Abstract
Spontaneous regression (SR) of cancer implies the partial or complete disappearance of malignant disease without or with adequate medical treatment. Typically, SR of cancer is a sporadic event, especially in non‐small cell lung cancer (NSCLC). Although the underlying mechanism of SR remains unknown, stimulation of an immunological response has been proposed. Herein, we report the case of a 56‐year‐old woman exhibiting SR of NSCLC with a mediastinal disease. Despite regression of the primary site after a lung biopsy, simultaneous progression of mediastinal lymph node metastasis occurred. Specimens obtained by surgical resection pathologically confirmed both primary and metastatic sites. Reportedly, primary and metastatic tumors shrink synchronously in SR of metastatic NSCLCs. Thus, the fact that the SR of NSCLC can present inconsistent development in primary and metastatic sites should be considered, and direct intervention is recommended if physicians diagnose this phenomenon.
Keywords: Biopsy, mediastinal lymph node metastasis, non‐small cell lung cancer, spontaneous regression, surgery
Introduction
Spontaneous regression (SR) implies the complete or partial, temporary or permanent disappearance of some or all parameters of malignant disease without or with adequate medical treatment.1 SR of metastatic non‐small cell lung cancer (NSCLC) is typically characterized by the synchronous reduction of primary and metastatic tumors.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Herein, we present a rare case of the SR pattern in a patient with NSCLC and autoimmune disease in whom the primary tumor regressed after biopsy, but metastasis of mediastinal lymph nodes progressed.
Case report
A 56‐year‐old woman was accidentally diagnosed by her previous physician via computed tomography (CT) with a pulmonary nodule on the superior segment of the right lower lobe. Her medical history comprised 26 years of smoking (90 pack‐years), scleroderma, interstitial pneumonia, and autoimmune hepatitis (treated with 5 mg/day of methylprednisolone). The CT scan revealed a solid peripheral tumor in the right lower lobe and enlarged mediastinal lymph nodes in the right lower paratracheal station (4R) (Fig 1). Positron emission tomography‐CT revealed accumulations of fluorodeoxyglucose with standard uptake values of 15.8 in the lung tumor and 9.9 in the 4R lymph node (Fig 2). Laboratory examinations were unremarkable, except for high KL‐6 (1872 U/mL). CT‐guided needle biopsy on the lung tumor from the back in the prone position revealed squamous cell carcinoma (SCC). A 4R lymph node biopsy was not performed.
Figure 1.
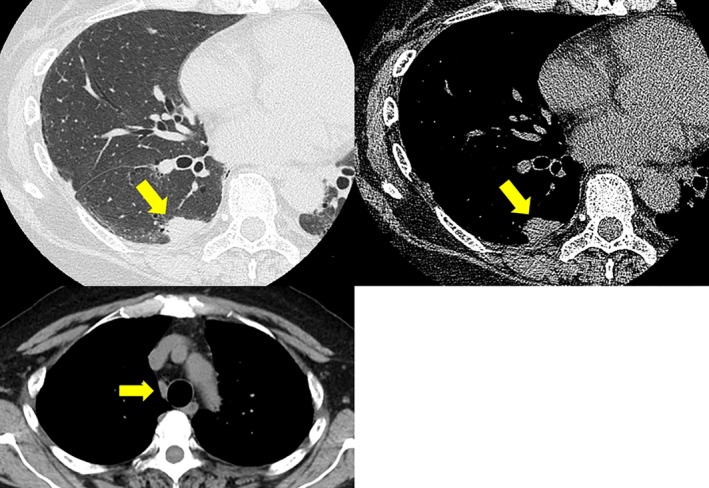
Initial computed tomography shows the tumor (diameter: 1.4 cm) in the right lower lobe and an enlarged mediastinal lymph node (diameter: 1.0 cm) in the right lower paratracheal station.
Figure 2.
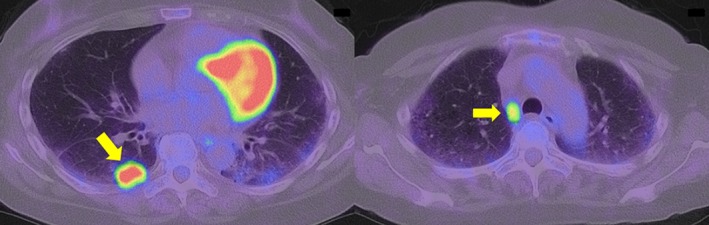
Positron emission tomography‐computed tomography reveals an accumulation of fluorodeoxyglucose with standard uptake values of 15.8 in the lung tumor and 9.9 in the 4R lymph node.
Six weeks after the biopsy was taken, the patient was referred to our hospital for treatment where we reviewed the CT findings. Remarkably, the lung tumor had shrunk from 1.4 to 0.5 cm in diameter, whereas the 4R lymph node had further progressed from 1.0 to 1.5 cm in diameter (Fig 3). Transbronchial needle aspiration of the 4R lymph node revealed metastatic SCC. Based on these findings, the patient was clinically diagnosed with advanced lung SCC (c‐T1aN2M0, stage IIIA, Union for International Cancer Control, 7th edition). As interstitial pneumonia rendered multimodality therapy risky, we proceeded with surgical resection and dissected the right lower lobe with the mediastinal lymph nodes (ND2a‐2). The resected lung tumor specimen was pathologically diagnosed with moderately differentiated SCC surrounded by fibrotic change with inflammatory cell infiltration (Fig 4a). The patient exhibited remarkable metastasis of the tumor cells in the 4R lymph nodes (Fig 4b). Finally, she was diagnosed with primary lung SCC with mediastinal lymph node metastases (p‐T1aN2M0, stage IIIA). Her postoperative course did not include adjuvant therapy and remained uneventful, and no recurrence was observed one year after surgery.
Figure 3.
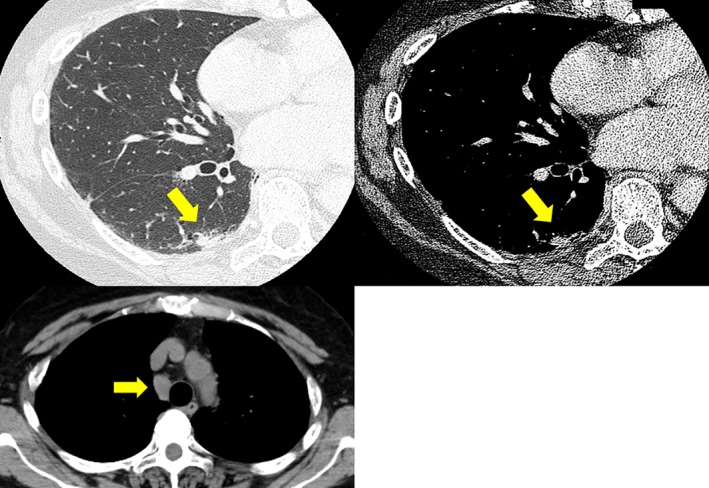
Preoperative computed tomography shows lung tumor shrinkage (from 1.4 to 0.5 cm in diameter) and mediastinal lymph node progression (from 1.0 to 1.5 cm in diameter).
Figure 4.
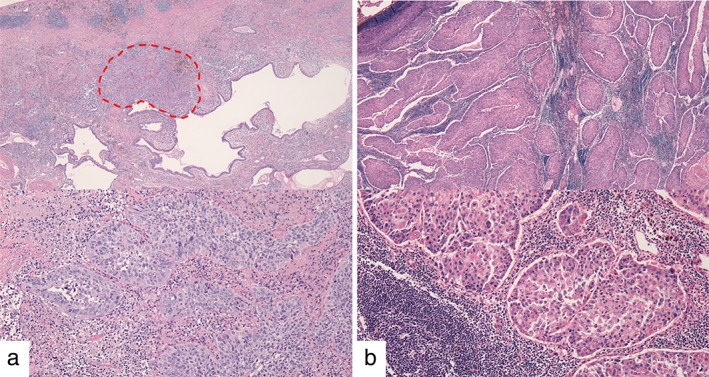
Hematoxylin staining of specimens obtained by surgical resection; magnification 40× (top), 200× (bottom). (a) Lung tumor cells are only present in the limited area of 0.2 cm × 0.1 cm in the right lower lobe (surrounded by a red dotted line). Rich fibrous tissue and inflammatory cells infiltrated the interstitium (around the area surrounded by a red dotted line), suggesting local regression of the tumor. (b) Remarkable metastases of tumor cells to the mediastinal lymph nodes are observed.
Discussion
Cole and Everson postulated the most common criteria for SR of cancer, based on which we categorized our case as partial SR.1 Although the actual incidence of SR remains unclear, Cole estimated it as 1 in 60 000–100 000 patients with cancer.12 SR is more uncommon in NSCLC than in any other cancer.13 To the best of our knowledge, to date only 11 SR cases of metastatic NSCLC have been identified,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 14 and in all of these, SR occurred after biopsy or surgical intervention of primary and/or metastatic lesions, with diagnosis and follow‐up assisted by CT. Both the primary and metastatic lesions simultaneously regressed in most of these cases; however, one case of primary lung cancer exhibited pathologically complete SR, but metachronous metastasis later occurred in the adrenal gland.14 Conversely, in our case, despite SR of the lung tumor, the patient concurrently experienced progression in the mediastinal lymph node. Examination of surgically resected specimens confirmed the presence of tumor cells at both sites. Our patient experienced simultaneous regression in the primary site and progression at the metastatic site.
An array of clinical features of SR suggest multiple biological mechanisms that might cause it.15 Recently, reports have hypothesized that the systemic immunological response contributes to SR,5, 16, 17 which could explain why all lesions, including primary and metastatic sites, shrink simultaneously, even when a biopsy is only performed on one lesion.2, 5, 7, 9, 11 In our case, SR of the primary site is attributable to a biopsy‐induced local immunological reaction and the contradictory behavior of metastatic lesions could be illustrated by the locally limited reaction. Our patient had received steroid therapy to suppress the systemic immune response to control autoimmune disease before the lung cancer had manifested, which could have resulted in the varying behavior of primary and metastatic lesions. In fact, a previous SR case revealed spontaneous tumor regression and subsequent rapid progression after the initiation of glucocorticoid treatment for rheumatoid arthritis.18
In conclusion, our case highlights SR cases in which primary and metastatic sites behave differently and indicates that further intervention, including surgical resection, should be guided by proper diagnosis.
Disclosure
No authors report any conflict of interest.
References
- 1. Cole WH, Everson TC. Spontaneous regression of cancer: Preliminary report. Ann Surg 1956; 144: 366–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kappauf H, Gallmeier WM, Wünsch PH. Complete spontaneous remission in a patient with metastatic non‐small‐cell lung cancer. Case report, review of literature, and discussion of possible biological pathways involved. Ann Oncol 1997; 8: 1031–9. [DOI] [PubMed] [Google Scholar]
- 3. Sperduto P, Vaezy A, Bringman A, Wilkie L. Spontaneous regression of squamous cell lung carcinoma with adrenal metastasis. Chest 1988; 94: 887–9. [DOI] [PubMed] [Google Scholar]
- 4. Miyazaki K, Masuko H, Satoh H, Ohtsuka M. Lung cancer with spontaneous regression of scalp metastasis. Respir Med Extra 2007; 3: 83–5. [Google Scholar]
- 5. Nakamura Y, Noguchi Y, Satoh E et al Spontaneous remission of a non‐small cell lung cancer possibly caused by anti‐NY‐ESO‐1 immunity. Lung Cancer 2009; 65: 119–22. [DOI] [PubMed] [Google Scholar]
- 6. Gladwish A, Clarke K, Bezjak A. Spontaneous regression in advanced non‐small cell lung cancer. BMJ Case Rep 2010. 10.1136/bcr.07.2010.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haruki T, Nakamura H, Taniguchi Y et al Spontaneous regression of lung adenocarcinoma: Report of a case. Surg Today 2010; 40: 1155–8. [DOI] [PubMed] [Google Scholar]
- 8. Hwang ED, Kim YJ, Leem AY et al Spontaneous regression of non‐small cell lung cancer in a patient with idiopathic pulmonary fibrosis: A case report. Tuberc Respir Dis (Seoul) 2013; 75: 214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez‐Pastorini A, Plones T, Brockmann M, Ludwig C, Beckers F, Stoelben E. Spontaneous regression of non‐small cell lung cancer after biopsy of a mediastinal lymph node metastasis: A case report. J Med Case Rep 2015; 9: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogawa R, Watanabe H, Yazaki K et al Lung cancer with spontaneous regression od primary and metastatic sites: A case report. Oncol Lett 2015; 10: 550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park YH, Park BM, Park SY et al Spontaneous regression in advanced squamous cell lung carcinoma. J Thorac Dis 2016; 8: E235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol 1981; 17: 201–9. [DOI] [PubMed] [Google Scholar]
- 13. Challis GB, Stam HJ. The spontaneous regression of cancer. A review of cases from 1900 to 1987. Acta Oncol 1990; 29: 545–50. [DOI] [PubMed] [Google Scholar]
- 14. Mizuno T, Usami N, Okasaka T, Kawaguchi K, Okagawa T, Yokoi K. Complete spontaneous regression of non‐small cell lung cancer followed by adrenal relapse. Chest 2011; 140: 527–8. [DOI] [PubMed] [Google Scholar]
- 15. Cafferata MA, Chiaramondia M, Monetti F, Ardizzoni A. Complete spontaneous remission of non‐small‐cell lung cancer: A case report. Lung Cancer 2004; 45: 263–6. [DOI] [PubMed] [Google Scholar]
- 16. Pujol JL, Godard AL, Jacot W, Labauge P. Spontaneous complete remission of a non‐small cell cancer associated with anti‐Hu antibody syndrome. J Thorac Oncol 2007; 2: 168–70. [DOI] [PubMed] [Google Scholar]
- 17. Moriyama C, Yamazaki K, Yokouchi H, Kikuchi E, Oizumi S, Nishimura M. A case of spontaneous remission of large cell carcinoma of the lung with brain metastasis. Jpn J Lung Cancer 2008; 48: 112–7. [Google Scholar]
- 18. Tomizawa K, Suda K, Takemoto T et al Progression after spontaneous regression in lung large cell neuroendocrine carcinoma: Report of a curative resection. Thorac Cancer 2015; 6: 655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


