Abstract
Background
Although a clinical complete response (cCR) after chemoradiotherapy (CRT) could lead to a better prognosis, the choice of a following strategy, such as surgical or non‐surgical approach, remains controversial.
Methods
All articles relevant to a comparison of surgical and non‐surgical treatment (including further definitive chemoradiotherapy or active surveillance) for esophageal carcinoma patients with a cCR after CRT were retrieved for meta‐analysis. The final date for data retrieval was 30 June 2018.
Results
Four retrospective studies including 648 patients met the inclusion criteria: 620 with squamous cell carcinoma and 28 with adenocarcinoma. The CRT + surgery group had an advantage over the non‐surgery group in regard to two‐year disease‐free survival (DFS); however, the two groups showed similar results in five‐year DFS. The CRT + surgery group had an advantage over the non‐surgery group in two‐year overall survival (OS); nevertheless, the two groups showed similar results in five‐year OS.
Conclusions
Based on the available evidence, the addition of surgery to thoracic locally advanced esophageal carcinoma patients with a cCR after neoadjuvant CRT provided no advantage to long‐term survival. As an exception, the two‐year DFS and OS could be improved. This research conclusion might be more suitable to patients with squamous cell carcinoma.
Keywords: Chemoradiotherapy, clinical complete response, esophageal carcinoma
Introduction
Esophageal carcinoma is the eighth most common malignancy worldwide, affecting more than 450 000 people per year. The overall five‐year survival rate is estimated at 15–25%.1, 3 Esophagectomy remains the cornerstone of treatment for resectable esophageal carcinoma. Unfortunately, the cure rate with esophagectomy is still poor and the procedure has been associated with the risk of higher morbidity and mortality. Its use is limited to patients who can tolerate the procedure and in whom all gross disease can be resected.4
Chemoradiotherapy (CRT) is the standard therapy for unresectable esophageal cancer and may also be performed in patients who refuse or cannot tolerate surgery.5, 6 However, there is no definitive answer as to whether this approach could offer similar cure rates with lower complications and mortality.
A clinical complete response (cCR) is defined as tumor residue not visible on esophagogram, computed tomography (CT), endoscopy, positron emission tomography (PET)‐CT, and other non‐surgical methods after CRT. Neoadjuvant chemoradiotherapy (nCRT) has been shown to improve survival in patients with locally advanced esophageal carcinoma. Monjazeb et al. demonstrated that a cCR after concurrent CRT could lead to a better prognosis.7 Patients with residual tumor cells after neoadjuvant therapy primarily experience relapse within the first two postoperative years; however, there have been reports of patients with complete remission who experienced late relapses four years after surgery. After curative surgery in a trimodality, the histological type and response to neoadjuvant therapy predicted different prognoses.8 The CROSS trial demonstrated median overall survival (OS) of 49.4 months in the nCRT + surgery group compared to 24 months in the surgery alone cohort (P = 0.003).9, 10 The five‐year survival rates were 47% and 34%, respectively, and 29% of patients reached a pathological complete response (pCR).
A number of studies have investigated whether every esophageal carcinoma patient should undergo esophagectomy and how to select the patients best suited to CRT alone. Two randomized trials of CRT with or without surgery demonstrated reduced local recurrence with trimodality therapy.11, 12 However, these trials failed to demonstrate a survival improvement with surgery, likely because of an increase in treatment‐related mortality. Some research has shown the opposite result.13 Surgery should be applied when CRT is ineffective; however, when CRT is effective, especially for patients with cCR, there is no agreement on whether to choose surgery or other methods, such as further definitive chemoradiotherapy or active surveillance.14, 17 Whether cCR after CRT is able to guide the subsequent treatment regimen requires further study, although only 30% of cCR patients achieve pCR.
Studies of thoracic esophageal carcinoma patients with a cCR after CRT were screened. Eligible patients were divided into the CRT + surgery and non‐surgery groups. A meta‐analysis was conducted to explore whether esophagectomy is always necessary in this cohort.
Methods
Search strategy and selection criteria
Articles published in databases, including PubMed, the Cochrane Library, and Embase, relevant to comparative analysis of surgical and non‐surgical strategies (including further definitive chemoradiotherapy or active surveillance) for patients with a cCR after concurrent chemoradiotherapy in thoracic esophageal carcinoma were retrieved. The keywords used were as follows: “esophageal or oesophageal” and “carcinoma or cancer or neoplasm” and “neoadjuvant or induction or preoperative” and “chemoradiotherapy.” The final date of data retrieval was 30 June 2018. After retrieval, we filtered the articles manually by reading the abstracts or full texts.
The selection criteria included: (i) precision radiotherapy, such as three‐dimensional (3D) conformal irradiation and intensity‐modulated radiotherapy had been performed (articles that applied 2D radiotherapy techniques or missed concurrent CRT were excluded); (ii) the original data were detailed, including a curative effect evaluation after CRT; (iii) patients with a cCR were classified into surgery and non‐surgery groups (further definitive CRT or active surveillance in which patients were subjected to serial clinical investigations after completion of CRT) and relevant contrastive data was provided; (iv) articles included an accurate statistical method, valid data, and clear conclusions; and (v) hazard ratios (HRs) and 95% confidence intervals (CI) were provided or could be calculated.
Data extraction
The data extracted included the first author's name, study year, journal, study period, number of patients, radiotherapy and chemotherapy regimens, and methods of response evaluation. Outcome data included two and five‐year OS and disease‐free survival (DFS).
Quality evaluation
The case‐control study evaluation guideline was applied in order to evaluate the quality of each manuscript from the following aspects: (i) whether the gender, age, and tumor location were clearly stated; (ii) whether the comparability of the two groups was analyzed; and (iii) whether the statistical method was appropriate (e.g. whether the OS or DFS was calculated using the Kaplan–Meier method and log‐rank testing had been performed); (iv) whether the test was designed as a prospective randomized control study; and (v) whether the biases in the study were discussed.18 A score was assigned for each of the five items. A total score of ≥ 3 indicates reliable quality. Two researchers independently reviewed the literature according to the unified quality standard. The results were then crosschecked. Cases of disagreement were resolved through discussion or by enlisting assistance from a third researcher.
Statistical analysis
Meta‐analysis was conducted using Stata version 11.0 provided by the Cochrane collaboration website (http://www.cochrane-handbook.org). The effect size was reflected by HR and 95% CI. A Q test was applied to test the heterogeneity of the results. For P ≤ 0.05, the result was considered to be heterogeneous, and the random effect model was used for statistical consolidation. For P > 0.05, the result was not considered heterogeneous, and the fixed effect model was used. The combined effect size was tested by z test.
Funnel plots were created to evaluate the risk of publication bias. An asymmetrically shaped funnel indicated the presence of publication bias, and Egger's regression method was conducted to test the publication bias.
Results
Literature search and study selection
The initial database screening yielded 2696 articles; 281 were filtered out by reading the titles and abstracts. After reading the entire text, the following were excluded: 725 articles because of missing contrastive analysis; 964 referred to esophageal gastric junction carcinoma patients; 390 articles did not apply concurrent CRT; 226 articles had missing therapeutic effect evaluation data; 104 articles had incomplete original data or uncollectible data; and 2 articles did not include non‐cCR after CRT. Finally, four articles were selected for this study, including 648 esophageal carcinoma patients. A flow diagram is shown in Figure 1. Of the 648 patients, 620 patients had squamous cell carcinoma (SCC) and 28 patients had adenocarcinoma. The basic characteristics and clinical data of the four articles are shown in Tables 1 and 2.
Figure 1.
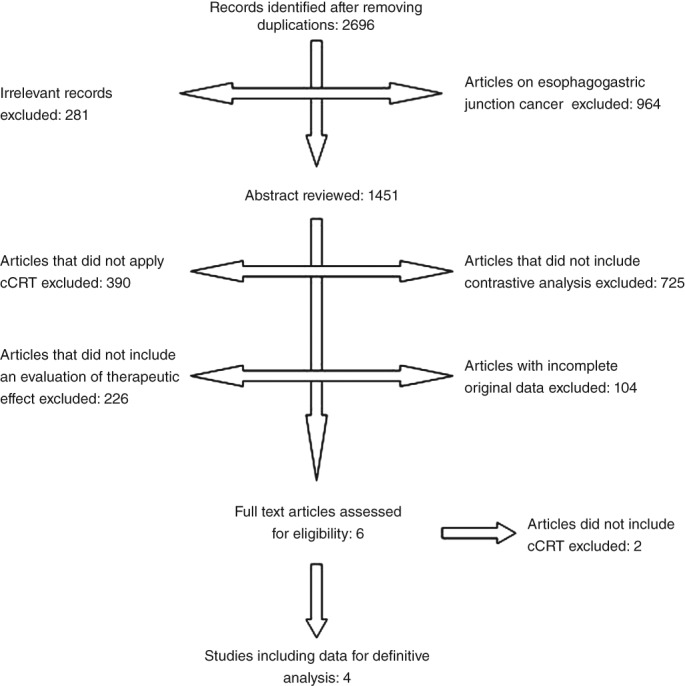
Study selection process.
Table 1.
Basic characteristicsof the included studies
| Author | Year | Nation | Case number | Regimen of radiotherapy | Surgery | Therapeutic effect evaluation | Quality evaluation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||||
| Castoro et al.14 | 2013 | Italy | 77 | Standard RT was usually performed in 1.8 Gy daily fractions for a total dose of 45–50 Gy. The PTV included the primary tumor, with 5cm longitudinal margins; metastatic nodes, with 2cm margins; supraclavicular fovea; and mediastinum | Surgery was performed 4–6 weeks after the completion of neoadjuvant treatment | CR for the primary tumor was defined as the disappearance of the tumor lesion, ulceration, and the absence of carcinoma cells in biopsy specimens upon endoscopic observation of the entire esophagus. CR for lymph nodes was defined according to the RECIST. CT (and PET/CT from 2005) scans were performed to rule out distant metastasis | 1 | 1 | 1 | 0 | 1 |
| Piessen et al.15 | 2013 | France | 257 | All patients underwent standardized radiotherapy: GTV was determined on the basis of clinical examination, planning CT, endoscopy, and EUS. All patients were treated with conformational 3D RT, with more than 6 MV X‐ray. Dose distribution was calculated by treatment planning system. Portal imaging was performed once a week | Curative surgical resection consisted of a transthoracic en bloc esophagectomy, including an abdominal and an extended mediastinal lymphadenectomy | cCR was defined when all of the following were present: absence of tumor residue visible by endoscopy, negative endoscopic biopsy; and on CT scan, the absence of a residual tumor, lymph nodes of more than 10mm in diameter, and metastasis. When a PET scan was performed, CMR was considered when having a physiologic level of SUVmax or when the SUVmax was higher than normal but was distributed in an esophagitis pattern | 1 | 1 | 1 | 1 | 0 |
| Chao et al.16 | 2013 | Taiwan | 160 | Radiation therapy between days 8 and 29 consisted of a total dose of 30 Gy, administered in daily fractions of 200 Gy, five days/week | The standard surgical approach was limited thoracotomy on the right side and intrathoracic gastric tube reconstruction (Ivor–Lewis procedure) for lesions of the middle and lower one‐third of the esophagus. Lesions of the upper one‐third of the esophagus/cervical lesions were treated by neck anastomosis (McKeown procedure) | Endoscopic CR was tentatively defined upon endoscopic observation of the entire esophagus as: the disappearance of the tumor lesion and ulceration, and the absence of carcinoma cells in biopsy specimens plus image evaluation of the regional lymph node and distant sites (by RECIST) | 1 | 1 | 1 | 0 | 0 |
| Jeong et al.17 | 2014 | Korea | 154 | The total radiation dose was 46 Gy for preoperative CRT and 54 Gy for definitive CRT (2 Gy per daily fraction, five days per week). In the definitive CRT group, a boost dose of 10 Gy in 5 fractions was added to the volume containing the GTV with 2cm of radial and longitudinal margins after administration of 44 Gy in 22 fractions | Of the 73 patients in the trimodality group, 72 underwent transthoracic esophagectomy and 1 underwent transhiatal esophagectomy | Metabolic complete remission (PET‐CR) was defined as FDG uptake of the primary tumor and lymph nodes were decreased and were indistinguishable from the surrounding normal tissue | 1 | 1 | 1 | 1 | 1 |
cCR, clinical complete response (cCR); CMR, complete metabolic response; CT, computed tomography; EUS, endoscopic ultrasound; FDG, 18F‐fluorodeoxyglucose; GTV, gross tumor volume; PET, positron emission tomography; PTV, planning target volume; RECIST, Response Evaluation Criteria in Solid Tumors; RT, radiotherapy; SUVmax, maximum standardized uptake value.
Table 2.
Survival data selected for analysis
| Author | Pretherapeutic stage | Group (Case Number) | Two‐year OS | Five‐year OS | Two‐year DFS | Five‐year DFS |
|---|---|---|---|---|---|---|
| Castoro et al.14 | I–IV†† | A (39) | 72.6% | 50% | 62.3% | 55.5% |
| B (38) | 72.6% | 57% | 40.0% | 34.6% | ||
| Piessen et al.15 | II–III | A (118) | 83.7% | 68.0% | 74.0% | 57.4% |
| B (59) | 58.6% | 40.5% | 52.6% | 33.4% | ||
| Chao et al.16 | II–IV†† | A (71) | — | 41% | — | 44% |
| B (79) | — | 39% | — | 45% | ||
| Jeong et al.17 | II–IV† | A (73) | 60.3% | — | 72.4% | — |
| B (81) | 37% | — | 35.6% | — |
Patients with distant metastasis at diagnosis were excluded, while those with stage IV disease resulting from nodal involvement were included. Patients were staged according to the 6th Amercian Joint Committee for Cancer Tumor Node Metastasis classification.
A, CRT + Surgery; B, non‐surgical approach; DFS, disease‐free survival; OS, overall survival.
Effects of treatment regimens on overall survival (OS)
Two‐year OS
Three articles analyzed the effects of two treatment regimens on two‐year OS. The results showed that the CRT + surgery group had an advantage over the non‐surgery group in two‐year OS (HR 2.108, 95% CI 0.981–4.530; P = 0.056). However, the differences were not statistically significant. The results are shown in Figure 2.
Figure 2.
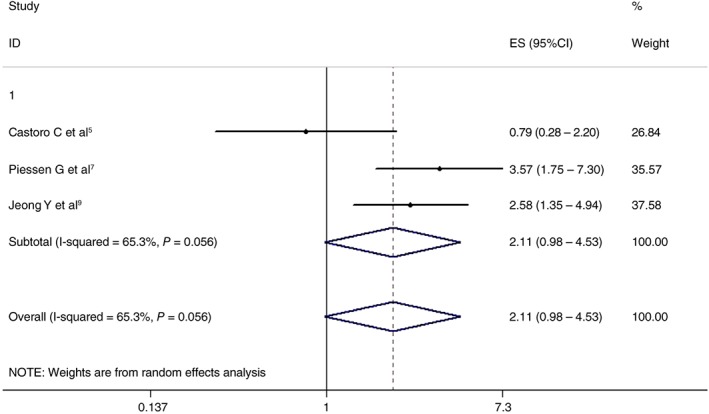
Effects of chemoradiotherapy + surgery and a non‐surgical approach on two‐year overall survival. CI, confidence interval; ES, effect size.
Five‐year OS
Three articles analyzed the effects of the two treatment regimens on five‐year OS. The CRT + surgery and non‐surgery groups showed similar results (HR 1.361, 95% CI 0.572–3.239; P = 0.486). The differences were not statistically significant. The results are shown in Figure 3.
Figure 3.
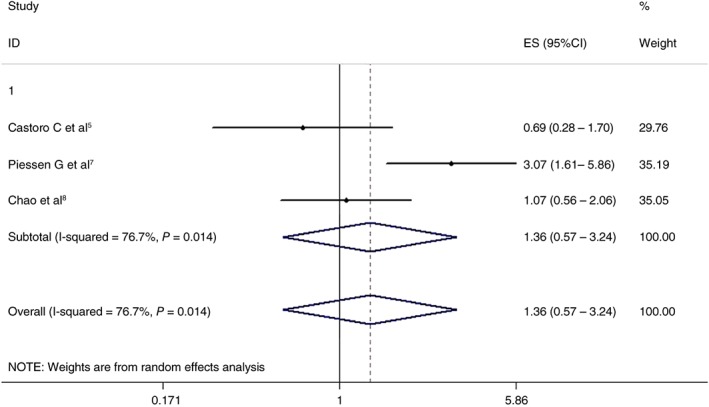
Effects of chemoradiotherapy+ surgery and a non‐surgical approach on five‐year overall survival. CI, confidence interval; ES, effect size.
Effects of treatment regimens on disease‐free survival (DFS)
Two‐year DFS
Three articles analyzed the effects of the two treatment regimens on two‐year DFS. The results showed that the CRT + surgery group had an advantage over the non‐surgery group (HR 3.186, 95% CI 2.071–4.901; P = 0.000). The differences were statistically significant. The results are shown in Figure 4.
Figure 4.
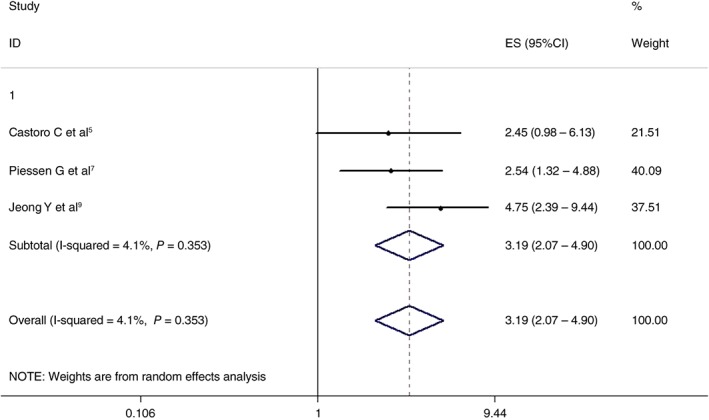
Effects of chemoradiotherapy + surgery and a non‐surgical approach on two‐year disease‐free survival. CI, confidence interval; ES, effect size.
Five‐year DFS
Three articles analyzed the effects of the two treatment regimens on five‐year DFS. The CRT + surgery and non‐surgery groups showed similar results (HR 1.780, 95% CI 0.866–3.657; P = 0.117). The differences were not statistically significant. The results are shown in Figure 5.
Figure 5.
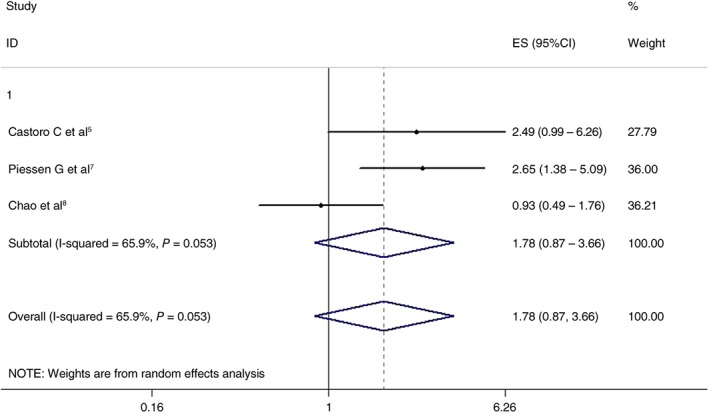
Effects of chemoradiotherapy + surgery and a non‐surgical approach on five‐year disease‐free survival. CI, confidence interval; ES, effect size.
Sensitivity analysis
Articles with similar HR results with combined HR were deleted in order to conduct sensitivity analysis. The results are shown in Table 3. After deletion, the combined HR was very close to the original HR. There was no material impact on the outcome.
Table 3.
Sensitivity analysis
| Item | Deleted Article | HR | 95% CI | P | |
|---|---|---|---|---|---|
| Two‐year OS | Jeong et al.17 | 1.759 | 0.402 | 7.699 | 0.453 |
| Five‐year OS | Piessen et al.15 | 0.919 | 0.542 | 1.560 | 0.755 |
| Two‐year DFS | Piessen et al.15 | 3.670 | 1.950 | 6.908 | 0.000 |
| Five‐year DFS | Castoro et al.14 | 1.565 | 0.558 | 4.390 | 0.394 |
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival.
Publication bias analysis
Publication bias of the selected articles was evaluated based on a funnel plot. Egger's regression test was conducted to analyze the symmetry of the funnel plot. As shown in Table 4, none of the articles had publication bias. All of the P values were > 0.05.
Table 4.
Publication bias results of the selected articles
| Evaluation Items | t | 95% CI | P | |
|---|---|---|---|---|
| Two‐year OS | −1.85 | −53.427 | 39.835 | 0.315 |
| Five‐year OS | −0.89 | −120.902 | 105.122 | 0.538 |
| Two‐year DFS | −0.33 | −74.295 | 70.575 | 0.799 |
| Five‐year DFS | 0.43 | −102.668 | 109.804 | 0.743 |
CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival.
Discussion
Preoperative CRT followed by surgery and definitive CRT are both therapeutic methods for locally advanced esophageal carcinoma. Clinical trials and meta‐analyses have shown that a CRT + surgery regimen could significantly improve the survival of locally advanced esophageal carcinoma patients compared to surgery alone.9, 10, 13 However, there is no consensus on whether CRT + surgery has an advantage over CRT alone. Stahl et al. conducted a prospective RCT study and divided esophageal SCC patients into two groups.11 In one group, surgery was conducted after induction chemotherapy followed by CRT (40 Gy), while in the other, induction chemotherapy was followed by CRT (at least 65 Gy) without surgery. The survival of the definitive CRT group was similar to the nCRT + surgery group, but the local control rate of the CRT + surgery group was better than in the CRT alone group (P < 0.05). In both of the groups, the survival rate in chemotherapy responders was higher than in non‐responders. Rawat et al. selected patients who underwent concurrent CRT (50 Gy, 40 mg/m2 of cisplatin per week) and evaluated the therapeutic effect after six weeks.19 Patients with resectable tumors underwent surgery, while the remainder was classified as the observation group (active surveillance). There was no statistical difference in DFS or OS between the groups. In patients who did not exhibit a response to induction therapy, in‐time surgery was more meaningful. It is generally believed that patients who are responsive to induction therapy have a better prognosis. However, in such patients, the choice of treatment regimen after induction therapy, such as surgery, definitive CRT, or active surveillance, remains controversial.20
The therapeutic effect of CRT has high heterogeneity, thus some scholars have conducted subgroup analysis of a more specific patient group: cCR after CRT. Although CR after CRT could lead to a better prognosis, whether esophagectomy is necessary is still controversial. Jeong et al. classified esophageal SCC patients with cCR after CRT into surgery and definitive CRT groups.17 Their results showed that the surgery group had an advantage over the definitive CRT group in two‐year OS, local recurrence‐free survival, and DFS. However, a similar study by Castoro et al. revealed no statistical differences in five‐year OS and DFS between surgery and active surveillance groups with cCR after nCRT.14 Chao et al. showed that there was no difference between five‐year OS and disease‐specific survival (DSS) between nCRT + esophagectomy groups and further definitive CRT when patients achieved cCR after nCRT.16 Controversy remains over whether is there a role for surgery in patients with a cCR after CRT for esophageal cancer.
It is noteworthy that although a “wait and see” policy has been proposed by some authors, data from patients who underwent scheduled esophagectomy following CRT have clearly demonstrated that cCR does not accurately reflect the presence of a pCR. Furthermore, the radiation dose used in nCRT ranged from 30 to 46 Gy, while the radiation dose used in definitive CRT ranged from 50.4 to 60 Gy. Different radiation doses affect local control, cCR and pCR rates, progression‐free survival, and OS, thus these methods cannot be directly compared. Therefore, an intensive surveillance strategy should be applied to detect cancer regrowth as early as possible before the tumor is unresectable, especially in patients who have undergone nCRT.
Based on the screening criteria, only four articles were selected for this meta‐analysis. After intensive reading and data extraction, four items could be statistically analyzed: two and five‐year OS and DFS. In patients with cCR after CRT, the CRT + surgery group had an advantage over the non‐surgery group in two‐year OS (HR 2.108, 95% CI 0.981–4.530; P = 0.056); however, the two groups showed similar results for five‐year OS. The difference was not statistically significant (HR 1.361, 95% CI 0.572–3.239; P = 0.486). The CRT + surgery group had an advantage over the non‐surgery group in two‐year DFS (HR 3.186, 95% CI 2.071–4.901; P = 0.000), but the two groups showed similar results for five‐year DFS (HR 1.780, 95% CI 0.866–3.657; P = 0.117). Based on this meta‐analysis, the addition of esophagectomy to thoracic locally advanced esophageal carcinoma patients with cCR after CRT could improve the short‐term therapeutic effect; however, it failed to show a benefit on long‐term survival. Our results show that application of an active surveillance strategy or further definitive chemoradiotherapy in patients with a cCR after CRT may reduce the need for an esophagectomy. Surgery may be only necessary in patients with suspected or proven locoregional regrowth/residual disease, without signs of distant dissemination.
There is no unified standard for the diagnosis of cCR. The four articles involved in this study used different diagnostic methods. Chao et al. used routine imageological examination and endoscopy (tumor disappearance, ulcer disappearance, and carcinoma cell absence in biopsy) to diagnose CR.16 Castoro et al. used CT (PET‐CT for some patients) and endoscopy (ulcer disappearance and carcinoma cell absence in biopsy) to diagnose CR.14 Jeong et al. used PET to define CR; the 18F‐fluorodeoxyglucose (FDG)‐PET procedure has been described previously.17 Metabolic complete remission (PET‐CR) was defined as a decrease in the FDG uptake of the primary tumor and lymph nodes to a level indistinguishable to that of the surrounding normal tissue. Piessen et al. used CT (PET‐CT for some patients) and endoscopy (ulcer disappearance and carcinoma cell absence in biopsy) to diagnose CR.15 When a PET scan was performed, a complete metabolic response was considered when the physiologic level of maximum standardized uptake value (SUVmax) was reaching or when the SUVmax was higher than normal but it was existed in an esophagitis pattern. Endoscopic biopsy, endoscopic ultrasonography, MRI (at various sequences), and PET‐CT all had shortcomings for evaluating cCR and the therapeutic effect of nCRT. Studies have shown that up to 6% of patients are unable to undergo endoscopy because of luminal stenosis, which would undoubtedly lead to inconclusive staging results.21, 22 In the meantime, just like CT examination, endoscopy cannot effectively distinguish residual tumors from inflammation, edema, and fibrosis. In addition, during endoscopy, samples must be collected at different times from multiple spots, which leads to heterogeneity in space and time and inconsistency in diagnostic results. Furthermore, PET/CT spatial resolution is limited, because there is currently no standard evaluation for SUV. 18F‐FDG‐PET has advantages in using a metabolic complete response (mCR) to predict pCR. However, research articles have differing opinions on how to define the mCR of treatments. Monjazeb et al. considered that an SUV ≤ 3 could be defined as mCR,7 while Stiles et al. considered that merely 0 SUV or no uptake was defined as mCR,23 and Jeong et al. considered that mCR could be recognized when the uptake of the primary lesion and metastatic lymph node were reduced to the same level as the surrounding normal tissue.17
More accurate restaging protocols are warranted to improve decision‐making on treatment strategy after CR with CRT. The main study parameter of the preSANO trial was the correlation between the clinical response during evaluation and the pathological response exhibited in the resection specimen.24 The trial aimed to determine the accuracy of detecting the presence or absence of residual disease after CRT, which would allow doctors to select the patients who would benefit most from surgery. Some patients might avoid surgery on the premise of locoregional control, and thus enjoy better quality of life with entire functioning organs. In addition, although multiple diagnostic methods were conducted to comprehensively evaluate cCR, the results were still somewhat inconsistent with pCR results. A significant number of cCR patients failed to reach pCR after surgery.15 This might be the major reason why the local recurrence rate in the CRT group was higher, while the two‐year DFS was lower than the surgery group. Adams et al. prospectively collected data from the United Kingdom carcinoma network and showed that the two and five‐year OS rates were similar in the CRT + surgery and CRT groups.25 However, patients administered non‐surgical‐based therapies had more than double the incidence of local relapse compared to patients administered surgical‐based therapies. Blackham et al. identified patients treated with CRT + surgery from a prospectively maintained database in a single institution (1996–2013); 73% of all recurrences occurred within 18 months of surgery.26 Prospective research by Stahl et al. showed better local PFS in the CRT + surgery (two‐year PFS 64.3%, 95% CI 52.1–76.5%) than in the CRT group (two‐year PFS 40.7%, 95% CI 28.9–52.5%; P = 0.003).11 Furthermore, some studies on esophageal SCC discovered that the amount of residual cancer, as measured by tumor regression grade, increased significantly after a longer interval between nCRT and surgery.27, 28 This may have been one of the reasons why the short‐term outcome was less favorable in the non‐surgery than in the surgery group.
Sensitivity analysis was conducted in order to explore heterogeneity. Articles that showed similar HR results with combined HR were deleted. After deletion, the new results were consistent with the original results (Table 3). Publication bias may also influence the viability of meta‐analysis; therefore statistical analysis was also conducted to test the four selected articles. Egger's analysis showed that all P values were > 0.05 (Table 4), which indicates that publication bias was not significant in this study. The viability of this study was further enhanced.
This study had some limitations that require consideration when interpreting the results. The meta‐analysis was limited to published studies and all of the articles were written in English. Thus, publication and language bias might affect the results. In addition, all of the selected studies were retrospective. Selection bias between surgery and non‐surgery patients could not be avoided. Two of the four studies spanned more than 15 years, which might result in greater heterogeneity on several parameters, such as the quality control of radiotherapy and surgery.14, 15 Admittedly, variation in practice habits evolves over time and standard treatment regimens would have been different at the beginning compared to the end of the study period; these result in low‐quality evidence‐based results. The survival data of the present study were completely based on original literature, and separate subgroup analyses of esophageal adenocarcinoma and SCC could not be performed. Although we focused on a strategy of patients who achieved cCR because SCC is more chemoradiosensitive than adenocarcinoma, more data is required to analyze the regimen based on histological subtypes in future.
In conclusion, based on the available evidence, additional esophagectomy in patients with cCR after CRT for thoracic locally advanced esophageal carcinoma provided no advantage to OS, while two‐year DFS could be improved. Because 95.7% of the sample were esophageal SCC patients, this research conclusion might be more suitable to SCC patients. Thus, more randomized clinical trials are needed to confirm our conclusions.
Disclosure
No authors report any conflict of interest.
Contributor Information
Jun Wang, Email: wangjunzr@163.com.
Jianjun Qin, Email: qinjianjun73@aliyun.com.
References
- 1. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Esophageal carcinoma. The Lancet 2013; 381: 400–12. [DOI] [PubMed] [Google Scholar]
- 2. Zhang YW. Epidemiology of esophageal cancer. World J Gastroenterol 2013; 19: 5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 4. Wald O, Smaglo B, Mok H, Groth SS. Future directions in esophageal carcinoma therapy. Ann Cardiothorac Surg 2017; 6: 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper JS, Guo MD, Herskovic A et al Chemoradiotherapy of locally advanced esophageal cancer: Long‐term follow‐up of a prospective randomized trial (RTOG 8501). Radiation Therapy Oncology Group. JAMA 1999; 281: 1623–7. [DOI] [PubMed] [Google Scholar]
- 6. Minsky BD, Pajak TF, Ginsberg RJ et al INT 0123 (Radiation Therapy Oncology Group 94‐05) phase III trial of combined‐modality therapy for esophageal cancer: High‐dose versus standard‐dose radiation therapy. J Clin Oncol 2002; 20: 1167–74. [DOI] [PubMed] [Google Scholar]
- 7. Monjazeb AM, Riedlinger G, Aklilu M et al Outcomes of patients with esophageal carcinoma staged with [18F]Fluorodeoxyglucose positron emission tomography (FDG‐PET): Could postchemoradiotherapy (FDG‐PET) predict the utility of resection? J Clin Oncol 2010; 28: 4714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steffen T, Dietrich D, Schnider A. Recurrence patterns and long‐term results after induction chemotherapy, chemoradiotherapy, and curative surgery in patients with locally advanced esophageal cancer. Ann Surg 2017. 10.1097/SLA.0000000000002435 [DOI] [PubMed] [Google Scholar]
- 9. Van Hagen P, Hulshof MC, van Lanschot JJ et al Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366: 2074–84. [DOI] [PubMed] [Google Scholar]
- 10. Shapiro J, van Lanschot JJB, Hulshof MCCM et al Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for esophageal or junctional carcinoma (CROSS): Long‐term results of a randomised controlled trial. Lancet Oncol 2015; 16: 1090–8. [DOI] [PubMed] [Google Scholar]
- 11. Stahl M, Stuschke M, Lehmann N et al Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005; 23: 2310–7. [DOI] [PubMed] [Google Scholar]
- 12. Bedenne L, Michel P, Bouche O et al Chemoradiation followed by surgery compared with chemoradiotherapy alone in squamous carcinoma of the esophagus: FFCD 9102. J Clin Oncol 2007; 25: 1160–8. [DOI] [PubMed] [Google Scholar]
- 13. Sjoquist KM, Burmeister BH, Smithers BM et al Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable esophageal carcinoma: An updated meta‐analysis. Lancet Oncol 2011; 12: 681–92. [DOI] [PubMed] [Google Scholar]
- 14. Castoro C, Scarpa M, Cagol M et al Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell carcinoma of the thoracic oesophagus: Was surgery always necessary? J Gastrointest Surg 2013; 17: 1375–81. [DOI] [PubMed] [Google Scholar]
- 15. Piessen G, Messager M, Mirabel X et al Is there a role for surgery for patients with a complete clinical response after chemoradiotherapy for esophageal cancer? An intention‐to treat case‐control study. Ann Surg 2013; 258: 793–9. [DOI] [PubMed] [Google Scholar]
- 16. Chao YK, Tseng CK, Wen YW et al Using pretreatment tumor depth and length to select esophageal squamous cell carcinoma patients for nonoperative treatment after neoadjuvant chemoradiotherapy. Ann Surg Oncol 2013; 20: 3000–8. [DOI] [PubMed] [Google Scholar]
- 17. Jeong Y, Kim JH, Kim SB et al Role of surgical resection in complete responders on FDG‐PET after chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. J Surg Oncol 2014; 109: 472–7. [DOI] [PubMed] [Google Scholar]
- 18. Lichtenstein MJ, Mulrow CD, Elwood PC. Guidelines for reading case‐control studies. J Chronic Dis 1987; 40: 893–903. [DOI] [PubMed] [Google Scholar]
- 19. Rawat S, Kumar G, Kakria A, Sharma MK, Chauhan D. Chemoradiotherapy in the management of locally advanced squamous cell carcinoma esophagus: Was surgical resection required? J Gastrointest Cancer 2013; 44: 277–84. [DOI] [PubMed] [Google Scholar]
- 20. Vellayappan BA, Soon YY, Ku GY et al Chemoradiotherapy versus chemoradiotherapy plus surgery for esophageal cancer. Cochrane Database Syst Rev 2017; 8: CD010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonnetain F, Bouche O, Michel P et al A comparative longitudinal quality of life study using the Spizer quality of life index in a randomized multicenter phase III trial (FFCD 9102): Chemoradiotherapy followed by surgery compared with chemoradiotherapy alone in locally advanced squamous resectable thoracic esophageal cancer. Ann Oncol 2006; 17: 827–34. [DOI] [PubMed] [Google Scholar]
- 22. Westerterp M, van Westreenen HL, Reitsma JB et al Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy‐systematic review. Radiology 2005; 236: 841–51. [DOI] [PubMed] [Google Scholar]
- 23. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine‐needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal carcinoma after neoadjuvant chemoradio therapy. J Thorac Cardiovasc Surg 2005; 129: 1232–41. [DOI] [PubMed] [Google Scholar]
- 24. Stiles BM, Salzler G, Jorgensen A et al Complete metabolic response was not uniformly predictive of complete pathologic response after induction therapy for esophageal cancer. Ann Thorac Surg 2013; 96: 1820–5. [DOI] [PubMed] [Google Scholar]
- 25. Noordman BJ, Spaander MC, valkema R et al Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): A prospective multicentre, diagnostic cohort sutdy. Lancet Oncol 2018; 19: 965–74. [DOI] [PubMed] [Google Scholar]
- 26. Adams R, Morgan M, Mukherjee S et al A prospective comparison of multidisciplinary treatment of esophageal carcinoma with curative intent in a UK carcinoma network. Eur J Surg Oncol 2007; 33: 307–13. [DOI] [PubMed] [Google Scholar]
- 27. Blackham AU, H Naqvi SM, Schell MJ et al Recurrence patterns and associated factors of locoregional failure following neoadjuvant chemoradiation and surgery for esophageal cancer. J Surg Oncol 2018; 117: 150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chiu CH, Chao YK, Chang HK et al Interval between neoadjuvant chemoradiotherapy and surgery for esophageal squamous cell carcinoma: Dose delayed surgery impact outcome? Ann Surg Oncol 2013; 20: 4245–51. [DOI] [PubMed] [Google Scholar]


