) and CT values were calculated using a consistent thresholding value for each assay across all plates. Raw CT values were scaled to values for cel-miR-39-3p. Then, ΔΔCT values were expressed relative to values of miR-423-3p and used in subsequent analyses.
Statistical analyses
Means and standard deviations (SD) were used to describe distributions of continuous variables. Frequencies and percentages were used to describe distributions of categorical variables. Sociodemographic and pregnancy characteristics were summarized overall, by prepregnancy physical activity and by early-pregnancy physical activity. MiRNA CT values were log-transformed to achieve normal distribution. DNA methylation levels were normally distributed.
Linear regression was used to examine associations of LTPA (continuous and categorized according to recommendations) with levels of each candidate DNA methylation site and each candidate circulating miRNA, adjusting for maternal age and gestational age at blood draw. Models were run separately for prepregnancy and early-pregnancy physical activity. We also conducted sensitivity analyses with additional adjustment for prepregnancy BMI, smoking, education and marital status. Two-way multiplicative interaction terms were used to assess interactions of physical activity with offspring sex. Models were also run stratified by offspring sex. A two-sided alpha level of 0.05 was used for statistical significance in all analyses. All analyses were performed using SAS 9.4 (SAS Institute, Inc., NC, USA).
Results
Eighty-six percent of participants reported some prepregnancy LTPA (range: 0.1–30 h per week) and 73% reported some early-pregnancy LTPA (range: 0.5–10.5 h per week) (Table 1). Women who reported some prepregnancy LTPA and women who reported some early-pregnancy LTPA were more likely to have at least a high school education, be married and have a lower prepregnancy BMI.
Table 1. . Characteristics of Omega participants overall, by prepregnancy activity status and by early-pregnancy activity status.
| Characteristic | Total (n = 92) | Some prepregnancy LTPA† (n = 80) | No prepregnancy LTPA (n = 12) | Some early-pregnancy LTPA‡ (n = 69) | No early-pregnancy LTPA (n = 21) |
|---|---|---|---|---|---|
| Maternal age (years), mean (SD) | 33 (4) | 33 (4) | 33 (5) | 33 (4) | 33 (5) |
| Non-Hispanic white race, n (%) | 77 (84) | 67 (84) | 10 (83) | 58 (84) | 17 (81) |
| At least high school education, n (%) | 89 (97) | 79 (99) | 10 (83) | 68 (99) | 19 (90) |
| Married, n (%) | 83 (90) | 73 (91) | 10 (83) | 64 (93) | 17 (81) |
| Prepregnancy BMI (kg/m2), mean (SD) | 23 (4) | 22 (4) | 26 (6) | 22 (4) | 24 (5) |
| Nulliparous, n (%) | 50 (54) | 45 (56) | 5 (42) | 36 (52) | 13 (62) |
| Gestational age at blood draw (weeks), mean (SD) | 16 (3) | 16 (3) | 16 (2) | 16 (3) | 17 (2) |
| Smoked during pregnancy, n (%) | 7 (8) | 7 (9) | 0 (0) | 4 (6) | 3 (15) |
| Infant birthweight, mean (SD) | 3427 (508) | 3384 (503) | 3699 (467) | 3421 (540) | 3467 (363) |
†Range: 0.1–30 h per week.
‡Range: 0.5–10.5 h per week.
LTPA: Leisure time physical activity; SD: Standard deviation.
Each additional hour (duration) or MET hour (energy expenditure) of prepregnancy moderate/vigorous LTPA was associated with hypermethylation of a site in C1orf212 (β = 0.137, 95% CI: 0.004–0.270; p = 0.05 and β = 0.030, 95% CI: 0.001–0.059; p = 0.05, respectively; Table 2). Associations of prepregnancy physical activity with levels of DNA methylation in C1orf212 were similar in women who delivered male and female offspring (all interaction p-values >0.05; Supplementary Table 3).
Table 2. . Associations of pre- and early-pregnancy leisure time physical activity with DNA methylation.
| DNA methylation site | Prepregnancy | Early pregnancy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (hours per week) | Energy expenditure (MET hours per week) | Duration (hours per week) | Energy expenditure (MET hours per week) | |||||||||||||
| n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | |
| NSMAF | 92 | -0.020 | -0.055–0.015 | 0.28 | 92 | -0.004 | -0.012–0.004 | 0.27 | 90 | -0.004 | -0.057–0.049 | 0.89 | 90 | 0.060 | -0.03–0.150 | 0.20 |
| NXN | 90 | -0.057 | -0.165–0.051 | 0.30 | 90 | -0.009 | -0.033–0.015 | 0.46 | 88 | 0.097 | -0.058–0.252 | 0.22 | 88 | 0.125 | -0.144–0.394 | 0.37 |
| MSGN1 | 90 | -0.009 | -0.105–0.087 | 0.85 | 90 | -0.002 | -0.024–0.020 | 0.84 | 88 | -0.041 | -0.168–0.086 | 0.52 | 88 | -0.081 | -0.302–0.140 | 0.48 |
| C1orf212 | 92 | 0.137 | 0.004–0.270 | 0.05 | 92 | 0.030 | 0.001–0.059 | 0.05 | 90 | -0.149 | -0.343–0.045 | 0.13 | 90 | -0.109 | -0.448–0.230 | 0.53 |
| NOS2A | 92 | -0.047 | -0.118–0.024 | 0.20 | 92 | -0.010 | -0.026–0.006 | 0.23 | 90 | 0.003 | -0.097–0.103 | 0.96 | 90 | 0.078 | -0.096–0.252 | 0.38 |
| H19 | 92 | 0.053 | -0.055–0.161 | 0.34 | 92 | 0.016 | -0.008–0.040 | 0.20 | 90 | -0.010 | -0.165–0.145 | 0.90 | 90 | -0.035 | -0.304–0.234 | 0.80 |
| HSD11B2 | 92 | -0.036 | -0.110–0.038 | 0.34 | 92 | -0.007 | -0.023–0.009 | 0.43 | 90 | 0.012 | -0.094–0.118 | 0.82 | 90 | 0.048 | -0.136–0.232 | 0.61 |
| F2 | 92 | -0.006 | -0.069–0.057 | 0.84 | 92 | -0.004 | -0.018–0.010 | 0.61 | 90 | -0.075 | -0.165–0.015 | 0.11 | 90 | -0.065 | -0.224–0.094 | 0.42 |
Results were similar after additional adjustment for prepregnancy BMI and smoking.
†Outcome is percent methylation. Model is adjusted for maternal age (years) and gestational age at blood draw (weeks).
MET: Metabolic equivalent.
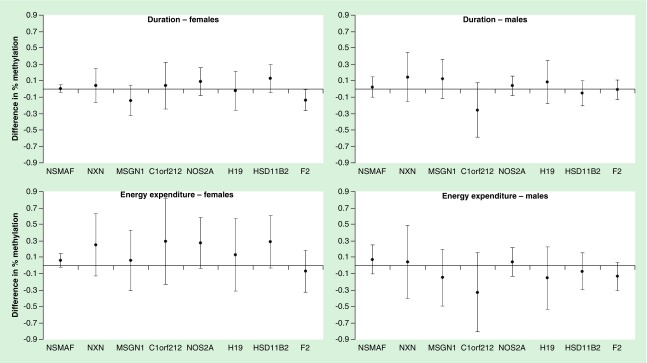
Early-pregnancy LTPA was not associated with levels of DNA methylation in candidate genes among all participants, however, associations of early-pregnancy LTPA energy expenditure with DNA methylation levels of sites in NOS2A and HSD11B2 differed by offspring sex (interaction p-value = 0.08 and 0.05, respectively; Figure 1 and Supplementary Table 4). Each additional MET hour of early-pregnancy physical activity energy expenditure was marginally associated with hypermethylation of sites in NOS2A and HSD11B2 (β = 0.272, 95% CI: -0.036–0.580; p = 0.08 and β = 0.287, 95% CI: -0.032–0.605; p = 0.08, respectively) among women who delivered female offspring, but not among women who delivered male offspring (corresponding estimates: β = 0.044, 95% CI: -0.131–0.218; p = 0.62 and β = -0.072, 95% CI: -0.295–0.152; p = 0.52, respectively).
Figure 1. . Associations of early-pregnancy physical activity with DNA methylation by offspring sex.
Each additional hour (duration) or MET hour (energy expenditure) of prepregnancy moderate/vigorous LTPA was associated with higher circulating levels of miR-146b-5p (β = 0.084, 95% CI: 0.017–0.151; p = 0.02 and β = 0.017, 95% CI: 0.001–0.033; p = 0.03, respectively; Table 3). Prepregnancy physical activity duration and energy expenditure were also marginally associated with higher circulating levels of miR-222-3p (β = 0.074, 95% CI: -0.002–0.150; p = 0.07 and β = 0.017, 95% CI: 0.001–0.033; p = 0.06, respectively). Associations of prepregnancy physical activity with circulating levels of miR-146b-5p and miR-222-3p were similar in women who delivered male and female offspring (all interaction p-values >0.05; Supplementary Table 5).
Table 3. . Associations of pre- and early-pregnancy physical activity and candidate miRNAs.
| miRNA | Prepregnancy | Early pregnancy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (hours per week) | Energy expenditure (MET hours per week) | Duration (hours per week) | Energy expenditure (MET hours per week) | |||||||||||||
| n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | n | Est† | 95% CI | p-value | |
| miR-146b-5p | 75 | 0.084 | 0.017–0.151 | 0.02 | 75 | 0.017 | 0.001–0.033 | 0.03 | 74 | 0.017 | -0.110–0.144 | 0.80 | 74 | -0.041 | -0.213–0.131 | 0.65 |
| miR-126-3p | 75 | 0.070 | -0.01–0.150 | 0.09 | 75 | 0.012 | -0.006–0.030 | 0.19 | 74 | -0.015 | -0.162–0.132 | 0.84 | 74 | 0.019 | -0.181–0.219 | 0.85 |
| miR-155-5p | 75 | 0.050 | -0.032–0.132 | 0.24 | 75 | 0.008 | -0.012–0.028 | 0.39 | 74 | 0.027 | -0.124–0.178 | 0.72 | 74 | 0.005 | -0.199–0.209 | 0.96 |
| miR-210-3p | 74 | 0.066 | -0.024–0.156 | 0.15 | 74 | 0.016 | -0.004–0.036 | 0.12 | 73 | 0.018 | -0.145–0.181 | 0.83 | 73 | -0.043 | -0.264–0.178 | 0.70 |
| miR-21-3p | 75 | 0.071 | -0.021–0.163 | 0.14 | 75 | 0.013 | -0.009–0.035 | 0.22 | 74 | 0.053 | -0.116–0.222 | 0.54 | 74 | 0.098 | -0.131–0.327 | 0.40 |
| miR-222-3p | 75 | 0.074 | -0.002–0.150 | 0.07 | 75 | 0.017 | -0.001–0.035 | 0.06 | 74 | 0.003 | -0.140–0.146 | 0.97 | 74 | -0.020 | -0.214–0.174 | 0.84 |
| miR-223-3p | 75 | 0.079 | -0.001–0.159 | 0.06 | 75 | 0.014 | -0.004–0.032 | 0.14 | 74 | -0.001 | -0.148–0.146 | 0.99 | 74 | 0.037 | -0.163–0.237 | 0.71 |
| miR-29a-3p | 75 | 0.055 | -0.031–0.141 | 0.22 | 75 | 0.013 | -0.007–0.033 | 0.20 | 74 | 0.023 | -0.134–0.180 | 0.78 | 74 | 0.009 | -0.205–0.223 | 0.93 |
| miR-517-5p | 73 | 0.060 | -0.032–0.152 | 0.20 | 73 | 0.013 | -0.009–0.035 | 0.22 | 72 | 0.021 | -0.148–0.190 | 0.81 | 72 | -0.156 | -0.379–0.067 | 0.17 |
| miR-518a-3p | 74 | 0.073 | -0.045–0.191 | 0.23 | 74 | 0.016 | -0.011–0.043 | 0.25 | 73 | 0.052 | -0.169–0.273 | 0.65 | 73 | -0.004 | -0.306–0.298 | 0.98 |
Results were similar after adjustment for prepregnancy BMI and smoking.
†Model is adjusted for maternal age (years) and gestational age at blood draw (weeks). Outcome is log transformed.
MET: Metabolic equivalent.
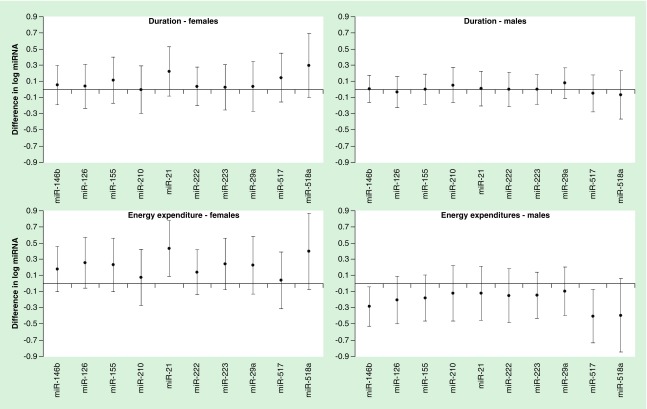
Early-pregnancy LTPA was not associated with levels of circulating miRNAs among all participants, however, associations of early-pregnancy LTPA energy expenditure with circulating levels of miR-146b-5p, miR21-3p and miR517-5p differed by offspring sex (interaction p-value = 0.09, 0.003 and 0.05, respectively; Figure 2 & Supplementary Table 6). Each additional MET hour of early-pregnancy physical activity energy expenditure was associated with lower circulating levels of miR-146b-5p and miR-517-5p (β = -0.285, 95% CI: -0.528 to -0.043; p = 0.02 and β = -0.406, 95% CI: -0.736 to -0.076; p = 0.02, respectively) among women who delivered male offspring, but not among women who delivered female offspring. Each additional MET hour of early-pregnancy physical activity energy expenditure was associated with higher circulating levels of miR-21-3p (β = 0.431, 95% CI: 0.089–0.772; p = 0.02) among women who delivered female offspring, but not among women who delivered male offspring. There was evidence for interaction of early-pregnancy physical activity duration with offspring sex on circulating levels of miR-21-3p and miR-517-5p (interaction p-value = 0.04 and 0.06, respectively) and of early-pregnancy physical activity energy expenditure with offspring sex on circulating levels of miR-126-3p, miR-155-5p, miR-222-3p and miR-223-3p (interaction p = 0.04, 0.02, 0.05 and 0.04, respectively), though offspring sex-stratified estimates were not statistically significant.
Figure 2. . Associations of early-pregnancy physical activity with candidate miRNAs by offspring sex.
Results were similar after adjustment for prepregnancy BMI, smoking, education and marital status. Prepregnancy or early-pregnancy physical activity, categorized according to recommendations for physical activity issued by the American College of Obstetricians and Gynecologists, was not associated with candidate DNA methylation or circulating miRNA level (Supplementary Tables 7 & 8).
Discussion
In the current study, prepregnancy LTPA was associated with hypermethylation at sites in C1orf212, and higher circulating levels of miR-146b-5p among all participants. We also observed marginally significant associations of prepregnancy physical activity with higher circulating levels of miR-222-3p among all participants. We observed marginally significant associations of early-pregnancy LTPA with hypermethylation at sites in NOS2A and HSD11B2 and significant associations of early-pregnancy physical activity with higher circulating levels of miR-21-3p among women who delivered female offspring. We also observed associations of early-pregnancy physical activity with lower circulating levels of miR-146b-5p and miR-517-5p among women who delivered male offspring.
A previous study of maternal physical activity during pregnancy and epigenetic regulation in newborn cord blood also found associations of physical activity and DNA methylation. McCullough et al. found associations between total maternal physical activity and methylation of an imprinted region of PLAG1 associated with fetal growth [15]. The study was nested within the Newborn Epigenetic Study, based in Durham County, NC, USA. Newborns of women in the highest quartile for total self-reported physical activity during pregnancy had 1.5% (absolute) lower methylation of PLAGL1 compared with newborns of women in the lowest quartile (p for trend = 0.01). This study did not assess interaction of maternal physical activity during pregnancy with offspring sex. Unlike the study by McCullough et al., we assessed methylation in maternal blood, not in newborn cord blood. We did find potential sex-specific associations of early-pregnancy moderate/vigorous physical activity with candidate DNA methylation and miRNA levels in maternal blood.
For observed associations of prepregnancy physical activity with candidate DNA methylation sites and miRNAs, associations were similar for physical activity duration and energy expenditure. For observed sex-specific associations of early-pregnancy physical activity with candidate DNA methylation sites and miRNAs, associations were mostly limited to energy expenditure. Physical activity energy expenditure considers intensity of the physical activity in addition to duration. In early pregnancy, intensity may play a stronger role in associations of physical activity with epigenetic biomarkers than before pregnancy.
Reports from previous studies in nonpregnant populations have indicated changes in levels of circulating miR-146, miR-222 and miR-21 in response to physical activity. In active men, acute aerobic physical activity was associated with upregulation of miR-21; sustained aerobic physical activity was associated with downregulation of miR-21 and upregulation of miR-146a; and, both acute and sustained aerobic physical activities were associated with upregulation miR-222 [13,14]. In another study of male athletes, Wardle et al. reported that endurance athletes (defined as those engaging in long-term aerobic physical activity) had higher levels of circulating miR-222 than nonexercising controls, whereas strength trained athletes had lower levels of miR-222 than nonexercising controls [26]. Wardle et al. also observed greater circulating levels of miR-146a and miR-21 in endurance athletes compared with strength trained athletes. Our results suggest that previously reported changes in circulating miR-146, miR-222 and miR-21 in male athletes in response to long-term aerobic physical activity may also be present in pregnant women in relation to LTPA. To our knowledge, physical activity and DNA methylation in C1orf212, NOS2A or HSD11B2 or levels of circulating miR-517 have not previously been studied.
Genetic polymorphisms in NOS2A and HSD11B2 have been associated with spontaneous preterm birth [27] and preeclampsia [28–30]. Methylation of the placental HSD11B2 promoter has been associated with fetal growth [31]. To our knowledge, C1orf212 has not been previously associated with pregnancy-related outcomes as no prior studies have evaluated associations between C1orf212 and pregnancy-related conditions. C1orf212 may play a role in the development of gestational hypertension, as C1orf212 is regulated by miR-520h, which is involved in the pathogenesis of gestational hypertension [32]. C1orf212 has also been associated with air pollution, a risk factor for pregnancy complications [33].
Targets of miR-146b include genes associated with inflammation (NFKB1, TRAF6), cell cycle regulation (CDKN1A), apoptosis (TRAF6) and extracellular matrix degradation (MMP16) [34]. Targets of miR-222 also include genes associated with cell cycle regulation (CDKN1B, CDKN1C), apoptosis (STAT5A, FOXO3) and extracellular matrix degradation (MMP1) [34]. Targets of miR-21 include genes associated with amino acid biosynthesis (MAT2A, MAT2B) [34]. Targets of miR-517 include genes associated with DNA methylation (KDM5A), protection from apoptosis (DNAJB9), muscular growth (FOXO1), RNA interference and transcriptional gene silencing (AGO1), and inflammation (SAMD9L) [34]. These pathways play important roles in the normal progression of pregnancy. miR-222, miR-21 and miR-517 have also been related to pregnancy complications (miR-222, miR-21 and miR-517 with preeclampsia [35–37]) and fetal growth (miR-21 with macrosomia [38] and miR-517 with growth restriction [39]).
Higher levels of circulating miR-21 were associated with a greater level of DNA methylation at sites in C1orf212, NOS2A and HSD11B2 (Supplementary Table 9). Associations between circulating miR-146b and DNA methylation sites in C1orf212 were marginal. Levels of circulating miR-222 and miR-517 were not associated with levels of DNA methylation in any candidate genes. These findings suggest potential regulatory relationships between DNA methylation and circulating miRNAs. However, due to the cross-sectional nature of these analyses, future studies are needed.
Sex-specific associations of HSD11B2 methylation and expression in relation to maternal exposures have been reported in rodent models and human placentas. In a study of folic acid supplementation during pregnancy, increased methylation of a site in HSD11B2 was observed in placentas of female, but not male, fetuses of folic acid supplemented pregnant rats [40]. Glucocorticoid exposure during pregnancy in mice increased HSD11B2 expression in placentas of female fetuses only [41]. In humans, placental HSD11B2 activity was greater in response to prenatal glucocorticoid exposure (bethamethasone treatment) in female, compared with male, infants [42]. Placental HSD11B2 converts maternal cortisol to its inactive form, cortisone, acting as a barrier to protect the fetus from high levels of maternal glucocorticoids [43]. Methylation of the HSD11B2 gene promoter, which results in reduction of HSD11B2 expression, has been associated with intrauterine growth restriction [31]. The sex-specific association of early-pregnancy physical activity with methylation of sites in HSD11B2 observed in this study was also limited to female fetuses. This suggests female fetuses may be more sensitive to changes in pathways related to maternal stress response, with subsequent implications for intrauterine growth. To our knowledge, no prior studies have evaluated sex-specific associations of maternal exposures and NOS2A methylation or expression. NOS2A polymorphisms have been associated with preeclampsia risk [29,30]. Previous studies have also reported an increased risk of preeclampsia in women carrying female fetuses [44,45]. The sex-specific association of early-pregnancy physical activity with NOS2A methylation observed in this study suggests maternal exposures may affect pathways related to immune response, in a sex-specific manner, with implications for preeclampsia.
Sex-specific associations of circulating levels of miR-146b, miR-21 and miR-517 with gestational diabetes have been reported. Greater maternal circulating levels of miR-146b, miR-21 and miR-517 were associated with gestational diabetes among women who delivered male offspring only [46]. We observed associations of maternal early-pregnancy physical activity with lower levels of miR-146b and miR-517 among women who delivered male offspring, and with higher levels of miR-21 among women who delivered female offspring. These results suggest that regulation of miR-146b and miR-517 may be one mechanism through which physical activity reduces the risk of gestational diabetes in women carrying male offspring. Lower levels of miR-146b and miR-517 may decrease inflammation, as targets of miR-146b and miR-517 include genes involved in pathways related to inflammation (NFKB1, TRAF6, SAMD9L) [34]. Reducing inflammation may reduce the risk of gestational diabetes, since gestational diabetes is a proinflammatory state [47]. Our results also suggest that mechanisms linking maternal physical activity during pregnancy with reduced risk of gestational diabetes may be different in women carrying male or female offspring, possibly through pathways involving other target genes of miR-146b and miR-517, such as cell cycle regulation (CDKN1A), extracellular matrix degradation (MMP16), DNA methylation (KDM5A), protection from apoptosis (DNAJB9), muscular growth (FOXO1) and RNA interference and transcriptional gene silencing (AGO1) [34].
Identifying circulating epigenetic biomarkers linking maternal physical activity with pregnancy complications and fetal growth may be useful for identifying women at high risk for pregnancy complications. Circulating miRNAs and DNA methylation in maternal peripheral blood, which are easily accessible and routinely collected during prenatal care, may be noninvasive biomarkers for early detection of systemic (tissue non-specific) or local (tissue-specific, such as placental) pathophysiologic processes. For instance, previous studies have identified placenta-specific epigenetic biomarkers in maternal blood [48], suggesting that epigenetic biomarkers from the placenta may be released or leak into maternal circulation during pregnancy.
Strengths of our study include assessment of perinatal physical activity in two time periods, before and during pregnancy, consideration of multiple types of epigenetic mechanisms of gene regulation (DNA methylation and miRNA level), and evaluation of sex-specific associations. We also confirmed the stability and suitability of our choice of miR-423 for normalization using a combination of three algorithms (geNorm [49], Normfinder [50] and Coefficient of Variation score) recommended for identifying miRNAs for normalization [24]. On the other hand, the candidate approach we used for selecting DNA methylation sites and circulating miRNAs of interest limited our capacity to identify novel DNA methylation sites or circulating miRNAs that have not been previously characterized. High-throughput sequencing can be used in future research to identify additional DNA methylation sites and circulating miRNAs that are associated with prepregnancy or early-pregnancy physical activity. The predictive capabilities of identified epigenetic biomarkers should also be evaluated in future research. Future research should also investigate the role of identified DNA methylation sites and miRNAs in associations of physical activity with pregnancy outcomes in more detail. We measured epigenetic biomarkers at one time point during pregnancy, so we are unable to assess longitudinal changes in epigenetic biomarkers across pregnancy. Future research should measure epigenetic biomarkers at multiple time points during pregnancy. Due to the relatively small size of our study, we had low statistical power. Although we used a candidate approach, type I error may explain our observed results. In sensitivity analyses, results were similar after adjustment for prepregnancy BMI, smoking, education and marital status (data not shown), suggesting that these variables were not strong confounders of observed associations. Self-report of LTPA may have introduced misclassification into our study. A similar interviewer-administered physical activity questionnaire had moderate to good validity compared with accelerometer data (Spearman correlation coefficient: 0.12–0.24) and good reliability (intraclass correlation coefficient: 0.82) for moderate to vigorous physical activity recall in early pregnancy for all domains of physical activity [51]. Our study population was composed of predominantly active, educated, white women and results of our study may not generalize to less active, more racially diverse populations.
Conclusion
In summary, overall, we observed associations of maternal prepregnancy or early-pregnancy physical activity with circulating miRNAs and peripheral blood DNA methylation. We also observed that these associations may differ by offspring sex. Our findings suggest that maternal physical activity may influence epigenetic regulation of genes involved in pathways that have been linked to pregnancy complications in an offspring sex-specific manner. Larger studies of differences in physical activity-related epigenetic regulation by offspring sex in the perinatal period are warranted. Findings from such studies will enhance understanding of mechanisms underlying associations of maternal physical activity with pregnancy complications and fetal growth.
Summary points.
Physical activity-related epigenetic changes during the perinatal period, a period of major physiologic changes in the mother, have not been well studied. To our knowledge, previous studies have not examined associations of maternal physical activity before or during pregnancy with peripheral blood DNA methylation or circulating miRNAs in maternal blood.
In our study, prepregnancy leisure time physical activity was associated with hypermethylation at sites in C1orf212 and higher circulating levels of miR-146b-5p in maternal peripheral blood during early–mid pregnancy.
We observed marginally significant associations of prepregnancy physical activity with higher circulating levels of miR-222-3.
We observed marginally significant associations of early-pregnancy leisure time physical activity with hypermethylation at sites in NOS2A and HSD11B2 and significant associations of early-pregnancy physical activity with higher circulating levels of miR-21-3p among women who delivered female offspring.
We observed associations of early-pregnancy physical activity with lower circulating levels of miR-146b-5p and miR-517-5p among women who delivered male offspring.
Our findings suggest that maternal physical activity may influence epigenetic regulation of genes involved in pathways that have been linked to pregnancy complications in an offspring sex-specific manner.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: https://www.futuremedicine.com/doi/suppl/10.2217/epi-2017-0169
Financial & competing interests disclosure
This work was supported by the NIH under grant T32HD052462, R01HD-32562, and K01HL103174 and by a pilot grant awarded by the Center for Ecogenetics and Environmental Health at the University of Washington, Seattle WA, through a program project (P30ES07033) funded by the National Institute of Environmental Health Sciences. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The Omega study was approved by the institutional review boards of Swedish Medical Center and Tacoma General Hospital. All participants gave written informed consent.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008. www.health.gov/paguidelines
- 2.Aune D, Sen A, Henriksen T, Saugstad OD, Tonstad S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose–response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 2016;31(10):967–997. doi: 10.1007/s10654-016-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Saugstad OD, Henriksen T, Tonstad S. Physical activity and the risk of preeclampsia: a systematic review and meta-analysis. Epidemiology. 2014;25(3):331–343. doi: 10.1097/EDE.0000000000000036. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe HW, Boule NG, Chari R, Davenport MH. The effect of supervised prenatal exercise on fetal growth: a meta-analysis. Obstet. Gynecol. 2015;125(5):1185–1194. doi: 10.1097/AOG.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 5.Badon SE, Wander PL, Qiu C, Miller RS, Williams MA, Enquobahrie DA. Maternal leisure time physical activity and infant birth size. Epidemiology. 2016;27(1):74–81. doi: 10.1097/EDE.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 6.Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events: inflammatory and metabolic mechanisms. Med. Sci. Sports Exerc. 2009;41(6):1206–1211. doi: 10.1249/MSS.0b013e3181971247. [DOI] [PubMed] [Google Scholar]
- 7.Genest DS, Falcao S, Gutkowska J, Lavoie JL. Impact of exercise training on preeclampsia: potential preventive mechanisms. Hypertension. 2012;60(5):1104–1109. doi: 10.1161/HYPERTENSIONAHA.112.194050. [DOI] [PubMed] [Google Scholar]
- 8.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Pre-gravid physical activity and reduced risk of glucose intolerance in pregnancy: the role of insulin sensitivity. Clin. Endocrinol. 2009;70(4):615–622. doi: 10.1111/j.1365-2265.2008.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell AP, Lamon S. Exercise, skeletal muscle and circulating microRNAs. Prog. Mol. Biol. Transl. Sci. 2015;135:471–496. doi: 10.1016/bs.pmbts.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barres R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Nitert MD, Dayeh T, Volkov P, et al. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61(12):3322–3332. doi: 10.2337/db11-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen S, Akerstrom T, Rinnov A, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE. 2014;9(2):e87308. doi: 10.1371/journal.pone.0087308. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reporting downregulation of miR-146a after acute aerobic physical activity and downregulation of miR-21 after sustained aerobic physical activity in active men.
- 14.Baggish AL, Hale A, Weiner RB, et al. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 2011;589(Pt 16):3983–3994. doi: 10.1113/jphysiol.2011.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reporting upregulation of miR-146a, miR-21 and miR-222 after an acute aerobic physical activity bout and upregulation of miR-146a and miR-222 after sustained aerobic physical activity.
- 15.McCullough LE, Mendez MA, Miller EE, Murtha AP, Murphy SK, Hoyo C. Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics. 2015;10(7):597–606. doi: 10.1080/15592294.2015.1045181. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study of maternal physical activity during pregnancy and epigenetic regulation of imprinted genes in newborn cord blood at birth showing physical activity during pregnancy was associated with lower methylation in an imprinted region of PLAGL1.
- 16.Wilhelm-Benartzi CS, Houseman EA, Maccani MA, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ. Health Perspect. 2012;120(2):296–302. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu G, Brkic J, Hayder H, Peng C. MicroRNAs in human placental development and pregnancy complications. Int. J. Mol. Sci. 2013;14(3):5519–5544. doi: 10.3390/ijms14035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu P, Farrell WE, Haworth KE, et al. Maternal genome-wide DNA methylation profiling in gestational diabetes shows distinctive disease-associated changes relative to matched healthy pregnancies. Epigenetics. 2018;13(2):122–128. doi: 10.1080/15592294.2016.1166321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsochandaridis M, Nasca L, Toga C, Levy-Mozziconacci A. Circulating microRNAs as clinical biomarkers in the predictions of pregnancy complications. BioMed. Res. Int. 2015:294954. doi: 10.1155/2015/294954. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Wen Y, Hu L, Miao T, Zhang M, Dong J. Serum microRNAs as diagnostic biomarkers for macrosomia. Reprod. Sci. 2015;22(6):664–671. doi: 10.1177/1933719114561557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl.):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Med. Sci. Sports Exerc. 2008;40(9):1581–1588. doi: 10.1249/MSS.0b013e31817cab1. [DOI] [PubMed] [Google Scholar]
- 23.Montoye HJ, Kemper HC, Saris WH, Washburn RA. Measuring Physical Activity and Energy Expenditure. Human Kinetics; Champaign, IL, USA: 1996. pp. 34–41. [Google Scholar]
- 24.Marabita F, de Candia P, Torri A, Tegner J, Abrignani S, Rossi RL. Normalization of circulating microRNA expression data obtained by quantitative real-time RT-PCR. Brief. Bioinform. 2016;17(2):204–212. doi: 10.1093/bib/bbv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts TC, Coenen-Stass AM, Wood MJ. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS ONE. 2014;9(2):e89237. doi: 10.1371/journal.pone.0089237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wardle SL, Bailey ME, Kilikevicius A, et al. Plasma microRNA levels differ between endurance and strength athletes. PLoS ONE. 2015;10(4):e0122107. doi: 10.1371/journal.pone.0122107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Finding greater circulating levels of miR-146a and miR-21 in endurance athletes compared with strength-trained athletes.
- 27.Gibson CS, MacLennan AH, Dekker GA, et al. Genetic polymorphisms and spontaneous preterm birth. Obstet. Gynecol. 2007;109(2 Pt 1):384–391. doi: 10.1097/01.AOG.0000252712.62241.1a. [DOI] [PubMed] [Google Scholar]
- 28.Shimodaira M, Nakayama T, Sato I, et al. Glucocorticoid synthesis-related genes: HSD11B1 and HSD11B2 in hypertensive disorders in pregnancy. Gynecol. Endocrinol. 2013;29(7):657–661. doi: 10.3109/09513590.2013.788623. [DOI] [PubMed] [Google Scholar]
- 29.Amaral LM, Palei AC, Sandrim VC, et al. Maternal iNOS genetic polymorphisms and hypertensive disorders of pregnancy. J. Hum. Hypertens. 2012;26(9):547–552. doi: 10.1038/jhh.2011.65. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar S, Bhattacharjee J, Vaid M, Madan T, Trivedi SS, Sarma PU. Inducible nitric oxide synthase (iNOS) gene polymorphism in pre-eclampsia: a pilot study in North India. Aust. NZJ Obstet. Gynaecol. 2007;47(6):477–482. doi: 10.1111/j.1479-828X.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhao Y, Gong X, Chen L, et al. Site-specific methylation of placental HSD11B2 gene promoter is related to intrauterine growth restriction. Eur. J. Hum. Genet. 2014;22(6):734–740. doi: 10.1038/ejhg.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hromadnikova I, Kotlabova K, Hympanova L, Doucha J, Krofta L. First trimester screening of circulating C19MC microRNAs can predict subsequent onset of gestational hypertension. PLoS ONE. 2014;9(12):e113735. doi: 10.1371/journal.pone.0113735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panni T, Mehta AJ, Schwartz JD, et al. Genome-wide analysis of DNA methylation and fine particulate matter air pollution in three study populations: KORA F3, KORA F4, and the normative aging study. Environ. Health Perspect. 2016;124(7):983–990. doi: 10.1289/ehp.1509966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou CH, Chang NW, Shrestha S, et al. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016;44(D1):D239–D247. doi: 10.1093/nar/gkv1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy MS, Casselman RC, Tayade C, Smith GN. Differential expression of plasma microRNA in preeclamptic patients at delivery and 1 year postpartum. Am. J. Obstet. Gynecol. 2015;213(3):367.e361–e369. doi: 10.1016/j.ajog.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Lasabova Z, Vazan M, Zibolenova J, Svecova I. Overexpression of miR-21 and miR-122 in preeclamptic placentas. Neuro Endocrinol. Lett. 2015;36(7):695–699. [PubMed] [Google Scholar]
- 37.Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol. Med. Rep. 2015;12(1):527–534. doi: 10.3892/mmr.2015.3414. [DOI] [PubMed] [Google Scholar]
- 38.Jiang H, Wu W, Zhang M, et al. Aberrant upregulation of miR-21 in placental tissues of macrosomia. J. Perinatol. 2014;34(9):658–663. doi: 10.1038/jp.2014.58. [DOI] [PubMed] [Google Scholar]
- 39.Hromadnikova I, Kotlabova K, Ondrackova M, et al. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA Cell Biol. 2015;34(6):437–457. doi: 10.1089/dna.2014.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penailillo R, Guajardo A, Llanos M, Hirsch S, Ronco AM. Folic acid supplementation during pregnancy induces sex-specific changes in methylation and expression of placental 11beta-hydroxysteroid dehydrogenase 2 in rats. PLoS ONE. 2015;10(3):e0121098. doi: 10.1371/journal.pone.0121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuffe JS, Dickinson H, Simmons DG, Moritz KM. Sex specific changes in placental growth and MAPK following short term maternal dexamethasone exposure in the mouse. Placenta. 2011;32(12):981–989. doi: 10.1016/j.placenta.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Stark MJ, Wright IM, Clifton VL. Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am. J. Physiol. 2009;297(2):R510–R514. doi: 10.1152/ajpregu.00175.2009. [DOI] [PubMed] [Google Scholar]
- 43.Bro-Rasmussen F, Buus O, Trolle D. Ratio cortisone/cortisol in mother and infant at birth. Acta Endocrinol. 1962;40:579–583. doi: 10.1530/acta.0.0400579. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Li G, Zhang W. Effect of fetal gender on pregnancy outcomes in Northern China. J. Matern. Fetal Neonatal. Med. 2017;30(7):858–863. doi: 10.1080/14767058.2016.1189527. [DOI] [PubMed] [Google Scholar]
- 45.Shiozaki A, Matsuda Y, Satoh S, Saito S. Impact of fetal sex in pregnancy-induced hypertension and preeclampsia in Japan. J. Reprod. Immunol. 2011;89(2):133–139. doi: 10.1016/j.jri.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Wander PL, Boyko EJ, Hevner K, et al. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res. Clin. Pract. 2017;132:1–9. doi: 10.1016/j.diabres.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf M, Sauk J, Shah A, et al. Inflammation and glucose intolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27(1):21–27. doi: 10.2337/diacare.27.1.21. [DOI] [PubMed] [Google Scholar]
- 48.Kotlabova K, Doucha J, Hromadnikova I. Placental-specific microRNA in maternal circulation–identification of appropriate pregnancy-associated microRNAs with diagnostic potential. J. Reprod. Immunol. 2011;89(2):185–191. doi: 10.1016/j.jri.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7) doi: 10.1186/gb-2002-3-7-research0034. Research 0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen CL, Jensen JL, Orntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64(15):5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 51.Evenson KR, Wen F. Measuring physical activity among pregnant women using a structured one-week recall questionnaire: evidence for validity and reliability. Int. J. Behav. Nutr. Phys. Act. 2010;7:21. doi: 10.1186/1479-5868-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.