Abstract
Background
Individuals with stress-related psychiatric disorders exhibit deficits in cognitive flexibility. We have shown that chronic intermittent cold stress induces deficits in reversal learning, a form of cognitive flexibility mediated in the orbitofrontal cortex, that was reversed by ketamine in male rats. Such effects have not been tested in females. In this study, we examined effects of chronic intermittent cold stress and ketamine on reversal learning in females.
Methods
Female Sprague-Dawley rats underwent 14 days of chronic intermittent cold and 3 days later received an injection of ketamine (10 mg/kg, i.p.). They were tested on reversal learning 24 hours post-injection. A separate cohort of female rats underwent 14 days of chronic intermittent cold. Three days later they received ketamine and were killed 2 hours post-injection for measurement of the synaptic marker PSD95 in orbitofrontal cortex.
Results
Chronic intermittent cold induced a reversal learning deficit in females comparable with that seen in males, which was corrected by ketamine. Moreover, chronic intermittent cold increased PSD95 expression in orbitofrontal cortex, but this increase was not seen in rats receiving ketamine.
Conclusions
Chronic intermittent cold stress and ketamine altered reversal learning in female rats similar to effects seen in males. Further, chronic intermittent cold increased PSD95 in orbitofrontal cortex of female rats, indicative of synaptic dysregulation. This effect was attenuated after ketamine administration.
Keywords: chronic stress, cognitive flexibility, females, ketamine, orbitofrontal cortex
Significance Statement
We present a viable animal model to study effects of stress on cognitive flexibility and treatment outcomes in females. Studying the effects of stress in both sexes is crucial to our understanding of the prevalence of stress-related diseases in women and may lead to improved treatments for both males and females.
Introduction
Depression affects 16% of individuals in the United States (Kessler et al., 2003). Existing pharmacotherapies for the treatment of depression are inadequate in that remission rates are high and maximal efficacy can require several weeks of treatment (Janicak et al., 1997). Even after effective treatment, residual symptoms can remain problematic, among the most prominent of which are cognitive deficits (Kurian et al., 2009). Thus, research has intensified toward finding new, more effective antidepressant treatments that act rapidly. Women are twice as likely to be diagnosed with depression than men (Noble, 2005). Biological as well as social factors may contribute to this difference in prevalence (Noble, 2005). In addition, women and men may vary in pharmacokinetics and side effects produced by antidepressants (Frackiewicz et al., 2000). Thus, it is important to include both sexes to study novel therapeutics using preclinical models of depression.
Chronic intermittent cold stress (CIC) induces a selective deficit in reversal learning, mediated in the orbitofrontal cortex (OFC), that can be corrected with an acute injection of the rapidly acting antidepressant ketamine (Patton et al., 2017). The cognitive-enhancing effects of ketamine have been associated with increases in expression of synaptic proteins in the medial prefrontal cortex (mPFC) (Li et al., 2010). However, Liston and colleagues (Liston et al., 2006) reported that chronic stress results in dendritic hyper-elaboration in the OFC. Thus, we tested whether CIC stress increases expression of the postsynaptic scaffolding protein PSD95 as an indirect indicator of synaptic density in the OFC.
The effects of chronic stress on cognitive flexibility, and the therapeutic effects of ketamine in reversing these deficits, have not been tested in females. In this study, we investigated effects of CIC stress and ketamine on reversal learning in female rats. Portions of this work have been presented in abstract form (Paredes et al., 2015).
Methods
Animals
A total of 52 adult female Sprague-Dawley rats (Envigo) weighing 220 to 240 g on arrival were used. Rats were initially group-housed (3 per cage) in 25×45×15-cm plastic cages on a 12-h-light/-dark cycle (lights on 7:00 am). Rats were housed individually prior to beginning experimental treatment. Experiments were conducted during the light phase, between 9:00 am and 5:00 pm. Food and water were available ad libitum except when rats were food restricted for the reversal learning test. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio and were consistent with NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize pain and the number of animals used.
Chronic Intermittent Cold (CIC) Stress
The procedure for CIC stress exposure was as described previously (Lapiz-Bluhm et al., 2009). Rats were randomly assigned to either control or stressed conditions. Rats in the CIC group were transported in their home cages, with food, water, and bedding, into a cold room (4°C) for 6 hours, then returned to the housing room every day for 14 consecutive days. Control rats remained undisturbed in the housing facility for days 1 to 7. On days 7 to 14, animals in both the CIC and control group were handled daily for 1 to 2 minutes. Beginning on day 10 of CIC or control treatment, all rats were restricted to 7 g/d food with water freely available.
Reversal Learning Test and Drug Administration
Behavioral procedures for reversal learning began the day after the end of CIC stress, as previously described (Patton et al., 2017). The testing arena was a white wooden box (75×44×30 cm) containing a start gate at one end and 2 terracotta pots (diameter 7 cm, depth 6 cm) separated by a Plexiglas wall at the other end. The day after the end of CIC, rats were trained to dig in sawdust to retrieve a food reward, one-half of a Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN), from the pots. On day 2, they were trained to locate the reward in one of the pots by discriminating cues in 2 stimulus dimensions, an odor applied to the rim of each pot, and the digging medium that filled each pot. On day 3, animals received a single injection of ketamine (10mg/kg, i.p.) or saline. On day 4, the rats were tested on a series of discrimination tasks in which a criterion of 6 consecutive correct responses was required to proceed to the next task. In the first task (simple discrimination), they learned to associate a cue in one stimulus dimension (either odor or digging medium) with the reward location. On the second task (compound discrimination), they continued to discriminate based on the same dimension, and the second irrelevant dimension was introduced as a distractor. In the reversal learning task, the relevant dimension was the same, but the cue/reward association was switched such that the previously non-rewarded cue was now positive and the previously positive cue was negative. The dependent measure was the number of trials required to meet criterion on the reversal learning task. Animals that did not dig within 10 minutes on 6 consecutive trials or did not reach criterion within 50 trials were excluded from analysis.
Western Blotting
Three days after the final session of CIC stress or control procedures, a separate cohort of rats received an injection of ketamine (10 mg/kg, i.p.) or vehicle and were killed 2 hours later via rapid decapitation. The OFC was dissected on ice from a 2-mm coronal slab cut from 2 to 4 mm caudal to the frontal pole. A wedge of tissue containing the OFC was dissected from the lateral margin of the brain to the medial boundary of the forceps minor, ventral to the forceps minor, and dorsal to the rhinal sulcus and frozen in isopentane on dry ice. Western blots were performed as previously described (Patton et al., 2017). After transfer, membranes were incubated with polyclonal antibodies to PSD95 (1:10000) and GAPDH (1:10000) to normalize for loading. They were subsequently incubated with anti-rabbit (PSD95, 1:5000, GAPDH, 1:5000) HRP-conjugated secondary antibody (Cell Signaling), then exposed to Prime ECL reagent (GE Healthcare, Little Chalfont, UK). Chemiluminescence signals were quantified using the G:BOX-XT4 Chemi system (Syngene, Frederick, MD). For analysis, ratios of PSD95/GAPDH were calculated for each sample.
Data Analysis
For experiment 1, data were analyzed using an unpaired t test. For experiments 2 and 3, data were analyzed by ANOVA. Pairwise comparisons to identify specific group differences were made using the Newman Keuls test. Significance was determined at P<.05.
Results
Experiment 1: CIC Stress Induces a Reversal Learning Deficit in Female Rats
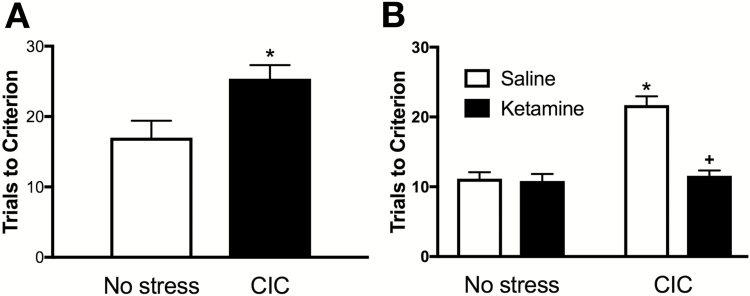
CIC stress significantly increased trials to criterion on the reversal learning task (Figure 1A) compared with controls (t7=-2.77, P<.05, n=4–6 per group).
Figure 1.
Chronic intermittent cold (CIC) stress induced a reversal learning deficit in female rats that was corrected by acute ketamine treatment. (A) In experiment 1, CIC stress increased the number of trials required to reach criterion in the reversal learning task compared with nonstressed controls (*P<.05). Data presented as mean±SEM (n=4–6 per group). (B) In experiment 2, CIC stress again increased the number of trials required to reach criterion in the reversal learning test (*P<.01 compared with nonstressed vehicle-treated controls). Acute systemic injection of ketamine (10mg/kg, i.p.) given 24hours prior to testing corrected this deficit (+P<.01 compared with CIC-stressed vehicle-treated group). Data presented as mean±SEM (n=6–7 per group).
Experiment 2: Acute Ketamine Treatment Corrects the CIC-Induced Deficit on Reversal Learning in Female Rats
In replication of experiment 1, CIC stress again significantly impaired reversal learning in female rats (Figure 1B; n=6–7/group). ANOVA revealed a significant main effect of stress (F(1,20)=28.69, P<.01), drug (F(1,20)=18.36, P<.01) and a stress × drug interaction (F(1,20)=15.83, P<.01). Posthoc comparisons revealed that acute injection of ketamine 24 hours prior to testing corrected the CIC-induced deficit in reversal learning, as stressed animals that received ketamine required significantly fewer trials to meet criterion compared with stressed animals that received saline (P<.01).
Experiment 3: CIC Stress Increases PSD95 Expression in the OFC of Female Rats, and the Effect of Stress Is Attenuated by Ketamine
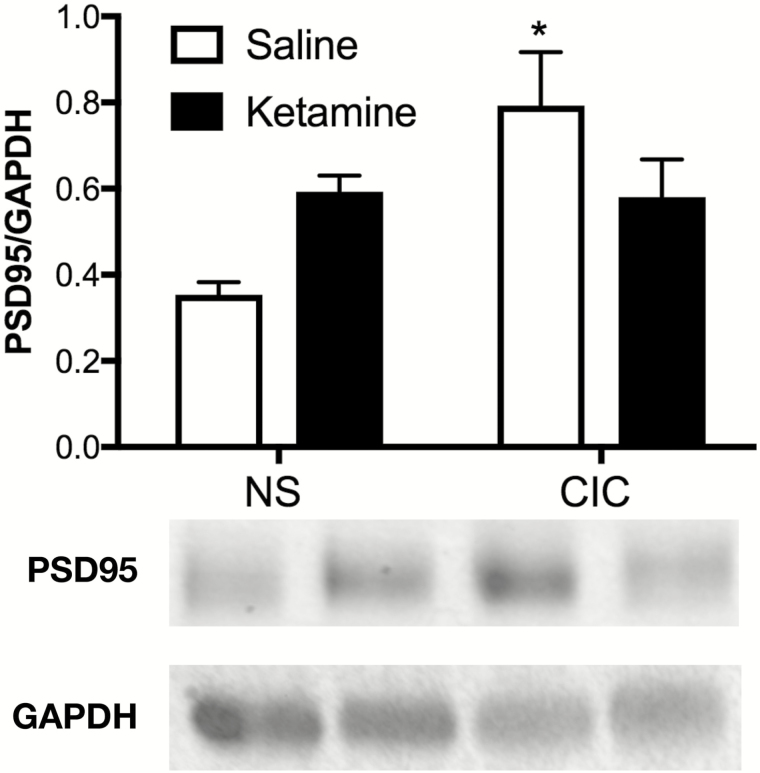
CIC stress increased PSD95 expression in the OFC, and this effect was attenuated by ketamine (Figure 2; n=3–5/group). ANOVA revealed a significant main of stress (F(1,11)=6.22, P<.05), and a stress × drug interaction (F(1,11)=7.00, P<.05). Pairwise comparisons showed that CIC stress increased PSD95 in the OFC compared with unstressed vehicle controls (P<.02). Ketamine alone did not increase PSD95 in the OFC of unstressed rats (P=.16). However, ketamine attenuated the effect of stress. PSD95 in the OFC of rats receiving ketamine after stress was not different from that in unstressed vehicle controls (P=.09) or from that in unstressed rats receiving ketamine (P=.92), although PSD95 in stress ketamine rats was also not significantly different from that in stress vehicle rats (P=.23).
Figure 2.
Chronic intermittent cold (CIC) stress increased the expression of PSD95 in the orbitofrontal cortex (OFC) of female rats, and this effect was attenuated after ketamine administration. PSD95 protein expression was significantly elevated in the OFC of CIC-stressed female rats (*P<.02 compared with nonstressed vehicle-treated controls). Ketamine attenuated this effect, as 2 hours after acute systemic ketamine administration (10mg/kg, i.p.), PSD95 expression in the OFC of stressed rats was not different from that in either unstressed rats receiving ketamine (P=.92) or in unstressed vehicle controls (P=.09). Data presented as mean±SEM (n=3–5 per group).
Discussion
Preclinical studies have described sex differences in the effects of chronic stress on cognition and memory (Galea et al., 1997; Luine, 2002; Bowman et al., 2003). For example, female rats that underwent chronic restraint stress performed better than males in a radial arm maze task, and these effects were attributed to estradiol (Bowman et al., 2003). Further, following stress, female rats showed changes in glucocorticoids that were influenced by ovarian hormones (Viau and Meaney, 1991). The behavioral model described in the current study could serve as a useful platform to investigate mechanistic similarities and differences between sexes as well as the interacting influences of sex and stress specifically on cognitive flexibility, an important component of stress-related psychiatric disorders such as depression.
Our results show that CIC stress induced a deficit in cognitive flexibility on a reversal learning task in female rats consistent with our prior observations in male rats (Lapiz-Bluhm et al., 2009), validating the use of CIC stress to study chronic stress-induced reversal learning deficits in females as well as males. In addition, we showed that the CIC-induced deficit in reversal learning in females was corrected by an acute injection of the NMDA-receptor antagonist ketamine given 24 hours before testing, also consistent with our previous observations in males (Patton et al., 2017). For comparison, the same dose of ketamine that was effective in males was used in this study (10 mg/kg). It has been reported that females are more sensitive to the effects of ketamine, showing effects on the forced swim test and novelty suppressed feeding test at a dose (2.5 mg/kg) that had no effect in males (Carrier and Kabbaj, 2013). It is therefore possible that females may also respond to a lower dose of ketamine in correcting stress-compromised reversal learning. A direct comparison of full-dose response curves in males and females will be required to test this.
In the present study, CIC stress increased the expression of PSD95 in the OFC of female rats, and this increase was attenuated in rats given ketamine 2 hours before sacrifice. These findings are congruent with the observation in males that repeated restraint stress increased spine elaboration in the OFC (Liston et al., 2006) and with our previous observation that ketamine depressed afferent-evoked field potential responses in the OFC (Patton et al., 2017). However, in male rats, an acute injection of ketamine increased the expression of synaptic proteins such as PSD95 in the medial prefrontal cortex (Li et al., 2010), whereas in the present study, ketamine alone did not increase expression of PSD95 in the OFC of either nonstressed or stressed females. This may reflect a sex difference, as our result is consistent with a previous report that ketamine also did not increase expression of PSD95 and other synaptic proteins in the medial prefrontal cortex of female rats (Sarkar and Kabbaj, 2016). On the other hand, this may instead reflect a regional difference between mPFC and OFC in the response to ketamine, similar to the regional difference in the response to stress reported by Liston et al. (Liston et al., 2006). Future studies will be required to directly compare the molecular and cellular responses to ketamine in the OFC and mPFC and the resulting circuit-level plasticity in female and male rats.
It has been reported that synaptic spine density in the medial prefrontal cortex of female rats is lower in diestrus than in proestrus (Sarkar and Kabbaj, 2016). It is unknown if such differences occur in the OFC across the estrus cycle. However, in that paper there were neither differences in the effects of either stress or ketamine on spine density or behavior in proestrus and diestrus, nor in effects on synaptic proteins, including PSD95. In the present study, we chose not to monitor the estrus cycle because the procedures involved with vaginal lavage have been reported to serve as a mild stressor and, importantly, to modify stress responses (Sfikakis et al., 1996) and behavior (Walker et al., 2002). Rather, testing in all groups was staggered over sequential days so that different stages of the estrus cycle would be equivalently represented in all groups. Because testing was conducted during the light phase, the stages would be limited to diestrus and proestrus, as estrus occurs during the dark phase. Thus, it is unlikely that any potential differences in spine density that may occur in the OFC across stages of the estrus cycle would have impacted the present results.
In sum, the results presented establish CIC stress as a valid model to study stress-induced impairments in reversal learning related to dysfunction in the OFC of female as well as male rats and to study potential sex differences in the mechanisms underlying stress-induced neuropathology and response to effective treatment.
Acknowledgments
We thank Lauren Hatherall for expert technical assistance. We thank Dr. Milena Girotti for guidance with the western blot experiments.
This work was supported by National Institute of Health research grant MH053851 and by Merit Award 1I01BX003512 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program. The contents of this paper do not represent the views of the Department of Veterans Affairs or the U.S. Government. The sponsors had no role in the design, analysis, or interpretation of the experiments described in this paper.
Statement of Interest
Ms. Paredes has no financial or other conflicts of interest to disclose. Ms. Silva has no conflicts of interest to disclose. Within the last 3 years, Dr. Morilak has served on a psychopharmacology advisory board for H. Lundbeck A/S and has received research funding from Lundbeck Research, USA, and from Alkermes. These activities have no relation to the work presented in this paper.
References
- Bowman RE, Beck KD, Luine VN (2003) Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav 43:48–59. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M(2013)Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Frackiewicz EJ, Sramek JJ, Cutler NR(2000)Gender differences in depression and antidepressant pharmacokinetics and adverse events. Ann Pharmacother 34:80–88. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS(1997)Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81:689–697. [DOI] [PubMed] [Google Scholar]
- Janicak PG, Davis JM, Preskorn SH, Ayd FJ(1997)Treatment with antidepressants. In: Principles and practice of psychopharmacotherapy 2nd ed, pp243–356. Baltimore: Williams & Wilkins. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication (2003)The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 289:3095–3105. [DOI] [PubMed] [Google Scholar]
- Kurian BT, Greer TL, Trivedi MH(2009)Strategies to enhance the therapeutic efficacy of antidepressants: targeting residual symptoms. Expert Rev Neurother 9:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm MD, Soto-Piña AE, Hensler JG, Morilak DA(2009)Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology (Berl) 202:329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS(2010)mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS(2006)Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine V.(2002)Sex differences in chronic stress effects on memory in rats. Stress 5:205–216. [DOI] [PubMed] [Google Scholar]
- Noble RE.(2005)Depression in women. Metabolism 54:49–52. [DOI] [PubMed] [Google Scholar]
- Paredes D, Silva JD, Morilak DA(2015)Effects of chronic unpredictable stress and chronic intermittent cold stress on cognitive flexibility in female rats. Soc Neurosci Abstr 41, Online program no. 812.04. [Google Scholar]
- Patton MS, Lodge DJ, Morilak DA, Girotti M(2017)Ketamine corrects stress-induced cognitive dysfunction through JAK2/STAT3 signaling in the orbitofrontal cortex. Neuropsychopharmacology 42:1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M(2016)Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 80:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfikakis A, Galanopoulou P, Konstandi M, Tsakayannis D(1996)Stress through handling for vaginal screening, serotonin, and ACTH response to ether. Pharmacol Biochem Behav 53:965–970. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ(1991)Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology 129:2503–2511. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM(2002)Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav 73:743–752. [DOI] [PubMed] [Google Scholar]