Abstract
Background
The prognostic value of surgery and postoperative radiotherapy (PORT) for primary thymic neuroendocrine tumors (TNETs) was estimated using the SEER database.
Methods
This retrospective study used SEER data of TNET patients between 1998 and 2015. Propensity score matching (PSM) was performed according to whether surgery was performed. The prognostic effects on overall survival (OS) and cancer‐specific survival (CSS) were evaluated using multivariate Cox regression.
Results
A total of 3947 patients were included: 293 (7.4%) TNET, 2788 (70.6%) thymoma, and 866 (21.9%) thymic carcinoma. Compared to other subtypes, TNET patients were younger, included a larger proportion of men, had a well or moderately differentiated histological grade, higher disease stage at diagnosis, and were more likely to have regional lymph node metastasis. The median OS and CSS for TNET were 82.9 (95% confidence interval 74.3–91.4) and 101.9 (95% confidence interval 91.9–111.8) months, respectively, significantly shorter than for thymomas. In the matched cohort of TNET patients, multivariate analysis of OS and CSS revealed a significantly poorer prognosis in the non‐surgery group (P < 0.001). Compared to total/radical resection, TNET patients who underwent debulking resection had significantly inferior outcomes (P < 0.05). Postoperative radiotherapy favorably impacted OS and CSS in Masaoka–Koga stage III–IV TNET patients; this OS impact was also observed in stage IIB patients.
Conclusion
TNETs are extremely rare with relatively dismal outcomes. This analysis revealed the role of complete surgical resection and the favorable effect of postoperative radiotherapy in specific TNET subgroups.
Keywords: Postoperative radiotherapy, primary thymic neuroendocrine tumor (TNET), SEER, surgery, thymic malignancy
Introduction
Primary thymic neuroendocrine tumors (TNETs) are extremely rare anterior mediastinal malignancies. Since the first report by Rosai and Higa in 1972,1 no more than 400 cases have been reported in the literature.2, 3 TNETs account for approximately 5% of all thymic neoplasms and are defined separately from thymomas. TNETs are categorized as a distinct entity according to the latest version of the World Health Organization (WHO) tumor classification.4 Similar to other neuroendocrine tumors, TNETs exhibit aggressive biological behavior and are always associated with endocrinopathies, such as Cushing's syndrome and multiple endocrine neoplasia type 1 (MEN‐1 syndrome).5, 6, 7 Compared to other thymic malignancies, TNETs have a poorer prognosis because of the high rate of lymph node and distant metastases at diagnosis.8, 9 Although surgical resection is the recommended option for curative‐intent treatment, prognosis remains poor.10 In published cases series, the five‐year overall survival (OS) of TNETs after surgery varied from 28% to 91.6%,3, 4, 9, 10, 11, 12 most likely because of the high risk of recurrence. Moreover, existing data are limited by the sample size, and the exact role of surgery requires validation. Postoperative radiotherapy (PORT) has been suggested as an adjuvant therapy to prevent local‐regional recurrence after surgical resection in thymoma and thymic carcinoma.13, 14, 15 However, the effect of PORT on TNET patients remains controversial,10, 16, 17 despite reports of its possible beneficial role in limited‐stage disease. Therefore, we conducted a retrospective study based on the Surveillance, Epidemiology and End Results (SEER) database to estimate the outcomes of patients treated with different types of surgery as well as PORT, and to confirm their prognostic value for TNET patients. We also compared the clinicopathological characteristics and survival outcomes of TNETs with those of other subtypes of thymic malignancies to benefit the decision‐making process in clinical practice.
Methods
Patient sample
This study was based on the SEER 18‐Registry databases (1973–2015), covering approximately 30% of the American population.18 We identified all patients with a primary site labeled as thymus (C37.9) between 1998 and 2015. The eligibility criteria included the following: (i) age > 18 years and (ii) survival duration ≥ 1 month; cases with a death certificate or autopsy were excluded. Tumors were classified into three categories according to International Classification of Disease (ICD‐O‐3) codes: TNETs (8150–8157, 8240–8246, and 8249), thymoma (8580–8585), and thymic carcinoma (8070, 8123, 8430, 8082, 8310, 8033, 8260, 8200, 8480, 8140, 8023, 8560, 8576, 8586, 8588, and 8589). We collected the demographic features and clinicopathological characteristics of these patients, such as age at diagnosis, gender, primary site, race, treatment for the primary site, survival duration, and vital status. Because information about microscopic capsular invasion is not included in the SEER database, stage I patients could not be distinguished from stage IIA patients. Referring to previously published SEER‐based studies, patients were categorized into four staging groups based on the Masaoka–Koga (M–K) staging system: stage I/IIA (invasive tumor confined to the thymus), stage IIB (tumor adjacent connective tissue), stage III/IV (tumor adjacent organs or further contiguous extension or lymph node metastasis occurs), and unknown (unknown extent of disease).15, 19 The pathologic M–K stage was determined by surgical specimen when resection was performed, or based on physical and imaging examinations or other non‐invasive clinical evidence in patients who did not undergo surgery.20 Using the variable “surgery of primary site (1998+),” the types of cancer‐directed surgical resection were categorized into total resection, radical surgery, partial removal, local excision, debulking surgery and surgery, and not otherwise specified. The institutional review board and independent ethics committee of Fudan University Shanghai Cancer Center approved this study.
Statistical analysis
Continuous data were analyzed by Student's t‐test, and categorical variables were compared using chi‐square or Fisher's exact tests. To estimate the difference between patients who did or did not undergo surgery, we conducted 1:1 propensity‐matched analysis. The PSM model was based on the clinicopathologic characteristics in this study. Kaplan–Meier (K–M) survival curves were assessed by the log‐rank test. OS was measured from the date of initial treatment to the date of death or last follow‐up. Cancer‐specific survival (CSS) was measured from diagnosis until death from the specific tumor. Univariate analyses were conducted on all variables included in the study, and variables of P < 0.2 were entered into multivariate analyses. The prognostic effects of the covariate on OS and CSS were evaluated using the multivariate Cox regression model. All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). Two‐tailed P values < 0.05 were considered statistically significant.
Results
Baseline characteristics
Based on the eligibility criteria, a total of 3947 thymus tumor patients diagnosed between 1998 and 2015 were included: 293 (7.4%) were diagnosed with TNETs, 2788 (70.6%) with thymoma, and 866 (21.9%) with thymic carcinoma. Compared to other subtypes, TNET patients were younger at diagnosis (mean age 56.6 ± 15.9) and included a higher proportion of men (202, 68.9%). These patients were also predominantly Caucasian (208, 71.0%) and mainly from the Pacific coast (164, 56.0%). TNET patients had a well or moderately differentiated histological grade (107, 67.3%), higher disease stage at diagnosis (84, 29.5%), were more likely to have regional lymph node metastasis (P < 0.001), and less likely to receive radiotherapy (129, 44.0%). The characteristics and demographics of the cohort are shown in Table 1.
Table 1.
Basic characteristics of thymic neuroendocrine tumors, thymoma, and thymic carcinoma
| Characteristics | TNET | Thymoma† | Thymic carcinoma | P | |||
|---|---|---|---|---|---|---|---|
| (n = 293) | % | (n = 2788) | % | (n = 866) | % | ||
| Gender | <0.001 | ||||||
| Male | 202 | 68.9 | 1412 | 50.6 | 518 | 59.8 | |
| Female | 91 | 31.1 | 1376 | 49.4 | 348 | 40.2 | |
| Age (years) | <0.001 | ||||||
| Median (range) | 59 | 19‐93 | 60 | 18‐94 | 62 | 19‐92 | |
| Mean (SD) | 56.6 | ±15.9 | 59.0 | ±15.1 | 60.9 | ±14.1 | |
| Ethnicity | 0.021 | ||||||
| Caucasian | 208 | 71.0 | 1928 | 69.2 | 609 | 70.3 | |
| African | 25 | 8.5 | 415 | 14.9 | 116 | 13.4 | |
| Other | 60 | 20.5 | 445 | 16 | 141 | 16.3 | |
| Marital status | 0.3 | ||||||
| Married | 182 | 62.1 | 1624 | 58.2 | 522 | 60.3 | |
| Unmarried | 111 | 37.9 | 1164 | 41.8 | 344 | 39.7 | |
| Region (CHSDA) | <0.001 | ||||||
| East | 95 | 32.4 | 1138 | 40.8 | 287 | 33.1 | |
| Pacific coast | 164 | 56.0 | 1343 | 48.2 | 462 | 53.3 | |
| Northern plains | 22 | 7.5 | 211 | 7.6 | 85 | 9.8 | |
| Southwest | 12 | 4.1 | 96 | 3.4 | 32 | 3.7 | |
| Grade | <0.001 | ||||||
| Well | 66 | 41.5 | — | — | 35 | 8.1 | |
| Moderate | 41 | 25.8 | — | — | 59 | 13.6 | |
| Poor | 34 | 21.4 | — | — | 281 | 64.7 | |
| Undifferentiated | 18 | 11.3 | — | — | 59 | 13.6 | |
| Unknown | 134 | — | — | 432 | |||
| Masaoka–Koga stage | <0.001 | ||||||
| I–IIA | 73 | 25.6 | 837 | 31.8 | 147 | 17.8 | |
| IIB | 128 | 44.9 | 1267 | 48.1 | 392 | 47.4 | |
| III–IV | 84 | 29.5 | 528 | 20.1 | 288 | 34.8 | |
| Unstaged | 8 | 156 | 39 | ||||
| Tumor size (cm) | 0.15 | ||||||
| Median (range) | 7.5 | 0.2‐27.0 | 6.5 | 0.2‐22.0 | 6.5 | 0.1‐20.2 | |
| Regional lymph node metastases | <0.001 | ||||||
| No | 43 | 43.0 | 702 | 88.9 | 197 | 67.0 | |
| Yes | 57 | 57.0 | 88 | 11.1 | 97 | 33.0 | |
| Unknown/not performed | 193 | 1998 | 572 | ||||
| Surgery | <0.001 | ||||||
| Yes | 196 | 66.9 | 2181 | 78.2 | 508 | 58.7 | |
| No | 97 | 33.1 | 607 | 21.8 | 358 | 41.3 | |
| Surgery type | <0.001 | ||||||
| Surgery not performed | 97 | 33.1 | 607 | 21.8 | 358 | 41.3 | |
| Radical surgery | 34 | 11.6 | 401 | 14.4 | 108 | 12.5 | |
| Total resection | 72 | 24.6 | 867 | 31.1 | 158 | 18.2 | |
| Partial removal | 39 | 13.3 | 454 | 16.3 | 100 | 11.5 | |
| Local excision | 35 | 11.9 | 319 | 11.4 | 91 | 10.5 | |
| Debulking | 11 | 3.8 | 64 | 2.3 | 34 | 3.9 | |
| Surgery, NOS | 5 | 1.7 | 76 | 2.7 | 17 | 2 | |
| Radiotherapy | <0.001 | ||||||
| Yes | 129 | 44.0 | 1305 | 46.9 | 472 | 54.5 | |
| No | 164 | 56.0 | 1483 | 53.2 | 394 | 45.5 | |
For thymoma, the number of patients with specific histological type instead of histological grade was identified using the code Histologic Type ICD‐O‐3 and listed as follows: type A = 227, type AB = 413, type B1 = 324, type B2 = 341, type B3 = 455, and thymoma not otherwise specified (NOS) = 1028.
CHSDA, contract health service delivery areas; SD, standard deviation; TNET, thymic neuroendocrine tumor.
We then investigated the clinicopathological characteristics of TNET patients across M–K stages (Table 2). Patients with M–K stage III–IV were similar in race and marital status but included a higher proportion of women than at other stages. These patients were more likely to have a higher histological grade, larger tumor size, and lymph node metastasis (P < 0.001 for all). A greater number of patients with M–K stage I–IIB underwent surgical resection compared to stage III–IV (P < 0.001). However, most patients diagnosed with advanced disease (M–K stage IIB–IV) underwent radiotherapy (P < 0.001).
Table 2.
TNET patient characteristics by Masaoka–Koga stage
| Characteristics | Masaoka–Koga stage I–IIA | Masaoka–Koga stage IIB | Masaoka–Koga stage III–IV | P | |||
|---|---|---|---|---|---|---|---|
| (n = 73) | % | (n = 128) | % | (n = 84) | % | ||
| Gender | 0.008 | ||||||
| Male | 49 | 67.1 | 99 | 77.3 | 48 | 57.1 | |
| Female | 24 | 32.9 | 29 | 22.7 | 36 | 42.8 | |
| Age (years) | 0.111 | ||||||
| Median (range) | 61 | 24–88 | 58 | 18–88 | 56 | 18–83 | |
| Mean (SD) | 59.22 | ±17.18 | 56.27 | ±14.48 | 53.92 | ±16.32 | |
| Ethnicity | 0.726 | ||||||
| Caucasian | 49 | 67.1 | 94 | 73.4 | 58 | 69 | |
| African | 7 | 9.6 | 12 | 9.4 | 6 | 7.1 | |
| Other | 17 | 23.3 | 22 | 17.2 | 20 | 23.8 | |
| Marital status | 0.687 | ||||||
| Married | 44 | 60.3 | 83 | 64.8 | 50 | 59.5 | |
| Unmarried | 29 | 39.7 | 45 | 35.2 | 34 | 40.5 | |
| Region (CHSDA) | 0.039 | ||||||
| East | 18 | 24.7 | 49 | 38.3 | 23 | 27.4 | |
| Pacific coast | 40 | 54.8 | 66 | 51.6 | 56 | 66.7 | |
| Northern plains | 10 | 13.7 | 8 | 6.3 | 3 | 3.6 | |
| Southwest | 5 | 6.8 | 5 | 3.9 | 2 | 2.4 | |
| Grade | 0.001 | ||||||
| Well | 21 | 61.8 | 33 | 42.9 | 11 | 24.4 | |
| Moderate | 9 | 26.5 | 22 | 28.6 | 10 | 22.2 | |
| Poor | 3 | 8.8 | 11 | 14.3 | 18 | 40.0 | |
| Undifferentiated | 1 | 2.9 | 11 | 14.3 | 6 | 13.3 | |
| Unknown | 39 | 51 | 39 | ||||
| Tumor size (cm) | 0.001 | ||||||
| ≤ 7.5 | 34 | 73.9 | 42 | 50.0 | 17 | 37.0 | |
| > 7.5 | 12 | 26.1 | 42 | 50.0 | 29 | 63.0 | |
| Unknown | 27 | 44 | 38 | ||||
| Regional lymph nodemetastases | < 0.001 | ||||||
| No | 22 | 100 | 14 | 27.5 | 7 | 25.9 | |
| Yes | 0 | 0 | 37 | 72.5 | 20 | 74.1 | |
| Unknown/not performed | 51 | 77 | 57 | ||||
| Surgery | < 0.001 | ||||||
| Yes | 62 | 84.9 | 98 | 76.6 | 34 | 40.5 | |
| No | 11 | 15.1 | 30 | 23.4 | 50 | 59.5 | |
| Surgery type | < 0.001 | ||||||
| Surgery not performed | 11 | 15.1 | 30 | 23.4 | 50 | 59.5 | |
| Total/radical resection | 31 | 42.5 | 55 | 43.0 | 20 | 23.8 | |
| Local excision/partial removal | 28 | 38.4 | 38 | 29.7 | 7 | 8.3 | |
| Debulking/NOS | 3 | 4.1 | 5 | 3.9 | 7 | 8.3 | |
| Radiotherapy | < 0.001 | ||||||
| Yes† | 16 | 21.9 | 65 | 50.8 | 47 | 56.0 | |
| No | 57 | 78.1 | 63 | 49.2 | 37 | 44.0 | |
Stage I–IIA: radiotherapy (RT) only = 4, postoperative radiotherapy (PORT) = 9, neoadjuvant radiotherapy (NRT) = 3; stage IIB: RT only = 15, PORT = 49, NRT = 1; stage III–IV: RT only = 28, PORT = 17, NRT = 2.
CHSDA, contract health service delivery areas; NOS, not otherwise specified; SD, standard deviation; TNET, thymic neuroendocrine tumor.
Survival analysis
The median follow‐up duration of TNET patients was 38 months (range 1–174). The median OS and CSS were 82.9 (95% confidence interval [CI] 74.3–91.4) and 101.9 (95% CI: 91.9–111.8) months, respectively. We analyzed the OS and CSS differences among TNET, thymoma, and thymic carcinoma patients. OS in TNET patients was better than in thymic carcinoma (hazard ratio [HR] 1.287, 95% CI 1.064–1.557; P = 0.009), but worse than in thymoma patients (HR 0.563, 95% CI 0.471–0.673; P < 0.001). The K–M curves of CSS showed that the prognosis of thymoma was superior to the other two histological subtypes (see Fig 1). The K–M curves of survival stratified by surgery or radiotherapy are shown in Figure S1. Among patients who underwent surgical resection, thymoma had better OS and CSS than other subtypes, whereas there was no significant difference in outcomes between TNETs and thymic carcinoma. Similar results were observed in the radiotherapy category. We further investigated the survival of TNET patients according to the specific SEER summary stage. As shown in Figure 2, patients with advanced stages (III–IV) had significantly inferior OS and CSS than those with early stages (I–IIB).
Figure 1.
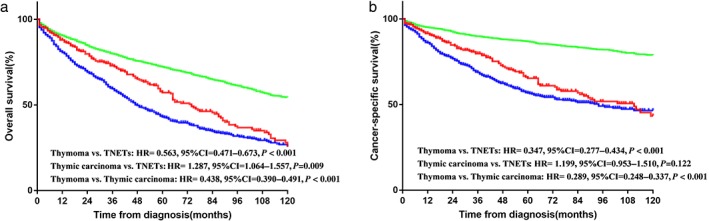
(a) Overall and (b) cancer‐specific survival in patients with thymic neuroendocrine tumors (TNETs), thymoma, and thymic carcinoma. CI, confidence interval; HR, hazard ratio. ( ) TNETs, (
) TNETs, ( ) Thymoma and (
) Thymoma and ( ) Thymic carcinoma
) Thymic carcinoma
Figure 2.
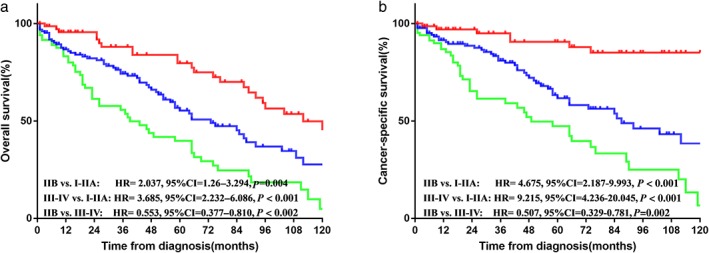
(a) Overall and (b) cancer‐specific survival in thymic neuroendocrine tumor (TNET) patients with Masaoka–Koga stage I–IIA, IIB, and III–IV. CI, confidence interval; HR, hazard ratio. ( ) I–IIA, (
) I–IIA, ( ) IIB, and (
) IIB, and ( ) III–IV
) III–IV
Prognostic value of surgery and postoperative radiotherapy for thymic neuroendocrine tumor patients
In the entire cohort, most patients (66.9%) received cancer‐directed surgery, particularly those with early‐stage disease. Patients with poorly differentiated tumors were less likely to undergo surgery. To minimize selection bias, we performed 1:1 nearest PSM analysis between TNET patients with or without surgery based on categorized variables. The balance of each variable in the unmatched and matched population is shown in Table S1. Ninety‐seven patients who underwent surgical resection were matched with 97 patients who did not undergo surgery. No significant difference in clinical characteristics was observed in the matched population. In the matched cohort, both the median OS and CSS were significantly better than those of patients who underwent surgery (yes vs. no, OS 103.4 vs. 50.0 months, P < 0.001; CSS 118.5 vs. 61.6 months, P < 0.001) (Fig 3). In univariate analysis of CSS, gender, age, marital status, histological grade, and surgery were prognostic factors (Table S2). Multivariate Cox analysis for CSS showed that women (HR 1.324, 95% CI 1.109–2.877; P = 0.047) and patients who did not undergo surgery (HR 3.429, 95% CI 1.482–7.933; P = 0.004) had a significantly poorer prognosis.
Figure 3.
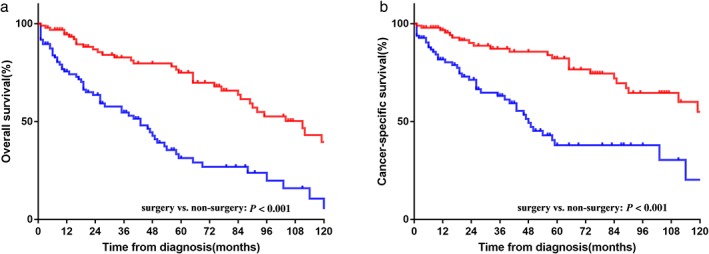
(a) Overall and (b) cancer‐specific survival in thymic neuroendocrine tumor (TNET) patients with and without surgery in the matched cohort. ( ) surgery and (
) surgery and ( ) non‐surgery
) non‐surgery
Because our results demonstrated the prognostic value of surgery, we estimated the difference in survival between surgery types. In the entire cohort, surgery was performed on 196 patients. The results of univariate and multivariate analyses are listed in Table 3. Significantly shorter OS was observed in women (P = 0.040) and patients who underwent debulking tumor resection (P = 0.003). We also found that age at diagnosis (HR 1.975, 95% CI 1.957–1.994; P = 0.006) and debulking tumor resection (HR 2.894, 95% CI 1.21–6.924; P = 0.017) were independent prognostic factors in multivariate analysis for CSS. Of note, postoperative radiotherapy (PORT) was not an independent prognostic factor in multivariate Cox regression analysis for either OS or CSS. We then conducted subgroup analysis to estimate the PORT value in TNETs stratified by the M–K staging system. For TNET patients with M–K stage IIB, the median OS was 92 months in the PORT group and 65 months in the non‐PORT group. The OS rate was higher in the PORT group than that in the control group (yes vs. no, five‐year OS 67.6% vs.54.6%; P = 0.036) (Fig 4a,b). PORT had a favorable survival impact in patients with M–K stage III–IV (yes vs. no, five‐year OS 77.4% vs. 35.2%, P = 0.005; five‐year CSS 77.4% vs.57.3%, P = 0.029) (Fig 4c,d). Unfortunately, no significant difference in survival between the PORT and non‐PORT groups was observed in patients with stage I–IIA (figures not shown).
Table 3.
Univariate and multivariate Cox regression analysis of clinical characteristics for OS and CSS in TNET patients who underwent surgery
| Characteristics | OS | CSS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | 95%CI lower | 95%CI upper | P | Multivariate analysis | 95%CI lower | 95%CI upper | P | Univariate analysis | 95%CI lower | 95%CI upper | P | Multivariate analysis | 95%CI lower | 95%CI upper | P | |
| Gender | ||||||||||||||||
| Male | 1 | — | — | 1 | — | — | 1 | — | — | 1 | — | — | ||||
| Female | 1.410 | 0.868 | 2.291 | 0.166 | 1.665 | 1.120 | 2.475 | 0.040 | 1.244 | 0.675 | 2.294 | 0.184 | 1.666 | 0.876 | 3.166 | 0.119 |
| Age (years) | 1 | 0.986 | 1.015 | 0.961 | — | — | — | — | 1.977 | 1.960 | 1.994 | 0.008 | 1.975 | 1.957 | 1.994 | 0.006 |
| Ethnicity | ||||||||||||||||
| Caucasian | 1 | — | — | — | — | — | 1 | — | — | — | — | — | ||||
| African | 0.754 | 0.302 | 1.880 | 0.545 | — | — | — | — | 0.863 | 0.308 | 2.416 | 0.779 | — | — | — | — |
| Other | 0.921 | 0.531 | 1.598 | 0.769 | — | — | — | — | 0.750 | 0.365 | 1.542 | 0.434 | — | — | — | — |
| Marital status | ||||||||||||||||
| Married | 1 | — | — | — | — | — | 1 | — | — | — | — | — | ||||
| Unmarried | 1.020 | 0.648 | 1.605 | 0.932 | — | — | — | — | 0.830 | 0.468 | 1.472 | 0.523 | — | — | — | — |
| CHSDA | ||||||||||||||||
| East | 1 | — | — | 1 | — | — | 1 | — | — | — | — | — | ||||
| Pacific coast | 1.203 | 0.732 | 1.977 | 0.465 | 1.065 | 0.638 | 1.777 | 0.810 | 0.876 | 0.491 | 1.565 | 0.655 | — | — | — | — |
| Northern plains | 1.037 | 0.464 | 2.319 | 0.929 | 1.211 | 0.482 | 3.043 | 0.685 | 0.653 | 0.222 | 1.920 | 0.439 | — | — | — | — |
| Southwest | 2.008 | 0.763 | 5.285 | 0.158 | 2.602 | 0.941 | 7.195 | 0.064 | 1.931 | 0.656 | 5.684 | 0.232 | — | — | — | — |
| Grade | ||||||||||||||||
| Well | 1 | — | — | 1 | — | — | 1 | — | — | 1 | — | — | ||||
| Moderate | 2.193 | 0.882 | 5.452 | 0.091 | 1.740 | 0.655 | 4.622 | 0.310 | 2.776 | 0.866 | 8.896 | 0.086 | 2.484 | 0.720 | 8.562 | 0.150 |
| Poor/Undifferentiated | 1.869 | 0.740 | 4.718 | 0.186 | 1.750 | 0.680 | 4.504 | 0.258 | 2.493 | 0.776 | 8.008 | 0.125 | 2.464 | 0.741 | 8.198 | 0.141 |
| Masaoka–Koga stage | ||||||||||||||||
| I–IIA | 1 | — | — | 1 | — | — | 1 | — | — | 1 | — | — | ||||
| IIB | 2.117 | 1.220 | 3.671 | 0.008 | 2.733 | 1.479 | 5.050 | 0.001 | 6.454 | 2.495 | 16.691 | 0.001 | 6.802 | 2.604 | 17.772 | 0.001 |
| III–IV | 4.152 | 2.176 | 7.925 | <0.001 | 5.375 | 2.432 | 11.879 | 0.001 | 13.827 | 4.983 | 38.367 | 0.001 | 12.851 | 4.289 | 38.507 | 0.001 |
| Tumor size (cm) | ||||||||||||||||
| ≤ 7.5 | 1 | — | — | — | — | — | — | — | — | — | — | — | ||||
| > 7.5 | 1.442 | 0.751 | 2.769 | 0.271 | — | — | — | — | 1.398 | 0.642 | 3.046 | 0.399 | — | — | — | — |
| Surgery | ||||||||||||||||
| Total/radical resection | 1 | — | — | 1 | — | — | 1 | — | — | 1 | — | — | ||||
| Local excision/partial removal | 1.284 | 0.806 | 2.047 | 0.293 | 1.543 | 0.932 | 2.555 | 0.094 | 0.957 | 0.528 | 1.733 | 0.883 | 1.124 | 0.606 | 2.086 | 0.710 |
| Debulking/NOS | 2.574 | 1.246 | 5.318 | 0.011 | 3.091 | 1.440 | 6.635 | 0.003 | 2.748 | 1.200 | 6.292 | 0.017 | 2.894 | 1.210 | 6.924 | 0.017 |
| Radiation | ||||||||||||||||
| Yes | 1 | — | — | — | — | — | 1 | — | — | — | — | — | ||||
| No | 1.075 | 0.686 | 1.686 | 0.752 | — | — | — | — | 0.647 | 0.381 | 1.098 | 0.306 | — | — | — | — |
CHSDA, contract health service delivery areas; CI, confidence interval; CSS, cancer‐specific survival; NOS, not otherwise specified; OS, overall survival; TNET, thymic neuroendocrine tumor.
Figure 4.
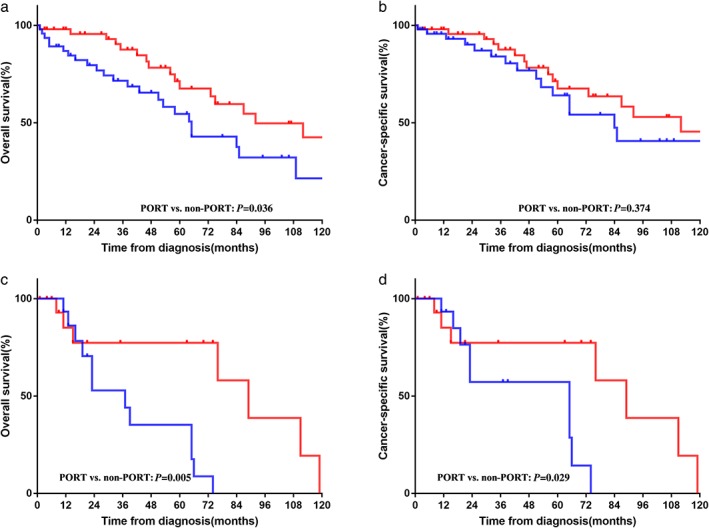
Overall and cancer‐specific survival in Masaoka–Koga stage (a, b) IIB and (c, d) III–IV thymic neuroendocrine tumor (TNET) patients with and without postoperative radiotherapy (PORT). ( ) PORT and (
) PORT and ( ) non‐PORT
) non‐PORT
Discussion
In the present study, we analyzed the survival outcomes of TNET patients over the past two decades using data from the SEER database. We found that surgery was associated with a better prognosis both before and after PSM and further found a favorable survival impact of complete resection in TNET patients. In subgroup analysis, PORT improved both OS and CSS in patients with M–K stage III–IV and improved OS in patients with M–K stage IIB.
Our results suggested a male predominance among TNET patients (men‐to‐women ratio 2.2:1), and multivariate analysis showed that women were associated with a poorer prognosis than men. The impact of gender on survival has been reported for gastrointestinal malignancies21, 22, 23 but has not yet been observed in thymic tumors. One explanation may be that in this study, men were more likely to receive surgery than women in the both entire population and the matched cohort. Among TNET patients, the median age at diagnosis was younger than in patients with other subtypes of thymic neoplasms, which was also observed in previous reports.12, 24 Because our study was based on a database of a Western population, Caucasian patients accounted for the largest proportion and the majority of patients were from East and Pacific coast regions, likely because of the advanced medical insurance systems in these regions.25
In this study, SEER histologic grade information was used to classify TNETs, including well, moderately, and poorly differentiated, and undifferentiated, which was used in a prior publication.26 Poorly differentiated and undifferentiated were combined into one category in the regression models because of the limited number of cases. Although the impact of histological grade was proposed in several small‐sized retrospective analyses,10, 11, 16 a large‐scale database study by Fillosso et al. suggested that it had no effect on survival (P = 0.19).3 Our analysis included more recently diagnosed patients and found that histological grade was not an independent prognostic factor for either OS or CSS in Cox regression analysis; therefore, the prognostic value of histological grade among TNET patients remains controversial.
Similar to published reports, we found that TNETs were larger than thymomas and thymic carcinomas, but were not significantly different (P = 0.15).6, 27 Moreover, compared to other thymic tumors, TNETs were more likely to present with lymph node metastasis. Weksler et al. analyzed thymic carcinoma and TNET patients who underwent lymph node dissection and suggested that nodal metastases may be a negative prognostic factor in these patients.28 However, their sample of TNET patients was much smaller than their sample of thymic carcinoma patients (1:3.3). Furthermore, in final multivariate analysis they did not separate TNET from non‐TNET patients; therefore, the true impact of the nodal status of survival in TNETs could be misunderstood. In univariate analysis performed in the present study, no significant difference in the status of lymph node metastasis of either OS or CSS (P > 0.2) was observed. Because of the limited data on lymph node metastases (65.9% missing), we did not use this variable in multivariate analysis. Our findings, together with those of a previous study, indicate that the association between lymph node metastasis and inferior outcomes should be questioned, and further studies are warranted to understand the impact of nodal metastases in TNETs.17
We also confirmed the predictive impact of M–K stage in TNETs. Patients with advanced‐stage disease had inferior outcomes compared to those with early‐stage disease. Treatments for patients with advanced‐stage disease were more likely to be of palliative‐intent, and management of these patients remains a challenge in clinical practice.9, 29 Recently, an updated tumor node metastasis (TNM) staging system was proposed to better suit thymic malignancies and provide comprehensive information, which could benefit decision‐making regarding the treatment strategy.30 However, because of the limitations of the SEER database, we could not compare the newest TNM staging system with the classic M–K staging system.
We performed PSM to minimize the potential selection bias and verified that surgery was the mainstay treatment option for TNET patients, which was consistent with the results of prior studies.2, 3, 9, 31 Compared to these studies, using multivariate analysis of subgroups, we further demonstrated the survival improvement for those who received complete resection (total/radical resection).16, 28 Debulking surgery was associated with inferior outcomes in our study; therefore, we suggest that radical surgery with a curative intent should be considered for patients with resectable disease. Sullivan et al. reported similar results after analyzing data of TNET patients between 1988 and 2011 in the SEER database.32 However, our study differs from this prior study because: (i) we analyzed more recently diagnosed patients, thus, the proportion of surgical resection was higher (nearly 70%) than that of any other SEER‐based study; (ii) PSM was performed to minimize the potential selection bias; and (iii) as information on the specific surgery type for the primary site was not routinely collected in the SEER database until 1998, our study classified the surgery type more accurately and not only discriminated surgical resection from debulking resection but also demonstrated the impact of complete and incomplete resection on survival outcomes. In addition, as discussed previously, the five‐year OS for patients who underwent surgery in the matched group was relatively high (nearly 75%), which was consistent with these two prior studies. Thus, CSS was also utilized as an outcome measure to more accurately validate the value of surgery, and CSS in patients who underwent surgical resection was higher than 80% (Fig 3b). Moreover, to our knowledge, this is the largest SEER‐based study to analyze the prognostic factors associated with disease‐specific survival of TNET patients.
Postoperative radiotherapy was recommended in earlier studies to eradicate residual tumors and improve local control. Lim et al. revealed the survival benefit of PORT in thymic carcinoma,15 and similar results were demonstrated for patients with thymoma in previous studies.14, 33, 34 However, the role of PORT for TNETs remains controversial. Some researchers found that PORT did not achieve a favorable survival impact,3, 32 and others reported a detrimental effect.10, 28 We found a trend of improved survival in TNET patients who received PORT and further revealed that PORT significantly improved survival in those with specific M–K stages. Sullivan et al. observed a trend of poor prognosis in the PORT group (P > 0.05); however, they did not perform subgroup analysis according to the M–K stage and found that patients with advanced‐stage disease were more likely to benefit from PORT.32 We enrolled more recently diagnosed patients in the present study, and subgroup analysis demonstrated the favorable impact of PORT among stage III–IV patients. In patients with M–K stage IIB, the PORT group had significantly better OS than the non‐PORT group. Although PORT showed a trend toward improved CSS, the improvement was not significant. To avoid selection bias of patients with lower postoperative mortality after PORT, we conducted additional sensitivity analysis using conditional survival. We compared OS and CSS for M–K stage IIB patients who survived the first three months. Conditional survival analysis showed that for patients who survived longer than three months, PORT improved OS (P = 0.036) and showed a trend toward improved CSS (P = 0.374, data not shown). Because of the lack of information on pathologic resection margins in the SEER database, we could not investigate the impact of PORT among those with positive margins who were more likely to suffer from a poor prognosis. Thus, further studies elucidating the exact role of PORT in this group are needed.
In the present study, the survival of patients with TNETs was significantly shorter than that of patients with thymomas, whereas there was no difference between patients with TNETs and thymic carcinoma, consistent with previous reports.27, 28 Considering the difference in biological behavior, the latest WHO tumor classification also distinguished TNETs from thymoma. Thus, our results confirm that the malignancy features of TNETs are similar to those in thymic carcinoma but significantly different from those of thymoma.
Similar to other SEER‐based studies, there were a few limitations in this study. First, information on lymph node metastases was unavailable for a large amount of patients, thus we could not include this variable in our final multivariate Cox analysis. Second, tumor size has always been considered to be a prognostic factor in other neoplasms, and patients with larger tumors are less likely to undergo surgery. After PSM analysis, although the difference between the surgery and non‐surgery group was not significant, patients with an “unknown” size comprised a relatively large proportion. Therefore, in final multivariate analysis, after excluding those with missing values (“unknown” covariate), we confirmed the prognostic values of surgery as well surgical types among TNET patients. Furthermore, the newest American Joint Committee on Cancer TNM staging system was proposed to better classify TNETs. However, the SEER database does not record TNM stage, which limited our ability to verify the prognostic value of this method. Other systemic treatment options, such as cytotoxic chemotherapy, somatostatin analogs, and everolimus, have been recommended to achieve prolonged local control and reduce the risk of recurrence.33, 35 Unfortunately, we could not estimate the effect of chemotherapy because of the lack of information. Recurrence rates and details of the radiotherapy methods, including the total dose, daily fraction, and treatment techniques, were also not available. Finally, as discussed above, selection biases may result in inconsistent survival analysis of PORT among M–K stage IIB patients; however, information on radiotherapy techniques and PORT toxicity are not recorded in the SEER database. Additional prospective randomized trials are warranted to confirm the role of PORT in this group.
In conclusion, using a population‐based approach, our study analyzed the management and prognosis of TNETs. To our knowledge, this is the first propensity‐matched analysis of SEER data, which not only demonstrated the role of complete surgical resection but also the favorable effect of PORT in specific subgroups of TNET patients. In addition, for the first time, prognostic factors were estimated and associated with CSS in a large cancer registry. However, because of the rarity of this disease, prospective analysis is still lacking and further investigation is necessary.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1. Overall and cancer‐specific survival for thymic neuroendocrine tumor (TNET), thymoma and thymic carcinoma patients who (a,b) underwent surgery and (c,d) received radiotherapy.
Table S1. Thymic neuroendocrine tumor (TNET) patient characteristics before and after propensity score matching.
Table S2. Univariate and multivariate Cox regression analysis of clinical characteristics for overall and cancer‐specific survival in thymic neuroendocrine tumor (TNET) patients in the matched population.
Acknowledgment
We thank American Journal Experts (https://www.aje.com/) for editing this manuscript.
References
- 1. Rosai J, Higa E. Mediastinal endocrine neoplasm, of probable thymic origin, related to carcinoid tumor. Clinicopathologic study of 8 cases. Cancer 1972; 29: 1061–74. [DOI] [PubMed] [Google Scholar]
- 2. Tiffet O, Nicholson AG, Ladas G, Sheppard MN, Goldstraw P. A clinicopathologic study of 12 neuroendocrine tumors arising in the thymus. Chest 2003; 124: 141–6. [DOI] [PubMed] [Google Scholar]
- 3. Filosso PL, Yao X, Ahmad U et al Outcome of primary neuroendocrine tumors of the thymus: A joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg 2015; 149: 103–109.e102. [DOI] [PubMed] [Google Scholar]
- 4. Cardillo G, Rea F, Lucchi M et al Primary neuroendocrine tumors of the thymus: A multicenter experience of 35 patients. Ann Thorac Surg 2012; 94: 241–5. [DOI] [PubMed] [Google Scholar]
- 5. de Montpreville VT, Macchiarini P, Dulmet E. Thymic neuroendocrine carcinoma (carcinoid): A clinicopathologic study of fourteen cases. J Thorac Cardiovasc Surg 1996; 111: 134–41. [DOI] [PubMed] [Google Scholar]
- 6. Teh BT. Thymic carcinoids in multiple endocrine neoplasia type 1. J Intern Med 1998; 243: 501–4. [DOI] [PubMed] [Google Scholar]
- 7. Takagi J, Otake K, Morishita M et al Multiple endocrine neoplasia type I and Cushing's syndrome due to an aggressive ACTH producing thymic carcinoid. Intern Med (Tokyo, Japan) 2006; 45: 81–6. [DOI] [PubMed] [Google Scholar]
- 8. Modlin IM, Lye KD, Kidd M. A 5‐decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97: 934–59. [DOI] [PubMed] [Google Scholar]
- 9. Crona J, Bjorklund P, Welin S, Kozlovacki G, Oberg K, Granberg D. Treatment, prognostic markers and survival in thymic neuroendocrine tumours. A study from a single tertiary referral centre. Lung Cancer (Amsterdam, Netherlands) 2013; 79: 289–93. [DOI] [PubMed] [Google Scholar]
- 10. Cardillo G, Treggiari S, Paul MA et al Primary neuroendocrine tumours of the thymus: A clinicopathologic and prognostic study in 19 patients. Eur J Cardiothorac Surg 2010; 37: 814–8. [DOI] [PubMed] [Google Scholar]
- 11. Moran CA, Suster S. Neuroendocrine carcinomas (carcinoid tumor) of the thymus. A clinicopathologic analysis of 80 cases. Am J Clin Pathol 2000; 114: 100–10. [DOI] [PubMed] [Google Scholar]
- 12. Yao JC, Hassan M, Phan A et al One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063–72. [DOI] [PubMed] [Google Scholar]
- 13. Forquer JA, Rong N, Fakiris AJ, Loehrer PJ Sr, Johnstone PA. Postoperative radiotherapy after surgical resection of thymoma: Differing roles in localized and regional disease. Int J Radiat Oncol Biol Phys 2010; 76: 440–5. [DOI] [PubMed] [Google Scholar]
- 14. Patel S, Macdonald OK, Nagda S, Bittner N, Suntharalingam M. Evaluation of the role of radiation therapy in the management of malignant thymoma. Int J Radiat Oncol Biol Phys 2012; 82: 1797–801. [DOI] [PubMed] [Google Scholar]
- 15. Lim YJ, Song C, Kim JS. Improved survival with postoperative radiotherapy in thymic carcinoma: A propensity‐matched analysis of Surveillance, Epidemiology, and End Results (SEER) database. Lung Cancer (Amsterdam, Netherlands) 2017; 108: 161–7. [DOI] [PubMed] [Google Scholar]
- 16. Gaur P, Leary C, Yao JC. Thymic neuroendocrine tumors: A SEER database analysis of 160 patients. Ann Surg 2010; 251: 1117–21. [DOI] [PubMed] [Google Scholar]
- 17. Filosso PL, Ruffini E, Solidoro P et al Neuroendocrine tumors of the thymus. J Thorac Dis 2017; 9 (Suppl 15): S1484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Surveillance, Epidemiology and End Results Program . About the SEER Program, 2018. [Cited 5 May 2018.] Available from URL: http://seer.cancer.gov/about
- 19. Fernandes AT, Shinohara ET, Guo M et al The role of radiation therapy in malignant thymoma: A surveillance, epidemiology, and end results database analysis. J Thorac Oncol 2010; 5: 1454–60. [DOI] [PubMed] [Google Scholar]
- 20. Adamo M, Dickie L, Ruhl J. In: National Cancer Institute (ed.). SEER Program Coding and Staging Manual 2018. National Cancer Institute, Bethesda, Maryland: 2016. [Google Scholar]
- 21. Wichmann MW, Muller C, Hornung HM, Lau‐Werner U, Schildberg FW. Gender differences in long‐term survival of patients with colorectal cancer. Br J Surg 2001; 88: 1092–8. [DOI] [PubMed] [Google Scholar]
- 22. Bohanes P, Yang D, Chhibar RS et al Influence of sex on the survival of patients with esophageal cancer. J Clin Oncol 2012; 30: 2265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hidaka H, Hotokezaka M, Nakashima S, Uchiyama S, Maehara N, Chijiiwa K. Sex difference in survival of patients treated by surgical resection for esophageal cancer. World J Surg 2007; 31: 1982–7. [DOI] [PubMed] [Google Scholar]
- 24. Dusmet ME, McKneally MF. Pulmonary and thymic carcinoid tumors. World J Surg 1996; 20: 189–95. [DOI] [PubMed] [Google Scholar]
- 25. He J, Shen J, Pan H, Huang J, Liang W, He J. Pulmonary lymphoepithelioma‐like carcinoma: A surveillance, epidemiology, and end results database analysis. J Thorac Dis 2015; 7: 2330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dasari A, Shen C, Halperin D et al Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017; 3: 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Filosso PL, Yao X, Ruffini E et al Comparison of outcomes between neuroendocrine thymic tumours and other subtypes of thymic carcinomas: A joint analysis of the European Society of Thoracic Surgeons and the international Thymic malignancy interest group. Eur J Cardiothorac Surg 2016; 50: 766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weksler B, Holden A, Sullivan JL. Impact of positive nodal metastases in patients with Thymic carcinoma and Thymic neuroendocrine tumors. J Thorac Oncol 2015; 10: 1642–7. [DOI] [PubMed] [Google Scholar]
- 29. Detterbeck FC, Nicholson AG, Kondo K, Van Schil P, Moran C. The Masaoka‐Koga stage classification for thymic malignancies: Clarification and definition of terms. J Thorac Oncol 2011; 6 (7 Suppl. 3): S1710–6. [DOI] [PubMed] [Google Scholar]
- 30. Detterbeck FC, Stratton K, Giroux D et al The IASLC/ITMIG Thymic epithelial tumors staging project: Proposal for an evidence‐based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014; 9 (9 Suppl. 2): S65–72. [DOI] [PubMed] [Google Scholar]
- 31. Huang J, Rizk NP, Travis WD et al Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg 2007; 134: 1477–83. [DOI] [PubMed] [Google Scholar]
- 32. Sullivan J, Weksler B. Neuroendocrine tumors of the thymus: Analysis of factors affecting survival in 254 patients. Ann Thorac Surg 2017; 103: 935–9. [DOI] [PubMed] [Google Scholar]
- 33. Girard N, Ruffini E, Marx A, Faivre‐Finn C, Peters S. Thymic epithelial tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology : Official Journal of the European Society for Medical Oncology 2015; 26 ((Suppl 5)): v40–55. [DOI] [PubMed] [Google Scholar]
- 34. Rimner A, Yao X, Huang J et al Postoperative radiation therapy is associated with longer overall survival in completely resected stage II and III Thymoma‐an analysis of the international Thymic malignancies interest group retrospective database. J Thorac Oncol 2016; 11: 1785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Comprehensive Cancer Network . Neuroendocrine Tumors (Version 4) 2018. [Cited 5 Sep 2018.] Available from URL: http://www.nccn.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overall and cancer‐specific survival for thymic neuroendocrine tumor (TNET), thymoma and thymic carcinoma patients who (a,b) underwent surgery and (c,d) received radiotherapy.
Table S1. Thymic neuroendocrine tumor (TNET) patient characteristics before and after propensity score matching.
Table S2. Univariate and multivariate Cox regression analysis of clinical characteristics for overall and cancer‐specific survival in thymic neuroendocrine tumor (TNET) patients in the matched population.


