Abstract
Immunotherapy has revolutionized the treatment of non‐small cell lung cancer; however, its role in the treatment response of lung brain metastasis is unknown. Understanding immunotherapy activity in the central nervous system is important in order to avoid additional toxicity, such as that associated with the use of cerebral radiotherapy. We present two cases with clinical and radiological progression with increases in size and perilesional edema of brain lesions after treatment with a combination of ipilimumab and nivolumab. The increasing use of immunotherapy in lung cancer requires increased knowledge of new patterns of radiological response, such as pseudoprogression.
Keywords: Brain metastasis, immunotherapy, pseudoprogression, lung cancer
Introduction
The brain is a common site of lung cancer metastasis and increasing incidence has been observed in recent years; up to 50% of non‐small cell lung cancer (NSCLC) patients develop metastasis in the brain.1 The new generation of EGFR and ALK inhibitors, such as osimertinib and alectinib, has shown impressive activity in NSCLC patients with brain metastasis (BM)2, 3 and could change the future management of these patients.
However, most patients do not have molecular target alteration. Chemotherapy, and more recently, immunotherapy are the standards of care. The role of immunotherapy in the treatment of BM is unknown because most trials exclude patients with active brain lesions. As such, the role of immunotherapy is now the subject of ongoing investigation. A retrospective analysis of a phase II trial of ipilimumab was the first study to investigate the effect of immunotherapy on BM in patients with metastatic melanoma, and reported a response in 5 out of 12 patients.4 Early analyses of phase II trials of pembrolizumab in metastatic melanoma or NSCLC with untreated BM have shown activity in BM, suggesting that there could be a role for systemic immunotherapy in these cases.5
Pseudoprogression is the initial rapid increase in the tumor volume or the number of lesions, followed by tumor shrinkage or stable disease.6 It does not usually affect the general state of the patient. Pseudoprogression is mainly observed in patients with advanced melanoma,7 and is less common in patients with NSCLC. We present the cases of two NSCLC patients with BM who developed brain pseudoprogression after treatment with the PD‐1 inhibitor nivolumab in combination with the CTLA‐4 inhibitor ipilimumab.
Case report
Case 1
In May 2017, a 47‐year‐old man consulted the emergency department because of a loss of upper limb strength that caused difficulty when attempting to perform tasks. He had no previous medical history and had been a smoker for 30 years. Brain magnetic resonance imagining (MRI) showed a left temporal lobe lesion (Fig 1). Positron emission tomography and computerized axial tomography scans revealed a mass in the right upper lobe, hypermetabolic lymph node enlargements in the mediastinum, retro tracheal, and upper right paratracheal areas, and liver metastasis. A biopsy determined NSCLC EGFR negative, with no ALK rearrangement and PD‐L1 < 1%. BM was treated with stereotactic radiosurgery (SRS).
Figure 1.
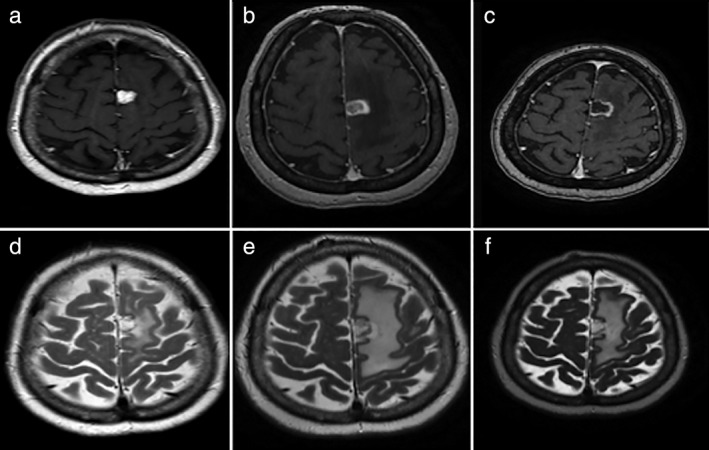
Magnetic resonance imaging before and after treatment. (a,b) Post‐contrast T1 and T2 weighted images revealed a metastatic lesion and edema within the brain parenchyma, respectively, prior to the initiation of treatment. (c,d) Post‐contrast T1 and T2 weighted images acquired one week after the onset of treatment with nivolumab and ipilimumab revealed an increase in size and the edema of the metastatic lesion, respectively, with a target‐like appearance. (e,f) Post‐contrast T1 and T2 weighted images acquired eight weeks after the onset of treatment revealed stable size and a decrease of the edema of the metastatic lesion, respectively.
In July 2017, the patient was included in a clinical trial (CA209‐817) that evaluated the efficacy of the combination of nivolumab and ipilimumab (240 mg, 1 mg/kg, respectively). One week after the first treatment, he presented with dysarthria, aphasia, and right hemiparesis. An MRI showed increased perilesional edema and the lesion in the parietal region had increased in size since the earlier imaging, now measuring 16.8 × 15.1 mm. We initiated treatment of 4 mg dexamethasone every eight hours and observed excellent improvement after one week. In this case, we considered brain pseudoprogression as a possibility.
Finally, we decided to continue immunotherapy treatment. After six weeks, a control MRI showed a lower minimum volume and surrounding edema of the lesion. In the perfusion study, the necrotic component was predominant.
Case 2
In August 2016, a 57‐year‐old woman was diagnosed with NSCLC. She had been a smoker for 40 years but had no previous medical history. Pneumonectomy with a lymphadenectomy was performed. The cancer was a pT4 (mediastinal infiltration) N1 M0, EGFR negative, with no ALK rearrangement, and PD‐L1 < 5%. The patient was treated with adjuvant chemotherapy based on cisplatin‐vinorelbine for four cycles and displayed excellent tolerance.
In March 2017, she exhibited pleural and brain disease progression. An MRI showed a left frontal brain lesion with significant perilesional edema, which produced a mass effect and suggested metastasis (Fig 2). The patient was treated with SRS.
Figure 2.
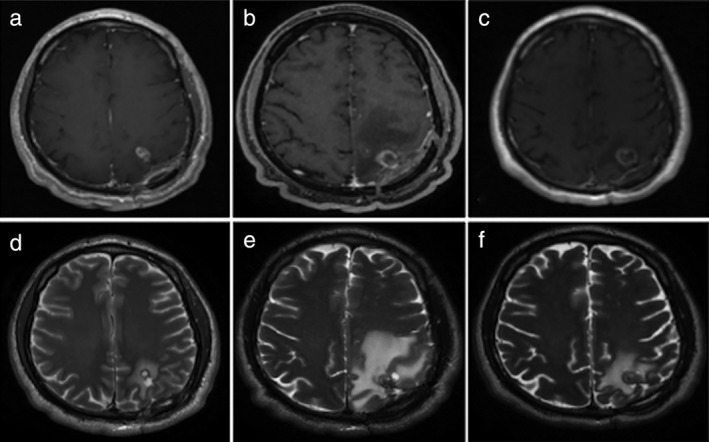
Magnetic resonance imaging before and after treatment. (a,b) Post‐contrast T1 and T2 weighted images revealed a frontal enhancing solid metastatic lesion with peripheral edema within the brain parenchyma, respectively, prior to initiation of treatment. (c,d) Post‐contrast T1 and T2 weighted images obtained one week after the onset of treatment with nivolumab and ipilimumab revealed an increase in the size and the edema of the metastatic lesion, respectively, with a target‐like appearance. (e,f) Post‐contrast T1 and T2 weighted images obtained eight weeks after the onset of treatment revealed cystic like appearance with thin peripheral enhancement of the metastatic lesion and decrease of the edema, respectively.
One month later, she was included in clinical trial CA209‐817. She received the first cycle of treatment with excellent tolerance.
Seven days later, the patient developed dysarthria and a loss of strength in the right hemisphere. An MRI showed enlargement of central nervous system lesions with intense central enhancement and diffuse perilesional edema. The patient was administered 4 mg dexamethasone every eight hours and showed a rapid clinical response after one week of treatment.
Because the speed of growth was atypical of tumor progression, we considered BM pseudoprogression. We decided to continue the nivolumab and ipilimumab treatment. After four cycles (six weeks), a control MRI of the brain showed decreased volume of the left frontal metastasis and a significant reduction of the perilesional edema.
Finally, we decided to continue treatment with immunotherapy. Currently, after 10 cycles, the patient's pleural disease is in partial response, there is no progression in the brain lesion, and she does not require steroids.
Discussion
Significant progress has been made in the treatment of NSCLC with immunomodulatory antibodies, which have shown improved overall survival compared to standard therapies. Different tumor response patterns have been shown. The most important are pseudoprogression and hyperprogression, which should be distinguished.
While pseudoprogression is a biological effect unique to immune agents that consists of an increase in tumor burden followed by tumor regression, hyperprogression is not unique to immunotherapy. It can develop with cytotoxic agents and is defined as an increase in tumor growth kinetics.8
Pseudoprogression is not real tumor growth, but rather radiographic progression explained pathologically by inflammatory cell infiltrates, edema, and necrosis.9 Clinically, pseudoprogression is not accompanied by deterioration in clinical or performance status. However, if pseudoprogression occurs in the brain with an increase in perilesional edema, neurological symptoms can appear, as evident in our cases. Conversely, hyperprogression is accompanied by a clear worsening of the general state and seems to be correlated with poor prognosis.8
Response Evaluation Criteria in Solid Tumors have been modified to include immune‐related response criteria (irRC). According to the irRC, confirmation of progressive disease requires a repeat, consecutive assessment six weeks from the date of the first documented progression.10 MRI is the best method to determine a response to BM; however, the BM response to immunotherapy and pseudoprogression has not been well studied.
Our cases demonstrate that rapid lesion enlargement associated with increased perilesional edema on MRI after nivolumab and ipilimumab treatment does not always imply disease progression. There is controversy over the use of steroids in patients treated with immunotherapy. A study by Downey et al. that evaluated anticancer activity by CTLA4 blockade in melanoma patients showed no statistically significant difference in survival in responders treated with or without steroids.11
Pseudoprogression in immune checkpoint inhibitor therapy has mainly been studied in patients with melanoma;6, 12, 13 thus it is not well known in lung cancer. Studies with a specific design including irRC are required to avoid underestimating pseudoprogression. The correct diagnosis of pseudoprogression may have a significant impact on patient prognosis. If diagnosed correctly, the optimal treatment regime can be applied.
It is important to note the presence of prior cerebral radiotherapy in our two cases. Data suggest that responses might be further improved by combining immune checkpoint inhibitors with radiation. Several studies have evaluated the combination in melanoma with BM.14, 15, 16 The median survival reported was 21.3 months in patients receiving ipilimumab and SRS versus 4.9 months in patients treated with SRS alone.15 Several mechanisms have been described to explain this situation. Radiation can upregulate PD‐L117 and could upregulate inflammatory cytokines, such as TNFα, IFN‐γ, and CXCL16, facilitating T cell infiltration;18, 19 thus supporting the use of radiotherapy combined with immunotherapy in this cohort.20
Recognizing the possibility of pseudoprogression is a challenge for physicians. It is crucial to keep pseudoprogression in mind when evaluating the response to immunotherapy, including in the brain. Our cases show two important points. Firstly, there is growing evidence that immunotherapy can cross the blood‐brain barrier and can play an important role in BM treatment. Secondly, these cases present the appearance of pseudoprogression in BM, not previously described in NSCLC.
Disclosure
No authors report any conflict of interest.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 97(Suppl. 12): 3133–24. [Google Scholar]
- 2. Peters S, Camidge DR, Shaw AT et al Alectinib versus Crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med 2017; 377: 829–38. [DOI] [PubMed] [Google Scholar]
- 3. Soria JC, Ohe Y, Vansteenkiste J et al 2017 Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 4. Weber JS, Amin A, Minor D, Siegel J, Berman D, O'Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospec‐tive analysis of data from a phase 2 trial. Melanoma Res 2011; 21: 530–4. [DOI] [PubMed] [Google Scholar]
- 5. Goldberg SB, Gettinger SN, Mahajan A et al Pembrolizumab for patients with melanoma or non‐small‐cell lung cancer and untreated brain metastases: Early analysis of a non‐randomised, open‐label, phase 2 trial. Lancet Oncol 2016; 17: 976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Di Giacomo AM, Danielli R, Guidoboni M et al Therapeutic efficacy of ipilimumab, an anti‐CTLA‐4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: Clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009; 58: 1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen JV, Alomari AK, Vortmeyer AO et al Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016; 4: 179–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saâda‐Bouzid E, Defaucheux C, Karabajakian A et al Hyperprogression during anti PD1/PD‐L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017; 28: 1605–11. [DOI] [PubMed] [Google Scholar]
- 9. Chiou VL, Burotto M. Pseudoprogression and immune‐related response in solid tumors. J Clin Oncol 2015; 33: 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wolchok JD, Hoos A, O'Day S et al Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009; 15: 7412–20. [DOI] [PubMed] [Google Scholar]
- 11. Downey SG, Klapper JA, Smith FO et al Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL‐associated Antigen‐4 blockade. Clin Cancer Res 2007; 13: 6681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodi FS, Ribas A, Daud A et al Evaluation of immune‐related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti‐PD‐1 monoclonal antibody MK‐3475. J Clin Oncol 2014; 32(Suppl 15): Abstract 3006. [Google Scholar]
- 13. Hodi S, Sznol M, Kluger HM et al Long‐term survival of ipilimumab‐naive patients with advanced melanoma (Mel) treated with nivolumab (anti‐Programmed Death‐1; Anti‐Pd‐1; Bms‐ 936558; Ono‐4538) in a phase I trial. Asia‐Pac J Clin Oncol 2014; 10(Suppl 15): 177. [Google Scholar]
- 14. Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg 2012; 117: 227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel KR, Lawson DH, Kudchadkar RR et al Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. Neuro Oncol 2015; 17: 1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mathew M, Tam M, Ott PA et al Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res 2013; 23: 191–5. [DOI] [PubMed] [Google Scholar]
- 17. Twyman‐Saint Victor C, Rech AJ, Maity A et al Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 2015; 520: 373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frey B, Rubner Y, Kulzer L et al Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother 2014; 63: 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee Y, Auh SL, Wang Y et al Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009; 114: 589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Postow MA, Callahan MK, Barker CA et al Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


