Abstract
Background
Antimicrobial prophylaxis is indicated to prevent Pneumocystis jirovecii pneumonia (PJP) in profoundly immunosuppressed children. The incidence of PJP infection in children with chronic glucocorticoid exposure is unknown, and PJP prophylaxis has been associated with adverse events. We hypothesized that PJP infection is rare in children without human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), cancer, or a transplant history who are using chronic glucocorticoids and that those exposed to PJP prophylaxis are more likely to experience a cutaneous hypersensitivity reaction or myelosuppression than unexposed patients.
Methods
This study involved a retrospective cohort from the Clinformatics Data Mart Database (OptumInsight, Eden Prairie, MN). We identified patients ≤18 years of age who received at least 2 prescriptions for a systemic glucocorticoid within a 60-day period and excluded patients with a history of PJP infection, an oncologic diagnosis, transplant, or HIV/AIDS. PJP prophylaxis exposure was identified by using national drug codes. Cutaneous hypersensitivity reaction or myelosuppression was identified by using International Classification of Diseases, 9th Revision (ICD-9), codes. We used a discrete time-failure model to examine the association between exposure and outcome.
Results
We identified 119399 children on glucocorticoids, 10% of whom received PJP prophylaxis. The incidences of PJP were 0.61 and 0.53 per 10000 patient-years in children exposed and those unexposed to PJP prophylaxis, respectively. In a multivariable model, trimethoprim-sulfamethoxazole was associated with cutaneous hypersensitivity reaction (odds ratio, 3.20; 95% confidence interval, 2.62–3.92) and myelosuppression (odds ratio, 1.85; 95% confidence interval, 1.56–2.20).
Conclusions
PJP infection was rare in children using glucocorticoids chronically, and PJP prophylaxis–associated cutaneous hypersensitivity reactions and myelosuppression are more common. The use of PJP chemoprophylaxis in children without HIV/AIDS, cancer, or a transplant history who are taking glucocorticoids chronically should be considered carefully.
Keywords: chronic glucocorticoids, opportunistic infections, pharmacoepidemiology, Pneumocystis jirovecii
Children with a chronic inflammatory disease often require immunosuppression, including glucocorticoid therapy [1–3]. For example, approximately 50% of children with inflammatory bowel disease [4] and 30% of children with nephrotic syndrome [5] are classified as steroid dependent, and children with juvenile dermatomyositis can receive glucocorticoids for 2 years or more as part of their induction [1] and might remain on therapy longer if their disease is refractory [6]. For such patients, clinicians must balance the benefits of these agents with glucocorticoid-related adverse effects, the most concerning of which are opportunistic infections [7, 8].
Pneumocystis jirovecii pneumonia (PJP) is an opportunistic infection that can be devastating in immunosuppressed children, particularly among those with human immunodeficiency virus (HIV) [9], cancer [10], or the risk after organ transplantation [11]. Chemoprophylaxis against PJP with antibiotics is effective in these patients; in 1 study, an attributed 85% incidence rate reduction in pediatric transplant and oncology patients using trimethoprim-sulfamethoxazole (TMP-SMX) was found [12]. However, the use of antimicrobial prophylaxis is associated with adverse effects such as severe cutaneous hypersensitivity reactions including Stevens Johnson syndrome and toxic epidermal necrolysis [13].
Although glucocorticoids are a risk factor for PJP infection [14], the incidence of PJP infection in children who are using glucocorticoids but do not have HIV, cancer, or history of an organ transplant is unknown. Furthermore, despite the common use of antimicrobial prophylaxis in children using glucocorticoids, little is known about the risks and benefits of this practice. Therefore, the objectives of this study were to determine the incidence of PJP infection in children who were using glucocorticoids but did not have HIV, cancer, or history of an organ transplant and to examine the risk of adverse events related to TMP-SMX use in this population, including cutaneous hypersensitivity reaction and myelosuppression.
MATERIALS AND METHODS
Study Design and Data Source
A retrospective cohort study was performed using the Clinformatics Data Mart Database (OptumInsight, Eden Prairie, MN). OptumInsight’s Clinformatics Data Mart is a deidentified administrative claims database from a nationally representative large private health care insurer. The data include demographic, diagnostic, and medical information, pharmacy claims, and some clinical laboratory results. Patients are assigned a unique identifier so if they drop insurance coverage and re-enroll, they are not counted erroneously as a new subject.
Study Population
We identified eligible patients 18 years of age or younger who had received at least 2 prescriptions for a systemic glucocorticoid (defined as prescriptions for oral or intravenous prednisone, prednisolone, methylprednisolone, dexamethasone, hydrocortisone, or budesonide) within a 60-day period. Patients were eligible for entry into the cohort regardless of the number of days for which the glucocorticoid was prescribed. Medications were identified by using national drug codes (NDCs), which are unique 11-digit codes included on all over-the-counter and prescription medications in the United States that identify medication manufacturer, strength, and dosage form [15] (see Supplementary Appendix for code list). Patients were required to have an antecedent glucocorticoid exposure-free window of at least 90 days and a minimum follow-up time of 90 days after cohort entry. Patients were considered glucocorticoid exposed from the start of the second prescription until 60 days after the end of the last prescription. Glucocorticoid duration was calculated using the prescription fill date in combination with the days’ supply provided and the next fill date to create start and stop dates for glucocorticoid exposure. If multiple glucocorticoid prescriptions were filled on the same day, we used the one with the higher days’ supply for calculations. Patients who met the inclusion criteria after stopping steroid use could re-enter the cohort up to 5 times. Patients who entered and left the database more than 5 times were considered glucocorticoid exposed from the first inclusion date until they no longer met our steroid-exposure definition; such patients accounted for approximately 1% of the cohort.
We excluded patients who had a history of PJP infection, oncologic diagnosis, solid organ or bone marrow transplant, HIV, or acquired immunodeficiency syndrome (AIDS) using International Classification of Diseases, 9th Revision (ICD-9), codes (see Supplementary Appendix for code list), patients with a prescription for TMP-SMX with a concurrent diagnosis code for a urinary tract infection, and patients who had the outcome of interest in the baseline period.
Study Time Period
Data from May 2000 to June 2013 were analyzed. Cohort entry occurred on the day of the second glucocorticoid prescription. Patients were followed until 90 days after their last glucocorticoid prescription ended (calculated as days’ supply plus 90 days of observation), the end of enrollment in the health plan or data collection (June 2013), or if they experienced one of the outcomes of interest (PJP diagnosis or adverse event).
Exposure Definition: PJP Prophylaxis
We examined prescriptions for PJP prophylaxis agents, including TMP-SMX, atovaquone, dapsone, and pentamidine (inhaled or intravenous). Patients were classified as exposed during the month in which they received a prescription for a prophylaxis medication until 30 days after the last prescription ended.
Covariates
Demographic covariates measured before cohort entry included sex, race (white, black, Hispanic, or Asian), and geographic region of residence (Northeast, South, Midwest, or West). Age (in years) was assessed at the date of inclusion in the cohort. Time-varying covariates included hospitalization and immunosuppressive medications, including tumor necrosis factor inhibitors (adalimumab, certolizumab, etanercept, golimumab, and infliximab), interleukin 1 blockers (anakinra, canakinumab, rilonacept), an interleukin 6 blocker (tocilizumab), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) fusion protein (abatacept), a Janus kinase inhibitor (tofacitinib), an alkylating agent (cyclophosphamide), disease-modifying antirheumatic drugs (azathioprine, 6-mercaptopurine, methotrexate, mycophenolate mofetil, sulfasalazine, and thalidomide), B-cell therapy (belimumab and rituximab), and T-cell therapy (basiliximab, cyclosporine, everolimus, sirolimus, and tacrolimus [defined by NDC codes; see Supplementary Appendix]). Patients were considered exposed to one these medications from the first date of the prescription until 30 days after the prescription ended. Patients were considered exposed to rituximab for 180 days after their infusion.
Outcomes
Outcomes of interest were identified using ICD-9 codes entered at the time of an outpatient encounter or on the day of discharge from hospitalization. PJP was identified using ICD-9 code 136.3. Cutaneous hypersensitivity reactions were identified using the codes for Stevens-Johnson syndrome (965.13), toxic epidermal necrolysis (695.15), Stevens-Johnson syndrome–toxic epidermal necrolysis overlap (695.14), dermatitis due to drugs or substances taken internally (693.0, 693.8, and 693.9), erythema multiforme minor (695.11), erythema multiforme major (695.12), erythema multiforme unspecified or other (695.10 and 695.19), and exfoliation due to erythematous condition involving less than 10% to 90% or more of the body surface (695.50, 695.51, 695.52, 695.53, 695.54, 695.55, 695.56, 695.57, 695.58, and 695.59). Previous studies used similar algorithms [16]. Myelosuppression was identified using ICD-9 codes for anemia (285.8 and 285.9), leukocytopenia (288.5), lymphocytopenia (288.51), neutropenia (288 and 288.09), drug-induced neutropenia (288.03), and thrombocytopenia (287.49 and 287.5).
Statistical Analysis
Descriptive analyses were used to examine differences between exposure groups. The incidences of PJP infection, hypersensitivity, and myelosuppression were calculated as the number of events divided by the total person time. For hypersensitivity reaction and myelosuppression, the number needed to harm was then calculated as 1 over the absolute risk difference (difference in incidence between exposed and unexposed patients).
Discrete time-failure models were used to examine the association between TMP-SMX use and the outcomes of interest during each month of follow-up. This model allows for time-varying exposure and covariates. Discrete time-failure models use logistic regression models that included time as a discrete covariate in the model (which we categorized into 6-month intervals) and clustered according to patient. We used this method because our data were inherently lumped into finite intervals, and event times were tied [17]. Univariate regression was used to examine the association between the individual covariates and outcome (clustering according to patient and accounting for time interval). Variables with a P value of <.05 were included in our multivariable model along with covariates of interest. Then, we fit a multivariate discrete time-failure model using logistic regression (clustering according to patient and accounting for the time interval). This analysis resulted in an odds ratio (OR) for the outcome among the exposed at 1 point in time compared to those who were unexposed. All analyses were performed using Stata 13.1 (Stata Corp, College Station, TX).
Sensitivity Analyses
Patients who had the outcome of interest during the first time interval in the cohort were excluded, because hypersensitivity reaction can be treated with glucocorticoids, and we therefore wanted to ensure the correct sequence of events. The outcome definition was restricted to inpatient codes for cutaneous hypersensitivity reaction, because a severe reaction would likely result in hospital admission.
RESULTS
Characterization of the Cohort
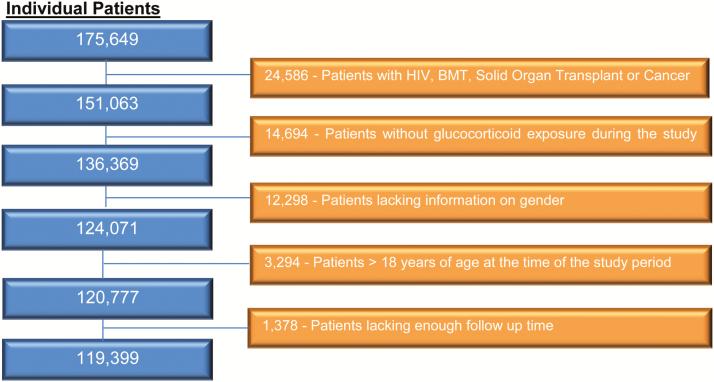
A total of 175649 patients between the ages of 0 and 18 years with chronic glucocorticoid exposure were identified. After applying our exclusion criteria, 119399 patients remained (Figure 1); the total number of glucocorticoid prescriptions received was 174403. The majority of PJP prophylaxis prescriptions were for TMP-SMX (n = 16750 [96%]). Other prescriptions included dapsone (n = 272 [2%]), pentamidine (n = 187 [1%]), and atovaquone (n = 94 [1%]). Because of the relative infrequent use of other PJP prophylactic medications, the analysis was restricted to adverse events related to TMP-SMX. Thus, in the final cohort, 10% (n = 12064) of the patients were ever exposed to TMP-SMX, and 107335 (90%) patients were unexposed.
Figure 1.
Evaluation of eligibility for final glucocorticoid-exposed patient cohort. Abbreviations: BMT, bone marrow transplant; HIV, human immunodeficiency virus.
Patient characteristics are shown in Table 1. Patients exposed to TMP-SMX were younger, more often female, and more commonly white than were the unexposed patients. In the TMP-SMX–exposed group (versus the TMP-SMX–unexposed group), the median cohort time was greater (12 vs 8 months, respectively), the duration of glucocorticoid exposure was longer (10 vs 6 months, respectively), and the duration of additional immunosuppressant exposure was longer (10 vs 8 months, respectively). Relatively few patients (3%) received immunosuppressive therapy in addition to glucocorticoids (see Supplementary Table 1).
Table 1.
Cohort Demographics and Outcomes According to TMP-SMX Exposure
| Characteristic | TMP-SMX Exposed (n = 12064) | TMP-SMX Unexposed (n = 107335) |
|---|---|---|
| Baseline age | ||
| Median (IQR) (y) | 2.5 (0–9) | 3 (0–9) |
| Range (mo) | 0–18 | 0–18 |
| Sex, female (n [%]) | 5782 (48) | 41561 (39) |
| Race (n [%]) | ||
| White | 9142 (76) | 78637 (73) |
| Black | 1375 (11) | 11850 (11) |
| Hispanic | 1250 (10) | 12901 (12) |
| Asian | 297 (2) | 3950 (4) |
| Geographic region (n [%])a | ||
| 1 | 724 (6) | 11944 (11) |
| 2 | 3047 (25) | 30644 (29) |
| 3 | 7331 (61) | 53830 (50) |
| 4 | 962 (8) | 10920 (10) |
| Cohort time | ||
| Median (IQR) (mo) | 12 (7–20) | 8 (5–13) |
| Range (mo) | 1–133 | 1–143 |
| Glucocorticoid exposure | ||
| Median (IQR) (mo) | 10 (6–16) | 6 (4–11) |
| Range (mo) | 1–131 | 1–140 |
| Hospitalization time | ||
| Median (IQR) (mo) | 1 (1–1) | 1 (1–1) |
| Range (mo) | 1–5 | 1–5 |
| TMP-SMX exposure | ||
| Median (IQR) (mo) | 3 (2–4) | N/A |
| Range (mo) | 1–82 | N/A |
| Additional immunosuppressant exposure | ||
| Median (IQR) (mo) | 10 (5–19) | 8 (5–14) |
| Range (mo) | 1–82 | 1–81 |
Abbreviations: IQR, interquartile range; N/A, not applicable; TMP-SMX, trimethoprim-sulfamethoxazole.
aRegions according to the US Census: Region 1, CT, MA, ME, NH, NJ, NY, PA, RI, and VT; region 2, IA, IL, IN, KS, MI, MN, MO, NE, ND, OH, SD, and WI; Region 3, AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, and VA; and Region 4, AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, WA, and WY.
Overall, only 6 children experienced PJP infection (incidence, 0.5 per 10000 person-years [PY]), and the incidence of PJP was similar in the patients who were exposed to TMP-SMX prophylaxis (Table 2). Among the patients exposed to TMP-SMX, cutaneous hypersensitivity reaction occurred in 205 patients (incidence, 125 cases per 10000 PY), compared to 808 of the unexposed patients (incidence, 86 cases per 10000 PY). Myelosuppression occurred in 386 patients in the TMP-SMX–exposed group (incidence, 235 per 10000 PY) compared to 1842 unexposed patients (incidence, 197 per 10000 PY). Among those who developed a cutaneous hypersensitivity reaction, the median time to outcome from cohort entry was 2 months (IQR, 0–14 months), and for those who experienced myelosuppression, the median time was 4 months (IQR, 1–16 months). Among those who developed a cutaneous hypersensitivity reaction in the TMP-SMX–exposed group, 22% of the events occurred in the first month, 41% within the first 3 months, and 43% within the first 6 months from the date of their first prescription. Among those who developed myelosuppression in the TMP-SMX–exposed group, 13% of the events occurred in the first month, 25% within the first 3 months, and 39% within the first 6 months from the date of their first prescription (Supplementary Figures 1 and 2).
Table 2.
Incidence of Outcomes
| Outcome | TMP-SMX Exposed (n = 12064) | TMP-SMX Unexposed (n = 107335) | ||
|---|---|---|---|---|
| Cases (n) | Incidencea (per 10000 PY) | Cases (N) | Incidencea (per 10000 PY) | |
| Pneumocystis jirovecii infectiona | 1 | 0.61 | 5 | 0.53 |
| Cutaneous hypersensitivity reaction | 205 | 125 | 808 | 86 |
| Myelosuppression | 386 | 235 | 1842 | 197 |
Abbreviations: IQR, interquartile range; PY, person-years; TMP-SMX, trimethoprim-sulfamethoxazole.
aOne patient in the TMP-SMX–unexposed group was treated with an alternative prophylaxis agent before P jirovecii pneumonia infection. The patient then was treated with TMP-SMX after developing P jirovecii pneumonia infection.
In a univariate model, cutaneous hypersensitivity reaction occurred more often in TMP-SMX–exposed than in unexposed patients (OR, 3.39 [95% confidence interval (CI) 2.77–4.15]), less often in black than in white patients (OR, 0.65 [95% CI, 0.52–0.82]), and less often in female than in male patients (OR, 0.78 [95% CI, 0.69–0.88]) (Table 3). In a multivariable model that was adjusted for current glucocorticoid exposure, female sex, and race, hypersensitivity reactions were significantly associated with TMP-SMX exposure (OR, 3.20 [95% CI, 2.62–3.92]). In a sensitivity analysis in which the outcome was restricted to hypersensitivity reactions that occurred during hospitalization, TMP-SMX exposure continued to be significantly related to the outcome (OR, 5.21 [95% CI, 2.89–9.39]). Excluding hypersensitivity reactions that occurred during the first-time month of cohort entry did not substantially change our results. No interaction between TMP-SMX and glucocorticoid exposures was found. The number needed to harm for cutaneous hypersensitivity reactions was 259 patients.
Table 3.
Predictors of Cutaneous Hypersensitivity Reaction in Children Chronically on Glucocorticoid Therapy
| Variable | Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P |
|---|---|---|---|---|
| Trimethoprim- sulfamethoxazole exposure | 3.39 (2.77–4.15) | <.001 | 3.20 (2.62–3.92) | <.001 |
| Current glucocorticoid exposurea | 3.53 (2.69–4.64) | <.001 | 3.49 (2.66–4.58) | <.001 |
| Additional immunosuppressant exposure | 1.01 (0.64–1.58) | .97 | ||
| Age (continuous) | 1.00 (0.98–1.01) | .47 | ||
| Sex (female) | 0.78 (0.69–0.88) | <.001 | 0.80 (0.70–0.90) | <.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.65 (0.52–0.82) | <.001 | 0.65 (0.52–0.82) | <.001 |
| Hispanic | 0.83 (0.68–1.02) | .08 | 0.84 (0.68–1.03) | .09 |
| Asian | 0.90 (0.64–1.28) | .56 | 0.93 (0.65–1.32) | .68 |
| Geographic regionb | ||||
| 1 | Reference | |||
| 2 | 0.84 (0.67–1.04) | .11 | ||
| 3 | 0.89 (0.73–1.09) | .26 | ||
| 4 | 1.03 (0.79–1.33) | .84 | ||
aCurrent glucocorticoid exposure indicates that the patient filled a prescription for glucocorticoid in the same month in which they had the outcome.
bRegions according to the US Census: Region 1, CT, MA, ME, NH, NJ, NY, PA, RI, and VT; region 2, IA, IL, IN, KS, MI, MN, MO, NE, ND, OH, SD, and WI; Region 3, AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, and VA; and Region 4, AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, WA, and WY.
In univariate analysis, we observed a significant association between myelosuppression and TMP-SMX exposure (OR, 2.01 [95% CI, 1.70–2.38]). Glucocorticoid exposure, immunosuppressant use, older age, and nonwhite race also were associated with myelosuppression while female sex and geographic regions were associated with a lower likelihood of developing myleosuppression (Table 4). In the multivariable model, myelosuppression was associated significantly with TMP-SMX exposure (OR, 1.85 [95% CI, 1.56–2.20]). Immunosuppressant use (OR, 6.75 [95% CI, 5.78–7.88]), older age (OR, 1.02 [95% CI, 1.01–1.03]), and nonwhite race (all P < .001) also were associated with development of myelosuppression. Female sex (OR, 0.78 [95% CI, 0.72–0.85]) and residence in a geographic region outside of the northeastern United States (all P < .001) were associated with a lower likelihood of developing myelosuppression. In a sensitivity analysis that excluded myelosuppression in the first month of cohort entry, the results were unchanged. The number needed to harm for myelosuppression was 269 patients.
Table 4.
Predictors of Myelosuppression in Children Chronically on Glucocorticoids
| Variable | Univariate OR (95% CI) | P | Multivariate OR (95% CI) | P |
|---|---|---|---|---|
| Trimethoprim-sulfamethoxazole exposure | 2.01 (1.70–2.38) | <.001 | 1.85 (1.56–2.20) | <.001 |
| Current glucocorticoid exposurea | 1.77 (1.55–2.03) | <.001 | 1.66 (1.44–1.90) | <.001 |
| Additional immunosuppressant exposure | 8.34 (7.24–9.61) | <.001 | 6.75 (5.78–7.88) | <.001 |
| Age | 1.04 (1.03–1.05) | <.001 | 1.02 (1.01–1.03) | <.001 |
| Sex (female) | 0.72 (0.66–0.78) | <.001 | 0.78 (0.72–0.85) | <.001 |
| Race | ||||
| White | Reference | Reference | ||
| Black | 1.33 (1.18–1.51) | <.001 | 1.35 (1.19–1.54) | <.001 |
| Hispanic | 1.82 (1.63–2.03) | <.001 | 1.91 (1.70–2.14) | <.001 |
| Asian | 1.57 (1.28–1.92) | <.001 | 1.66 (1.35–2.04) | <.001 |
| Geographic regionb | ||||
| 1 | Reference | Reference | ||
| 2 | 0.66 (0.58–0.76) | <.001 | 0.70 (0.60–0.80) | <.001 |
| 3 | 0.72 (0.63–0.81) | <.001 | 0.73 (0.64–0.82) | <.001 |
| 4 | 0.68 (0.57–0.81) | <.001 | 0.62 (0.52–0.74) | <.001 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aCurrent glucocorticoid exposure indicates that the patient filled a prescription for glucocorticoid in the same month in which they had the outcome.
bRegions according to the US Census: Region 1, CT, MA, ME, NH, NJ, NY, PA, RI, and VT; region 2, IA, IL, IN, KS, MI, MN, MO, NE, ND, OH, SD, and WI; Region 3, AL, AR, DE, DC, FL, GA, KY, LA, MD, MS, NC, OK, SC, TN, TX, and VA; and Region 4, AK, AZ, CA, CO, HI, ID, MT, NM, NV, OR, WA, and WY.
DISCUSSION
Many children chronically require glucocorticoids, yet epidemiologic data about the PJP infection risk and adverse events associated with PJP prophylaxis outside of the HIV/AIDS, transplant, and oncology populations are lacking. Prophylactic therapy might be warranted for children at high risk for development of this potentially fatal infection. In contrast, cutaneous hypersensitivity reactions associated with prophylaxis, including Stevens-Johnson syndrome and toxic epidermal necrolysis, result in high mortality rates (25% and 35%, respectively) [18]. Thus, decisions about when and to whom to recommend antibiotic prophylaxis are particularly challenging. Such a scenario is relatively common in the management of children with an inflammatory, allergic, or autoimmune disorder, because chronic glucocorticoid use and immunosuppression are thought to be risk factors for PJP infection [19]. To our knowledge, ours is the first report on the incidence of PJP infection in a large cohort of children treated with glucocorticoids. We found a low incidence of PJP infection in children on glucocorticoids regardless of whether they were exposed or unexposed to TMP-SMX. In addition, we found an increased risk of adverse events associated with TMP-SMX exposure, including cutaneous hypersensitivity reaction and myelosuppression. Given the very low incidence of PJP infection and the relatively high risk for adverse events, the use of PJP prophylaxis should be considered carefully.
We found PJP infection in our cohort to be an extremely rare outcome (overall incidence, 0.5 cases per 10000 PY). Most of what is known about PJP infection in children is isolated to the HIV/AIDS, transplant, and oncology populations. In 1977, a sentinel controlled trial of children with leukemia who were not receiving prophylaxis reported a 21% incidence rate of PJP infection, and the rate was 0% for those who were receiving PJP prophylaxis [20]. The modern incidence rate is <0.5 cases per 100 child-years in children with HIV on combined antiretroviral therapy [21]. Both of these studies reported incidence rates similar to those from our study. PJP infection occurs outside of this population, such as in patients with autoimmune disease [22–25], but a paucity of data exists, which is why we focused on them. The majority of cases we found were in patients with a history of pulmonary disease or a primary or secondary immunosuppressing condition (see Supplementary Table 2). It is interesting that the incidence of PJP was the same in the TMP-SMX–exposed and unexposed arms, but given the small absolute number of outcomes in each arm and the lack of prophylaxis-adherence data, it is difficult to comment on the efficacy of prophylaxis. Furthermore, because 10% of the cohort received TMP-SMX, it is unclear how many cases might have been prevented, and that was not the aim of our study. A limitation of our study is that it was not designed to address the complex question of PJP prophylaxis effectiveness.
We found a rate lower than that reported by Beukelman et al [26] in an observational cohort of 8503 children with juvenile idiopathic arthritis in which a single case of PJP was identified (incidence rate, 7 per 13990 PY [95% CI, 0.2–39 per 13990 PY]). This patient population was similar but different than ours; all of our patients were on glucocorticoid therapy, and some of them took additional immunosuppressants. The rituximab in antineutrophil cytoplasmic antibody-associated vasculitis (RAVE) trial compared cyclophosphamide to rituximab for adults with antineutrophil cytoplasmic antibody–positive vasculitis and provided PJP prophylaxis to all patients; the authors of the report did not comment on any cases of infection occurring [27]. Additional longitudinal studies reporting the incidence of PJP infection in children are lacking. Although limited, our data show that patients chronically on glucocorticoids (and frequently also on other immunosuppressive medications) have a low incidence of PJP despite prophylaxis being used in a minority of patients.
Previous studies also found that the use of TMP-SMX can lead to adverse events in adults. In this population, we reported a lower incidence of myelosuppression and a higher incidence of cutaneous hypersensitivity [28–30]. In 1996, a randomized double-blind study in which 3 PJP treatment regimens were compared in adults with PJP infection reported rates of adverse events associated with treatment. Among patients in the TMP-SMX arm, 19% reported a rash, 6% had a grade III rash that they defined as vesiculation, moist desquamation, or ulceration, and no one had an exfoliative dermatitis [29]. The authors also reported a 5% incidence of neutropenia (absolute neutrophil count, <750) and 5% incidence of anemia (hemoglobin level, <8 g/dL). In a meta-analysis of TMP-SMX versus placebo, the relative risk for leukopenia was reported to be 1.96 (95% CI, 0.18–20.97) [30]. Few data have been reported about the risk of adverse events attributed to TMP-SMX in children. A 2004 literature review by a urology group of the incidence of cutaneous reactions associated with TMP-SMX in children reported 7.4 events per 100 years of risk in children younger than 2 years and 1.4 events per 100 years in children aged 2 through 15 years [28]. Our study revealed a slightly lower incidence in our population (125 cases per 10000 PY). It might be that glucocorticoids are protective because they are used to treat hypersensitivity reaction; however, we did not assess this aspect, because we did not have a glucocorticoid-unexposed control group.
Strengths of this study include our access to a claims database with a large sample size and ability to capture exposures and outcomes across the spectrum of medical care. We also had a large sample size sufficient to detect differences between exposures. Our study also has limitations. First, a risk for misclassification of the outcomes of interest exists. We did not validate the diagnosis codes for PJP infection or the codes for hypersensitivity in the OptumInsight database. Previous studies found positive predictive values between 53.7% and 59.6% by using the unique ICD-8 or ICD-9 code (695.1) for erythema multiforme to identify Stevens-Johnson syndrome or toxic epidermal necrolysis in various data sources [16]. Prophylaxis regimens have ranged from 1 to 3 times per week [31], and the evidence suggests that higher rates of adverse drug reactions occur with higher TMP-SMX dosages [32]. The database used for our study does not provide drug instruction information but, rather, medication strength, formulation, and number of pills dispensed, which limited our ability to determine specific drug regimens. Furthermore, given the absence of patient weights, we were unable to quantify daily glucocorticoid dose and, thus, unable to examine the risk for PJP according to subgroups of daily glucocorticoid exposure. However, regardless of dose, the incidence of PJP in this large representative population of patients without cancer or HIV was very low. Because no formal guidelines for prophylaxis in children on chronic glucocorticoids exist, patients who receive PJP prophylaxis might be sicker and deemed to be at greater risk for PJP infection. Although we attempted to adjust for this potential confounding by indication, unmeasured confounders might remain, and we lacked objective methods to gauge disease activity using our administrative data. In addition, the median duration of glucocorticoid exposure was much longer than the median TMP-SMX exposure, which might explain the similar incidence rates of PJP infection between the 2 TMP-SMX–exposed groups. Finally, although TMP-SMX was associated in our study with hypersensitivity reaction and myelosuppression, additional immunosuppressive use was strongly associated also with myelosuppression. We are unable to discern whether myelosuppression was the result of more aggressive disease requiring the immunosuppression or whether the immunosuppression resulted in the myelosuppression.
In conclusion, we report here that, overall, PJP infection is rare in children on chronic glucocorticoid therapy and that TMP-SMX is associated with hypersensitivity reaction and myelosuppression. The risk of disease and adverse events needs to be weighed carefully when deciding whether to provide prophylaxis to such children.
Supplementary Material
Notes
Financial support. This work was supported by the National Institutes of Health. Dr Basiaga was supported by National Institutes of Health (NIH) grant T32 GM075766-09, and Dr Ogdie is supported by NIH grant K23 AR063764.
Potential conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ramanan AV, Campbell-Webster N, Ota S, et al. The effectiveness of treating juvenile dermatomyositis with methotrexate and aggressively tapered corticosteroids. Arthritis Rheum 2005; 52:3570–8. [DOI] [PubMed] [Google Scholar]
- 2. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007; 369:767–78. [DOI] [PubMed] [Google Scholar]
- 3. Ruemmele FM, Veres G, Kolho KL, et al. ; European Crohn’s and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 2014; 8:1179–207. [DOI] [PubMed] [Google Scholar]
- 4. Tung J, Loftus EV Jr, Freese DK, et al. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 2006; 12:1093–100. [DOI] [PubMed] [Google Scholar]
- 5. Skrzypczyk P, Panczyk-Tomaszewska M, Roszkowska-Blaim M, et al. Long-term outcomes in idiopathic nephrotic syndrome: from childhood to adulthood. Clin Nephrol 2014; 81:166–73. [DOI] [PubMed] [Google Scholar]
- 6. Oddis CV, Reed AM, Aggarwal R, et al. ; RIM Study Group Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum 2013; 65:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee SJ, Kavanaugh A. Adverse reactions to biologic agents: focus on autoimmune disease therapies. J Allergy Clin Immunol 2005; 116:900–5. [DOI] [PubMed] [Google Scholar]
- 8. McLean LP, Cross RK. Adverse events in IBD: to stop or continue immune suppressant and biologic treatment. Expert Rev Gastroenterol Hepatol 2014; 8:223–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos AN Jr, Matida LH, Hearst N, Heukelbach J. Opportunistic illnesses in Brazilian children with AIDS: results from two national cohort studies, 1983–2007. AIDS Res Ther 2011; 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solodokin LJ, Klejmont LM, Scipione MR, et al. Safety and effectiveness of intravenous pentamidine for prophylaxis of Pneumocystis jirovecii pneumonia in pediatric hematology/oncology patients. J Pediatr Hematol Oncol 2016; 38:e180–5. [DOI] [PubMed] [Google Scholar]
- 11. Martin SI, Fishman JA; AST Infectious Diseases Community of Practice Pneumocystis pneumonia in solid organ transplantation. Am J Transplant 2013; 13(Suppl 4):272–9. [DOI] [PubMed] [Google Scholar]
- 12. Stern A, Green H, Paul M, et al. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014; 10:CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995; 333:1600–7. [DOI] [PubMed] [Google Scholar]
- 14. Roblot F, Godet C, Le Moal G, et al. Analysis of underlying diseases and prognosis factors associated with Pneumocystis carinii pneumonia in immunocompromised HIV-negative patients. Eur J Clin Microbiol Infect Dis 2002; 21:523–31. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration. National Drug Code Directory http://www.fda.gov/Drugs/InformationOnDrugs/ucm142438.htm. Accessed 15 September 2015.
- 16. Schneider G, Kachroo S, Jones N, et al. A systematic review of validated methods for identifying erythema multiforme major/minor/not otherwise specified, Stevens-Johnson syndrome, or toxic epidermal necrolysis using administrative and claims data. Pharmacoepidemiol Drug Saf 2012; 21(Suppl 1):236–9. [DOI] [PubMed] [Google Scholar]
- 17. Fahrmeir L. Discrete failure time models. 1997. https://epub.ub.uni-muenchen.de/1483/1/paper_91.pdf. [Google Scholar]
- 18. Miliszewski MA, Kirchhof MG, Sikora S, et al. Stevens-johnson syndrome and toxic epidermal necrolysis: an analysis of triggers and implications for improving prevention. Am J Med 2016; 129:1221–5. [DOI] [PubMed] [Google Scholar]
- 19. Cutolo M, Seriolo B, Pizzorni C, et al. Use of glucocorticoids and risk of infections. Autoimmun Rev 2008; 8:153–5. [DOI] [PubMed] [Google Scholar]
- 20. Hughes WT, Kuhn S, Chaudhary S, et al. Successful chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med 1977; 297:1419–26. [DOI] [PubMed] [Google Scholar]
- 21. Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA 2006; 296:292–300. [DOI] [PubMed] [Google Scholar]
- 22. Ognibene FP, Shelhamer JH, Hoffman GS, et al. Pneumocystis carinii pneumonia: a major complication of immunosuppressive therapy in patients with Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:795–9. [DOI] [PubMed] [Google Scholar]
- 23. Gerrard JG. Pneumocystis carinii pneumonia in HIV-negative immunocompromised adults. Med J Aust 1995; 162:233–5. [DOI] [PubMed] [Google Scholar]
- 24. Godeau B, Coutant-Perronne V, Le Thi Huong D, et al. Pneumocystis carinii pneumonia in the course of connective tissue disease: report of 34 cases. J Rheumatol 1994; 21:246–51. [PubMed] [Google Scholar]
- 25. Ling C, Qian S, Wang Q, et al. Pneumocystis pneumonia in non-HIV children: a 10-year retrospective study. Clin Respir J 2016. doi: 10.1111/crj.12467. [DOI] [PubMed] [Google Scholar]
- 26. Beukelman T, Xie F, Baddley JW, et al. ; SABER Collaboration Brief report: incidence of selected opportunistic infections among children with juvenile idiopathic arthritis. Arthritis Rheum 2013; 65:1384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stone JH, Merkel PA, Spiera R, et al. ; RAVE-ITN Research Group Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karpman E, Kurzrock EA. Adverse reactions of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol 2004; 172:448–53. [DOI] [PubMed] [Google Scholar]
- 29. Safrin S, Finkelstein DM, Feinberg J, et al. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS. A double-blind, randomized, trial of oral trimethoprim-sulfamethoxazole, dapsone-trimethoprim, and clindamycin-primaquine. ACTG 108 Study Group. Ann Intern Med 1996; 124:792–802. [DOI] [PubMed] [Google Scholar]
- 30. Stern A, Green H, Paul M, et al. Prophylaxis for Pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev 2014:CD005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 2014; 44:1350–63. [DOI] [PubMed] [Google Scholar]
- 32. Nguyen AT, Gentry CA, Furrh RZ. A comparison of adverse drug reactions between high- and standard-dose trimethoprim-sulfamethoxazole in the ambulatory setting. Curr Drug Saf 2013; 8:114–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.