Abstract
Neuronal excitotoxicity induced by spreading depolarization occurs during multiple brain diseases. The subsequent extensive releasing of neuronal transmitter glutamate results in over activation of the ionic glutamate receptors and then triggers neuronal cell death. The N-methyl-D-aspartate (NMDA) receptor is one major type of excitatory ionic glutamate receptors in the central nervous system, and it exerts vital functions on the membrane of neurons. Distinct subtypes of the NMDA receptor play different roles and their expression was dynamically regulated according to both physiological and pathological stimulations. During neuronal excitotoxicity the expression of the GluN2A-containing NMDA receptor is specifically up-regulated, and as a result, the ratio of GluN2A- versus GluN2B-containing NMDA receptors is altered. However the physiological significance of this phenomenon is still not clear. In this research, we specifically inhibited the increase of the GluN2A-containing NMDA receptor by a peptide without affecting the basic expression of both GluN2A- and GluN2B-containing NMDA receptors, and found that the oxidative stress of neurons was intensified, with increased endogenous reactive oxygen species (ROS), loss of mitochondrial membrane potential, and elevated expressions of Bcl-2-associated X protein (Bax) and apoptosis-inducing factor (AIF). Furthermore, the phosphorylation of Akt and ERK were also inhibited. These results indicated that the dynamic expression of the GluN2A-containing NMDA receptor played crucial roles in protecting neurons from excitotoxicity.
Keywords: Biochemistry, Cell biology, Molecular biology, Neuroscience, Physiology
1. Introduction
Neuronal excitotoxicity can occur during multiple brain diseases such as epilepsy, cerebral ischemia, Alzheimer's disease, multiple sclerosis, amyotrophic lateral sclerosis, as well as acute traumatic brain injury or spinal cord injury [1, 2, 3]. During this pathological process extensive amount of glutamate was released as the consequence of spreading depolarization, and resulted in over-activation of excitatory glutamatergic receptors which may cause cell death [2, 4, 5].
The NMDA receptor is a primary kind of excitatory ionic glutamate receptor in the central nervous system which participates in the neuronal excitotoxicity [6, 7, 8]. NMDA receptors expressed in frontal cortex and hippocampus are usually composed of two GluN1 subunits and two GluN2A or GluN2B subunits, and thus can be divided into two subtypes, the GluN2A-containing NMDA receptor and the GluN2B-containing NMDA receptor [9, 10]. The two subtypes exhibit different properties and perform distinct functions during excitotoxicity. It has been suggested that the GluN2A-containing NMDA receptor which mainly located within synapse couples to neuronal survival, whereas the extrasynaptic located GluN2B-containing NMDA receptor relates to neuronal death [11, 12]. This is partly due to the fact that the intracellular C-terminus of GluN2A and GluN2B coupled to different signaling pathways and that the influx of Ca2+ through these two NMDA receptor subtypes from different sub-regions of neurons activate diverse Ca2+-dependent downstream events [5, 13, 14].
Another reason that the NMDA receptor is a major mediator of excitotoxicity is because its activation leads to elevated intracellular Ca2+ which was buffered by mitochondria. Ca2+ overload depolarizes the mitochondrial membrane and initiate the oxidative stress mediated by mitochondria pathway [15, 16]. Although that all the ionic glutamate receptors are permeable to calcium but individual type of receptors (NMDA and non-NMDA) seems played different roles in oxidative stress, and NMDA receptor caused a stronger mitochondrial depolarization and ROS generation compared with kainite (KA) receptor [8]. However the contribution of different subtypes of the NMDA receptor in oxidative stress was not clearly investigated. More importantly, Zhao et al, recently found that the depolarization of neurons during ischemia stroke would specifically up-regulate the GluN2A-containing NMDA receptors [17], but whether this selective and dynamic regulation of the GluN2A-containing subtype potentiates neuronal protection or neuronal death is still not clear.
In this work we studied this problem by specifically inhibit the dynamic increase of the GluN2A-containing NMDA receptor after depolarization. Our results confirmed that the surface expression of the GluN2A-containing NMDA receptor could be selectively enhanced during depolarization induced excitotoxicity of the culture cortical neurons, and we also found that inhibition of this increment intensified the oxidative stress through caspase independent mitochondria pathway. Our data indicated a novel function of the GluN2A-containing NMDA receptor in excitotoxicity.
2. Methods
2.1. Primary cortical neuron culture and transfection
Primary cortical neuron cultures were prepared from E18 rats following the protocol described previously [18]. Neurons were transfected at DIV10 with GFP-GluN2A or GFP-GluN2B plasmids respectively using Lipofectamine LTX with PLUS (Invitrogen). Plasmids were first mixed with PLUS and then mixed with Lipofectamine LTX and incubated for 30 min at room temperature. The final mixture was added to cortical neuron culture for 3 hr. 2 μg of plasmid was used for each 35 mm dish. The transfected neurons were used for experiments at DIV14.
2.2. Pharmacological treatment
At DIV 14 the neurons were rinsed three times in ECS and incubated in high K+ solution (90 mM KCl) for 5, 10 or 20 min. After that, the neurons were rinsed by pre-cold ECS twice and fixed in 4% paraformaldehyde for further staining or Western blot test. APV (20 μM) was pre-incubated with the neurons for 2 hr before depolarization to block the activity of the NMDA receptor. And PEP-I (10 μM) was pre-incubated with the neurons for 2 hr before depolarization. PEP-I was derived from the ATD domain of the GluN2A subunit and its sequence was as followed: YGRKKRRQRRRRSLGLTGYDFF.
2.3. Neuron staining
Cortical neurons plated on slides were rinsed three times in ECS, fixed in 4% paraformaldehyde for 5 min, and blocked with 5% BSA in ECS for 10 min. For surface staining, the neurons were incubated in rabbit anti-GFP primary antibody (Abcam, 1:500) for 15 min, rinsed three times in ECS, and incubated with Alexa-546-conjugated secondary antibody (Abcam, 1:1 000) for another 15 min. For intracellular staining, next the neurons were blocked and permeabilized with 0.1% Triton X-100 in PBS containing 5% BSA, then incubated in primary and secondary antibodies for 1 hr each with three times of rinse. Then the neurons were then examined under a 60×, 1.4 numerical aperture oil-immersion objective on an Olympus confocal microscope equipped with FV1000 software. Surface and intracellular staining were analyzed using Metamorph 5.0 software, and the ratio of surface/intracellular signals after stimulation was normalized to the ratio in transfected neurons before stimulation.
2.4. Protein extraction
The proteins were extracted as previously described [18]. The cortical cultures were rinsed three times in ECS, lysed with RIPA buffer (50 mM Tris.HCl, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.1 mM PMSF, 1 mM aprotinin, pH 7.4) and collected in 1.5 ml effendorp tubes, and then the neurons were ultrasonicated twice for 8 sec each followed by incubation at 4 °C for 2 hr. Samples were then centrifuged at 12 000× g for 10 min at 4 °C and the supernatant was used for SDS PAGE.
2.5. Measurement of endogenous ROS
The formation of endogenous ROS was detected using the non-fluorescent probe DCFH-DA (Invitrogen). DCFH-DA was applied to the cultured cortical neurons at DIV14 at a final concentration of 10 μM and incubated with neurons for 20 min at 37 °C. Then the fluorescent was measured by Zeiss living cell imaging system.
2.6. Surface biotinylation
Cortical cultures were rinsed twice with ice cold PBS (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, pH 7.4) with 1 mM MgCl2 and 0.5 mM CaCl2, then incubated with biotin (1 mg/ml sulfo-NHS-LC-biotin in PBS) for 30 min at 4 °C. 100 μM glycine in PBS was added to quench the biotin binding for 15 min at 4 °C. Cells were then lysed in ice-cold RIPA buffer after rinsing twice with PBS, and incubated at 4 °C for 30 min. Cells were concentrated at 12 000× g for 10 min, and incubated with NeutrAvidin beads (Thermo Fisher Scientific) for 2 hr at 4 °C. The beads were then washed three times with RIPA buffer, and proteins were extracted with 2× sample buffer and boiled at 100 °C for 10 min before use for western blot.
2.7. JC-10 mitochondrial membrane potential assay
Mitochondrial membrane potential was measured according to the manufacturer's instruction (Sigma). Briefly, cortical neurons were culture in 96-well plates until DIV 14. Thirty minutes before the end of the treatment, 50μl/well of JC-10 Dye Loading Solution was added into each well, and the cells were incubated at 37 °C and protected from light for 30 min. Another 50μl/well of Assay Buffer B was added into each well. The fluorescence intensity of monomer (Ex/Em = 490/525) and aggregator (Ex/Em = 540/590) was measured. And the ratio between aggregate (Em = 525) and monomeric forms (Em = 590) was calculated to determine mitochondrial membrane potential. An increase of the ratio indicates mitochondrial membrane depolarization.
2.8. Statistical analysis
All data were analyzed using GraphPad prism 5 software and are represented as mean ± SEM. Data from multiple groups were quantified using one-way ANOVA, and comparisons of two groups were quantified by the student's t-test.
3. Results
3.1. Depolarization induced a selective increase of the GluN2A-containing NMDA receptor
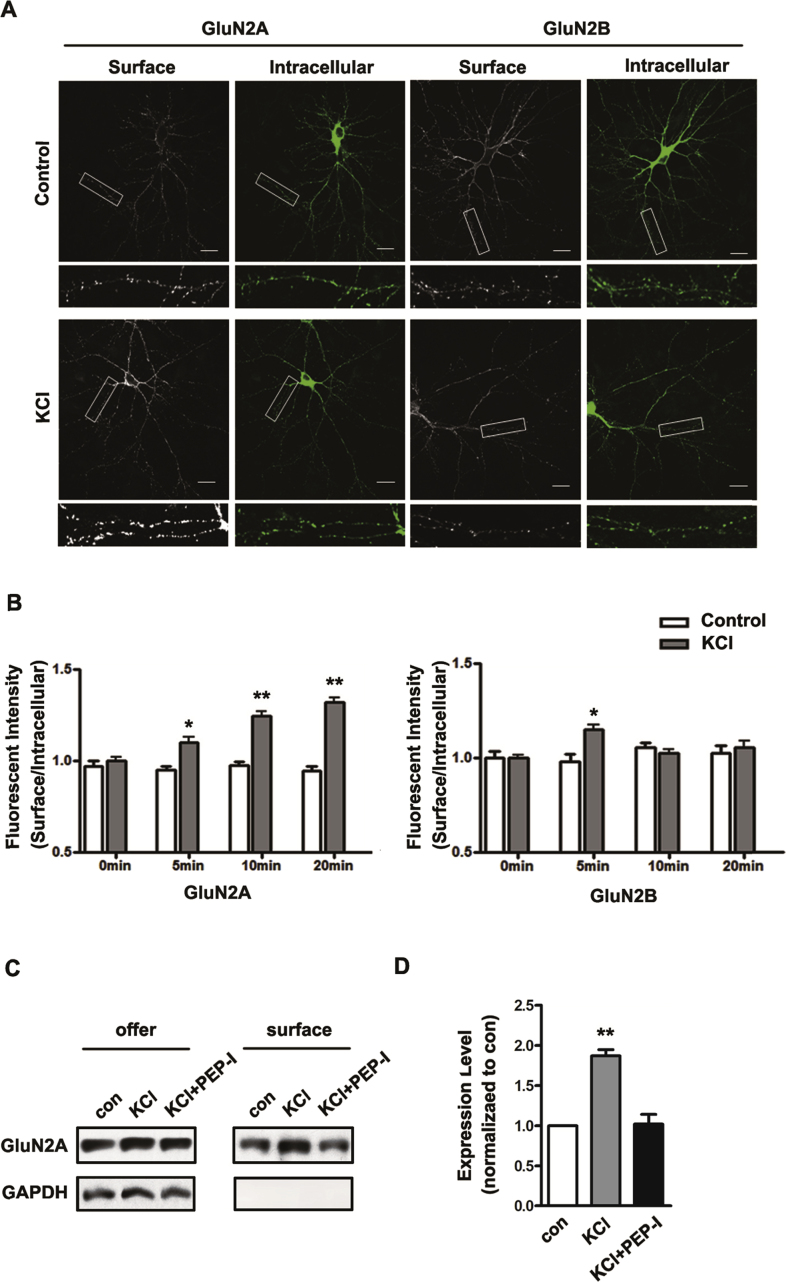
We first examined the surface expression of the GluN2A-containing NMDA receptor after bath incubating cultured cortical neurons in high K+ solution (90mM KCl) for 5, 10 and 20 min at DIV 14, which is accepted as a chemical method to induce depolarization of neurons [19, 20]. The surface expressions of the GluN2A- and GluN2B-containing NMDA receptors were measured by immunocytochemistry. And we found that GluN2A increased continuously while GluN2B increased transiently at 5 min and then decreased to the basal level (Fig. 1A and B). To confirm this result, we further evaluated the membrane fraction of GluN2A by surface biotinylation 20 min after depolarization and again we found the surface expression of GluN2A significantly increased (Fig. 1C and D). Consistent with other in vivo studies [17], these data showed distinct expression pattern of the GluN2A- and GluN2B- containing NMDA receptor after depolarization. However in this study, we are interested in the impact of this dynamic expression of GluN2A-containing NMDA receptor on excitotoxicity induced by depolarization thus we inhibited the increase of GluN2A with a formerly developed peptide, while the NMDA receptor non-selective antagonist APV was used as a comparing manipulation other than control treatment.
Fig. 1.
Depolarization of cortical neurons by KCl increased the surface expression of the GluN2A-containing NMDA receptor. A. The GFP-GluN2A or GFP-GluN2B was transfected into cultured cortical neurons at DIV 10, and neurons were depolarized by 90 mM KCl at DIV14. The surface fraction and intracellular fraction of GFP-GluN2A and GFP-GluN2B were shown by surface staining (Red) and transmembrane staining (Green). The scale bar represents 20μm. B. The ratio of surface fraction and intracellular fraction of GFP-GluN2A and GFP-GluN2B was measured continuously (at 5 min, 10 min, 15 min and 20 min) after depolarization (n = 30). All data are presented as mean ± SEM. *P < 0.05, **P < 0.01. C. The membrane expression of GluN2A was measured by surface biotinylation at 20 min after ECS (con), KCl or KCl with PEP-I treatment. Full, non-adjusted blots are available in Supplementary Fig. 1. All data are presented as mean ± SEM. **P < 0.01. D. The statistical analysis of membrane expression GluN2A after 20 min incubation with ECS (con) KCl or KCl with PEP-I treatment. All data are presented as mean ± SEM. **P < 0.01.
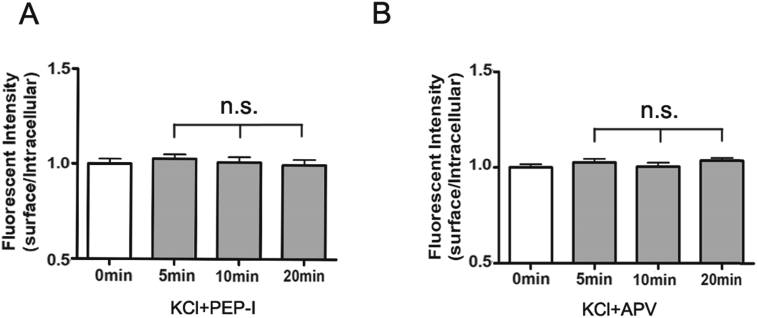
In our former study, we developed a peptide (PEP-I) derived from the ATD domain of the GluN2A subunit which locates at the interaction interface between GluN2A and Bip. Since this interaction is required for the maturation and assembly of GluN2A in the endoplasmic reticulum, this peptide can inhibit the dynamic synaptic increase of GluN2A under neuronal plasticity stimulus without affecting its basic membrane expression [18]. We first evaluated whether the peptide was also effective in our current depolarization model of cultured cortical neurons. We incubated the cortical neurons with PEP-I for 2 hr before depolarization and then measured the expression of the GluN2A-containing NMDA receptor by immunocytochemistry. As a result, we found no change of the GluN2A expression before or after depolarization (Fig. 1C and D, Fig. 2A) which indicated that the dynamic trafficking of the GluN2A-containing NMDA receptor was effectively inhibited by PEP-I. And we further found that pre-incubation with APV for 2 hr also inhibited the membrane increase of GluN2A (Fig. 2B), this result suggested that the depolarization-induced dynamic expression of GluN2A depends on the activity of NMDA receptor and also indicated that APV could be used as a comparing manipulation since it not only inhibits the expression of GluN2A but also blocks the overall activity of the NMDA receptor.
Fig. 2.
The increasing of the surface expression of the GluN2A-containing NMDA receptor after depolarization could be abolished by PEP-I and NMDA receptor antagonist APV. A. The cultured cortical neurons were incubated with the inhibiting peptide (PEP-I) for 2 hr before depolarization. And the surface expression changes of GFP-GluN2A were measured continuously (at 0 min, 5 min, 10 min and 20 min) after depolarization (n = 30). All data are presented as mean ± SEM. n.s. means no significant differences. B. The cultured cortical neurons were incubated with APV for 2 hr before depolarization. And the surface expression changes of GFP-GluN2A were measured continuously (at 0 min, 5 min, 10 min and 20 min) after depolarization (n = 30). All data are presented as mean ± SEM. n.s. means no significant differences.
3.2. Depolarization resulted in the oxidative stress through mitochondria pathway which was intensified by PEP-I
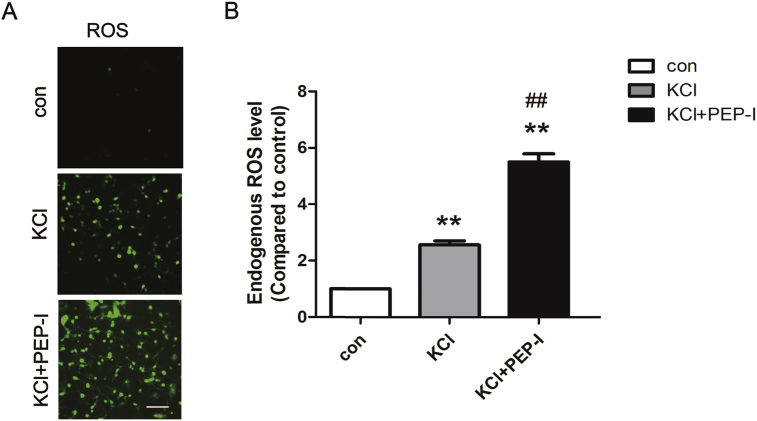
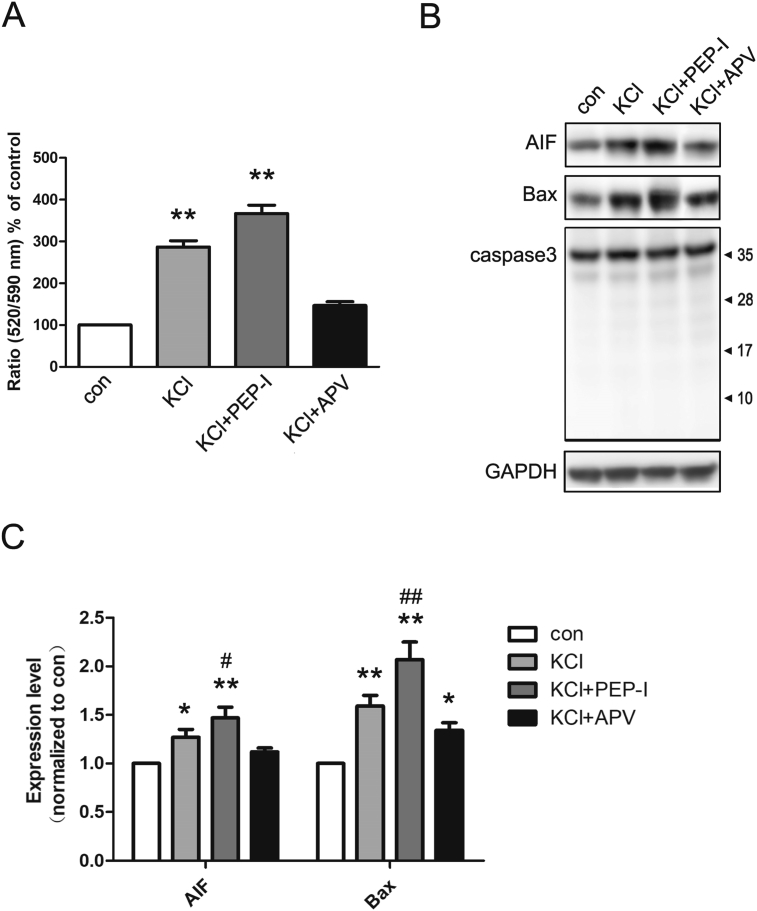
Next, we explored the function of the GluN2A-containing NMDA receptor in oxidative stress of neurons induced by depolarization. We first measured the endogenous ROS level after depolarization and we found ROS increased significantly while inhibiting the surface increase of the GluN2A-containing NMDA receptor by PEP-I resulted in a further increase of ROS compared with the depolarized neurons (Fig. 3A and B). Since the increase of endogenous ROS was resulted from the malfunction of mitochondria [21, 22], we next evaluated the status of mitochondria by measuring its membrane potential and we found the ratio of monomer/aggregator of JC 10 was elevated after depolarization while PEP-I incubation group showed a highest ratio compared with the control and KCl treatment group which suggested a stronger mitochondrial depolarization (Fig. 4A). We also measured the expression of Bax and AIF which participate in mitochondria mediated oxidative stress [2, 23]. We found both Bax and AIF increased significantly after depolarization of neurons for 20 min which further suggested an impaired function of mitochondria after depolarization. And consistent with former results, the expressions of Bax and AIF also increased after PEP-I incubation (Fig. 4B and C). Because it has been suggested that during oxidative stress and the following apoptosis, AIF functions independent on caspase, therefore we evaluated the cleavage of caspase 3. And as a result, no obvious cleavage of caspase 3 was seen after depolarization or PEP-I treatment (Fig. 4B and C). These data revealed that the oxidative stress induced by depolarization was mediated by caspase-independent mitochondria pathway and requires the activity of the NMDA receptor, whereas the dynamic increasing of the membrane GluN2A-containing NMDA receptor during this process was triggered to protect neurons from apoptosis.
Fig. 3.
Increased endogenous ROS generation after depolarization was intensified by PEP-I pre-incubation. A. The total endogenous ROS were measured 20 min after depolarization by live cell imaging. The scale bar represents 100 μm. B. The statistical analysis of the total ROS generation (n = 6).The pre-incubation of neurons with PEP-I for 2hr before depolarization further increased the endogenous ROS generation. All data are presented as mean ± SEM. **P < 0.01, compared to control, ##P < 0.01, compared to KCl treatment.
Fig. 4.
Depolarization induced oxidative stress in cultured cortical neurons. A. The mitochondrial membrane potential was measured in control, KCl, KCl + PEP-I and KCl + APV groups (n = 6). The ratio of JC-10 aggregated (Em = 525) and monomeric (Em = 590) form was measured. The ratio significantly increase after KCl or KCl + PEP-I treatment indicating depolarization of the mitochondrial membrane. *P < 0.05, **P < 0.01 compared to control. #P < 0.05, ##P < 0.01 compared to KCl treatment. B. Representative blots of the expression of AIF, Bax, caspase 3 and GAPDH at DIV 14 in control, KCl, KCl + PEP-I and KCl + APV groups. GAPDH was used as negative control. The expression of AIF and Bax increased after KCl and KCl + PEP-I incubation. C. The statistical analysis of AIF and Bax, normalized to GAPDH (n = 6). The expression of AIF and Bax increased significantly after depolarization. Pre-incubating with PEP-I for 2 hr resulted in a further increase of Bax and AIF expression. No cleavage of caspase 3 was found in all four groups. *P < 0.05, compared to control. #P < 0.05, ##P < 0.01 compared to KCl treatment. Full, non-adjusted blots are available in Supplementary Fig. 1.
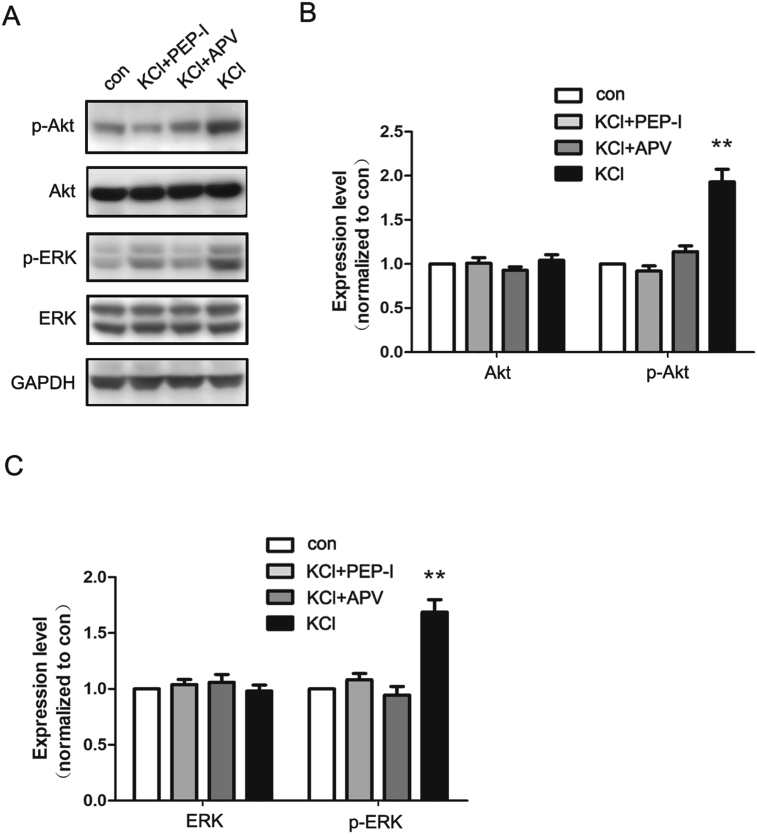
3.3. Depolarization activated Akt and ERK pathway mediated by the GluN2A-containing NMDA receptor
Through previous experiments, we have found that inhibiting the increase of the GluN2A-containing NMDA receptor intensified the oxidative stress of neurons during excitotoxicity which suggested a protective role of GluN2A. To confirm this result, we further investigated the pro-survival signaling pathways coupled to GluN2A. We evaluated the expression and phosphorylation of Akt and ERK after depolarization with and without PEP-I or APV treatment. We found that both the phosphorylation of Akt and ERK increased after depolarization, and were fully blocked by both PEP-I or APV (Fig. 5A, B and C). And there was no significant difference between the inhibitory effects of PEP-I and APV. Therefore, these data showed that the increased expression of the GluN2A-containing NMDA receptor exerted crucial protective role through activating Akt and ERK, and the strengthened oxidative stress after PEP-I treatment might be caused by the inhibition of these two kinase pathways.
Fig. 5.
Depolarization induced activation of Akt and ERK which could be inhibited by both APV and PEP-I. A. Representative blots of the expression of total Akt, phosphorylated Akt (p-Akt), total ERK, phosphorylated ERK (p-ERK) and GAPDH at DIV 14 after incubation with ECS, KCl, KCl with APV or KCl with PEP-I for 20 min. GAPDH was used as negative control. B. The statistical analysis of Akt and p-Akt. The expression of Akt was normalized to GAPDH first can then to control. The expression of p-Akt was normalized to Akt. Both APV and PEP-I could inhibit the phosphorylation of Akt induced by depolarization (n = 6). **P < 0.01. C. The statistical analysis of ERK and p-ERK. The expression of ERK was normalized to GAPDH first can then to control. The expression of p-ERK was normalized to ERK. Both APV and PEP-I could inhibit the phosphorylation of ERK induced by depolarization (n = 6). **P < 0.01.
4. Discussion
The spreading depolarization occurs during multiple brain diseases, it causes an excessive activation of ionic glutamatergic receptors and leads to excitotoxicity [24]. The NMDA receptor is one of the prominent ionic glutamatergic receptors that participate in this pathological process. Because the Ca2+ entry was the major mediator of excitotoxicity and the NMDA receptor was the primary source of the Ca2+ during glutamate exposure [25, 26, 27], the NMDA receptor antagonist is able to attenuated the glutamate neurotoxicity caused by depolarization [27]. Consistently we found in our study that acute application of APV attenuated the mitochondrial membrane depolarization and inhibited the mitochondria-mediated apoptosis, these data suggest an eliminating of oxidative stress during excitotoxicity by inhibiting the activity of NMDA receptors. However a large amount of in vivo studies have shown that broad inhibition of the NMDA receptor could enhance neuronal injury and even lead to neuronal death because the NMDA receptor plays dual roles during excitotoxicity, both pro-death and pro-survival [28]. Consistently we found in our study that the pro-survival pathway was inhibited by APV. This controversy is called “NMDA paradox” and one explanation for this phenomenon is that the NMDA receptor located in the synaptic or extrasynaptic region of neurons couples with pro-survival or pro-death pathway separately [29]. And because the GluN2A-containing NMDA receptors primarily located within the synapse while GluN2B-containing NMDA receptors mainly in the extrasynaptic area, an alternative explanation is that the different subtypes of the NMDA receptor play separate roles during excitotoxicity that one triggers neuronal protection and the other one mediates neuronal apoptosis [28].
In addition, the subunit composition of the NMDA receptor is dynamically regulated according to both physiological and pathological stimulations [17, 30, 31]. It is well acknowledged that the plasticity of the NMDA receptor composition exhibits critical functions in the cognitive development, metaplasticity and NMDA receptor-mediated long term potentiation (LTPNMDA) [10, 28, 30], however its role in excitotoxicity is not well studied. In our current study, in order to address this problem a peptide (PEP-I) derived from the ATD of GluN2A was used. In our former research we identified the interacting interface between GluN2A and Bip and designed a peptide that was able to disrupt the binding of GluN2A to Bip during its synthesis [18]. Acute application of this peptide to cultured neurons could interfere with the dynamic trafficking of GluN2A. As a result, the dynamic increase of the GluN2A-containing NMDA receptor on the neuronal membrane was eliminated while the basic expression of both NMDA receptor subtypes was not changed. We found that if the neurons were incubated with PEP-I before depolarization, then the oxidative stress was significantly intensified. We tested the mitochondrial pathway of apoptosis induced by Ca2+ overload and found strengthened mitochondrial depolarization, elevated intracellular level of ROS, and increased expression of Bax and AIF. However the cleavage of caspase 3 was not found after depolarization or PEP-I and APV treatment which consistent with the hypothesis that AIF could function as a caspase-independent death effector [32], and our data also confirmed that the NMDA receptor mediated excitotoxicity is mitochondrial dependent but caspase independent. Furthermore, since the basic expression of GluN2A-containing NMDA receptor was not altered by the peptide, whereas both the Akt and ERK pathways were inhibited by PEP-I to the level that was similar to the effect of APV, it seems that the dynamic expression change of GluN2A is required for the activation of pro-survival signaling pathways in neurons. Thus our data for the first time proved that the increased portion of GluN2A during excitotoxicity is critical for the neuronal protection, and also proved the importance of the dynamic trafficking of GluN2A during excitotoxicity. Moreover because the NMDA receptor could mediate both pro-death and pro-survival signaling cascades, the low-affinity, broad-spectrum NMDA receptor antagonists usually show only modest clinical benefits. Our data also showed that the function of PEP-I and APV were different in oxidative stress, these results indicated that more specific targeting therapeutic strategies are required. Although our peptide caused more severe injury to neurons during excitotoxicity, it could still be a possible therapeutic device in other potential pathological processes mediated by the GluN2A-containing NMDA receptor.
In conclusion, we found in this study that the surface expression of the GluN2A-containing NMDA receptor was elevated during strong depolarization of cultured cortical neurons and we proved for the first time that this dynamic trafficking of this NMDA receptor subtype was critical for the neuroprotection during excitotoxicity.
Declarations
Author contribution statement
Jia Zhu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Shilian Xu: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Shengpei Li: Performed the experiments; Analyzed and interpreted the data.
Xueling Yang, Xianhui Yu: Performed the experiments.
Xiaomin Zhang: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Natural Science Foundation of China (Grant No. 31460258 to Xiaomin Zhang, 31360245 to Shilian Xu, and 81501043 to Jia Zhu), One hundred talented young scholars programme of Kunming Medical University (Grant No. 60117190404 to Xiaomin Zhang), and Foundation of Science and Technology innovative team building of Kunming Medical University (Grant No. CXTD201807 and No. CXTD 201601 to Xiaomin Zhang).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999 doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 2.Sevrioukova I.F. Apoptosis-inducing factor: structure, function, and redox regulation. Antioxid. Redox Signal. 2011 doi: 10.1089/ars.2010.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bano D., Ankarcrona M. Beyond the critical point: an overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci. Lett. 2018 doi: 10.1016/j.neulet.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 4.Choi D.W. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci. 1995 [PubMed] [Google Scholar]
- 5.Baxter P.S., Bell K.F.S., Hasel P., Kaindl A.M., Fricker M., Thomson D., Cregan S.P., Gillingwater T.H., Hardingham G.E. Synaptic NMDA receptor activity is coupled to the transcriptional control of the glutathione system. Nat. Commun. 2015 doi: 10.1038/ncomms7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frandsen A., Drejer J., Schousboe A. Direct evidence that excitotoxicity in cultured neurons is mediated via N-Methyl-D-Aspartate (NMDA) as well as non-NMDA receptors. J. Neurochem. 1989 doi: 10.1111/j.1471-4159.1989.tb07327.x. [DOI] [PubMed] [Google Scholar]
- 7.Hardingham G.E., Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003 doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 8.Sanganahalli B.G., Joshi P.G., Joshi N.B. NMDA and non-NMDA receptors stimulation causes differential oxidative stress in rat cortical slices. Neurochem. Int. 2006 doi: 10.1016/j.neuint.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Cull-Candy S., Brickley S., Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001 doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 10.Yashiro K., Philpot B.D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wyllie D.J.A., Livesey M.R., Hardingham G.E. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X., Chen Z., Yun W., Ren J., Li C., Wang H. Extrasynaptic NMDA receptor in excitotoxicity: function revisited. Neuroscientist. 2015 doi: 10.1177/1073858414548724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardingham G.E. Pro-survival signalling from the NMDA receptor. Biochem. Soc. Trans. 2006 doi: 10.1042/BST0340936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Wong T.P., Aarts M., Rooyakkers A., Liu L., Lai T.W., Wu D.C., Lu J., Tymianski M., Craig A.M., Wang Y.T. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007 doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polster B.M., Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J. Neurochem. 2004 doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- 16.a Waxman E., Lynch D.R. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005 doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C., Du C.P., Peng Y., Xu Z., Sun C.C., Liu Y., Hou X.Y. The upregulation of NR2A-containing N-Methyl-d-Aspartate receptor function by tyrosine phosphorylation of postsynaptic density 95 via facilitating Src/proline-rich tyrosine kinase 2 activation. Mol. Neurobiol. 2015 doi: 10.1007/s12035-014-8796-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X.M., Yan X.Y., Zhang B., Yang Q., Ye M., Cao W., Bin Qiang W., Zhu L.J., Du Y.L., Xu X.X., Wang J.S., Xu F., Lu W., Qiu S., Yang W., Luo J.H. Activity-induced synaptic delivery of the GluN2A-containing NMDA receptor is dependent on endoplasmic reticulum chaperone Bip and involved in fear memory. Cell Res. 2015 doi: 10.1038/cr.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreier J.P., Major S., Pannek H.W., Woitzik J., Scheel M., Wiesenthal D., Martus P., Winkler M.K.L., Hartings J.A., Fabricius M., Speckmann E.J., Gorji A. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain. 2012 doi: 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farkas E., Pratt R., Sengpiel F., Obrenovitch T.P. Direct, live imaging of cortical spreading depression and anoxic depolarisation using a fluorescent, voltage-sensitive dye. J. Cereb. Blood Flow Metab. 2008 doi: 10.1038/sj.jcbfm.9600569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budd S.L., Tenneti L., Lishnak T., Lipton S.A. Mitochondrial and extramitochondrial apoptotic signaling pathways in cerebrocortical neurons. Proc. Natl. Acad. Sci. 2000 doi: 10.1073/pnas.100121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls D.G., Budd S.L. Mitochondria and neuronal survival. Physiol. Rev. 2000 doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 23.Polster B.M. AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem. Int. 2013 doi: 10.1016/j.neuint.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siesjö B.K., Zhao Q., Pahlmark K., Siesjö P., ichiro Katsura K., Folbergrová J. Glutamate, calcium, and free radicals as mediators of ischemic brain damage. Ann. Thorac. Surg. 1995 doi: 10.1016/0003-4975(95)00077-x. [DOI] [PubMed] [Google Scholar]
- 25.Choi D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987 doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi D.W., Maulucci-Gedde M., Kriegstein A.R. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987 doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi D.W., Koh J.Y., Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J. Neurosci. 1988 doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013 doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 29.Hardingham G.E., Fukunaga Y., Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002 doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 30.Dumas T.C. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog. Neurobiol. 2005 doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y., Zhao J., Gu Q.H., Chen R.Q., Xu Z., Yan J.Z., Wang S.H., Liu S.Y., Chen Z., Lu W. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus. 2010 doi: 10.1002/hipo.20654. [DOI] [PubMed] [Google Scholar]
- 32.Candé C., Cohen I., Daugas E., Ravagnan L., Larochette N., Zamzami N., Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002 doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.