Abstract
Background
Social isolation in the elderly is one of the principal health risks in an aging society. Physical environmental enrichment is shown to improve sensory, cognitive, and motor functions, but it is unknown whether environmental enrichment can protect against brain impairments caused by social isolation.
Methods
Eighteen-month-old mice were housed, either grouped or isolated, in a standard or enriched environment for 2 months, respectively. Behavioral tests were performed to evaluate cognitive functional and social interaction ability. Synaptic protein levels, myelination, neuroinflammation, brain derived neurotrophic factor, and NOD-like receptor protein 3 inflammasome signaling pathways were examined in the medial prefrontal cortex and hippocampus.
Results
Isolated aged mice exhibited declines in spatial memory and social memory compared with age-matched littermates living within group housing. The aforementioned memory malfunctions were mitigated in isolated aged mice that were housed in a large cage with a running wheel and novel toys. Enriched housing prevented synaptic protein loss, myelination defects, and downregulation of brain derived neurotrophic factor, while also increasing interleukin 1 beta and tumor necrosis factor alpha in the medial prefrontal cortex and hippocampus of isolated mice. In addition, activation of glial cells and NOD-like receptor protein 3 inflammasomes was partially ameliorated in the hippocampus of isolated mice treated with physical environmental enrichment.
Conclusions
These results suggest that an enriched physical environment program may serve as a nonpharmacological intervention candidate to help maintain healthy brain function of elderly people living alone.
Keywords: aged mice, social isolation, environmental enrichment, cognitive function, neuroinflammation
Significance Statement
Social isolation (SI) in the elderly is one of the principal health risks in an aging society. However, there is limited research on how to reduce its deleterious consequences. This study was designed to investigate whether an enriched physical environment would protect against brain impairments in aged, socially isolated, mice. The results reveal that after 2 months of isolation, 20-month-old male mice displayed apparent declines in spatial and social memory, with increased synaptic protein loss, myelin damage, reactive gliosis, neuroinflammation, and disturbances in BDNF and NLRP3 inflammasome pathways in the medial prefrontal cortex and hippocampus. These changes in aged SI mice were partially diminished by enriched housing. This experimental evidence highlights the potential of an enriched physical environment acting as nonpharmacological therapy for maintaining cognitive function in the elderly suffering from SI.
Introduction
The brain is the chief regulatory center of the body, and delaying brain aging is critical for improving quality of life of the elderly. In the process of aging, there are not only declines in learning, memory, and cognitive abilities, but also obstacles in social and personal relationships (Mora et al., 2007; Singer et al., 2014; Arendt et al., 2017). The exact mechanism of brain aging has not been fully elucidated. Nevertheless, the role of external environmental and social factors in this process cannot be ignored (Seeman, 2000).
Social isolation (SI) exerts negative effects on human physical and mental health, while also increasing the risk of death (Holt-Lunstad et al., 2015). Notably, modern social lifestyle intensifies the risks of SI in seniors. Epidemiological studies have shown that nearly one-quarter of people over the age of 60 years suffer from SI (Sundström et al., 2009; Shimada et al., 2014). SI not only aggravates the aging process but also increases the incidence of Alzheimer’s disease (AD) and other neurological diseases (Eng et al., 2002; Iliffe et al., 2007; Friedler et al., 2015; Holt-Lunstad et al., 2015). Therefore, it is significant to explore effective interventions to alleviate, or potentially reverse, negative effects of SI on brain function during aging and aging-related diseases.
Environmental enrichment (EE) involves brain stimulation by its physical and social surroundings (Van Praag et al., 2000). Engaging in cognitive stimulation, team activities, and party games has been shown to play a beneficial effect on cognitive reserve in the elderly (Ben-Sadoun et al., 2016; Narme, 2016). Paradigms of EE in rodent studies have consisted of increased numbers of cage-mates, large cages with tunnels, running wheels, and objects varying in color and texture that are changed over time (Kotloski and Sutula, 2015). This nurturing environment is demonstrated to improve social ability, spatial cognition, and brain plasticity in healthy animals (Fares et al., 2013; Hannan, 2014; Garthe et al., 2016). EE also exerts positive effects on various animal models of neurological disorders including AD (Wolf et al., 2006; Salmin et al., 2017), Parkinson’s disease (Hilario et al., 2016), stroke (Livingston-Thomas et al., 2016), traumatic brain injury (Radabaugh et al., 2017), and Huntington’s disease (Skillings et al., 2014). EE includes not only physical factors, such as exercise and sensorial and visual stimulations, but also improved social interaction between individuals (Van Praag et al., 2000; Nithianantharajah and Hannan, 2006). However, it remains unclear whether these nonsocial stimulating factors are sufficient to mitigate, or even eliminate, the adverse effects of SI on the aging brain.
To address this issue, we investigated the effects of physical EE on spatial and social cognitive functions of aged SI mice via increased feeding space and the addition of novel items introduced into the cage. This study has provided evidence for the establishment of physical EE as a nonpharmacological therapy option for improving brain functions in the elderly suffering from SI.
Materials and Methods
Animals and Experimental Groups
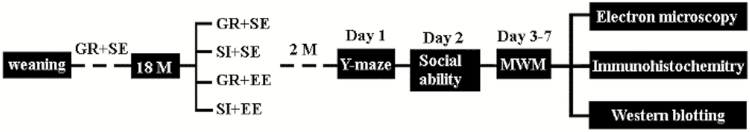
On postnatal day 30, weaned male C57BL/6 mice from each litter were separated and housed 4 mice per cage. Mice were purchased from Animal Research Center of Nanjing Medical University (NMU). At 18 months old, mice were divided randomly into 4 groups (n=16 mice per group): group housing in a standard environment (GR+SE), socially isolated housing in a standard environment (SI+SE), group housing with an enriched environment (GR+EE), and social isolation housing with an enriched environment (SI+EE). Housing protocols for each group were as previously described (Cao et al., 2017). Briefly, mice in the GR+EE and SI+EE groups were housed in large cages (47×30×23 cm) with various kinds of objects contained within the cages, such as teeterboards, tunnels, hanging plat-forms, running wheels, wooden houses, and ladders to increase somatosensory, visual, and motor stimulation. These objects were changed every 3 to 4 days to remain novel. Animals in the GR+SE and SI+SE groups were housed in standard plastic cages (31×22×15 cm) without stimulation. Mice in the GR+SE and GR+EE groups were kept 4 per cage, while 1 mouse was kept isolated in each cage in both the SI+SE and SI+EE groups. All animals were maintained in their respective experimental conditions until behavioral experiments were performed 2 months later (Figure 1).
Figure 1.
Diagram depicting animal grouping and experimental procedures. At 18 months old, group housing male C57/BL6 mice were divided randomly into 4 groups (n=16 mice per group): group housing in a standard environment (GR+SE), socially isolated housing in a standard environment (SI+SE), group housing with an enriched environment (GR+EE), and socially isolated housing with an enriched environment (SI+EE). Two months later, these animals received behavioral tests, followed by immunohistochemical, electron microscopic, or immunoblotting analyses.
This study was carried out in accordance with the recommendations of the Institutional Animal Care and Use Committee of NMU. The protocol was approved by the Animal Ethical and Welfare Committee of NMU (approval no. IACUC-1601106).
Behavioral Evaluations
Y-Maze Test
Short-term spatial memory of mice was determined by the Y-maze test, as previously described (Huang et al., 2015). During the 5-minute training stage, one arm, the novel arm (NA), was blocked by a black barrier, and mice were allowed to move freely only in the other 2 arms. Two hours later, the barrier was removed and mice could freely move in all 3 arms during the 5-minute testing stage. The time spent in the NA and the numbers of entries into the NA were calculated.
Morris Water Maze (MWM)
The MWM protocol for investigating hippocampus-dependent spatial learning and memory has been utilized previously in our laboratory (Liu et al., 2012a). The maze consists of a black plastic pool (diameter: 100 cm, height: 50 cm) filled with water (depth: 40 cm; temperature: 22°C) and divided into 4 quadrants. The platform was placed in one quadrant (target quadrant), and submerged 1 cm below the surface of the water. Training was conducted over 4 consecutive days, with 4 trials per day. Escape latency, swimming distance, and swimming speed was recorded every day. A probe trial was conducted on the 5th day, and the platform was removed, allowing the testing mouse to swim freely in the pool for 60 seconds. The percentage of total time spent in each quadrant and the number of crossing the target quadrant were calculated.
Social Interaction and Memory Test
The apparatus for detecting mouse social behavior consisted of 3 plastic compartments (40×40×30 cm) (Cao et al., 2017). During the first habituation phase, each mouse was allowed to explore the apparatus freely for 10 min/d, on 2 consecutive days. During the sociability test, an unfamiliar male mouse of the same background strain and age (stranger 1) was enclosed in a wire cup within the side chamber. The testing mouse was placed into the central compartment and allowed to explore the apparatus for 5 minutes. Time spent in each chamber was calculated and expressed as a percentage. Social memory testing was conducted 10 minutes afterwards. During this phase, a novel male mouse of the same background strain (stranger 2) was enclosed in the wire cup that had been empty during the sociability phase. The percentage of time spent in each chamber was calculated once again.
Mouse’s activity in the above behavioral apparatuses was collected using a digital video camera connected to a computer-controlled system (Beijing Sunny Instruments Co. Ltd, China). All tests were performed by 2 independent experimenters who were blind to animal groups.
Section Preparation
Following behavioral testing, mice were anesthetized with an i.p. injection of 40 mg/kg of pentobarbital sodium and perfused with 0.9% saline transcardially by perfusion pump for 5 minutes, followed by 4% paraformaldehyde without or with 0.5% glutaraldedyde (for electron microscopy) (Cao et al., 2017). Brains were removed, postfixed overnight at 4°C, dehydrated in a series of graded ethanol solutions, then embedded in paraffin. Coronal brain sections containing the medial prefrontal cortex (mPFC) and hippocampus were serially cut at 5 μm for immunohistochemical staining. For electron microscopy, brain tissues were cut into coronal sections at 100 μm using a vibratome (Leica VT1200S, Germany). The mPFC and hippocampus were trimmed, postfixed in 2% osmium, rinsed, dehydrated, and embedded in Epon 812. Ultrathin sections were cut at 70 nm, stained with uranyl acetate and leadcitrate, and examined using an electron microscope (Jeol 1200EX, Japan).
Immunohistochemistry
Immunohistochemical staining was performed as previously described (Huang et al., 2015). Following deparaffinization and rehydration, tissue sections were incubated with mouse monoclonal antibody against glial fibrillary acidic protein (GFAP) (1:1000; Sigma-Aldrich) or rabbit polyclonal antibody against ionized calcium-binding adaptor molecule 1 (Iba-1; 1:1000; Wako) at 4°C overnight. The next day, sections were incubated with biotinylated-conjugated goat anti-mouse (1:200, Zhong Shan Jin Qiao Biotechnology) or rabbit IgG (1:200, Zhong Shan Jin Qiao Biotechnology) for 1 hour at 37°C and visualized with diaminobenzidine (Sigma-Aldrich).
Image Analysis
For analysis of GFAP and Iba-1 positive signal percentage area, corresponding sections containing the mPFC and hippocampus were captured at 100× magnification using a digital microscope (LeicaDM4000B, Germany) with constant exposure time, offset, and gain. Each image was analyzed using NIH Image J software as described previously (Xu et al., 2015). Briefly, the mPFC or hippocampal area was manually delineated. The area of positive signal was measured using the interest grayscale threshold analysis. In addition, a minimum of 6 electron micrographs per animal, taken at 10000×, were randomly collected from the hippocampal lacunar molecular layer and mPFC, respectively. These micrographs were used to determine the extent of axonal myelination. The g-ratio of the myelinated axons was calculated as the diameter of the axon divided by the diameter of the entire myelinated fiber, as previously described (Marcus et al. 2006). Fifty myelinated axons per area of interest on each animal were analyzed.
Western Blotting
Homogenized tissues of the mPFC and hippocampus were centrifuged at 4°C and 12000 rpm for 15 minutes. Quantification proteins (7 μg/μL and 28 μg) were loaded. The samples were resolved on SDS-PAGE, then transferred onto PVDF membranes. After blocking in 5% nonfat milk/TBST for 1 hour, the membranes were incubated at 4°C overnight with one of the following primary antibodies: apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (1:500; Santa Cruz), brain derived neurotrophic factor (BDNF) (1:1000; Abcam), caspase-1 (1:1000; Millipore), cAMP response-element binding protein (CREB) (1:1000; Cell Signaling Technology), inhibitor of nuclear factor kappa-B kinase (IKKβ) (1:1000; Cell Signaling Technology), interleukin 1 beta (IL-1β) (1:1000; Millipore), IL-6 (1:1000; Abcam), NOD-like receptor containing a pyrin domain 3 (NLRP3) (1:1000; AdipoGen), p-CREB (1:1000; Cell Signaling Technology), p-IKKβ (1:1000; Cell Signaling Technology), p-P65 (1:1000; Cell Signaling Technology), P65 (1:1000; Cell Signaling Technology), postsynaptic density protein 95 (PSD-95) (1:1000; Abcam), procaspase-1 (1:500; Millipore), ProIL-1β (1:1000; Millipore), synaptophysin (SYN) (1:1000; Abcam), tropomyosin receptor kinase B (TrkB) (1:1000; Abcam), tumor necrosis factor-α (TNF-α) (1:1000; Abcam), or mouse monoclonal antibody against β-actin (1:500; Boster). Horseradish peroxidase-conjugated secondary antibodies goat anti-mouse IgG (1:200, Zhong Shan Jin Qiao Biotechnology) or goat anti-rabbit IgG (1:200, Zhong Shan Jin Qiao Biotechnology) were used, and bands were visualized using ECL Western Blotting Substrate (Thermo Fisher Scientific). β-Actin was used as an internal reference for protein loading and transfer efficiency. Four mice per group, in duplicate experiments, were averaged to provide a mean value for each group.
Statistical Analysis
All data were expressed as mean±SEM. The data from MWM platform trainings were analyzed by repeated-measures ANOVA with day of training serving as the within-subject variable and housing (group housing vs isolated housing) and environment (standard environment vs enriched environment) as the between-subject factors. Other data were analyzed using 2-tailed Student’s t test or 2-way ANOVA with housing and environment as factors, followed by Tukey’s posthoc test.
Results
Physical Enrichment Restores Spatial Learning and Memory Decline of Aged SI Mice
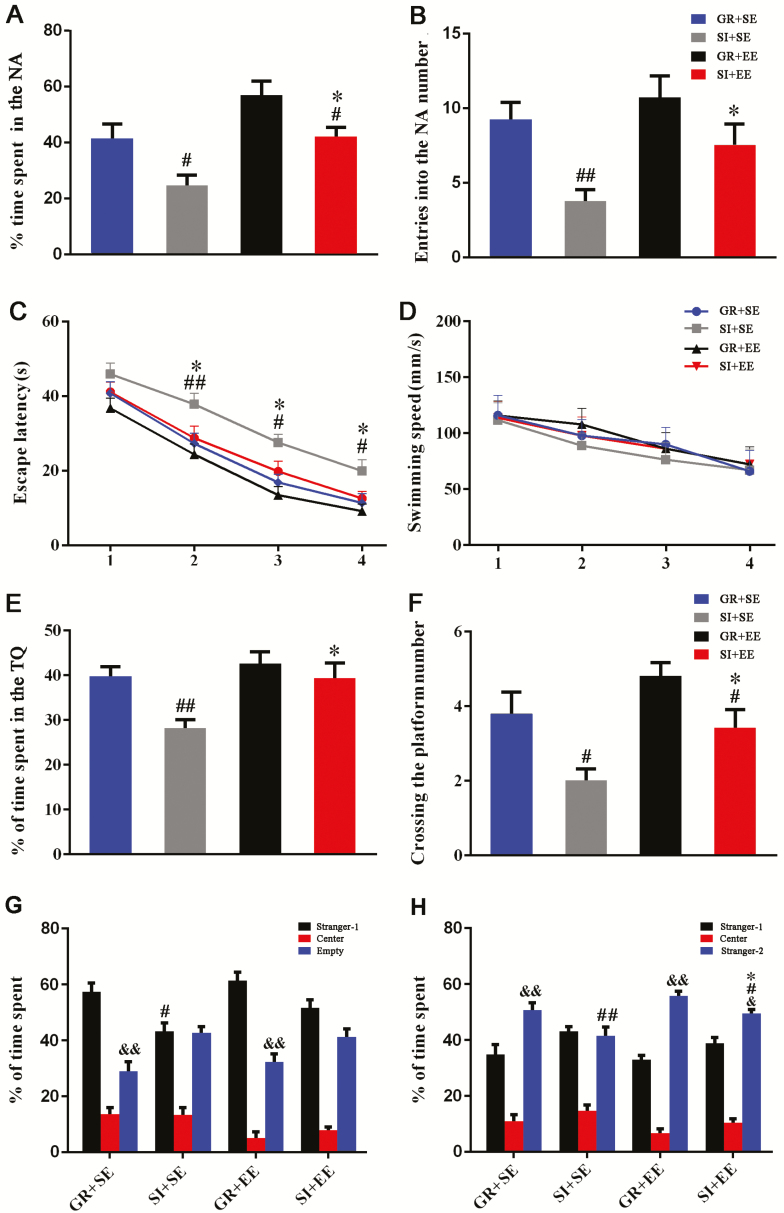
Hippocampus-dependent spatial learning and memory was tested by the Y maze and MWM. The percentage of time in the NA and numbers of NA entrances were significantly reduced in SI+SE mice compared with those in GR+SE mice (P=.0157, P=.0088, respectively). SI+EE mice exhibited higher percentages of time spent in the NA and numbers of entrances into the NA than SI+SE mice (P=.0103, P=.0465, respectively) (Figure 2A, B).
Figure 2.
Enriched housing attenuated declines of spatial cognition and social interaction ability of socially isolated aged mice. (A-B) The Y-maze test. (A) The percentage of time spent in the novel arm (NA). (B) The number of entries into the NA. Social isolation reduced the percentage of time spent in and entering numbers into the NA, which was reversed by exposure to enriched physical environment. Housing (time: F1,60=12.398, P=.001; number: F1,60=9.556, P=.003); environment (time: F1,60=11.056, P=.002; number: F1,60=5.687, P=.020); interaction effects (time: F1,60=0.000, P=.995; number: F1,60=0.146, P=.703). (C–F) The Morris water maze test. (C) The mean escape latency during training period. Enriched housing improved spatial learning abilities of isolated mice, as illustrated by reduced time to reach the hidden platform, relative to isolated controls that were reared in a standard environment. Housing (F3,240=18.772, P<.001); environment (F3,240=11.279, P<.001); interaction effects (F3,240=1.779, P=.187). (D) Swimming speed. There was no significant difference in swimming speed among the 4 groups. Housing (F3,240=0.506, P=.480); environment (F3,240=0.499, P=.483); interaction effects (F3,240=0.066, P=.799). (E) The percentage of time spent in the target quadrant in the probe test. (F) The platform area crossing number. Isolated housing significantly reduced the percentage of time spent in the target quadrant and platform area crossing number, which were greatly mitigated by with novel object supplement within the cage. Housing (time: F1,60=9.571, P=.003; number: F1,60=11.699, P=.001); environment (time: F1,60=6.055, P=.017; number: F1,60=6.665, P=.012); interaction effects (time: F1,36=1.976, P=.165; number: F1,36=0.122, P=.728, respectively). (G) The percentage of time spent in the chamber during the sociability test. Mice housed in a group showed more time spent in the compartment containing unfamiliar mouse (stranger 1) compared with socially isolated mice. Housing (F1,60=8.200, P=.006); environment (F1,60=2.316, P=.133); interaction effects (F1,60=0.053, P=.818). #P<.05, socially isolated housing (SI) vs group housing (GR). (H) The percentage of time spent in the chamber during the social memory test. Isolated mice housed in an enriched environment (EE) showed a high percentage of time spent in the compartment containing a novel mouse of the same background strain (stranger 2) relative to isolated controls in a standard environment. Housing (F1,60=14.519, P<.01); environment (F1,60=4.643; P=.035; P<.01, GR+SE vs SI+SE; P=.05, SI+EE vs SI+SE); interaction effects (F1,60=0.180; P=.672). #P<.05; ##P<.01, SI vs GR; *P<.05, EE vs standard environment (SE). Data in C and D were analyzed by repeated-measures ANOVA. &&P<.01, stranger 1 vs empty (G); &P<.05; &&P<.01, stranger 1 vs empty or stranger 2 (H), Student’s t test. Data in other figures were analyzed by 2-way ANOVA with Tukey’s posthoc test. Data were expressed as mean±SEM from 16 mice per group.
Consistently, physical EE restored spatial learning deficits of aged SI mice. Escape latency required to reach the platform of SI+SE mice was longer during the 4-day training in the MWM than that of GR+SE mice (F3, 120 =18.772, P<.001) and SI+EE mice (F3, 120 =11.279, P=.001) (Figure 2C). Swimming speed was comparable with each other (Figure 2D), indicating that differences in spatial learning ability among groups were not associated with the swimming ability.
During the probe trial of the MWM, both the percentage of time spent in the target quadrant and platform area crossing numbers decreased in isolated aged mice (P=.0055, P=.0139, SI+SE vs GR+SE, respectively) but was normalized by physical EE (P=.0144, P=.0379, SI+SE vs SI+EE, respectively) (Figure 2E, F). The above results demonstrated that 18-month-old mice, placed in isolated housing for 2 months, exhibited poor working memory, spatial learning, and cognitive performance, which was restored by physical EE.
Physical Enrichment Improves Social Memory Ability of SI Aging Mice
During sociability testing, GR+SE mice and GR+EE mice demonstrated a preference for staying in the compartment containing the unfamiliar mouse (stranger 1) relative to the empty one (both P<.01). However, both SI+SE mice and SI+EE mice showed no differences in time spent in the 2 compartments (both P>.05), suggesting an occurrence of mild social withdrawal. Moreover, SI resulted in less time spent with stranger 1 (P=.0164, SI+SE vs GR+SE), which was not mitigated following the EE exposure (P=.1158, SI+SE vs SI+EE) (Figure 2G).
In subsequent social memory testing, SI+SE mice did not display a preference for stranger 2 relative to stranger 1 (P>.05). By contrast, GR+SE, GR+EE, and SI+EE mice showed a preference for the novel (stranger 2) relative compared with stranger 1 (P<.01, P<.01, P<.05, respectively). Time spent with stranger 2 was decreased by SI (P=.0021, SI+SE vs GR+SE) and increased by physical EE (P=.0171, SI+SE vs SI+EE) (Figure 2H). These data suggested that physical enrichment improves social memory ability of SI aging mice.
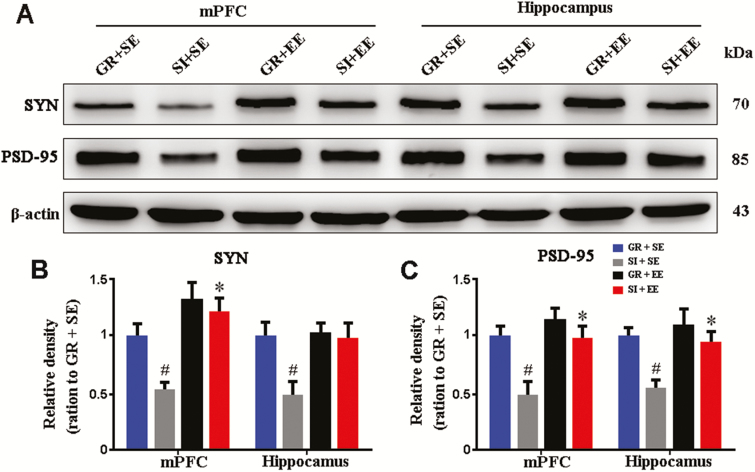
Physical Enrichment Increases Synaptic Proteins in Aged SI Mice
The maintenance of learning and memory functions depends on cortical and hippocampal synaptic proteins (Fields, 2008; Wang and Peng, 2016). Thus, to clarify the molecular basis of the beneficial effects of EE in aged SI mice, expression levels of SYN and PSD-95 in the mPFC and hippocampus were analyzed by western blot. SI significantly reduced mPFC and hippocampal SYN (P=.0299, P=.0486, SI+SE vs GR+SE, respectively) as well as PSD-95 expression levels (P=.0115, P=.0144, SI+SE vs GR+SE, respectively). Isolated mice raised in physical EE increased PSD-95 in both the mPFC and hippocampus (P=.0109, P=.0182, SI+SE vs SI+EE, respectively), but increased SYN only in the mPFC (P=.0123, SI+SE vs SI+EE) (Figure 3A–C).
Figure 3.
Enriched housing improved synaptic protein expression in the medial prefrontal cortext (mPFC) and hippocampus of socially isolated (SI) aged mice. (A) Representative western-blot bands for synaptophysin (SYN) and PSD-95 in the mPFC and hippocampus of mice with 4 different housing patterns. (B,C) Densitometry analysis of the expression levels of synaptophysin (SYN) (B) and PSD-95 (C). Enriched housing increased SYN and PSD-95 to normal levels in the mPFC and hippocampus of isolated mice. Housing (SYN: mPFC, F1,12=5.510, P=.037; hippocampus, F1,12=5.674, P=.035; PSD-95: mPFC, F1,12=11.377, P=.006; hippocampus, F1,12=9.434, P=.010); environment (SYN: mPFC, F1,12=12.432, P=.004; hippocampus, F1,12=4.872, P=.048; PSD-95: mPFC, F1,12=9.573, P=.009; hippocampus, F1,12=6.606, P=.025); interaction effects (SYN: mPFC, F1,12=0.887, P=.365; hippocampus, F1,12=1.229, P=.289; PSD-95: mPFC, F1,12=3.235, P=.097; hippocampus, F1,12=2.145, P=.169). #P<.05, SI housing vs group housing (GR); *P<.05, enriched environment (EE) vs standard environment (SE). Data were analyzed by 2-ways ANOVA with Tukey’s posthoc test and expressed as mean±SEM from 4 mice per group.
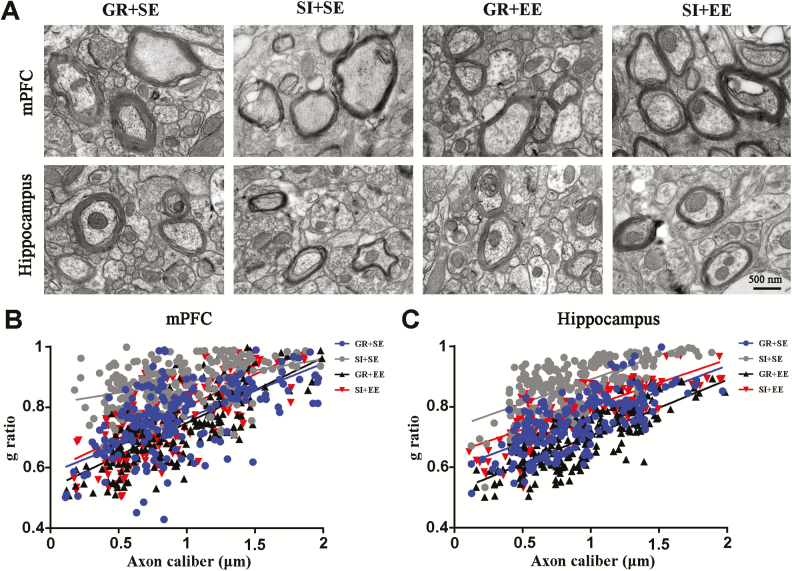
Physical Enrichment Protects Myelination of Aged SI Mice
SI not only impairs the development and formation of myelination in the mPFC of young mice (Makinodan et al., 2012) but also hampers long-term maintenance of mature myelin sheaths in adult mice (Liu et al., 2012b). Conversely, EE improves postnatal development and long-term maintenance of myelin sheaths (Pusic and Kraig, 2014; Pusic et al., 2016). Therefore, we compared the thickness of myelin sheaths among mice in different groups. SI in aged mice resulted in thinner myelin, revealed by an increased g ratioin the mPFC and hippocampus (both P<.05, SI+SE vs GR+SE). Physical EE completely normalized myelin thickness in the mPFC and hippocampus of aged SI mice (both P<.01, SI+EE vs SI+SE, respectively) (Figure 4A–C).
Figure 4.
Enriched housing rescued myelination defects of socially isolated (SI) aged mice. (A) Representative electron micrographs of myelin sheaths in the II/III layer of the medial prefrontal cortex (mPFC) and lacunar molecular layer in the hippocampus of mice with different housing patterns. (B,C) Histogram of g ratio values of myelin sheaths in the mPFC (B) and hippocampus (C). Enriched housing significantly increased the thickness of myelin sheaths of isolated mice, revealed by decreases in g ratio, relative to isolated controls in standard environment. Housing (mPFC, F1,12=20.547, P=.001; hippocampus, F1,12=30.722, P<.001); environment (mPFC, F1,12=21.548, P=.001; hippocampus, F1,12=13.006, P=.004); interaction effects (mPFC, F1,12=0.519, P=.485; hippocampus, F1,12=0.433, P=.523). #P<.05; ##P<.01, socially isolated housing (SI) vs group housing (GR); *P<.05; **P<.01, enriched environment (EE) vs standard environment (SE). Data were analyzed by 2-way ANOVA with Tukey’s posthoc test and expressed as mean±SEM from 50 axons per mice, and 4 mice per group.
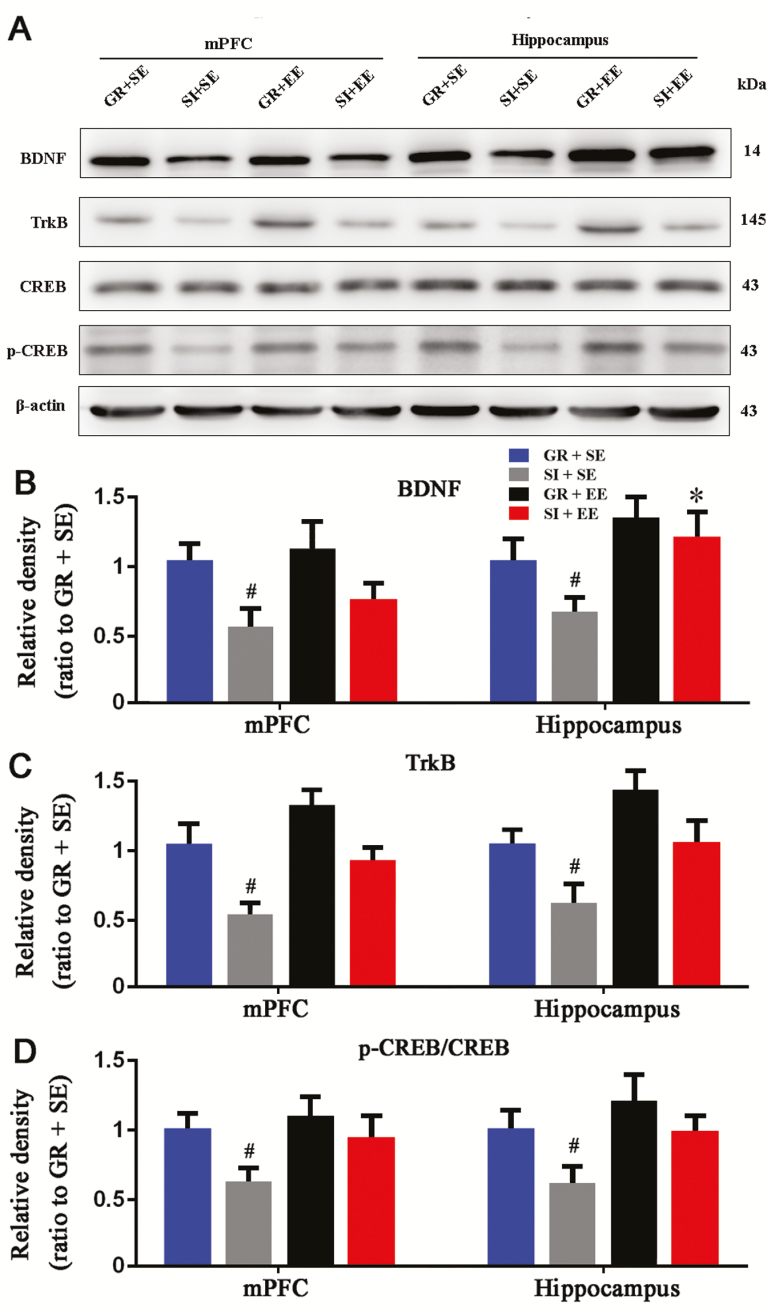
Physical EE Ameliorates Downregulation of the BDNF-TrkB-CREB Singling Pathways of Aged SI Mice
BDNF is known to maintain synaptic plasticity by combining with its receptor TrkB, triggering an intracellular signal cascade response, and subsequently leading to the phosphorylation of CREB and CREB-mediated gene transcription (Carlezon et al., 2005; Novkovic et al., 2015). We examined the effects of SI or/and EE on activation of BDNF-TrkB-CREB signal pathway in the aged brain. SI decreased mPFC and hippocampal BDNF (P=.0119, P=.0240, SI+SE vs GR+SE, respectively), TrkB expression levels (P=.0467, P=.0461, SI+SE vs GR+SE, respectively), and p-CREB/CREB ratios (P=.0106, P=.0474, SI+SE vs GR+SE, respectively). These deleterious effects were partially corrected by physical EE exposure in the mPFC and hippocampus (BDNF: P=.0313, P=.0974; TrkB: P=.0656, P=.1066; p-CREB/CREB: P=.0606, P=.0402; SI+SE vs SI+EE, respectively) (Figure 5A–D).
Figure 5.
Enriched housing attenuated downregulation of brain derived neurotrophic factor (BDNF)- tropomyosin receptor kinase B (TrkB)- cAMP response-element binding protein (CREB) signaling pathway in socially isolated (SI) aged mice. (A) Representative western-blot bands for BDNF, TrkB, p-CREB, and CREB in the medial prefrontal cortex (mPFC) and hippocampus of mice with 4 different housing patterns. (B–D) Densitometry analysis of the expression levels of BDNF (B) and TrkB (C) and p-CREB/CREB ratio (D). The enriched housing attenuated reduced expression levels of BDNF and TrkB as well as p-CREB/CREB ratios caused by social isolation. Housing (BDNF: mPFC, F1,12=10.512, P=.007; hippocampus, F1,12=6.042, P=.030; TrkB: mPFC, F1,12=10.096, P=.008; hippocampus, F1,12=6.918, P=.022; p-CREB/CREB: mPFC, F1,12=7.027, P=.021; hippocampus, F1,12=7.682, P=.017); environment (BDNF: mPFC, F1,12=1.928, P=.190; hippocampus, F1,12=11.260, P=.006; TrkB: mPFC, F1,12=6.078, P=.030; hippocampus, F1,12=6.408, P=.026; p-CREB/CREB: mPFC, F1,12=3.239, P=.097; hippocampus, F1,12=6.637, P=.024); interaction effects (BDNF: mPFC, F1,12=0.802, P=.388; hippocampus, F1,12=0.736, P=.408; TrkB: mPFC, F1,12=0.169, P=.688; hippocampus, F1,12=0.005, P=.947; p-CREB/CREB: mPFC, F1,12=1.125, P=.310; hippocampus, F1,12=0.365, P=.557.). #P<.05, SI housing vs group housing (GR); *P<.05, enriched environment (EE) vs standard environment (SE). Data were analyzed by 2-way ANOVA with Tukey’s posthoc test and expressed as mean±SEM from 4 mice per group.
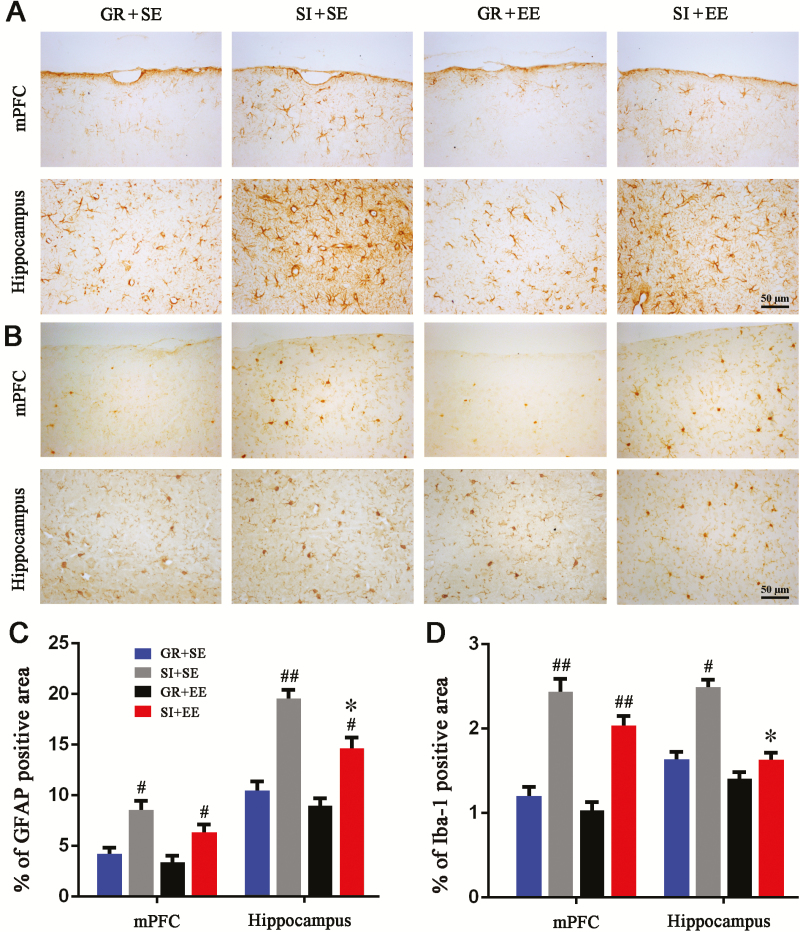
Physical EE Partially Reduces Glial Cell Activation of Aged SI Mice
Life stresses, including SI, can induce inflammatory responses in the adult rodent brain (Khandaker et al., 2014; Lacey et al., 2014), while EE has been shown to mitigate activation of glial cells in the cerebral cortex and hippocampus of AD mouse models (Beauquis et al., 2013; Singhal et al., 2014). We further explored the interaction between SI and physical EE on neuroinflammation during the aging process. First, we found that GFAP positive astrocytes were activated in the mPFC and hippocampus of SI+SE mice compared with GR+SE controls, but astrogliosis was partially reduced in those raised in an enriched cage (Figure 6A). Compared with GR+SE mice, SI+SE mice showed increased percentages of GFAP positive area in the mPFC (P=.0211) and hippocampus (P=.0017), while a decreasing effect of physical EE was observed only in the hippocampus (P=.0318, SI+EE vs SI+SE) and not the mPFC (P=.1389, SI+EE vs SI+SE) (Figure 6C). Similarly, SI caused activation of Iba-1 positive microglia of the mPFC and hippocampus of aged mice (Figure 6B). The percentage of Iba-1 positive area was elevated by SI (P=.0182, SI+SE vs GR+SE) and decreased by physical EE (P=.0185, SI+EE vs SI+SE) in the hippocampus. However, for the mPFC, an alleviating effect of the physical EE on microglial activation was not observed (P=.0046, SI+SE vs GR+SE; P=.2807, SI+EE vs SI+SE) (Figure 6D).
Figure 6.
Enriched housing attenuated glial reactivation in the hippocampus of socially isolated (SI) aged mice. (A,B) Representative images showing expression and distribution of glial fibrillary acidic protein (GFAP)-positive astrocytes (A) and Iba-1 positive microglia (B) in the medial prefrontal cortex (mPFC) and hippocampus of mice with 4 different housing patterns. (C,D) The percentage of GFAP (C) and Iba-1 (D) positive areas in the mPFC and hippocampus, respectively. Enriched housing resulted in a significantly decreased percentage of positive area for GFAP or Iba-1 in the hippocampus but not mPFC of isolated mice. Housing (GFAP: mPFC, F1,12=16.780, P=.001; hippocampus, F1,12=39.400; Iba-1: mPFC, F1,12=31.869, P<.001; hippocampus, F1,12=10.057, P=.008); environment (GFAP: mPFC, F1,12=2.488, P=.141; hippocampus, F1,12=7.007, P=.021 Iba-1: mPFC, F1,12=2.597, P=.133; hippocampus, F1,12=10.674, P=.007); interaction effects (GFAP: mPFC, F1,12=0.595, P=.456; hippocampus, F1,12=1.553, P=.237; Iba-1: mPFC, F1,12=0.000, P=.994; hippocampus, F1,12=4.062, P=.067). #P<.05; ##P<.01, SI housing vs group housing (GR); *P<.05, enriched environment (EE) vs standard environment (SE). Data were analyzed by ANOVA with 2-way Tukey’s posthoc test and expressed as mean±SEM from 4 mice per group.
Physical EE Partially Reduces Neuroinflammation of SI Aging Mice
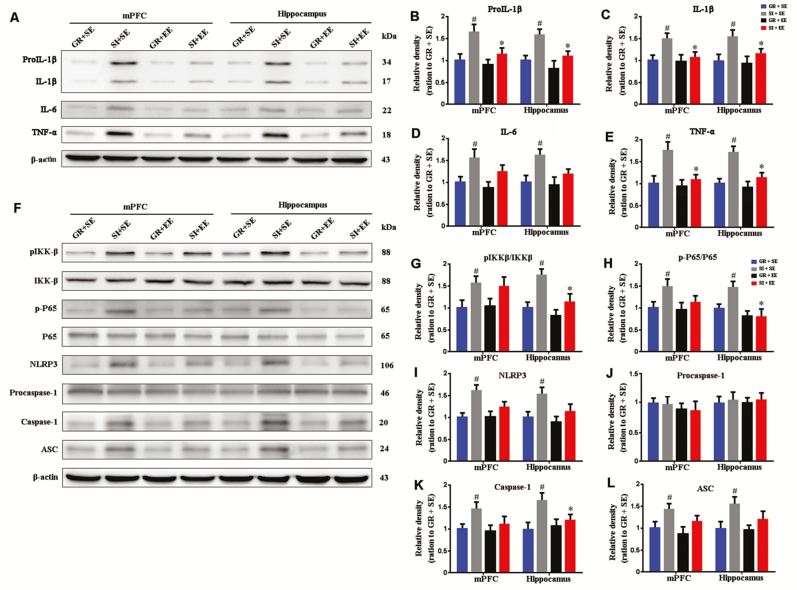
We examined levels of inflammatory factors in the mPFC and hippocampus of different groups by western blot (Figure 7A). When compared with GR+SE controls, SI+SE mice showed increased pro-IL-1β (mPFC, P=.0477; hippocampus, P=.0311) (Figure 7B), IL-1β (mPFC, P=.0393; hippocampus, P=.0239) (Figure 7C), IL-6 (mPFC, P=.0423; hippocampus, P=.0464) (Figure 7D), and TNF-α (mPFC, P=.0495; hippocampus, P=.0266) (Figure 7E). Isolated mice housed in an enriched environment had decreased levels of IL-1β and TNF-α, but not pro-IL-1β and IL-6, in the mPFC and hippocampus (pro-IL-1β: P=.0813, P=.0957; IL-1β: P=.0353; P=.0351; IL-6: P=.2583, P=.2168; TNF-α: P=.0440, P=.0484, respectively) (Figure 7B, C, and E).
Figure 7.
Enriched housing decreased brain proinflammatory factor levels and downregulated NOD-like receptor containing a pyrin domain 3 (NLRP3) inflammasome activation in the medial prefrontal cortex (mPFC) and hippocampus of socially isolated (SI) aged mice. (A) Representative western-blot bands for pro-interleukin (IL)-1β, IL-1β, IL-6, and tumor necrosis factor (TNF)-α in the mPFC and hippocampus of mice with 4 different housing patterns. (B–E) Densitometry analysis of the expression levels of pro-IL-1β (B), IL-1β (C), IL-6 (D), and TNF-α (E). Enriched housing significantly reduced expression levels of pro-IL-1β, IL-1β, and TNF-α in the mPFC and hippocampus of isolated mice. Housing (pro-IL-1β: mPFC, F1,12=7.379, P=.019; hippocampus, F1,12=8.650, P=.012; IL-1β: mPFC, F1,12=4.849, P=.048; hippocampus, F1,12=7.594, P=.017; IL-6: mPFC, F1,12=12.165, P=.004; hippocampus, F1,12=8.332, P=.014; TNF-α: mPFC, F1,12=5.455, P=.038; hippocampus, F1,12=7.881, P=.016); environment (pro-IL-1β: mPFC, F1,12=3.165, P=.101; hippocampus, F1,12=4.531, P=.055; IL-1β: mPFC, F1,12=3.950950, P=.070; hippocampus, F1,12=4.068, P=.067; IL-6: mPFC, F1,12=2.330, P=.153; hippocampus, F1,12=2.035, P=.179; TNF-α: mPFC, F1,12=4.226, P=.062; hippocampus, F1,12=5.643, P=.035); interaction effects (pro-IL-1β: mPFC, F1,12=1.685, P=.219; hippocampus, F1,12=0.982, P=.341; IL-1β: mPFC, F1,12=2.286, P=.156; hippocampus, F1,12=2.691, P=.127; IL-6: mPFC, F1,12=0.203, P=.660; hippocampus, F1,12=0.542, P=.476; TNF-α: mPFC, F1,12=2.877, P=.116; hippocampus, F1,12=2.969, P=.111) (F) Representative western-blot bands of phospho-P65 (p-P65), P65, phosphor-IKKβ (p-IKKβ), IKKβ, NLRP3, pro-caspase-1, and ASC in the mPFC and hippocampus of mice with 4 different housing patterns. (G–L) Densitometry analysis of the expression levels of p-P65/P65 (G), phosphor-IKKβ/IKKβ (H), NLRP3 (I), pro-caspase-1 (J), caspase-1 (K), and ASC (L). The enriched housing normalized p-P65/P65 ratios, p-IKKβ/IKKβ ratios, and caspase-1 in the hippocampus but not in the mPFC. Housing (p-IKKβ/IKKβ: mPFC, F1,12=7.294, P=.019; hippocampus, F1,12=19.763, P=.001; p-P65/P65: mPFC, F1,12=7.058, P=.021; hippocampus, F1,12=2.892, P=.115; NLRP3: mPFC, F1,12=10.149, P=.008; hippocampus, F1,12=7.626, P=.017; Caspase 1: mPFC, F1,12=5.014, P=.045; hippocampus, F1,12=7.001, P=.021; ASC: mPFC, F1,12=5.932, P=.031; hippocampus, F1,12=7.341, P=.019; procaspase-1: mPFC, F1,12=0.037, P=.851; hippocampus, F1,12=0.330, P=.576); environment (p-IKKβ/IKKβ: mPFC, F1,12=0.307, P=.590; hippocampus, F1,12=11.833, P=.005; p-P65/P65: mPFC, F1,12=0.680, P=.426; hippocampus, F1,12=5.620, P=.035; NLRP3: mPFC, F1,12=2.475, P=.142; hippocampus, F1,12=2.971, P=.110; Caspase 1: mPFC, F1,12=3.446, P=.088; hippocampus, F1,12=4.301, P=.060; ASC: mPFC, F1,12=1.808, P=.204; hippocampus, F1,12=2.269, P=.158; procaspase-1: mPFC, F1,12=0.694, P=.421; hippocampus, F1,12=0.002, P=.969); interaction effects (IKKβ/IKKβ: mPFC, F1,12=0.483, P=.500; hippocampus, F1,12=1.543, P=.238; p-P65/P65: mPFC, F1,12=0.743, P=.405; hippocampus, F1,12=2.862, P=.117; NLRP3: mPFC, F1,12=2.879, P=.116; hippocampus, F1,12=0.911, P=.359; Caspase 1: mPFC, F1,12=1.862, P=.197; hippocampus, F1,12=2.322, P=.153; ASC: mPFC, F1,12=0.370, P=.554; hippocampus, F1,12=1.081, P=.319; procaspase-1: mPFC, F1,12=0.010, P=.921; hippocampus, F1,12=0.021, P=.887). #P<.05, socially isolated housing (SI) vs grouping housing (GR); *P<.05, enriched environment (EE) vs standard environment (SE). Data were analyzed by 2-way ANOVA with Tukey’s posthoc test and expressed as mean±SEM from 4 mice per group.
Inflammasomes are multiprotein complexes that can induce inflammation in response to cellular infection or stress (Schroder and Tschopp, 2010). The NLRP3 inflammasome is well documented in various pathological conditions (Heneka et al., 2013). Activation of NLRP3 inflammasome requires 2 steps: NF-κB activation and NLRP3 complex formation (Mitchell et al., 2016). Thus, we examined levels of p-IKKβ, IKKβ, p-P65, and P65, several key markers involved in the NF-κB pathway, among different groups (Figure 7F). Compared with mice in the SI+SE group, GR+SE mice revealed increased p-IKKβ/IKKβ and p-P65/P65 ratios in the mPFC (P=.0124, P=.0468, respectively) and hippocampus (P=.0119, P=.0489, respectively) (Figure 7G, H). Isolated housing in an enriched environment decreased IKKβ/IKKβ and p-P65/P65 in the hippocampus (P=.0237, P=.0445, SI+EE vs SI+SE, respectively) but not the mPFC of aged mice (P=.2581, P=.2891, SI+EE vs SI+SE, respectively) (Figure 7G, H).
We also investigated several proteins involved in NLRP3 inflammasome formation. SI increased the expression levels of NLRP3, Caspase 1, and ASC in both the mPFC and hippocampus (NLRP3: P=.0142, P=.0335; Caspase 1: P=.0354, P=.0114; ASC: P=0.416, P=.0433, GR+SE vs SI+SE, respectively). However, the mitigating effect of physical EE was very limited, reflected by only downregulation of hippocampal Caspase-1 expression (P=.0343, SI+SE vs SI+EE), without changes in other parameters (Figure 7F, I-L).
Discussion
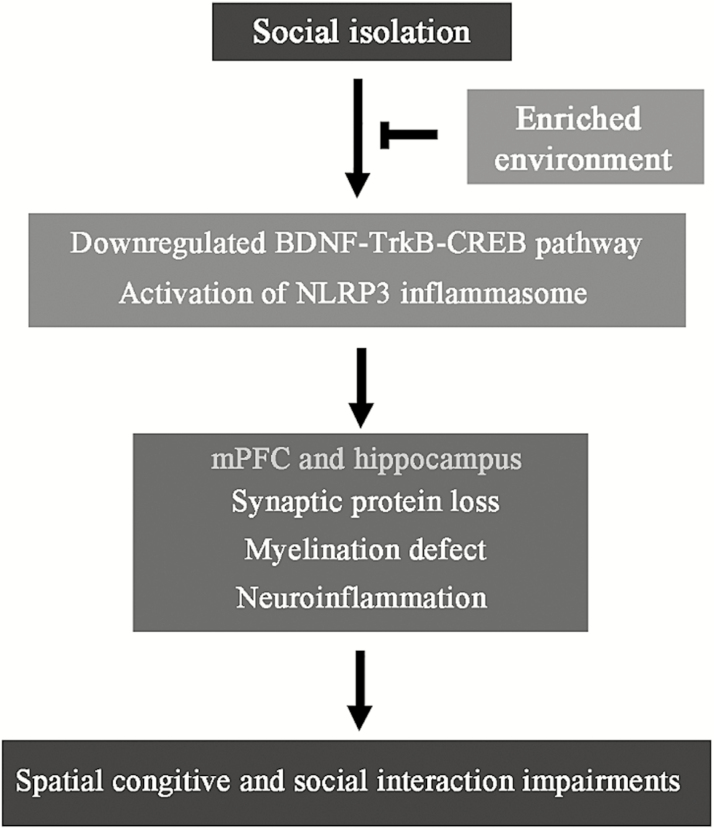
This study was designed to investigate the effects of SI on cognitive and social functions and histopathological characteristics of the mPFC and hippocampus of aged mice. We also examined whether physical EE would exert protection against these impairments. The results have shown that, after 2 months of isolation, 20-month-old mice display apparent declines in spatial learning and memory, as well as social interaction and memory, relative to age-matched littermates exposed to group housing. Consistently, the pathological and biochemical analyses have revealed that SI increases synaptic protein loss, myelin damage, reactive gliosis, and neuroinflammation in the mPFC and hippocampus. The functional and pathological changes of these brain areas are related to disturbances in BDNF-TrkB-CREB and NLRP3 inflammasome. These changes in aged SI mice are partially or completely mitigated by enriched housing (Figure 8). This finding reveals the effects and mechanisms of EE on cognitive improvement in aged SI mice, thus providing a fundamental basis that would allow establishment of a potential nonpharmacological therapeutic strategy against heightened aging process of the elderly with SI.
Figure 8.
Schematic diagram of potential mechanisms underlying the beneficiary effects of enriched housing on spatial and social memory of socially isolated (SI) aged mice.
Accumulative evidence reveals negative influences of SI on brain structure and function in young and adult rodents (Cacioppo et al., 2011; Huang et al., 2015; Cao et al., 2017). For example, protracted SI impairs myelination and ultrastructure of oligodendrocytes in the mPFC of adult mice (Liu et al., 2012b). Additionally, SI has been shown to aggravate the pathological process of AD model mice. Two to 3 months of isolation exacerbates cognitive impairment in young or aged APP/PS1 mice, which is associated with severe brain inflammation and Aβ pathology (Dong et al., 2004; Cacioppo et al., 2011; Powell et al., 2013; Huang et al., 2015). Each of these studies highlights that a normal social life is necessary for maintaining brain development and maturation as well as for delaying aging and neurodegenerative processes.
Furthermore, the present results reveal the influence of age on brain functional impairments of SI mice. For example, compared with our previous study on young adult mice (Cao et al., 2017), SI in aged mice exhibits more severe negative effects on behavioral performance in the Y-maze test, showing obvious decreases in time percentages (decreased by 19.05% in young adult mice vs 42.72% in aged mice) and entering numbers in the NA (decreased by 21.15% in young adult mice vs 59.91% in aged mice).
Previous studies suggest that EE produces a neuroprotective effect on young and adult rodents, improving the branch number and length of dendrites, synaptic plasticity, neurogenesis, and spatial memory capacity (Nithianantharajah and Hannan, 2006; Wolf et al., 2006; Lonetti et al., 2010; Williamson et al., 2012; Scholz et al., 2015; Bechard et al., 2016; Garthe et al., 2016; He et al., 2017; Soares et al., 2017). Our previous studies have also shown that 1-month-old mice raised in an EE for 8 weeks exhibit better spatial and social memory-related behavioral performances than those housed in a standard environment (Cao et al., 2017). Functional improvement of EE on young adult mice is supported by pathological evidence, displaying increases in SYN and PSD-95 levels, and thickness of myelin sheaths, in the mPFC and hippocampus. Consistently, the BDNF-TrkB-CREB signaling pathway is upregulated to some extent in the 2 brain regions.
Conversely, the present study found that 18-month-old mice in the group of GR exposure to EE for 2 months do not show improving effects on the spatial and social memory and expression levels of SYN and PSD-95 in the mPFC and hippocampus (Figure 3). Similarly, previous studies reported that exposure to EE during early adulthood of Tg2576 mice reduces the severity of AD-related cognitive deficits more effectively than in aged mice (Verret et al., 2013). Indeed, epidemiological studies highlight that education, enriched social experience, and professional training in early life exert long-lasting protection against brain aging and AD progression (Norton et al., 2012; Sattler et al., 2012). Together, these results suggest that brain structure and functional plasticity in response to the EE stimuli gradually decreases as age increases. However, the beneficial efficiency of EE on myelination remains in the mPFC and hippocampus of aged mice, revealed by decreases in g ratios. The mechanism underlying the differences in protective effects of enriched housing on neurons and myelin sheaths in aged mice needs further study.
It is worth noting that physical EE completely corrects the spatial memory decline caused by SI. Deficits in social interaction ability are also partially alleviated. In line with behavioral reverse, the loss of synaptic proteins and the damage to the myelin sheath are attenuated in the mPFC and hippocampus of SI+EE mice. Furthermore, the present study revealed that physical EE mitigates alterations of the BDNF-TrkB-CREB signaling pathway in these brain regions of aged isolated mice. Notably, our previous study discovered that physical EE attenuates synaptic protein loss, neuronal apoptosis, and myelination impairment in the hippocampus but not in the mPFC of young adult SI mice (Cao et al., 2017). In the present study, aged SI mice were exposed to EE for 2 months, and results found that the loss of synaptic proteins, damage of the myelin sheath, and decreased BDNF signal pathway are alleviated in both the mPFC and hippocampus. Accordingly, social behavior that is mainly controlled by the mPFC is partially improved in SI+EE aged mice. These results suggest that SI has more severe damaging effects on developing mice than aging ones.
There is growing evidence showing a close interrelation between SI stress and neuroinflammation (Van Praag et al., 2000). Consistently, the present study demonstrates glial cell activation and increases in inflammatory cytokine IL-1β, IL-6, and TNF-α accompanied with NLRP3 inflammasome activation in the mPFC and hippocampus of aged SI mice. Unlike the complete correction of synaptic and myelin damage in the above brain regions, physical EE ameliorates activation of astrocytes and microglia only in the hippocampus rather than in the mPFC of aged SI mice. NLRP-3 inflammasome activation is not attenuated in the mPFC of SI +EE mice compared with SI+SE controls. For the hippocampus, only the NF-κB pathway, the first signal of NLRP3 activation, has been normalized. The potential mechanism of these brain region differences needs to be further explored. In addition, physical EE decreases IL-1β and TNF-α but not IL-6 in the above brain regions, and the underlying mechanism remains unclear. Previous studies have reported that SI impairs remyelination via modulation of IL-6 (Makinodan et al., 2016). Apart from the brain, infiltrating cells from peripheral tissues are also a source of IL-6 after SI (Erta et al., 2012). Indeed, an elevation of systemic IL-6 levels has been observed in SI after experimental stroke (Venna et al., 2012) as well as in children who have an increased risk of developing depression and psychosis in young adulthood (Khandaker et al., 2014). The pathophysiological role of peripheral IL-6 in the isolated aged population warrants further investigation.
It is necessary to point out that EE enhances social, physical, and somatosensory stimulation via larger group housing, running wheels, and other extra novel objects (Van Praag et al., 2000). The present study has demonstrated that EE without social simulation can prevent cognitive dysfunction in aged SI mice. This protection could be the combined effect of both physical exercise and novel visual stimulation. It is well known that EE can enhance brain plasticity and cognitive reserves in the elderly (Kempermann et al., 2002; Leal-Galicia et al., 2008). However, social interaction alone has been shown to improve novel object location memory and reduces hippocampal inflammation (Smith et al., 2018). On the other hand, long-term exercise plays a positive effect on hippocampal neurogenesis throughout life (Hamilton et al., 2015), but exercise-induced neurogenesis can be significantly averted by SI (Stranahan et al., 2006; Leasure and Decker, 2009). Moreover, an early study has demonstrated increased neurogenesis following EE in the absence of exercise in a mouse model of AD independent of amyloid plaque load (Mirochnic et al., 2009). Recently, Birch and Kelly (2018) reported that lifelong EE without exercise protects the brain from age-related cognitive deficit, which is associated with increases in hippocampal apoptosis and proinflammatory markers and decreases in brain blood flow. Together, these data suggest somatosensory and visual stimulations, rather than physical exercise, may play a leading role in mitigating SI-induced cognitive dysfunction in aged mice. Further study is necessary to validate this hypothesis, and potential findings will help to establish a straightforward nonpharmacological strategy against brain aging.
Additionally, the current study focuses only on pathological and biochemical changes of the hippocampus and mPFC that are responsible for spatial cognition and social activity. Effects of SI and/or EE on other brain regions involved in anxiety and emotion control, such as the amygdala and nucleus accumbens, warrant further investigation (Floresco, 2015; Fareri and Tottenham, 2016; Gothard et al., 2018). Furthermore, the effects of SI and/or EE on the neuroendocrine system, which is crucial for regulation of aging process, are still unknown (Esquifino et al., 2007).
In conclusion, this study revealed mitigating effects of physical EE on brain impairments caused by SI in aged mice. This finding provides a fundamental basis for developing effective nonpharmacological strategies benefiting brain health in the elderly.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81801378, 81671070, and 81271210).
Acknowledgments
Thanks to Zhengrong Xia for providing excellent technical support for electron microscopy.
Statement of Interest
None.
References
- Arendt T, Ueberham U, Janitz M(2017)Non-coding transcriptome in brain aging. Aging (Albany NY) 9:1943–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauquis J, Pavía P, Pomilio C, Vinuesa A, Podlutskaya N, Galvan V, Saravia F(2013)Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of alzheimer’s disease. Exp Neurol 239:28–37. [DOI] [PubMed] [Google Scholar]
- Bechard AR, Cacodcar N, King MA, Lewis MH(2016)How does environmental enrichment reduce repetitive motor behaviors? Neuronal activation and dendritic morphology in the indirect basal ganglia pathway of a mouse model. Behav Brain Res 299:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sadoun G, Sacco G, Manera V, Bourgeois J, König A, Foulon P, Fosty B, Bremond F, d’Arripe-Longueville F, Robert P(2016)Physical and cognitive stimulation using an exergame in subjects with normal aging, mild and moderate cognitive impairment. J Alzheimers Dis 53:1299–1314. [DOI] [PubMed] [Google Scholar]
- Birch AM, Kelly ÁM(2018)Lifelong environmental enrichment in the absence of exercise protects the brain from age-related cognitive decline. Neuropharmacology doi: 10.1016/j.neuropharm.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG(2011)Social isolation. Ann N Y Acad Sci 1231:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Pu T, Wang L, Marshall C, He H, Hu G, Xiao M(2017)Early enriched physical environment reverses impairments of the hippocampus, but not medial prefrontal cortex, of socially-isolated mice. Brain Behav Immun 64:232–243. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Duman RS, Nestler EJ(2005)The many faces of CREB. Trends Neurosci 28:436–445. [DOI] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG(2004)Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in appsw (tg2576) mutant mice by isolation stress. Neuroscience 127:601–609. [DOI] [PubMed] [Google Scholar]
- Eng PM, Rimm EB, Fitzmaurice G, Kawachi I(2002)Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol 155:700–709. [DOI] [PubMed] [Google Scholar]
- Erta M, Quintana A, Hidalgo J(2012)Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 8:1254–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquifino AI, Cano P, Jiménez-Ortega V, Fernández-Mateos P, Cardinali DP(2007)Neuroendocrine-immune correlates of circadian physiology: studies in experimental models of arthritis, ethanol feeding, aging, social isolation, and calorie restriction. Endocrine 32:1–19. [DOI] [PubMed] [Google Scholar]
- Fareri DS, Tottenham N(2016)Effects of early life stress on amygdala and striatal development. Dev Cogn Neurosci 19:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fares RP, Belmeguenai A, Sanchez PE, Kouchi HY, Bodennec J, Morales A, Georges B, Bonnet C, Bouvard S, Sloviter RS, Bezin L(2013)Standardized environmental enrichment supports enhanced brain plasticity in healthy rats and prevents cognitive impairment in epileptic rats. Plos One 8:e53888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD.(2008)White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB.(2015)The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol 66:25–52. [DOI] [PubMed] [Google Scholar]
- Friedler B, Crapser J, McCullough L(2015)One is the deadliest number: the detrimental effects of social isolation on cerebrovascular diseases and cognition. Acta Neuropathol 129:493–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Roeder I, Kempermann G(2016)Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus 26:261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Mosher CP, Zimmerman PE, Putnam PT, Morrow JK, Fuglevand AJ(2018)New perspective on the neurophysiology of pirmate amygdala emerging from the study of naturalistic social behaviors. Wiley Interdiscip Rev Cogn Sci 9. doi: 10.1002/wcs.1449 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Rhodes JS(2015)Exercise regulation of cognitive function and neuroplasticity in the healthy and diseased brain. Prog Mol Biol Transl Sci 135:381–406. [DOI] [PubMed] [Google Scholar]
- Hannan AJ.(2014)Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol 40:13–25. [DOI] [PubMed] [Google Scholar]
- He C, Tsipis CP, LaManna JC, Xu K(2017)Environmental enrichment induces increased cerebral capillary density and improved cognitive function in mice. Adv Exp Med Biol 977:175–181. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT(2013)NLRP3 is activated in alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario WF, Herlinger AL, Areal LB, de Moraes LS, Ferreira TA, Andrade TE, Martins-Silva C, Pires RG(2016)Cholinergic and dopaminergic alterations in nigrostriatal neurons are involved in environmental enrichment motor protection in a mouse model of parkinson’s disease. J Mol Neurosci 60:453–464. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D(2015)Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 10:227–237. [DOI] [PubMed] [Google Scholar]
- Huang H, Wang L, Cao M, Marshall C, Gao J, Xiao N, Hu G, Xiao M(2015)Isolation housing exacerbates alzheimer’s disease-like pathophysiology in aged APP/PS1 mice. Int J Neuropsychopharmacol 18:pyu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliffe S, Kharicha K, Harari D, Swift C, Gillmann G, Stuck AE(2007)Health risk appraisal in older people 2: the implications for clinicians and commissioners of social isolation risk in older people. Br J Gen Pract 57:277–282. [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH(2002)Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 52:135–143. [DOI] [PubMed] [Google Scholar]
- Kotloski RJ, Sutula TP(2015)Environmental enrichment: evidence for an unexpected therapeutic influence. Exp Neurol 264:121–126. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB(2014)Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, Bartley M(2014)Social isolation in childhood and adult inflammation: evidence from the national child development study. Psychoneuroendocrinology 50:85–94. [DOI] [PubMed] [Google Scholar]
- Leal-Galicia P, Castañeda-Bueno M, Quiroz-Baez R, Arias C(2008)Long-term exposure to environmental enrichment since youth prevents recognition memory decline and increases synaptic plasticity markers in aging. Neurobiol Learn Mem 90:511–518. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Decker L(2009)Social isolation prevents exercise-induced proliferation of hippocampal progenitor cells in female rats. Hippocampus 19:907–912. [DOI] [PubMed] [Google Scholar]
- Liu L, Lu Y, Kong H, Li L, Marshall C, Xiao M, Ding J, Gao J, Hu G (2012a) Aquaporin-4 deficiency exacerbates brain oxidative damage and memory deficits induced by long-term ovarian hormone deprivation and D-galactose injection. Int J Neuropsychopharmacol 15:55–68. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P (2012b) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15:1621–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston-Thomas J, Nelson P, Karthikeyan S, Antonescu S, Jeffers MS, Marzolini S, Corbett D(2016)Exercise and environmental enrichment as enablers of task-specific neuroplasticity and stroke recovery. Neurotherapeutics 13:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T(2010)Early environmental enrichment moderates the behavioral and synaptic phenotype of mecp2 null mice. Biol Psychiatry 67:657–665. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Ikawa D, Miyamoto Y, Yamauchi J, Yamamuro K, Yamashita Y, Toritsuka M, Kimoto S, Okumura K, Yamauchi T, Fukami SI, Yoshino H, Wanaka A, Kishimoto T(2016)Social isolation impairs remyelination in mice through modulation of IL-6. Faseb J 30:4267–4274. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G(2012)A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337:1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL(2006)Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia 53:372–381. [DOI] [PubMed] [Google Scholar]
- Mirochnic S, Wolf S, Staufenbiel M, Kempermann G(2009)Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of alzheimer disease. Hippocampus 19:1008–1018. [DOI] [PubMed] [Google Scholar]
- Mitchell S, Vargas J, Hoffmann A(2016)Signaling via the nfκb system. Wiley Interdiscip Rev Syst Biol Med 8:227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A(2007)Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev 55:78–88. [DOI] [PubMed] [Google Scholar]
- Narme P.(2016)Benefits of game-based leisure activities in normal aging and dementia. Geriatr Psychol Neuropsychiatr Vieil 14:420–428. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ(2006)Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7:697–709. [DOI] [PubMed] [Google Scholar]
- Norton MC, Dew J, Smith H, Fauth E, Piercy KW, Breitner JC, Tschanz J, Wengreen H, Welsh-Bohmer K, Cache County Investigators (2012)Lifestyle behavior pattern is associated with different levels of risk for incident dementia and alzheimer’s disease: the cache county study. J Am Geriatr Soc 60:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novkovic T, Mittmann T, Manahan-Vaughan D(2015)BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus 25:1–15. [DOI] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JM, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, Cole SW(2013)Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci U S A 110:16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic AD, Kraig RP(2014)Youth and environmental enrichment generate serum exosomes containing mir-219 that promote CNS myelination. Glia 62:284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusic KM, Pusic AD, Kraig RP(2016)Environmental enrichment stimulates immune cell secretion of exosomes that promote CNS myelination and may regulate inflammation. Cell Mol Neurobiol 36:313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radabaugh HL, LaPorte MJ, Greene AM, Bondi CO, Lajud N, Kline AE(2017)Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury. Exp Neurol 294:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmin VV, Komleva YK, Kuvacheva NV, Morgun AV, Khilazheva ED, Lopatina OL, Pozhilenkova EA, Shapovalov KA, Uspenskaya YA, Salmina AB(2017)Differential roles of environmental enrichment in Alzheimer’s type of neurodegeneration and physiological aging. Front Aging Neurosci 9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler C, Toro P, Schönknecht P, Schröder J(2012)Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and alzheimer’s disease. Psychiatry Res 196:90–95. [DOI] [PubMed] [Google Scholar]
- Scholz J, Allemang-Grand R, Dazai J, Lerch JP(2015)Environmental enrichment is associated with rapid volumetric brain changes in adult mice. Neuroimage 109:190–198. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J(2010)The inflammasomes. Cell 140:821–832. [DOI] [PubMed] [Google Scholar]
- Seeman TE.(2000)Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot 14:362–370. [DOI] [PubMed] [Google Scholar]
- Shimada K, Yamazaki S, Nakano K, Ngoma AM, Takahashi R, Yasumura S(2014)Prevalence of social isolation in community-dwelling elderly by differences in household composition and related factors: from a social network perspective in urban japan. J Aging Health 26:807–823. [DOI] [PubMed] [Google Scholar]
- Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E(2014)Arterial stiffness, the brain and cognition: a systematic review. Ageing Res Rev 15:16–27. [DOI] [PubMed] [Google Scholar]
- Singhal G, Jaehne EJ, Corrigan F, Baune BT(2014)Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front Cell Neurosci 8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillings EA, Wood NI, Morton AJ(2014)Beneficial effects of environmental enrichment and food entrainment in the R6/2 mouse model of huntington’s disease. Brain Behav 4:675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BM, Yao X, Chen KS, Kirby ED(2018)A larger social network enhances novel object location memory and reduces hippocampal microgliosis in aged mice. Front Aging Neurosci 10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares RO, Horiquini-Barbosa E, Almeida SS, Lachat JJ(2017)Environmental enrichment protects spatial learning and hippocampal neurons from the long-lasting effects of protein malnutrition early in life. Behav Brain Res 335:55–62. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E(2006)Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci 9:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström G, Fransson E, Malmberg B, Davey A(2009)Loneliness among older europeans. Eur J Ageing 6:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH(2000)Neural consequences of environmental enrichment. Nat Rev Neurosci 1:191–198. [DOI] [PubMed] [Google Scholar]
- Venna VR, Weston G, Benashski SE, Tarabishy S, Liu F, Li J, Conti LH, McCullough LD(2012)NF-κb contributes to the detrimental effects of social isolation after experimental stroke. Acta Neuropathol 124:425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Krezymon A, Halley H, Trouche S, Zerwas M, Lazouret M, Lassalle JM, Rampon C(2013)Transient enriched housing before amyloidosis onset sustains cognitive improvement in tg2576 mice. Neurobiol Aging 34:211–225. [DOI] [PubMed] [Google Scholar]
- Wang H, Peng RY(2016)Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Mil Med Res 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LL, Chao A, Bilbo SD(2012)Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav Immun 26:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G(2006)Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of alzheimer’s disease. Biol Psychiatry 60:1314–1323. [DOI] [PubMed] [Google Scholar]
- Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, Cai Z, Wu T, Hu G, Xiao M(2015)Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain aβ accumulation and memory deficits. Mol Neurodegener 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]