Abstract
We report here the first pharmacokinetic–pharmacodynamic relationship for ceftaroline in a preterm infant born at <28 weeks’ gestational age who was given ceftaroline (8.5 mg/kg every 8 hours) for pneumonia attributable to methicillin-resistant Staphyloccocus aureus. This dose of ceftaroline was adequate to achieve the pharmacodynamic endpoint associated with efficacy for methicillin-resistant Staphyloccocus aureus.
Keywords: ceftaroline, pharmacodynamics, pharmacokinetics, preterm infant
Ceftaroline fosamil (Teflaro) is a cephalosporin antibiotic indicated for the treatment of acute bacterial skin and skin-structure infections and community-acquired bacterial pneumonia (CABP) in patients ≥2 months of age [1]. The prodrug, ceftaroline fosamil, is rapidly and completely hydrolyzed to ceftaroline by plasma phosphatase enzymes [1, 2]. Ceftaroline binds plasma proteins (15%–28%), is excreted primarily unchanged in urine, and undergoes minimal metabolism (~20%) to the biologically inert open-ring metabolite (ceftaroline M1) [1, 2]. Ceftaroline has broad-spectrum coverage that includes methicillin-resistant Staphyloccocus aureus (MRSA) and exhibits time-dependent bactericidal killing [1].
To date, 5 studies have been conducted in children receiving ceftaroline, 2 of which were pharmacokinetic (PK) studies in children between birth and 17 years of age [3]. Pediatric data were used to update a population PK model developed for adults, and simulations were performed to guide dosing recommendations in children aged ≥2 months [1, 3]. Eleven preterm neonates (born between 32 and 37 weeks’ gestational age [GA]) were included in the analysis, and results of simulations suggested that a dose of 6 mg/kg every 8 hours would be adequate in infants <2 months of age. Limited information is known about the optimal dose of ceftaroline in extremely preterm (<28 weeks’ GA) infants. To our knowledge, this is the first case report of a preterm infant born at <28 weeks' GA who received ceftaroline and for whom PK data were available.
CLINICAL CASE REPORT
A small-for-GA female infant who weighed 530 g was born at 24 weeks' GA via emergent cesarean section performed as a result of worsening decelerations and intolerance of labor. The pregnancy was complicated by prolonged preterm rupture of membranes and preterm labor. The infant’s mother tested negative for group B Streptococcus. Two doses of betamethasone and an infusion of magnesium sulfate were administered to the mother prior to delivery. The infant’s Apgar scores were 2, 5, and 6 at 1, 5, and 10 minutes, respectively. The infant was admitted to the North Carolina Children’s Newborn Critical Care Center (Chapel Hill, NC) for surfactant therapy and management of prematurity.
On postnatal day 39, the infant began to decompensate and experienced increasing respiratory needs and decreased urine output. Broad-spectrum antibiotic coverage was started, which included ampicillin, gentamicin, oxacillin, cefepime, and metronidazole, because of concern for neonatal sepsis and possible necrotizing enterocolitis; the plan was to narrow antibiotic coverage once blood culture results were available. The infant’s condition continued to worsen overnight as she experienced hypotension, hyponatremia, thrombocytopenia, decreased urine output, lactic acidosis, and acute kidney injury with an increase in her serum creatinine level from 0.45 to 0.96 mg/dL. Her white blood cell count was 23.8 × 109/L, her platelet count was 137 × 109/L, her absolute neutrophil count was 11.9 × 109/L, and her absolute monocyte count was 6.0 × 109/L. Ampicillin, gentamicin, and oxacillin were discontinued on postnatal day 40, and fluconazole and vancomycin were added. Results of a blood culture performed on postnatal day 39 were positive for MRSA and Staphylococcus capitis. Fluconazole and metronidazole were discontinued on postnatal day 41. Antibiotic coverage continued with cefepime and vancomycin, and 2 subtherapeutic vancomycin troughs of 7.5 and <5.0 mg/L observed while receiving on 15 mg/kg twice per day. Results of the lower respiratory tract culture collected on postnatal day 39 were positive for MRSA. Chest radiography performed on day of life 43 revealed progressive opacification of both lung fields.
Rifampin (20 mg/kg per day) was added on postnatal day 43 to treat persistent MRSA bacteremia. Due to the difficulty dosing vancomycin in the setting of declining renal function, an infectious diseases consult on postnatal day 43 resulted in a recommendation to discontinue vancomycin and cefepime, begin treatment with ceftaroline (8.5 mg/kg every 8 hours), and continue rifampin. Pediatric infectious diseases peers familiar with existing pediatric studies recommend this dose, which is slightly higher than the recommended 8 mg/kg dose (every 8 hours) for infants aged ≥2 months [1], because limited PK data for preterm infants exist. Blood culture results became negative on postnatal day 47, and the child clinically improved. Ceftaroline was continued for 3 weeks beyond the first negative blood culture result based on the pediatric infectious diseases consult recommendations. Her serum creatinine levels ranged from 0.25 to 0.46 mg/dL during ceftaroline treatment. At the age of 5 months, the infant died as a result of complications of prematurity (bronchopulmonary dysplasia and pulmonary artery hypertension) that were not related directly to her MRSA infection.
MATERIALS AND METHODS
This infant was enrolled in an off-label study approved by The University of North Carolina at Chapel Hill institutional review board. If an infant was receiving an off-label medication as part of clinical care, blood for PK testing was collected during times of routine blood draws, and clinical information was obtained from the medical chart. The study did not prescribe the therapeutic agent or the dose. Written informed consent was obtained from the patient’s parents.
Ceftaroline was administered intravenously (IV) at 8.5 mg/kg every 8 hours, with a total infusion duration of 2 hours, including the line flush. Samples were collected throughout the dosing interval (treatment days 1–20). Blood samples were obtained from arterial or capillary blood via skin puncture. Samples were available as plasma, scavenged serum, or plasma from scavenged whole blood. Blood was collected in an ethylenediaminetetraacetic acid (EDTA) microcontainer and spun at 2000g for 10 minutes at 4°C, and then it was frozen at −80°C within 30 minutes of collection. The scavenged samples were obtained from those collected for routine clinical laboratory tests and were processed in the hospital laboratory. The samples were shipped on dry ice to Keystone Bioanalytical, Inc. (North Wales, PA), and analyzed using a validated turbo ion-spray liquid chromatography–tandem mass spectrometry method for the simultaneous detection of ceftaroline and ceftaroline M1. This method uses protein precipitation with methanol to isolate the analytes from plasma. The calibration ranges were 50 to 50000 ng/mL for ceftaroline and 50 to 10000 ng/mL for ceftaroline M1. The interassay and intraassay coefficients of variation ranged from 2.14% to 3.39% and 0.68% to 1.64% for ceftaroline and 2.11% to 2.96% and 1.11% to 1.70% for ceftaroline M1, respectively.
The plasma concentrations of free ceftaroline and total ceftaroline M1 were plotted against time since the last dose on days 2 through 20 to ensure that the data were at a steady state. Mean adult data were obtained and plotted for comparison by digitizing published concentration data after 600 mg IV every 12 hours on day 14 using Plot Digitizer version 2.6.8 [2]. Unbound (free) ceftaroline concentrations were calculated using a protein-binding level of 20% obtained from adult studies [1, 4]. The percentage of time the unbound drug concentration exceeded the minimum inhibitory concentration (fT > MIC) associated with bactericidal activity was calculated in this infant for MICs of 1 and 2 µg/mL, because the MIC required to inhibit the growth of 90% (MIC90) of organisms for MRSA is 1 µg/mL [1].
RESULTS
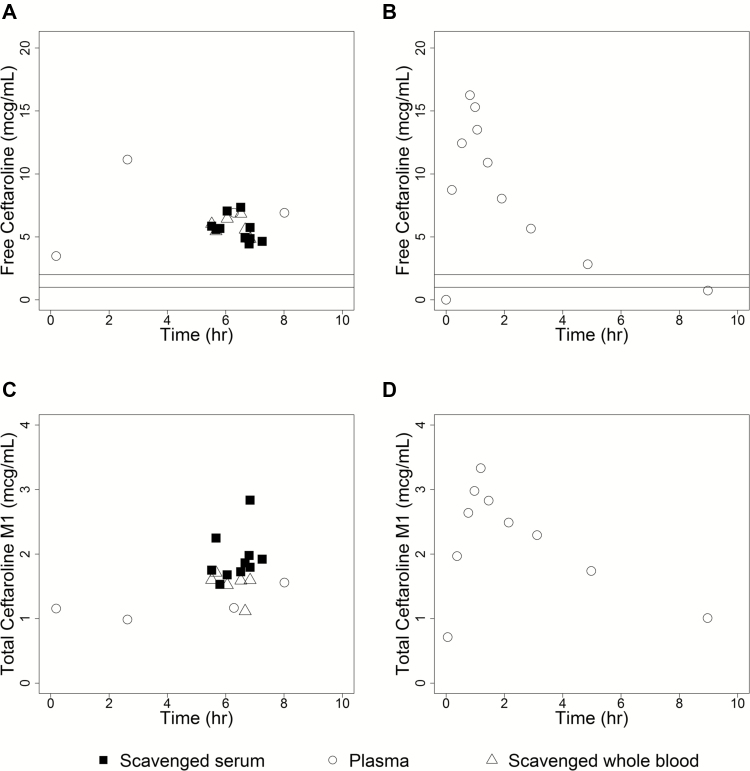
Twenty-two samples (6 plasma, 6 plasma from scavenged whole blood, and 10 scavenged serum) were collected, and the concentration–time profile was plotted for free ceftaroline and total ceftaroline M1 according to specimen type (Figure 1). The ratio of ceftaroline M1 to ceftaroline was 22% in this infant versus 20% in healthy adults [2]. Ceftaroline concentrations ranged from 4338 ng/mL (at 0.18 hours) to 13914 ng/mL (at 2.63 hours) in this preterm infant (Figure 1). In adults receiving the dose approved for CABP (600 mg IV every 12 hours), concentrations ranged from 0 (at 0 hours) to 21330 ng/mL (at 0.92 hours) [1, 2] (Figure 1). The fT > MIC was 100% for all concentrations available using a MIC of 1 µg/mL and 2 µg/mL.
Figure 1.
Plasma concentration data for free ceftaroline and total ceftaroline M1 levels versus time after the last dose stratified according to specimen type. Shown are free ceftaroline (in micrograms per milliliter) and total ceftaroline M1 (in micrograms per milliliter) levels after last dose on days 2 through 20 for this preterm infant (A and C, respectively) and for the digitized mean data obtained from 6 healthy adult volunteers on day 14 after receiving 600 mg IV every 12 hours [2] (B and D, respectively). Lines on the free ceftaroline concentration versus time plots correspond to MICs of 1 and 2 µg/mL for MRSA. One day 1 sample (2.83 hours after last dose; 11168 ng/mL) was excluded to ensure that samples were at steady state. Open circles represent plasma data, open triangles represent plasma data obtained from scavenged whole blood samples, and filled squares represent plasma data obtained from scavenged serum samples.
DISCUSSION
We have presented here the case of an extremely preterm infant with pneumonia attributable to MRSA who was treated with 8.5 mg/kg IV of ceftaroline every 8 hours. Ceftaroline was chosen for treatment given its known activity against MRSA, its penetration into lung tissue, and the challenge of achieving therapeutic vancomycin troughs in this infant. The PK profile of ceftaroline in this infant was similar to that in adults (Figure 1). We found no observable differences in the PK profiles according to specimen type (Figure 1). Scavenged samples are collected from blood remaining after routine laboratory tests as part of clinical care. The advantages of using scavenged samples include a reduction in total blood loss and risks from multiple blood sampling and possible facilitation of parental consent for clinical trials and PK studies [5].
The fT > MIC is associated with efficacy for ceftaroline, similar to other β-lactam antibiotics. The mean fT > MICs associated with 1- and 2-log kill of Staphylococcus aureus were 33% and 45% in a murine thigh and lung infection model, respectively [4]. This preterm infant achieved an fT > MIC of 100% throughout the dosing interval for all available data using MICs of 1 and 2 µg/mL for MRSA. One study which included preterm infants (32–37 weeks’ GA) simulated that >96% of infants <2 months of age are predicted to achieve a fT > MIC of 36% at an MIC of 2 mg/L, and 100% are predicted to achieve a fT > MIC of 44% at an MIC of 1 mg/L [3]. Ceftaroline at 6 mg/kg is currently being evaluated in neonates and young infants with late-onset sepsis [6]. In the case of this infant, ceftaroline (8.5 mg/kg IV every 8 hours) was adequate for achieving the pharmacodynamics endpoint associated with efficacy for MRSA.
Notes
Acknowledgments. We thank John Bradley and Michael Cohen-Wolkowiez for productive discussions regarding ceftaroline dosing.
Disclaimer. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. M. L. receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (grant FDA R01 FD005101 [principal investigator, M. L.], grant NHLBI R34 HL124038 [principal investigator, M. L.], and NICHD Pediatric Trials Network government contract HHSN267200700051C [principal investigator, Daniel K. Benjamin Jr.] under the Best Pharmaceuticals for Children Act). D. G. receives support for research from the National Institute for Child Health and Human Development (grant K23HD083465), from the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry (Cempra, Inc., and Jacobus Pharmaceutical Company, Inc.) for drug development in adults and children.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the contentof the manuscript have been disclosed.
References
- 1. Forest Pharmaceuticals, Inc. TEFLARO® (ceftaroline fosamil) injection for intravenous (IV) use. 2016. Available at: www.allergan.com/assets/pdf/teflaro_pi. Accessed February 9, 2017.
- 2. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/200327Orig1s000ClinPharmR.pdf. Accessed September 2, 2016.
- 3. Riccobene TA, Khariton T, Knebel W, et al. Population PK modeling and target attainment simulations to support dosing of ceftaroline fosamil in pediatric patients with acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. J Clin Pharmacol 2017; 57:345–55. [DOI] [PubMed] [Google Scholar]
- 4. Andes D, Craig WA. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 2006; 50:1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leroux S, Turner MA, Guellec CB, et al. ; TINN (Treat Infections in NeoNates) and GRiP (Global Research in Paediatrics) Consortiums Pharmacokinetic studies in neonates: the utility of an opportunistic sampling design. Clin Pharmacokinet 2015; 54:1273–85. [DOI] [PubMed] [Google Scholar]
- 6. ClinicalTrials.gov. Safety, tolerability and efficacy of ceftaroline in paediatrics with late-onset sepsis Available at: https://clinicaltrials.gov/ct2/show/NCT02424734. Accessed February 9, 2017.