Abstract
Background
Recent studies have highlighted the possible risk of neuropsychiatric adverse effects during treatment with lipid-lowering medications. However, there are still controversies that require a novel genetic-based approach to verify whether the impact of lipid-lowering drug treatment results in neuropsychiatric troubles including insomnia, depression, and neuroticism. Thus, we applied Mendelian randomization to assess any potential neuropsychiatric adverse effects of conventional lipid-lowering drugs such as statins, proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, and ezetimibe.
Methods
A 2-sample Mendelian randomization study was conducted based on summary statistics from genome-wide association studies for lipids, insomnia, depression, and neuroticism. Single-nucleotide polymorphisms located in or near drug target genes of HMGCR, PCSK9, and NPC1L1 were used as proxies for statins, PCSK9 inhibitors, and ezetimibe therapy, respectively. To assess the validity of the genetic risk score, their associations with coronary artery disease were used as a positive control.
Results
The Mendelian randomization analysis showed a statistically significant (P <.004) increased risk of depression after correcting for multiple testing with both statins (odds ratio=1.15, 95% CI: 1.04–1.19) and PCSK9 inhibitor treatment (odds ratio =1.19, 95%CI: 1.1–1.29). The risk of neuroticism was slightly reduced with statin therapy (odds ratio=0.9, 95%CI: 0.83–0.97). No significant adverse effects were associated with ezetimibe treatment. As expected, the 3 medications significantly reduced the risk of coronary artery disease.
Conclusion
Using a genetic-based approach, this study showed an increased risk of depression during statin and PCSK9 inhibitor therapy while their association with insomnia risk was not significant.
Keywords: statin, ezetimibe, PSCK9 inhibitor, neuropsychiatric adverse effect, Mendelian randomization
Significance Statement
Statins and PCSK9 inhibitors are significantly associated with increased risk of depression as evident by findings of a significant association between genetic variants within HMGCR and PCSK9 genes that are associated with lower LDL-C and higher risk of depression. This finding appears to be a result of on-target effects of both medications since reduction LDL-C through other biological pathways were not associated with such side effects.
Introduction
Statins are the main class of lipid-lowering medication that acts by competitively inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), a rate-limiting enzyme of the mevalonate pathway, which leads to downregulation of primary metabolism and results in a dose-response reduction in the risk of cardiovascular events (Cholesterol Treatment Trialists Collaboration, 2012). Statins were the most widely prescribed pharmacological class between 1999 and 2012, and their use is expected to increase after releasing the new American College of Cardiology and American Heart Association (ACC/AHA) guidelines for the management of cholesterol (Stone et al., 2014). Although statins are well tolerated with an excellent safety profile, severe adverse effects related to muscle and liver toxicity may rarely occur. Specifically, recent interest has been shifted toward potential risk of adverse neuropsychiatric impacts related to statins and other lipid-lowering medications including PCSK9 inhibitors (Tuccori et al., 2014; Cham et al., 2016). Importantly, the accumulating evidence from medical literature and case reports has led the Medicines and Healthcare products Regulatory Agency (MHRA; 2009) and European Medicine Agency (EMA; 2009) to update the patient information leaflet for all statins and to include depression, sleep disturbances, and memory loss as potential undesirable effects.
Studies designed to investigate specific neuropsychiatric adverse effects have yielded conflicting results due to differences in study methodology, population, and medications types. For instance, randomized-controlled trials (RCTs) that assessed the risk of depression with statins reported conflicting results, ranging from a significantly high risk of depression among the statins group (Hyyppä et al., 2003; Morales et al., 2006) opposed to a significant difference between statins and control groups (Muldoon et al., 2000; Stewart et al., 2000) to the finding of a protective effect of statins against the risk of depression (Kim et al., 2015). Similar inconsistency has been observed for the risk of sleep disturbances with a meta-analysis of 5 RCTs reporting no effects of statins on sleep quality (Broncel et al., 2015). However, based on data analysis from a postmarketing surveillance database, a strong signal of potential association between statins use and sleep disturbances has been found (Takada et al., 2014). Hence, this inconsistency between studies necessitates the use of a novel approach to clarify if statins cause these neuropsychiatric adverse effects.
Mendelian randomization (MR) is an alternative approach that has been utilized to predict drug adverse effects using genetic variants as instrument variable (IV) to assess the causal relationship of an exposure on an outcome (Bennett and Holmes, 2017). For detection of the unintended drug effects, MR study utilizes genetic variants located in the vicinity of the drug target gene (e.g., HMGCR) as IV for exposure to the medication (e.g., statins). For instance, concordant findings were reported between RCT studies and MR that suggested no beneficial effects of statins on the risk of Alzheimer’s disease (Feldman et al., 2010; Benn et al., 2017). A recent study has reported an MR study assessing the risk of dementia diseases using genetic risk score (GRS) that reflects lowering low-density lipoprotein cholesterol (LDL-C) specifically through HMGCR, PCSK9, or both (Benn et al., 2017). The null association for these genetic scores suggested lack of evidence for the roles of LDL-C in the pathogenesis of these diseases as well as pharmacological therapy that target HMGCR and PCSK9.
This study has aimed to construct GRSs that mimic the biological effects of inhibiting HMGCR (i.e., drug target gene of statin), PCSK9 (drug target gene of PCSK9 inhibitors), and NPC1L1 (drug target gene of ezetimibe) as well as an overall genetically lower LDL-C level to find out whether any of these GRSs is associated explicitly with risk of depression and sleep disturbances, as reported by MHRA and EMA reports. We have also assessed the association of these GRSs with the risk of neuroticism, a personality trait that is characterized by easily experiencing negative emotions such as anxiety and fear. HMGCR, PCSK9, and NPC1L1 GRS association with coronary artery disease (CAD) was used as a positive control.
Methods
Ethical Approval
This study used publically available summary results from genome-wide association studies (GWAS), which exempted the requirement of ethical approval. Ethical approval for the original studies was mentioned in the source studies. This present research adhered to the Declaration of Helsinki.
Study Population
Summary statistics obtained from the GWAS database were utilized for this study. In regards to statins effects, the Global Lipid Genetics Consortium (GLGC) summary results were used to estimate the reduction in LDL-C due to genetic variations as an instrumental variable (Willer et al., 2013). The GLGC studied lipid profile (high-density lipoprotein cholesterol [HDL-C], LDL-C, triglycerides, and total cholesterol) in more than 188000 individuals from 60 studies using a genome-wide array scan after adjusting for sex, age, genomic control inflation factor, and study-specific variables (Willer et al., 2013). Concerning insomnia, summary results were used from the Hammerschlag et al. (2017) study, which performed a GWAS in more than 113000 subjects from the UK BioBank Study. This GWAS focused on insomnia as measured by experiencing trouble falling asleep or waking up in the middle of the night. The participants answered a touch screen multiple-choice questionnaire including “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” A help button showed the following information: “If this varies a lot, answer this question in relation to the last 4 weeks.” The participants had 4 multiple choice answers to choose from: “never/rarely,” “sometimes,” “usually,” or “prefer not to answer.” Cases were defined as participants who answered “usually” and controls those answered with “never/rarely” or “sometimes.” Validation of the discriminative validity of this questionnaire in independent sample Netherlands Sleep Registry showed a good discriminative validity.
In regards to depression and neuroticism, the summary results were based on the Social Science Genetic Association Consortium (SSGAC) that performed a meta-analysis from 3 cohorts and conducted a GWAS of major depressive disorder (n=180866) and neuroticism (n=170911) by combining data from the Psychiatric Genomics Consortium with UK BioBank and Genetic Epidemiology Research on Aging (Okbay et al., 2016). Different survey instruments and surveys were used in each cohort for defining each phenotype as described in the supplemental material of the original study. However, estimating the pairwise genetic correlations between the different measures used by each cohort showed a high correlation (Okbay et al., 2016). Finally, summary results for CAD were based on the CARDIoGRAMplusC4D Consortium that conducted a meta-analysis of 185000 CAD cases and controls (Nikpay et al., 2015).
SNP Selection
Several single nucleotide polymorphisms (SNPs) were selected based on their significant association with the primary biomarker (i.e., LDL-C) and their genomic locations near lipid-lowering medication drug targets. First, a simple analysis was performed considering all the genetic variants that were significantly associated with LDL-C in GLGC, in which 54 variants were selected to be included in LDL-C GRS to assess the effect of genetically reduced LDL-C on each adverse effect (supplementary Table 1). Finding a significant association with this GRS implies that adverse effects may be a result of a biomarker effect rather than a specific mechanism effect. Second, we tested the presence of mechanism effects for each lipid-lowering medication by selecting genetic variants that are located in each class of gene-targeted drugs to serve as proxies for medication. For this reason, Ensembl Genome Browser was used to identify all genetic variants that are located within each gene region, including variants in HMGCR, NPC1L1, and PCSK9 genes (using the GRCh38.p10 Assembly) and were used as proxies for statins, ezetimibe, and PCSK9 inhibitors, respectively (supplementary Figure 1). Remarkably, a metabolic profiling study found a high correlation in the metabolic profile of statins and rs12916 in HMGCR, which confirms the validity of this variant to investigate the causal molecular effects of HMGCR inhibition (Würtz et al., 2016). From all the identified variants in each gene, only variants that are significantly associated with LDL-C were considered as candidate proxies for each medication class, and then their summary results were also extracted from each outcome GWAS (Willer et al., 2013; Nikpay et al., 2015; Okbay et al., 2016; Hammerschlag et al., 2017). To select only SNPs that are independently associated with LDL-C, an iterative exclusion was performed to include only SNPs in low linkage disequilibrium (r2<0.6 for all comparisons; using European population as a reference; supplementary Table 2). For all variants, the reported effect size was oriented to the allele associated with a decreased lipid fraction and expressed in 1 SD of reduced lipid fraction per allele. The same methods were applied to other secondary lipid fractions (HDL, total cholesterol, and triglycerides; supplementary Tables 3–5).
Statistical Analysis
All the statistical analyses were performed by 2-samples MR using inverse-variance weighted (IVW) methods with uncorrelated variants (Burgess et al., 2015). For all the selected genetic variants, the effect estimates of each variant on outcome were regressed on its effect on LDL-C. The estimates were then pooled using a random meta-analytic model to produce a summary measure of the effect of genetically reduced LDL-C on outcome risk. To check for the presence of pleiotropy that could bias the MR estimates (i.e., sensitivity analysis), we applied MR-Egger regression and weighted median methods (Bowden et al., 2015, 2016). Briefly, the MR regression utilizes the Egger regression method that is traditionally applied in meta-analysis literature review to check for publication bias (Egger et al., 1997). In this method, the SNP effect on LDL-C is plotted against its effect on the outcome, and the presence of pleiotropy is evident if the intercept is significantly distinct from the origin (Bowden et al., 2015). Importantly, this method can provide unbiased estimates even if all the chosen SNPs are invalid (Bowden et al., 2015). Furthermore, the presence of pleiotropy was assessed by performing a confirmatory MR analysis using the weighted median approach, which is assumed to provide unbiased estimates as long as up to 50% of the total weight comes from variants without pleiotropic effects (Bowden et al., 2016). Compared with the MR-Egger regression method, the weighted median is more robust to violation of IV assumptions and substantially improves precision. Hence, we utilized both methods as sensitivity analyses to assess whether pleiotropy had influenced our results (Bowden et al., 2016). All analysis were performed using the “TwoSampleMR” package for the R Statisical software platformhttps://cran.r-pro-ject.org/web/packages/MendelianRandomization/. The significant association was considered after correcting for multiple testing (P < .0031; 0.05/16 comparisons).
Results
The Impacts of Lipid Biomarkers with Neuropsychiatric Adverse Effects (Testing the Presence of Biomarker Effects)
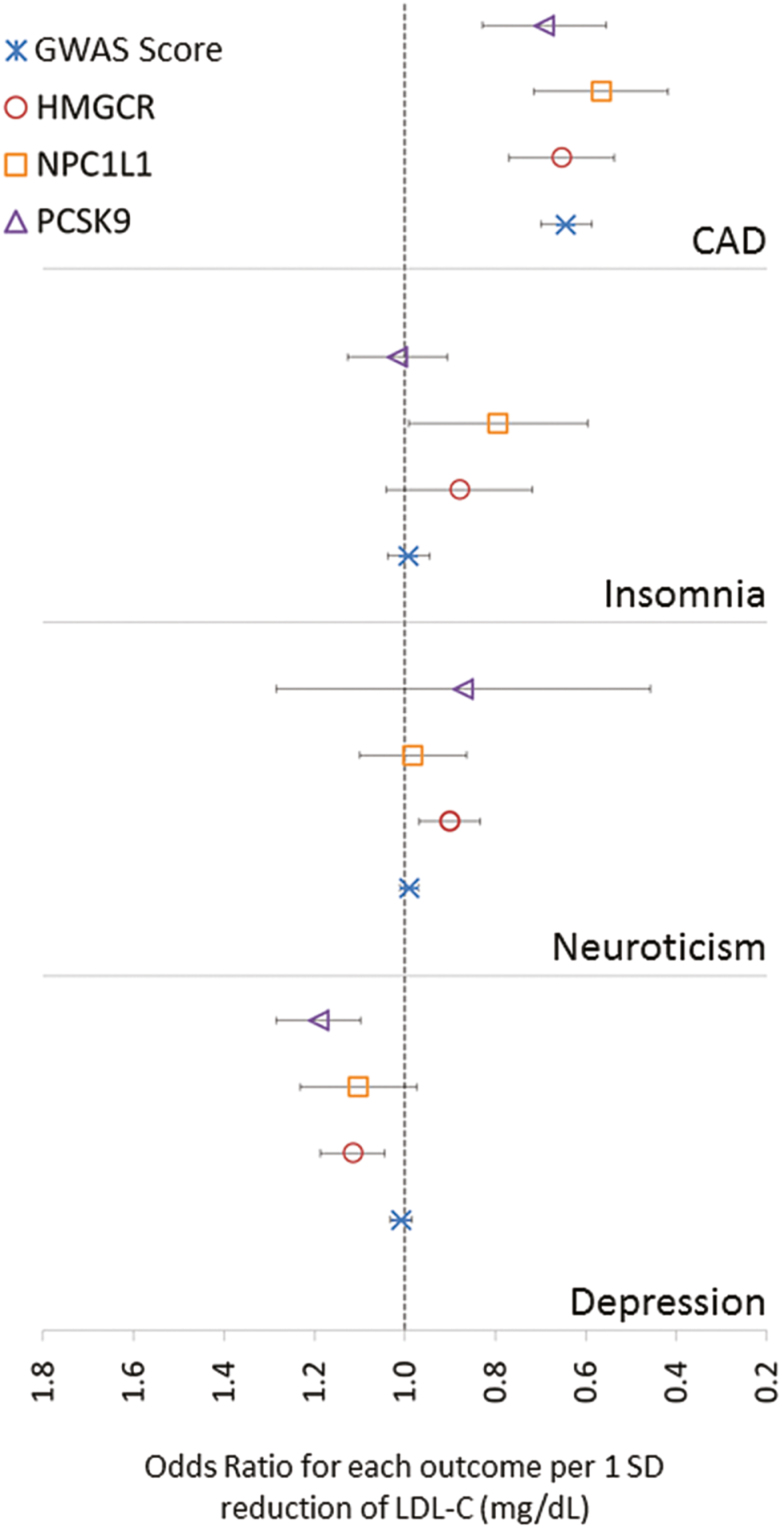
The association between LDL-C GRS, each neuropsychiatric adverse effects, and CAD showed a significant association with CAD only after correcting for multiple testing (P < .0031) (Table 1; Figure 1). Each 1-SD reduction in LDL-C was associated with a 36% reduction in CAD (OR=0.64, 95%CI: 0.55–0.73). Risk of CAD was also proportionally associated with total cholesterol and triglycerides and inversely associated with HDL-C (supplementary Tables 6–8). None of the 3 neuropsychiatric adverse effects (i.e., insomnia, depression, and neuroticism) were significantly associated with an LDL-C level, nor with any of the other lipid fractions. Heterogeneity was tested by Cochran’s Q statistics indicated no apparent heterogeneity in the estimates, except for CAD (P=2.73E-13). The slope of MR-Egger was consistent with the absence of directional pleiotropy, suggesting the validity of the IV assumptions in all the analyses, as evident by intercept that is insignificantly different from 0 (Table 1). The results obtained from the weighted median methods further confirmed the results of the IVW method (supplementary Figure 2). The association of the 4 GRSs with the other HDL-C, TG, and TC are shown in supplementary Figures 3–5.
Table 1.
MR Analysis of LDL-C and Genetic Proxies for Lipid Therapy with Neuropsychiatric Adverse Effects and CAD
| Genetic Score | MR Method | Depression | Neuroticism | Insomnia | Coronary Artery Disease | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) |
P | β (95% CI) |
P | β (95% CI) |
P | β (95% CI) |
P | ||
| LDL-C | IVW | 0.008 (-0.02–0.03) |
.51 | -0.1 (-0.03–0.01) |
.37 | -0.01 (-0.06–0.04) |
.72 |
-0.44
(-0.53–-0.35) |
9.82E-22 |
| Weighted median | 0.001 | .99 | 0.002 (-0.03–0.003) |
.91 | -0.03 (-0.11–0.04) |
.39 |
-0.45s
(-0.53–-0.36) |
2.67E-25 | |
| MR-Eggera | 0.003 (-0.04–0.04) |
.99 | -0.001 (-0.002–0.002) |
.64 | -0.02 (-0.09–0.06) |
.66 |
-0.56
(-0.70–-0.41) |
4.76E-10 | |
| Interceptb | 0.0006 (-0.002–0.003) |
.64 | -0.001 (-0.002–0.002) |
.91 | 0.001 (-0.004–0.005) |
.78 | 0.009 (0.0003–0.02) |
.05 | |
| HMGCR | IVW |
0.11
(0.04–0.18) |
1.15E-03 | -0.1 (-0.18–-0.03) |
.008 | -0.13 (-0.33–0.07) |
.21 |
-0.43
(-0.62–-0.23) |
1.89E-05 |
| Weighted median |
0.11
(0.03–0.18) |
3.01E-03 | -0.1 (-0.18–-0.02) |
.02 | -0.13 (-0.34–0.08) |
.23 |
-0.42
(-0.63–-0.21) |
1.06E-04 | |
| MR-Eggera | 0.04 (-0.49–0.56) |
.92 | 0.1 (-0.31–0.5) |
.72 | -0.21 (-1.36–0.94) |
.78 | -0.76 (-1.84–0.33) |
.4 | |
| Interceptb | -0.005 (-0.03–0.04) |
.828 | -0.013 (-0.04–0.02) |
.5 | 0.005 (-0.07–0.08) |
.91 | 0.022 (-0.048–0.091) |
.65 | |
| NPC1L1 | IVW | 0.1 (-0.03–0.22) |
.12 | 0.02 (-0.15–0.11) |
.79 | -0.23 (-0.52–0.06) |
.11 |
-0.57
(-0.87–-0.26) |
2.58E-04 |
| Weighted median | 0.09 (-0.06–0.23) |
.23 | 0.02 (-0.17–0.13) |
.79 | -0.22 (-0.55–0.12) |
.21 |
-0.57
(-0.94–-0.21) |
2.16E-03 | |
| MR-Eggera | 0.07 (-0.58–0.72) |
.85 | -0.04 (-0.56–0.65) |
.91 | -0.15 (-1.59–1.3) |
.86 | 0.06 (-1.43–1.54) |
.95 | |
| Interceptb | 0.001 (-0.02–0.03) |
.939 | 0.002 (-0.03–0.02) |
.87 | -0.003 (-0.06–0.05) |
.92 | -0.024 (-0.079–0.031) |
.49 | |
| PCSK9 | IVW |
0.17
(0.09–0.26) |
2.61E-05 | 0.14 (-0.78–0.5) |
.75 | 0.02 (-0.1–0.13) |
.78 |
-0.37
(-0.59–-0.15) |
9.98E-04 |
| Weighted median |
0.16
(0.06–0.26) |
2.02E-03 | 0.03 (-0.12–0.06) |
.49 | 0.04 (-0.09–0.16) |
.54 |
-0.47
(-0.66–-0.27) |
3.36E-06 | |
| MR-Eggera | -0.3 (-1.05–0.46) |
.58 | 0.03 (-0.11–0.04)) |
.38 | 0.05 (-0.12–0.22) |
.61 | -0.56 (-0.86–-0.26) |
.07 | |
| Interceptb | 0.028 (-0.02–0.07) |
.433 | -0.006 (-0.03–0.04) |
.8 | -0.005 (-0.02–0.01) |
.62 | 0.019 (-0.005–0.04) |
0.25 | |
Abbreviations: CAD, coronary artery disease; IVW, inverse-variance weighted; LDL-C, low-density lipoprotein cholesterol; MR, Mendelian randomization; Q pval, P value.
Estimates are in log(odds ratio) for the effect of 1-SD increase in (LDL-C); all SNPs were oriented to the LDL-C decreasing allele.
Summary statistics are based on genome-wide association studies (GWAS) from Social Science Genetic Association Consortium (SSGAC) for depression and neuroticism [PMID: 27089181], from Hammerschlag et al. GWAS for insomnia [PMID: 28604731], and from the CARDIoGRAMplusC4D for CAD.
Multiple testing is correcting P value equal to (0.05/12=.0041). Significant P values are in bold.
aMR-Egger is considered a sensitivity test; insignificant P value does not contradict the conventional estimates from IVW.
bMR-Egger intercept is the average pleiotropic effect of genetic variants included in the analysis, if the value of the intercept is significantly different from zero, then the conventional MR estimate is biased (either presence of directional pleiotropy, violation of MR assumption, or both).
Figure 1.
Effect of lowering low-density lipoprotein cholesterol (LDL-C) medicated by the 4 genetic risk scores. Circles represent the summary point estimates of effect for the association between each exposure genetic risk score (GRS) and outcome. Bars represent the 95%CI.
The Impact of Lipid-Lowering Drug Target Genetic Variants on Neuropsychiatric Adverse Effects (Testing the Presence of Specific Mechanisms Effects)
The association of lowering LDL-C specifically by the GRS of the 3 lipid-lowering drug target genes (i.e., HMGCR, NPC1L1, and PCSK9) showed a significant reduction in the risk of CAD (Table 1; Figure 1), confirming the validity of using CAD as a positive control for each drug proxy GRS. Specifically, the risk of CAD was reduced by 35%, 43%, and 31% with statins, ezetimibe, and PSCK9 inhibitors, respectively. The association of these GRSs with the 3 neuropsychiatric adverse effects showed an increased risk of depression with both statins and PCSK9 inhibitors by 12% (OR=1.12, 95%CI: 1.04–1.18, P=.0011) and 19% (OR=1.19, 95%CI 1.11–1.27, P=.00002). The risk of neuroticism was slightly reduced with statins treatment with a nominal P value (OR=0.9, 95%CI 0.83–0.97, P=.008). No significant neuropsychiatric adverse effects were associated with the GRS of NPC1L1, and sensitivity analysis showed no evidence of heterogeneity or directional pleiotropy in all the analyses, as evident by very close to zero MR-Egger intercept and insignificant Cochran’s Q statistics.
Discussion
This present MR study has provided unique evidence of the increased risk of depression during statin and PSCK9 inhibitor therapy as demonstrated by the significant association of depression with genetic inhibition of HMGCR and PCSK9. We showed that each 1-SD reduction (equal to 38 mg/dL) in LDL-C by GRSs of HMGCR and PCSK9 conferred 12% and 19% increased risk of depression, respectively. The validity of the tested genetic scores was established by finding statistically significant reduction in the risk of CAD with genetically lower level of LDL-C, as expected and reported by previous studies (Ference et al., 2012; White et al., 2016), which were also documented in several RCTs with lower risk of CAD after treatment with statins (LaRosa et al., 1999), PCSK9 inhibitors (Raal et al., 2014), and ezetimibe (Cannon et al., 2015). The null association between depression and the overall LDL-C GRS suggests that the increased risk of depression is likely to be an on-target effect of statins and PCSK9 inhibitors (i.e., mechanism effect) rather than an off-target effect or biomarker effect, as the aggregate reduction in LDL-C did not confer a higher risk of depression. Furthermore, this study increased the weight of evidence of the safety of the 3 lipid-lowering drug classes on the sleep quality and reported a suggestive benefit of statins on reducing neuroticism.
Several studies have reported inconsistent findings regarding the association of depression with statins. Two RCTs performed by Morales et al. (2006) and Hyyppä et al. (2003) have reported an increased risk of depression among simvastatin groups compared with placebo group. Furthermore, a case series of 12 participants reported a temporal association between statin initiation and mood change that was resolved after drug discontinuation, suggesting a causal connection (Cham et al., 2016). Moreover, an increased risk of depression with statins was documented by one systematic review (You et al., 2013). In contrary, 2 systematic reviews have concluded evidence of potential beneficial effects of statins on mood with a possible reduction in the depressive symptoms (O’Neil et al., 2012; Salagre et al., 2016). Remarkably, Swiger et al. (2014) have conducted a systematic review of 34 studies and suggested that evidence for assessing such adverse effects using the available literature is limited and weak due to differences in data collection and methodology. These inconsistent findings were even documented in the Swedish national cohort study that reported a 7% reduction in the risk of depression with simvastatin but an 11% increase in the risk of depression with atorvastatin (Redlich et al., 2014), suggesting that this protective effect is only with lipophilic statins. Smit et al. (2016) attributed this inconsistency to the finding that higher visit-to-visit LDL-C variability was associated with lower cognitive performance and greater white matter hyperintensity that are independent of LDL-C level or statin treatment. Other factors that may increase the risk of neuropsychiatric side effects include the intensity of statin regimens and patients underline characteristics, such as pre-existing cognitive impairment, older age, and pharmacogenetics interactions (Swiger and Martin, 2015).
Limited evidence is available to assess the risk of depression with PCSK9 inhibitors, as this class is considered as a new class with most of the RCTs in their early stages. Our finding of a significantly higher risk of depression with genetic inhibition of PCSK9 reported in this study represents a novel finding. Although Postmus et al. (2013) have reported a null association of rs11591147 of the PCSK9 gene with cognitive function, our study has specifically assessed the risk of depression with genetic inhibition of PCSK9 using 2-sample MR approach and unprecedented sample size (≈180000). Clinically, the Open-Label Study of Long-Term Evaluation against LDL Cholesterol 1 trial has reported higher incidence of neurocognitive adverse events among the PCSK9 inhibitor group (0.9%) compared with the standard of care group (0.3%), and that incidence of these adverse events did not appear to be related to the LDL-C level during treatment (Sabatine et al., 2015). Similarly, the ODYSSEY LONG TERM trial reported a higher incidence of neurocognitive adverse events among the PCSK9 inhibitor group (1.2%) compared with 0.5% in the placebo group (Robinson et al., 2015). Although the Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects study reported no significant difference on cognitive function, study subjects were followed-up for a relatively short period (<2 years) and were not assessed for depressive symptoms specifically (Giugliano et al., 2017). A recent meta-analysis of 8 RCTs reported a possibility of neurocognitive impairment with the use of PCSK9 inhibitors as evident by findings that larger sample size RCTs with longer follow-up showed a 2.0-fold increase of the incidence of neurocognitive adverse events (Khan et al., 2017). Importantly, the scarcity of the available evidence and the complexity of assessing neuropsychiatric adverse effects for such medications, taking the statin experience as an example, implies that traditional RCTs are insufficient to settle debate given their inherent focus on group averages (Swiger and Martin, 2015).
The risk of insomnia has also been reported by several studies with inconsistent findings. The highest evidence came from the Medicines and Healthcare products Regulatory Agency (2009), which published a report after assessing the available evidence suggesting that the use of statins is associated with sleep disturbance including insomnia. Also, one study that utilized multiple approaches and postmarketing surveillance databases found a significant association between statin use and hypnotic drugs in the prescription sequence symmetry analysis, specifically for lipophilic statins (Takada et al., 2014). However, a recent meta-analysis of 5 RCTs that used polysomnographic recording found no significant association between statin use and sleep duration, sleep efficiency, or entry into stage-1 sleep (Broncel et al., 2015). Our study findings are consistent with the findings of this meta-analysis and suggest that statins and other lipid-lowering medications have no significant adverse effects on sleep quality.
Neuroticism is the tendency to experience negative emotions and is considered as one of the main 5-factor personality traits. Although Okbay et al. (2016) has reported a high genetic correlation between depression and neuroticism (r2=0.75, SEM=0.027), neuroticism represents an independent trait that was found to be uniquely associated with pain catastrophizing and pain-related anxiety (Kadimpati et al., 2015), physical limitations (Goodwin and Friedman, 2006), and responding to antidepressant (Tang et al., 2009). Importantly, the improvement of neuroticism with an antidepressant was independent of their effect on depression, suggesting that a specific pharmacological effect plays a role that is distinct from its effect on depression (Tang et al., 2009). This study proposed a cause-correction hypothesis that antidepressant treatment may influence factors underlying neuroticism, leading to better depression treatment. From this perspective, a possible explanation of our findings is that the increased risk of depression observred with both PCSK9 inhibitors and statins is not through neurobiological factors that underlie neuroticism. Moreover, Sutin et al. (2010) reported a suggestive association between HDL-C and neuroticism that was null for the other lipid fractions. Interestingly, our secondary analyses showed that HDL-C was only significantly associated with neuroticism for HMGCR GRS (supplementary Table 7). Although this association was only at nominal P value (P=.006), it emphasizes the need for further studies to specifically investigate these findings.
The mechanism by which HMGCR and PCSK9 variants increase the risk of depression is unclear. Nevertheless, a low level of cholesterol has been correlated with depression, possibly through deregulations in serotonin neurotransmission (Vevera et al., 2005). In addition, as cholesterol presents the main component of lipid rafts that play an essential role in regulated exocytosis pathways, an inhibition of neuronal cholesterol biosynthesis with lovastatin has been demonstrated to result in the impairment of synaptic vesicle release (Mailman et al., 2011; Egawa et al., 2016). Furthermore, a significant reduction in brain serotonin level was found in animal studies after simvastatin treatment (Thomas et al., 2014). However, the findings of this study imply that this risk is on-target effects of both statins and PCSK9 inhibitors, supported by the null association observed with the GRSs of LDL-C and NPC1L1. Compared with statins, our MR models showed that the risk of depression is slightly higher and more significant with PCSK9 inhibitors, which is consistent with the higher reduction of LDL-C achieved by PCSK9 inhibitors but counter the very similar profile of risk reduction for CVD events (Ference et al., 2016). To explore these findings, we used the Genotype-Tissue Expression project database to evaluate further if HMGCR, PCSK9, and NPC1L1 are highly expressed in the specific tissue, particularly the brain and CNS (supplementary Figures 6 and 7). The Genotype-Tissue Expression database showed that unlike NPC1L1, both HMGCR and PCSK9 are highly expressed in the brain regions; HMGCR is highly expressed in the entire reported regions, whereas PCSK9 is highly expressed in regions of the cerebellar hemisphere and cerebellum only. The roles of these regions in emotional processing especially for negative emotional memories are well established (Fitzgerald et al., 2008; Monti et al., 2018). To determine whether the significantly increased risk of depression with statins or PCSK9 inhibitors is due to specific effects on these brain regions requires further studies. Specifically, animal and in vitro studies showed that statins decrease β-amyloid production, which has also been associated with depression (Friedhoff et al., 2001; Sun et al., 2008). Similarly, a study in PCSK9-/- mice emphasized the important role of PCSK9 in modulating the composition of brain lipids (Mannarino et al., 2018). Nevertheless, PCSK9 has been associated with several pleiotropic effects apart from their role in cholesterol traffickings, such as regulation of neuronal apoptosis, disposal of nonacetylated intermediates of the nascent membrane protein (BACE1), and degradation of other apolipoprotein E-binding receptors as well as possible roles in inflammation and immune functions (Banerjee et al., 2016; Mannarino et al., 2018).
This study used a novel method of MR and the largest currently available sources of data on genetic association with lipid fractions and with the neuropsychiatric traits to assess the question of causality of lipid-lowering medications on neuropsychiatric adverse effects. The MR method is assumed to provide robust evidence on the causal impact of intervening on the specific biological pathway since the effects of the genetic variants on that pathway are less likely to be confounded by environmental, lifestyle, or disease-related factors operating later in life (Walker et al., 2017). Furthermore, the MR method is less likely to be affected by nongenetic confounding such as confounding by indication and reverse causation. This is because the genetic variants used as a proxy for drug exposure are germline variants, which are unlikely to be consequence of the indication. It is also unlikely that the outcome will change the DNA sequence (Mokry et al., 2015). However, Lohoff et al. (2017) reported that alcohol exposure is associated with a decreased PCSK9 methylation and lower gene expression. Hence, such epigenetics factors may confound the MR estimates if alcohol exposure is not controlled for in the original study.
The strengths of this study are utilizing the MR approach using summary results from the largest available GWAS for each neuropsychiatric adverse and supporting the MR findings by replicating the previous findings using CAD as a positive control. Also, the sensitivity analyses as performed by MR Egger and Weighted median tests support the findings of the conventional MR analysis. Concordant findings resulted from these methods implying that statins and PCSK9 inhibitors may cause depression. Nevertheless, this study is limited by the same factors that limit most MR studies. First, despite the consistent findings from the different MR methods used in this study, we cannot rule out the possibility of residual pleiotropy. However, this effect is most likely minimal given that the MR-Egger intercept was centered at the origin. Second, estimates used in our analysis were based on summary results of population-based GWAS, and therefore these estimates may not be relevant to patient groups in whom particular therapy is indicated. Also, the genetic variants produce their effects over the course of the life, not like the inhibition of these molecular targets by short-term pharmacological therapy. Nevertheless, their impact is balanced by a small effect size, unlike the pharmacological treatment that produces a larger effect size over a shorter period.
In conclusion, this study provided evidence based on the genetic proxy of lipid-lowering drug targets suggesting that statins and PCSK9 inhibitors may increase the risk of depression, in particular through on-target inhibition of HMGCR and PCSK9. Ongoing clinical trials for PCSK9 should monitor depressive symptoms and postmarketing surveillance. Patients at higher risk of depression should be assessed regularly and preferably be prescribed alternative pharmacological classes. We also provided evidence that strengthens the weight of current evidence for the safety of statins, PCSK9 inhibitors, and ezetimibe lipid-lowering medication on sleep efficiency.
Supplementary Material
Acknowledgments
We thank the Global Lipid Genetics Consortium (GLGC) (http://csg.sph.umich.edu/abecasis/public/lipids2017/), CARDIoGRAMplusC4D Consortium (http://www.cardiogramplusc4d.org/), Social Science Genetic Association Consortium (SSGAC) (https://www.thessgac.org/), and Hammerschlag et al. 2017 GWAS (http://ctg.cncr.nl/software/summary_statistics) for making their GWAS summary statisitcs publicly available.
Statement of Interest
None.
References
- Banerjee Y, Santos RD, Al-Rasadi K, Rizzo M(2016)Targeting PCSK9 for therapeutic gains: have we addressed all the concerns?Atherosclerosis 248:62–75. [DOI] [PubMed] [Google Scholar]
- Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjærg-Hansen A(2017)Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer’s disease and Parkinson’s disease: mendelian randomisation study. Bmj 357:j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Holmes MV(2017)Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart 103:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Burgess S(2015)Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden J, Davey Smith G, Haycock PC, Burgess S(2016)Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broncel M, Gorzelak-Pabiś P, Sahebkar A, Serejko K, Ursoniu S, Rysz J, Corina Serban M, Możdżan M, Banach M, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group (2015)Sleep changes following statin therapy: a systematic review and meta-analysis of randomized placebo-controlled polysomnographic trials. Arch Med Sci 11:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG, EPIC- InterAct Consortium (2015)Using published data in mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol 30:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon CP, et al. , IMPROVE-IT Investigators (2015)Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372:2387–2397. [DOI] [PubMed] [Google Scholar]
- Cham S, Koslik HJ, Golomb BA(2016)Mood, personality, and behavior changes during treatment with statins: a case series. Drug Saf Case Rep 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists Collaboration , Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R, Baigent C(2012)The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa J, Pearn ML, Lemkuil BP, Patel PM, Head BP(2016)Membrane lipid rafts and neurobiology: age-related changes in membrane lipids and loss of neuronal function. J Physiol 594:4565–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C(1997)Bias in meta-analysis detected by a simple, graphical test. Bmj 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicine Agency (2009)PhVWP Report on 1 F F The association of HMG-CoA Reductase Inhibitors with the following safety concerns: sleep disturbances; memory loss; micturition disorders; sexual disturbance; depression; and interstitial pneumopathy Available at: https://www.bfarm.de/SharedDocs/Downloads/DE/Arzneimittel/Pharmakovigilanz/Risikoinformationen/RisikoBewVerf/s-z/statine/phvwp_report.pdf?__blob=publicationFile&v=3 Retrived 2 Apr 2018.
- Feldman HH, Doody RS, Kivipelto M, Sparks DL, Waters DD, Jones RW, Schwam E, Schindler R, Hey-Hadavi J, DeMicco DA, Breazna A, LEADe Investigators (2010)Randomized controlled trial of atorvastatin in mild to moderate alzheimer disease: leade. Neurology 74:956–964. [DOI] [PubMed] [Google Scholar]
- Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA Sr, Flack JM(2012)Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a mendelian randomization analysis. J Am Coll Cardiol 60:2631–2639. [DOI] [PubMed] [Google Scholar]
- Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS(2016)Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 375:2144–2153. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ(2008)A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedhoff LT, Cullen EI, Geoghagen NS, Buxbaum JD(2001)Treatment with controlled-release lovastatin decreases serum concentrations of human beta-amyloid (A beta) peptide. Int J Neuropsychopharmacol 4:127–130. [DOI] [PubMed] [Google Scholar]
- Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider J, Wang H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR, EBBINGHAUS Investigators (2017)Cognitive function in a randomized trial of evolocumab. N Engl J Med 377:633–643. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Friedman HS(2006)Health status and the five-factor personality traits in a nationally representative sample. J Health Psychol 11:643–654. [DOI] [PubMed] [Google Scholar]
- Hammerschlag AR, et al. (2017)Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet 49:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyyppä MT, Kronholm E, Virtanen A, Leino A, Jula A(2003)Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 28:181–194. [DOI] [PubMed] [Google Scholar]
- Kadimpati S, Zale EL, Hooten MW, Ditre JW, Warner DO(2015)Associations between neuroticism and depression in relation to catastrophizing and pain-related anxiety in chronic pain patients PLoS One 10:e0126351. [DOI] [PMC free article] [PubMed]
- Khan AR, Bavishi C, Riaz H, Farid TA, Khan S, Atlas M, Hirsch G, Ikram S, Bolli R(2017)Increased risk of adverse neurocognitive outcomes with proprotein convertase Subtilisin-Kexin type 9 inhibitors. Circ Cardiovasc Qual Outcomes 10:e003153. [DOI] [PubMed] [Google Scholar]
- Kim SW, Bae KY, Kim JM, Shin IS, Hong YJ, Ahn Y, Jeong MH, Berk M, Yoon JS(2015)The use of statins for the treatment of depression in patients with acute coronary syndrome. Transl Psychiatry 5:e620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa JC, He J, Vupputuri S(1999)Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. Jama 282:2340–2346. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, et al. (2017)Methylomic profiling and replication implicates deregulation of PCSK9 in alcohol use disorder. Mol Psychiatry. doi:10.1038/mp.2017.168 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailman T, Hariharan M, Karten B(2011)Inhibition of neuronal cholesterol biosynthesis with lovastatin leads to impaired synaptic vesicle release even in the presence of lipoproteins or geranylgeraniol. J Neurochem 119:1002–1015. [DOI] [PubMed] [Google Scholar]
- Mannarino MR, Sahebkar A, Bianconi V, Serban M-C, Banach M, Pirro M(2018)PCSK9 and neurocognitive function: Should it be still an issue after FOURIER and EBBINGHAUS results?J Clin Lipidol. In Press. [DOI] [PubMed] [Google Scholar]
- Medicines and Healthcare products Regulatory Agency (2009)MHRA PUBLIC ASSESSMENT REPORT Statins: updates to product safety information Available at: http://www.mhra.gov.uk/home/groups/s-par/documents/websiteresources/con079339.pdf. Retrieved 14 February 2018.
- Mokry LE, Ahmad O, Forgetta V, Thanassoulis G, Richards JB(2015)Mendelian randomisation applied to drug development in cardiovascular disease: a review. J Med Genet 52:71–79. [DOI] [PubMed] [Google Scholar]
- Monti DA, Tobia A, Stoner M, Wintering N, Matthews M, Conklin CJ, Mohamed FB, Chervoneva I, Newberg AB(2018)Changes in cerebellar functional connectivity and autonomic regulation in cancer patients treated with the neuro emotional technique for traumatic stress symptoms. J Cancer Surviv 12:145–153. [DOI] [PubMed] [Google Scholar]
- Morales K, Wittink M, Datto C, DiFilippo S, Cary M, TenHave T, Katz IR(2006)Simvastatin causes changes in affective processes in elderly volunteers. J Am Geriatr Soc 54:70–76. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB(2000)Effects of lovastatin on cognitive function and psychological well-being. Am J Med 108:538–546. [DOI] [PubMed] [Google Scholar]
- Nikpay M, et al. (2015)A comprehensive 1000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 47:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil A, Sanna L, Redlich C, Sanderson K, Jacka F, Williams LJ, Pasco JA, Berk M(2012)The impact of statins on psychological wellbeing: a systematic review and meta-analysis. BMC Med 10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A, et al. , LifeLines Cohort Study(2016)Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 48:624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postmus I, Trompet S, de Craen AJ, Buckley BM, Ford I, Stott DJ, Sattar N, Slagboom PE, Westendorp RG, Jukema JW(2013)PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res 54:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raal FJ, Giugliano RP, Sabatine MS, Koren MJ, Langslet G, Bays H, Blom D, Eriksson M, Dent R, Wasserman SM, Huang F, Xue A, Albizem M, Scott R, Stein EA(2014)Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1300 patients in 4 phase II trials. J Am Coll Cardiol 63:1278–1288. [DOI] [PubMed] [Google Scholar]
- Redlich C, Berk M, Williams LJ, Sundquist J, Sundquist K, Li X(2014)Statin use and risk of depression: a Swedish national cohort study. BMC Psychiatry 14:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, ODYSSEY LONG TERM Investigators (2015)Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 372:1489–1499. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA, Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators (2015)Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 372:1500–1509. [DOI] [PubMed] [Google Scholar]
- Salagre E, Fernandes BS, Dodd S, Brownstein DJ, Berk M(2016)Statins for the treatment of depression: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Affect Disord 200:235–242. [DOI] [PubMed] [Google Scholar]
- Smit RA, Trompet S, Sabayan B, le Cessie S, van der Grond J, van Buchem MA, de Craen AJ, Jukema JW(2016)Higher visit-to-visit low-density lipoprotein cholesterol variability is associated with lower cognitive performance, lower cerebral blood flow, and greater white matter hyperintensity load in older subjects. Circulation 134:212–221. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Sharples KJ, North FM, Menkes DB, Baker J, Simes J(2000)Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID study investigators. Arch Intern Med 160:3144–3152. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (2014)2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol 63:2889–2934. [DOI] [PubMed] [Google Scholar]
- Sun X, Steffens DC, Au R, Folstein M, Summergrad P, Yee J, Rosenberg I, Mwamburi DM, Qiu WQ(2008)Amyloid-associated depression: a prodromal depression of Alzheimer disease?Arch Gen Psychiatry 65:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin AR, Terracciano A, Deiana B, Uda M, Schlessinger D, Lakatta EG, Costa PT Jr(2010)Cholesterol, triglycerides, and the five-factor model of personality. Biol Psychol 84:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiger KJ, Manalac RJ, Blaha MJ, Blumenthal RS, Martin SS(2014)Statins, mood, sleep, and physical function: a systematic review. Eur J Clin Pharmacol 70:1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiger KJ, Martin SS(2015)PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf 38:519–526. [DOI] [PubMed] [Google Scholar]
- Takada M, Fujimoto M, Yamazaki K, Takamoto M, Hosomi K(2014)Association of statin use with sleep disturbances: data mining of a spontaneous reporting database and a prescription database. Drug Saf 37:421–431. [DOI] [PubMed] [Google Scholar]
- Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B(2009)Personality change during depression treatment: a placebo-controlled trial. Arch Gen Psychiatry 66:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Varkey J, Augustine B(2014)Association between serum cholesterol, brain serotonin, and anxiety: A study in simvastatin administered experimental animals. Int J Nutr Pharmacol Neurol Dis 4:69. [Google Scholar]
- Tuccori M, Montagnani S, Mantarro S, Capogrosso-Sansone A, Ruggiero E, Saporiti A, Antonioli L, Fornai M, Blandizzi C(2014)Neuropsychiatric adverse events associated with statins: epidemiology, pathophysiology, prevention and management. CNS Drugs 28:249–272. [DOI] [PubMed] [Google Scholar]
- Vevera J, Fisar Z, Kvasnicka T, Zdenek H, Stárková L, Ceska R, Papezová H(2005)Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res 133:197–203. [DOI] [PubMed] [Google Scholar]
- Walker VM, Davey Smith G, Davies NM, Martin RM(2017)Mendelian randomization: a novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int J Epidemiol 46:2078–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, Sattar N, Humphries SE, Hingorani AD, Holmes MV(2016)Association of lipid fractions with risks for coronary artery disease and diabetes. JAMA Cardiol 1:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, et al. , Global Lipids Genetics Consortium(2013)Discovery and refinement of loci associated with lipid levels. Nat Genet 45:1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würtz P, et al. (2016)Metabolomic profiling of statin use and genetic inhibition of HMG-CoA reductase. J Am Coll Cardiol 67:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Lu W, Zhao S, Hu Z, Zhang J(2013)The relationship between statins and depression: a review of the literature. Expert Opin Pharmacother 14:1467–1476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.