Abstract
Background
This study investigated the relationship between the neutrophil‐to‐lymphocyte ratio (NLR) and clinicopathological features and prognosis in patients with postoperative esophageal squamous cell carcinoma (ESCC).
Methods
The preoperative NLR was evaluated in 419 patients who underwent esophagectomy for ESCC. A receiver operating characteristic (ROC) curve was plotted to verify the accuracy of the NLR for predicting survival. Correlation between the NLR and clinicopathological characteristics was analyzed using the χ2 test. Prognostic influence was calculated by using the Kaplan–Meier method and the difference was assessed by log‐rank test. Multivariate Cox regression models were applied to evaluate the independent prognostic value.
Results
The cutoff value of the NLR was 2.998, the area under the curve was 0.735, and the sensitivity and specificity were 69.3% and 69.3%, respectively. Tumor length (P = 0.0317), lymph node metastasis (P = 0.0352), pathological tumor node metastasis (pTNM) stage (P = 0.0271), and postoperative radiotherapy (P = 0.0385) were significantly different between the groups. Multivariate analysis showed that pTNM stage (P = 0.0098), lymph node metastasis (P = 0.001), and NLR (P = 0.0022) were independent prognostic factors for survival. Moreover, when patients were stratified by TNM stage, the adverse effects of preoperative NLR on cancer‐specific survival were greater in patients with stage II and III ESCC and in patients with lymph node metastasis.
Conclusions
The preoperative NLR is significantly correlated with long‐term prognosis in postoperative patients with ESCC, particularly in patients with lymph node metastasis and stage II and III ESCC.
Keywords: Esophageal squamous cell carcinoma, neutrophil to lymphocyte ratio, prognosis, survival
Introduction
Esophageal cancer is the eighth most common cancer worldwide and the sixth leading cause of cancer death. Nearly 50% of esophageal cancer cases and deaths resulting from esophageal cancer occur in China.1, 2 Esophageal squamous cell carcinoma (ESCC) accounts for > 90% of all pathological types of esophageal cancer in China.3, 4 Surgery is the main treatment for resectable esophageal cancer. In recent years, with the development of medical science, surgical methods for treating esophageal cancer have made great progress, but the prognosis of esophageal cancer is still poor.5, 6 Therefore, investigating supplementary criteria to tumor node metastasis (TNM) staging in order to comprehensively evaluate esophageal cancer prognosis has been widely studied in recent years.
Since Virchow et al. first found white blood cells in the pathological tissues of malignant tumors in 1863, an increasing number of studies have confirmed the important role of inflammation in the development of tumors.7, 8 The systemic inflammatory response is related to tumor development, apoptosis inhibition, and angiogenesis and is an important factor leading to tumor progression and metastasis.9, 10 The serum neutrophil‐to‐lymphocyte ratio (NLR) is an economical and convenient indicator of systemic inflammation. Studies have reported that the preoperative NLR level is significantly correlated with the prognosis of patients with gastric cancer, liver cancer, and renal cell carcinoma.11, 12, 13 However, there are few studies on ESCC.
The aim of this study was to explore the prognostic value of the preoperative NLR and provide new indicators for prognosis in postoperative patients with ESCC.
Methods
Patient selection
We retrospectively analyzed the records of 789 patients with ESCC who underwent an esophagectomy at the First Hospital of the University of Science and Technology of China between January 2010 and December 2012. Patients were selected based on the following eligibility criteria: (i) a pathologic diagnosis of ESCC, (ii) Ivor–Lewis or McKeown esophagectomy and a modern two field lymphadenectomy (total mediastinal and perigastric lymph nodes) had been performed, and (iii) R0 resection. Patients were excluded based on the following criteria: (i) received anti‐inflammatory or nonsteroidal anti‐inflammatory drugs for one week, (ii) pT4 was proven after surgery, (iii) administered preoperative chemotherapy and/or radiotherapy, and (iv) had synchronous or metachronous cancer in other organs. Four hundred nineteen patients were included in the study.
The routine preoperative evaluation included chest radiographs, barium swallow examinations, Doppler ultrasonography examinations of the abdomen, computed tomography (CT) scans from the chest to the upper abdomen, endoscopy with biopsy, electrocardiograms, lung function tests, complete blood counts, blood biochemistry analyses, and liver and renal function evaluations. All patients were staged according to the TNM staging system of the American Joint Committee on Cancer (AJCC Staging Manual, 8th edition).
Data collection
The NLR was the ratio of the absolute neutrophil count (×109/L) and absolute lymphocyte count (×109/L). All blood samples were collected within a week before surgery, and the same patient underwent multiple routine blood examinations; the last sample taken before surgery was used in subsequent analyses. The following clinical characteristics and pathological findings were collected: age, gender, smoking history, history of alcohol consumption, tumor location, tumor differentiation, tumor length, depth of invasion, lymph node metastasis, TNM stage, and postoperative adjuvant therapy. Cancer‐specific survival (CSS) was defined as the interval from surgery to death as a result of cancer.
Follow‐up
Patients were followed up at the outpatient department every four months for the first year, every six months for two years, and annually thereafter. Evaluation included a clinical examination and a CT scan of the chest and abdomen. When clinical or radiographic evidence suggested local recurrence, an endoscopic biopsy was performed.
Statistical analysis
All statistical analyses were performed using SPSS version19.0 (SPSS Inc., Chicago, IL, USA), and the best cut‐off value of the NLR was determined via the receiver operating characteristic (ROC) curve. Correlation between the NLR and clinicopathological characteristics was analyzed by χ2 test. The Kaplan–Meier method was used to calculate the prognostic influence of the NLR and other clinicopathological factors on CSS, and the difference was assessed by the log‐rank test. To evaluate the independent prognostic value of NLR, multivariate Cox regression models were applied. Statistical significance was set at P < 0.05.
Results
Best cut‐off value
Based on the maximum Youden index, 2.998 was chosen as the best cut‐off value of the NLR, and the curve area was 0.735, with a sensitivity of 69.3% and specificity of 69.3% (Fig 1). Four hundred nineteen patients were divided into groups with a high (≥ 2.998; n = 167) and low (< 2.998; n = 252) NLR.
Figure 1.
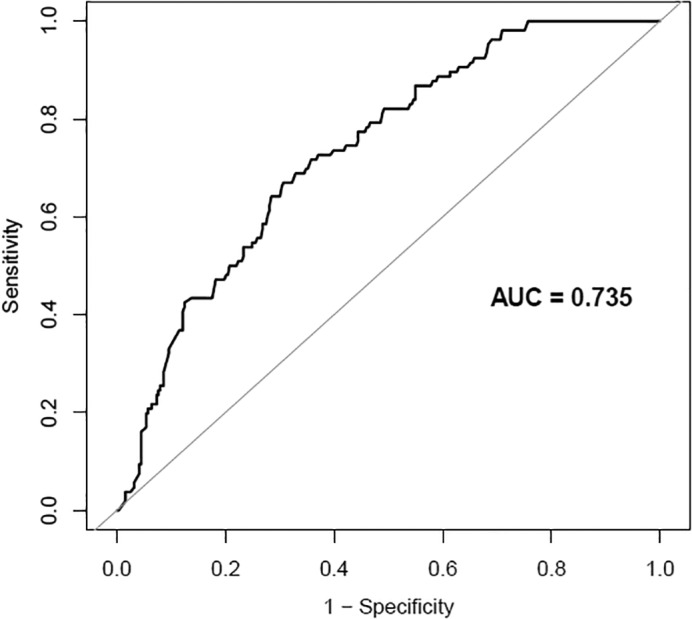
Receiver operating characteristic (ROC) curve analysis for the optimal cutoff value of the neutrophil‐to‐lymphocyte ratio (NLR). AUC, area under the curve.
Patient characteristics
Among the 419 patients, 91 were women and 328 were men. The NLR was associated with lymph node metastasis, pathological (p)TNM stage, tumor length, and radiotherapy, with a preoperative NLR > 2.998 associated with lymph node metastasis (P = 0.0352), radiotherapy (P = 0.0385), a higher pTNM stage (P = 0.0271), and tumor length (P = 0.0317) (Table 1).
Table 1.
Clinical characteristics according to NLR
| Characteristics | High NLR group (n = 167) | Low NLR group (n = 252) | χ2 | P |
|---|---|---|---|---|
| Gender | 0.1014 | 2.6838 | ||
| Male | 138 (82.63%) | 190 (75.4%) | — | — |
| Female | 29 (17.37%) | 62 (24.6%) | — | — |
| Age (years) | 1.5082 | 0.2194 | ||
| ≤ 65 | 67 (40.12%) | 85 (33.73%) | — | — |
| > 65 | 100 (59.88%) | 167 (66.27%) | — | — |
| Differentiation | 1.8578 | 0.3950 | ||
| Lower | 40 (23.95%) | 75 (29.76%) | — | — |
| Middle | 93 (55.69%) | 126 (50.00%) | — | — |
| High | 34 (20.36%) | 51 (20.24%) | — | — |
| Tumor location | 6.8438 | 0.3271 | ||
| Upper | 7 (4.19%) | 17 (6.75%) | — | — |
| Middle | 84 (50.3%) | 140 (55.56%) | — | — |
| Lower | 76 (45.51%) | 95 (37.7%) | — | — |
| Tumor diameter (cm) | 2.263 | 0.0317 | ||
| ≤ 3 | 58 (34.73%) | 148 (58.74%) | — | — |
| > 3 | 109 (65.27%) | 104 (41.26%) | — | — |
| pT | 0.3470 | 0.8407 | ||
| T1 | 31 (18.56%) | 46 (18.25%) | — | — |
| T2 | 91 (54.49%) | 144 (57.14%) | — | — |
| T3 | 45 (26.95%) | 62 (24.6%) | — | — |
| pN | 2.3842 | 0.0352 | ||
| N0 | 59 (35.33%) | 147 (41.67%) | — | — |
| N1–3 | 108 (64.67%) | 105 (58.33%) | — | — |
| TNM stage | 4.273 | 0.0271 | ||
| I | 49 (29.34%) | 105 (41.67%) | — | — |
| II | 86 (51.49%) | 109 (43.25%) | — | — |
| III | 32 (19.17%) | 38 (15.08%) | — | — |
| Smoking | 0.0050 | 0.9434 | ||
| Yes | 98 (58.68%) | 147 (58.33%) | — | — |
| No | 69 (41.32%) | 105 (41.67%) | — | — |
| Alcohol consumption | 0.3588 | 0.5492 | ||
| Yes | 97 (58.08%) | 155 (61.51%) | — | — |
| No | 70 (41.92%) | 97 (38.49%) | — | — |
| Chemotherapy | 0.1104 | 0.7396 | ||
| Yes | 110 (65.87%) | 162 (64.29%) | — | — |
| No | 57 (34.13%) | 90 (35.71%) | — | — |
| Radiotherapy | 0.365 | 0.0385 | ||
| Yes | 43 (25.75%) | 78 (30.95%) | — | — |
| No | 124 (74.25%) | 185 (69.05%) | — | — |
| Neutrophil count, 109 | 0.0021 | 0.9261 | ||
| ≥ 6.5 | 69 (41.32%) | 105 (41.67%) | — | — |
| < 6.5 | 98 (58.68%) | 147 (58.33%) | — | — |
| Lymphocyte count, 109 | 1.0830 | 0.2980 | ||
| ≥ 1 | 43 (25.75%) | 78 (30.95%) | — | — |
| < 1 | 124 (74.25%) | 174 (69.05%) | — | — |
NLR, neutrophil‐to‐lymphocyte ratio; TNM, tumor node metastasis.
Table 2.
Univariate analysis of prognostic factors influencing survival rate
| Characteristics | Cases | Median survival time (month) (95% CI) | Five‐year survival rate (%) | P |
|---|---|---|---|---|
| Gender | 0.1020 | |||
| Male | 328 | 26.67 (13.44, 33.32) | 65.36 | — |
| Female | 91 | 28.25 (12.44, 34.11) | 68.14 | — |
| Age (years) | 0.0646 | |||
| ≤ 65 | 152 | 32.93 (12.35, 44.25) | 53.27 | — |
| > 65 | 267 | 30.78 (11.76, 41.16) | 56.93 | — |
| Differentiation | 0.2638 | |||
| Lower | 115 | 28.83 (18.76, 35.83) | 65.27 | — |
| Middle | 219 | 26.24 (15.64, 25.56) | 62.83 | — |
| High | 85 | 25.89 (12.88, 23.52) | 59.63 | — |
| Tumor location | 0.5868 | |||
| Upper | 179 | 32.38 (19.38, 36.85) | 62.16 | — |
| Middle | 216 | 31.63 (17.38, 34.18) | 61.28 | — |
| Lower | 24 | 30.23 (15.93, 37.93) | 58.37 | — |
| Tumor diameter (cm) | 0.7060 | |||
| ≤ 3 | 298 | 33.89 (19.37, 37.12) | 70.28 | — |
| > 3 | 121 | 31.63 (17.37, 34.99) | 67.39 | — |
| pT | 0.4749 | |||
| T1 | 77 | 27.32 (13.68, 37.52) | 67.82 | — |
| T2 | 107 | 28.52 (11.61, 35.40) | 63.39 | — |
| T3 | 235 | 27.76 (9.81, 31.86) | 61.28 | — |
| pN | 0.0008 | |||
| N0 | 253 | 36.82 (19.23, 42.26) | 68.26 | — |
| N1–3 | 166 | 28.03 (15.43, 34.13) | 46.38 | — |
| TNM stage | ||||
| I | 87 | 36.28 (20.87, 48.25) | 65.12 | 0.0071 |
| II | 213 | 34.24 (17.05, 42.73) | 53.29 | — |
| III | 119 | 27.96 (13.26, 30.97) | 38.36 | — |
| Smoking | ||||
| Yes | 245 | 32.28 (17.63, 40.67) | 46.39 | 0.2345 |
| No | 174 | 34.03 (19.12, 42.23) | 52.91 | |
| Alcohol consumption | — | — | 0.1309 | |
| Yes | 252 | 31.93 (18.87, 39.46) | 59.39 | |
| No | 167 | 30.87 (20.11, 41.30) | 54.83 | |
| Chemotherapy | — | — | 0.314 | |
| Yes | 272 | 27.38 (14.35, 36.13) | 65.33 | |
| No | 147 | 26.84 (16.12, 35.60) | 55.27 | |
| Radiotherapy | — | — | 0.0236 | |
| Yes | 110 | 22.93 (12.23, 28.98) | 65.38 | |
| No | 309 | 26.38 (16.21, 34.36) | 43.47 | |
| Neutrophil count, 109 | — | — | 0.3300 | |
| ≥ 6.5 | 174 | 30.29 (23.87, 44.26) | 45.38 | |
| < 6.5 | 245 | 32.88 (25.60, 46.69) | 42.82 | |
| Lymphocyte count, 109 | — | — | 0.5957 | |
| ≥ 1 | 111 | 26.09 (13.63, 35.82) | 54.39 | |
| < 1 | 298 | 27.82 (11.04, 36.33) | 52.82 | |
| NLR | — | — | 0.0381 | |
| ≥ 2.9980 | 252 | 24.83 (20.74, 35.83) | 45.39 | |
| < 2.9980 | 167 | 28.94 (24.68, 38.96) | 41.28 |
CI, confidence interval; NLR, neutrophil‐to‐lymphocyte ratio; TNM, tumor node metastasis.
Prognostic factors
Univariate analyses showed that lymph node metastasis, TNM stage, postoperative radiotherapy, and the NLR were predictive of CSS. Multivariate analyses were performed using the Cox proportional hazards model. Multivariate analysis identified pTNM stage (P = 0.0098), lymph node metastasis (P = 0.001), and the NLR (P = 0.0022) as predictive of CSS (Tables 3).
Table 3.
Multivariate analysis of prognostic factors influencing survival rate
| Characteristics | P | HR (95% CI) |
|---|---|---|
| NLR | 0.0022 | (1.35, 3.95) |
| TNM stage | 0.0098 | (1.23, 4.56) |
| Gender | 0.5803 | (0.65, 2.14) |
| Age | 0.9428 | (0.62, 1.67) |
| Smoking | 0.8231 | (0.66, 1.72) |
| Alcohol consumption | 0.8032 | (0.66, 1.72) |
| Tumor location | 0.7491 | (0.56, 1.51) |
| Differentiation | 0.6571 | (0.64, 2.01) |
| Tumor diameter | 0.1727 | (0.86, 2.33) |
| pT | 0.1892 | (0.78, 3.55) |
| pN | 0.0010 | (1.39, 3.64) |
| Chemotherapy | 0.7705 | (0.65, 1.77) |
| Radiotherapy | 0.2740 | (0.74, 2.90) |
CI, confidence interval; NLR, neutrophil‐to‐lymphocyte ratio; TNM, tumor node metastasis.
Prognostic value of neutrophil‐to‐lymphocyte ratio
The one, three, and five‐year CSS were 82.52%, 74.27%, and 62.38% in the low NLR group and 77.37%, 56.84%, and 34.02% in high the NLR group, respectively. Patients with a high NLR had significantly poorer CSS compared to those with a low NLR (P < 0.001) (Fig 2).
Figure 2.
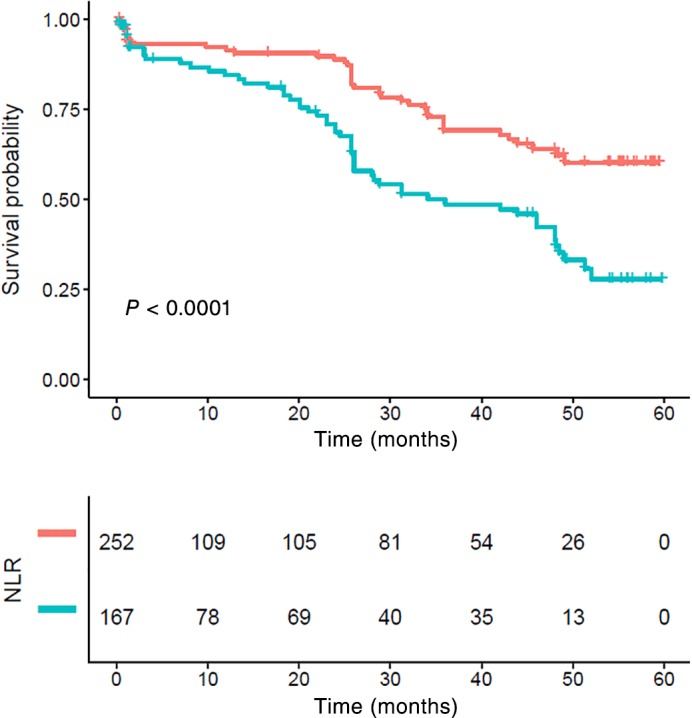
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) of the complete cohort. Patients with high NLR had significantly poorer cancer‐specific survival compared to those with lower NLR (P < 0.001). NLR ( ) <low group and (
) <low group and ( ) ≥ high group.
) ≥ high group.
Prognostic value of N stage
Patients with lymph node metastasis had significantly poorer CSS compared to those without lymph node metastasis (P < 0.001) (Fig 3). While stage N0 patients with a NLR > 2.998 also showed poor survival, the differences were not statistically significant (P = 0.067) (Fig 4). Patients who had lymph node metastasis with a high NLR had significantly poorer CSS compared to those with a low NLR (P = 0.009) (Fig 5).
Figure 3.
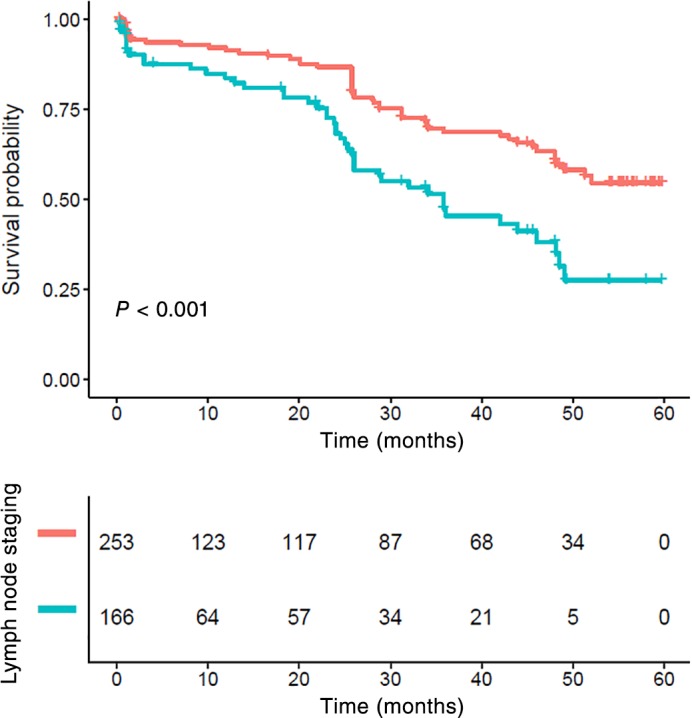
Kaplan–Meier survival curve and lymph node status of the complete cohort. Patients with lymph node metastasis had significantly poorer cancer‐specific survival compared to those without (P < 0.001). Lymph nodes staging ( ) N0 and (
) N0 and ( ) N1–3.
) N1–3.
Figure 4.
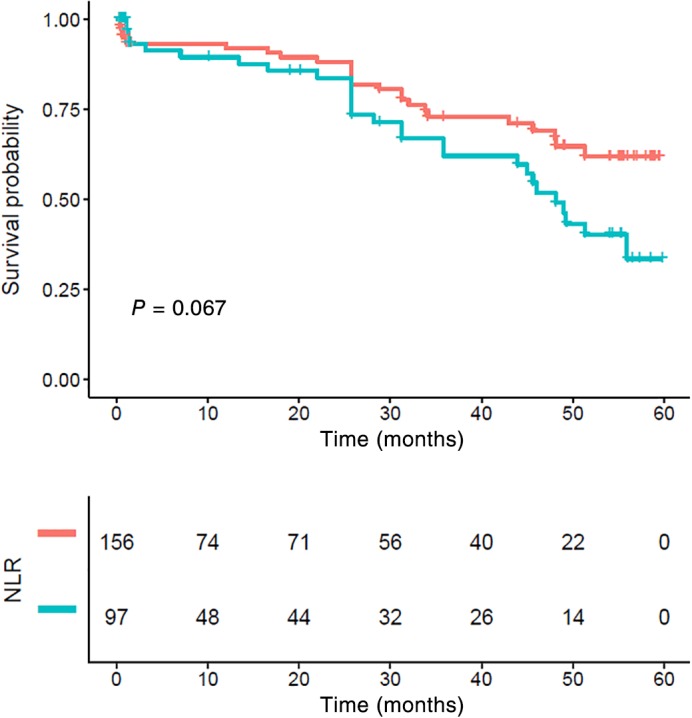
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) of N0. There were no statistically significant differences between the groups (P > 0.05). NLR ( ) <low group and (
) <low group and ( ) ≥high group.
) ≥high group.
Figure 5.
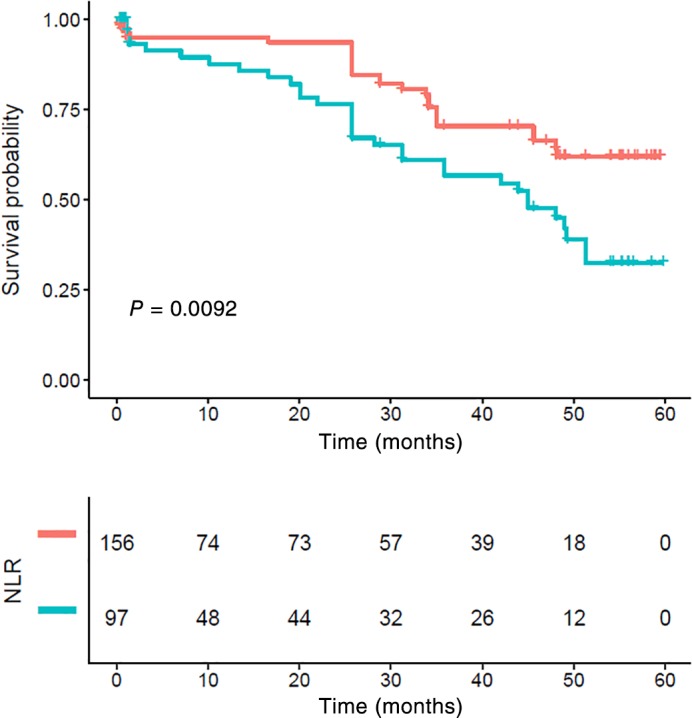
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) for N1–3. Patients with high NLR had significantly poorer cancer‐specific survival compared to those with lower NLR (P < 0.01). NLR ( ) low group and (
) low group and ( ) high group.
) high group.
Prognostic value of TNM stage
The five‐year CSS in patients with stage I, II, and III ESCC was 67.08%, 53.93%, and 49.27%, respectively (P = 0.014) (Fig 6). Stage I patients with an NLR > 2.998 also showed poor survival, but the difference was not statistically significant (P = 0.17) (Fig 7). Stage II and III patients with an NLR > 2.998 had significantly poorer CSS compared to those with a low NLR (P = 0.029) (Figs 8, 9).
Figure 6.
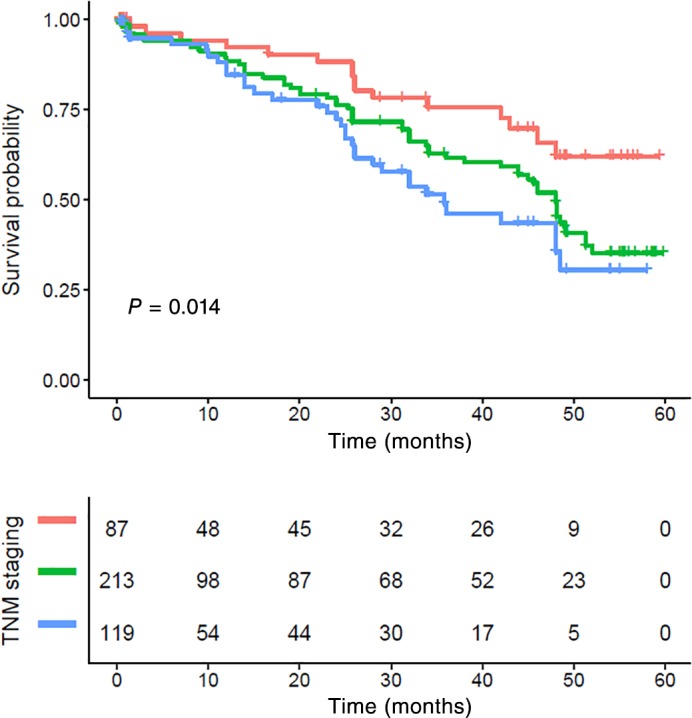
Kaplan–Meier survival curve and tumor node metastasis (TNM) staging of the complete cohort. The five‐year cancer‐specific survival rates in patients with stage I, II, and III were 67.08%, 53.93%, and 49.27%, respectively (P < 0.001). TNM staging ( ) I staging, (
) I staging, ( ) II staging, and (
) II staging, and ( ) III staging.
) III staging.
Figure 7.
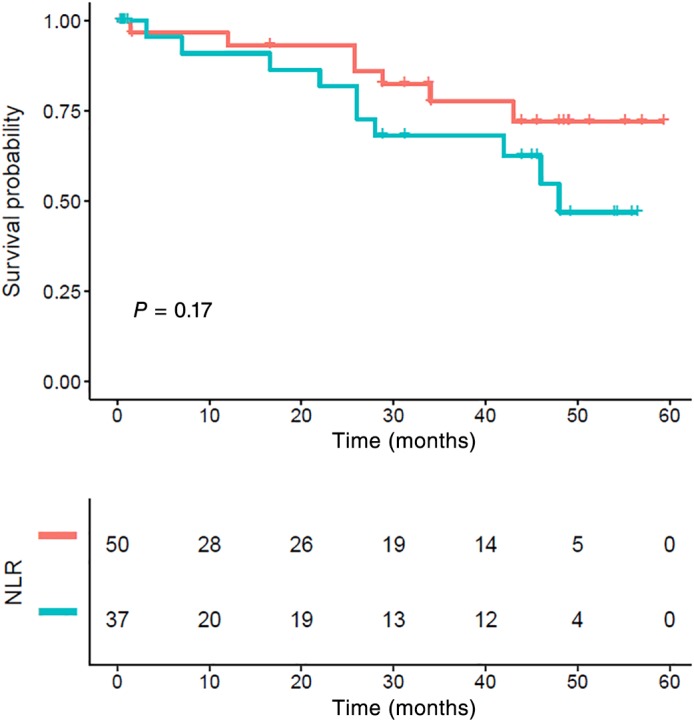
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) of stage I. There were no statistically significant differences between the groups (P > 0.05). NLR ( ) <low group and (
) <low group and ( ) ≥high group.
) ≥high group.
Figure 8.
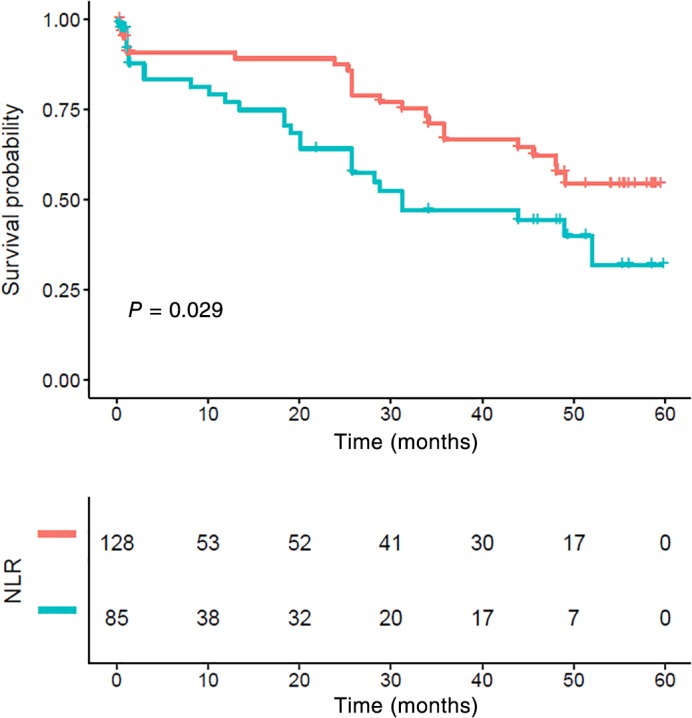
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) of stage II. Patients with high NLR had significantly poorer cancer‐specific survival compared to those with lower NLR (P < 0.001). NLR ( ) <low group and (
) <low group and ( ) ≥high group.
) ≥high group.
Figure 9.
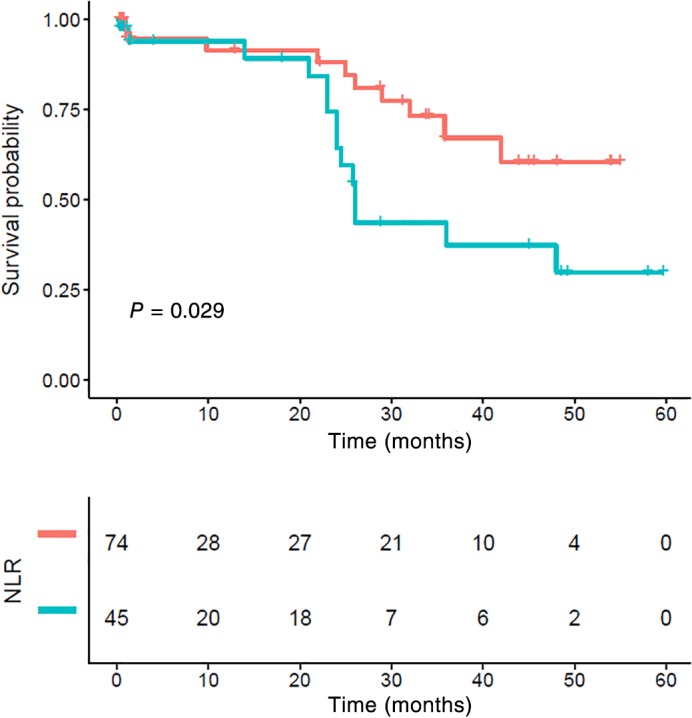
Kaplan–Meier survival curve and neutrophil‐to‐lymphocyte ratio (NLR) of stage III. Patients with high NLR had significantly poorer cancer‐specific survival compared to those with lower NLR (P < 0.001). NLR ( ) <low group and (
) <low group and ( ) ≥high group.
) ≥high group.
Discussion
Esophageal carcinoma is a highly aggressive disease with a poor prognosis and is usually fatal.14, 15 Currently, a determination of esophageal cancer prognosis is mainly based on the TNM stage, but because of tumor heterogeneity, patients at the same postoperative pathological stage administered the same treatment have different prognoses. Therefore, new indicators other than the TNM stage need to be determined to screen high‐risk patients with recurrence and metastasis, and early intervention should be implemented to improve the level of individualized treatment. The determination of esophageal cancer prognosis is changing from the detection of single biological markers to a comprehensive evaluation of multiple clinical pathological features. The NLR can be obtained from a simple routine blood test at low cost. This study found that preoperative NLR levels were significantly associated with CSS in postoperative patients with ESCC and that CSS was lower in patients with a high preoperative NLR. Multivariate analysis identified TNM stage, lymph node metastasis, and the NLR as independent prognostic factors in postoperative patients with ESCC. These results indicate that the NLR is a sensitive immune parameter for evaluating the prognosis of patients with ESCC.
As the major inflammatory cells, neutrophils are an important element in tumor progression. Neutrophils can promote tumor cell proliferation, angiogenesis, invasion, and metastasis by inhibiting T cells, secreting cytokines and other mechanisms, and have a direct killing effect or antibody‐dependent killing effect on tumor cells. Lymphocytes can prevent tumor development from immune compartments and can express recombined antigen receptors to regulate the immunosurveillance process, which can inhibit the proliferation and metastasis of tumors via cytotoxicity‐mediated cytotoxicity.16, 17, 18, 19 However, during tumor development, the inflammatory response induced by tumor cells usually inhibits the killing effect of lymphocytes, leading to immune escape. Lymphocyte reduction leads to suppression of the lymphocyte‐mediated immune response, which enhances the proliferation and metastasis of tumor cells. The lower the number of lymphocytes, the poorer the immune function, which contributes to tumor progression. However, the sensitivity of these two indicators is weak. In this study, the preoperative serum neutrophil and lymphocyte counts in most patients were in the normal range. Univariate and multivariate analyses showed that neutrophil and lymphocyte counts were not associated with CSS.
Based on these inflammatory responses, systemic inflammatory markers, which can reflect the inflammatory status of patients, such as the NLR, can predict mortality and recurrence in a variety of cancers.20, 21 Sharaiha et al. reviewed the records of 295 patients with esophageal cancer who underwent an esophagectomy and showed that the overall and disease‐free survival rates of patients with a high preoperative NLR were lower than those of patients with a low preoperative NLR, suggesting that the NLR is a sensitive index to judge the prognosis of esophageal cancer.22 Duan et al. reviewed 371 patients with esophageal cancer who underwent an esophagectomy and showed that tumor specific and recurrence‐free survival rates of patients with a high preoperative NLR were significantly lower than those of patients with a low preoperative NLR.23 Subgroup analysis showed that the NLR had the most significant predictive effect on patients at stage IIIA. This study found that the prognosis of patients with a high preoperative NLR was significantly lower than that of patients with a low preoperative NLR. We believe that the main reason for this result is that neutrophils are relatively high in patients with a high preoperative NLR, while lymphocytes are relatively low. The inflammatory response is relatively severe and the immune state is relatively poor. An increase in the inflammatory response and decrease in immune status are significant factors that accelerate ESCC progression.
The results of this study further demonstrate that the adverse effects of the NLR on CSS are greatest in patients with lymph node metastasis and stage II and III ESCC. This result suggests that the NLR is more sensitive as a prognostic factor in intermediate stage ESCC than in the other subgroups. The fact that the majority of patients in our study with an NLR > 2.998 had shorter CSS indicated that surgical resection alone was not sufficient. An evaluation of preoperative serum NLR may facilitate the selection of patients with a high risk of disease recurrence and cancer‐specific mortality to receive neoadjuvant or adjuvant therapy.
Although the results reveal the prognostic value of the preoperative serum NLR, this study had several limitations. First, our study was a single‐center retrospective analysis; therefore, the results of our study may be biased. Second, we excluded patients who had received preoperative chemotherapy and/or radiotherapy, which may have influenced our analysis. As such, further prospective studies with larger samples are required to confirm the predictive value of the preoperative NLR in postoperative patients with ESCC.
The preoperative NLR level is significantly correlated with long‐term prognosis in postoperative patients with ESCC, particularly in patients with lymph node metastasis and stage II and III ESCC. Therefore, patients with a high preoperative NLR should be followed‐up closely and intervention should be applied in a timely manner.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of Anhui Province (1708085MH179), Fundamental Research Funds for the Central Universities (WK9110000021), and the Department of Thoracic Surgery, the First Affiliated Hospital of University of Science and Technology of China (USTC), Division of Life Sciences and Medicine, USTC, Hefei City, Anhui Province, China.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 1: 25. [DOI] [PubMed] [Google Scholar]
- 2. Wang S‐M, Fan J‐H, Jia M‐M et al Body mass index and long‐term risk of death from esophageal squamous cell carcinoma in a Chinese population. Thoracic Cancer 2016; 7: 387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanemura T, Makino T, Miyazaki Y et al Distribution patterns of metastases in recurrent laryngeal nerve lymph nodes in patients with squamous cell esophageal cancer. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
- 4. Mu J‐W, Gao S‐G, Xue Q et al Comparison of short‐term outcomes and 3 year survival between total minimally invasive McKeown and dual‐incision esophagectomy. Thoracic Cancer 2017; 8: 80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oshikiri T, Yasuda T, Hasegawa H et al Short‐term outcomes and one surgeon's learning curve for thoracoscopic esophagectomy performed with the patient in the prone position. Surg Today 2017; 47: 313–9. [DOI] [PubMed] [Google Scholar]
- 6. Yang X, Huang Y, Feng JF, Liu JS. Prognostic significance of neutrophil‐to‐lymphocyte ratio in esophageal cancer: Meta‐analysis. Onco Targets Ther 2015; 8: 789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu JS, Huang Y, Yang X et al Anomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res 2015; 5: 2180–9. [PMC free article] [PubMed] [Google Scholar]
- 8. Grenader T, Plotkin Y, Mohammadi B et al Predictive value of the neutrophil‐lymphocyte ratio in peritoneal and/or metastatic disease a staging laparoscopy for gastric and esophageal adenocaroinoma. J Gastrointest Cancer 2015; 46: 267–71. [DOI] [PubMed] [Google Scholar]
- 9. Yoshimoto Y, Suzuki Y, Mimura K et al Radiotherapy‐induced anti‐tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA‐4 blockade in a mouse model. PLoS One 2014; 9: e92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baba Y, Ishimoto T, Harada K et al Molecular characteristics of basaloid squamous cell carcinoma of the esophagus: Analysis of KRAS, BRAF, and PIK3CA mutations and LINE‐1 methylation. Ann Surg Oncol 2015; 22: 3659–65. [DOI] [PubMed] [Google Scholar]
- 11. Choi WJ, Cleghorn MC, Jiang H et al Preoperative neutrophil‐to‐lymphocyte ratio is a better prognostic serum biomarker than platelet‐to‐lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol 2015; 22: 603–13. [DOI] [PubMed] [Google Scholar]
- 12. Wen RM, Zhang YJ, Ma S et al Preoperative neutrophil to lymphocyte ratio as a prognostic factor in patents with non‐metastatic renal cell carcinoma. Asian Pac J Cancer Prev 2015; 16: 3703–8. [DOI] [PubMed] [Google Scholar]
- 13. Wang SC, Chou JF, Stong VE et al Pretreatment neutrophil to lymphocyte ratio Independently predicts disease‐specific survivin resectable gastroesophageal junction and gastric adenocarcinoma. Ann Surg 2015; 263: 292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji WH, Jiang YH. Prechemotherapy neutrophil: Lymphocyte ratio is superior to the platelet: Lymphocyte ratio as a prognostic indicator for locally advanced esophageal squamous cell cancer treated with neoadjuvant chemotherapy. Dis Esophagus 2015; 10: 1–9. [DOI] [PubMed] [Google Scholar]
- 15. Diema S, Schmida S, Krapf M. Neutrophil‐to‐Lymphocyte ratio (NLR) and Platelet‐to‐Lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–81. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Li F, Ping Y et al Local production of the chemokines CCL5 and CXCL10 attracts CD8+ T lymphocytes into esophageal squamous cell carcinoma. Oncotarget 2015; 6: 24978–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu JY, Li F, Wang LP et al CTL‐ vs Treg lymphocyte‐attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with esophageal squamous cell carcinoma. Br J Cancer 2015; 113: 747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Jia Y, Wang N et al The clinical significance of tumor‐infiltrating neutrophils and neutrophil‐to‐CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med 2014; 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lv L, Pan K, Li XD et al The accumulation and prognosis value of tumor infiltrating IL‐17 producing cells in esophageal squamous cell carcinoma. PLoS One 2011; 6: e18219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakamura K, Nagasaka T, Nishida T et al Neutrophil to lymphocyte ratio in the pre‐treatment phase of final‐line chemotherapy predicts the outcome of patients with recurrent ovarian cancer. Oncol Lett 2016; 11: 3975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aino H, Sumie S, Niizeki T et al The systemic inflammatory response as a prognostic factor for advanced hepatocellular carcinoma with extrahepatic metastasis. Mol Clin Oncol 2016; 5: 83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sharaiha RZ, Halazun KJ, Mirza F et al Elevated preoperative neutrophil: Lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol 2011; 18: 3362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan H, Zhang X, Wang FX et al Prognostic role of neutrophil‐lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21: 5591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


