Abstract
Background
Previous evidence suggests that UNC9994 is a beta-arrestin2-selective agonist at the dopamine D2 receptor, lacking ability both to activate and antagonize G protein-dependent signaling. However, this has only been reported by one laboratory using a single assay.
Methods
We used G protein-coupled inward rectifier potassium channel activation in Xenopus oocytes to investigate UNC9994-induced modulation of G protein-dependent signaling at dopamine D2 receptor and dopamine D3 receptor.
Results
At dopamine D2 receptor, UNC9994 induced G protein-coupled inward rectifier potassium channel currents that were 15% of the maximal response to dopamine, with an EC50 of 185 nM. At dopamine D3 receptor, the ligand elicited 89% of the maximal dopamine response with an EC50 of 62 nM. Pertussis toxin abolished G protein-coupled inward rectifier potassium channel activation. Furthermore, UNC9994 antagonized dopamine-induced G protein-coupled inward rectifier potassium channel activation at dopamine D2 receptor.
Conclusions
UNC9994 modulates G protein-coupled inward rectifier potassium channel channel activation via pertussis toxin-sensitive G proteins at dopamine D2 receptor and dopamine D3 receptor. These findings may have implications for the interpretation of data obtained with this ligand.
Keywords: antipsychotic, arrestin, beta-arrestin2, biased ligand, electrophysiology
Significance Statement
The ability of G protein-coupled receptors to signal not only via G proteins but also via beta-arrestins has gained recent interest. Dopamine D2 receptor (D2R) ligands, which selectively modulate the arrestin pathway while leaving G protein signaling undisturbed, have been proposed as a strategy for developing antipsychotics with fewer side-effects and improved efficacy. The compound used in the present study, UNC9994, was reported to be devoid of agonist activity at the G protein pathway and unable to antagonize dopamine-evoked, G protein-dependent signaling. Here, we reassessed these properties of UNC9994 using GIRK channel activation in Xenopus oocytes, a time-resolved, G protein-dependent readout of D2R activity. We find that UNC9994 is a partial agonist at D2R-mediated GIRK channel activation and, as expected of a partial agonist, also antagonizes DA-mediated signaling in the same assay. These findings have implications for the interpretation of data obtained with this ligand, which has been used to interrogate arrestin-dependent dopaminergic signaling.
Introduction
A major challenge to effective treatment of psychotic disorders (including schizophrenia, schizoaffective disorder, and bipolar disorder with mania) is the occurrence of adverse reactions, such as extrapyramidal side-effects, to antipsychotic drugs. These side-effects lead to high rates of treatment nonadherence, thus limiting the clinical usefulness of current antipsychotics. Furthermore, whereas existing drugs show efficacy against the so-called positive symptoms of schizophrenia (i.e., delusions and hallucinations), current treatment does not adequately address the negative (e.g., social withdrawal, emotional flattening, avolition) and cognitive symptom domains (Miyamoto et al., 2012).
Current antipsychotics are antagonists or weak partial agonists (which may be functionally selective; see Urban et al., 2007) at dopamine D2 and D3 receptors (D2R and D3R). These receptors are known to signal via several downstream pathways, which include the classical G protein pathway and the more recently described arrestin pathway (Beaulieu and Gainetdinov, 2011). G protein-mediated actions include adenylate cyclase inhibition and activation of G protein-coupled inward rectifier (GIRK) channels, whereas arrestin recruitment to the receptor, apart from desensitization and internalization, initiates a distinct signaling cascade, which involves Akt/PKB and glycogen synthase-kinase 3. β-Arrestin2 (also known as arrestin3) has specifically been implicated as the arrestin isoform regulating D2R downstream signaling (Beaulieu et al., 2005).
The importance of arrestin signaling has come to the forefront of schizophrenia research in recent years (Freyberg et al., 2010). All clinically used antipsychotics antagonize arrestin recruitment to the D2R (Klewe et al., 2008; Masri et al., 2008), suggesting that this mechanism might be relevant for their therapeutic activity. Indeed, it has been proposed that the clinical benefits of antipsychotics are mediated mainly via inhibition of the arrestin pathway, whereas attenuation of G protein activation would be responsible for extrapyramidal side-effects (Allen et al., 2011). Accordingly, efforts are underway to develop D2R ligands that specifically modulate arrestin signaling (Urs et al., 2016; Männel et al., 2017; McCorvy et al., 2018).
Recent data suggest that “arrestin-biased” ligands, which act as partial agonists on the arrestin pathway without affecting G protein activity, such as UNC9994 (Allen et al., 2011), are able to restore rodent behavioral deficits induced by phencyclidine (PCP) and amphetamine; 2 pharmacological models frequently used for detecting antipsychotic efficacy (Urs et al., 2016). Acting via D2R, UNC9994 appears to activate arrestin-dependent signaling preferentially in cortex (Urs et al., 2016), presumably due to the high expression of both β-arrestin2 and G protein receptor kinase-2 (GRK2) in this brain region enhancing D2R coupling to this pathway (Urs et al., 2016; Sahlholm et al., 2017). The resulting action in the striatum, where β-arrestin2 and GRK2 expression is lower, remains less defined but appears to contribute to antipsychotic-like efficacy in the amphetamine and PCP models (Urs et al., 2016; Sahlholm et al., 2018). It has been suggested that such an action could substitute for the frontal cortical hypodopaminergia believed to underlie cognitive and negative symptoms in schizophrenia while simultaneously counterbalancing excessive D2R signaling in the striatum, thought to cause positive symptoms (Urs et al., 2016). Motor side-effects could potentially be avoided by leaving G protein signaling intact (Allen et al., 2011). However, there are some notable weaknesses and contradictions to these hypotheses; clinical evidence suggests that hypofunction of prefrontal cortical D1R, rather than D2R, may be involved in cognitive deficits in schizophrenia (Arnsten et al., 2017), and activation of both cortical and striatal D2Rs may contribute to the production of positive symptoms (Arnsten et al., 2015). In addition, aripiprazole, which does interfere with G-protein-dependent D2R signaling (Allen et al., 2011; Urs et al., 2016), has a very favorable motor side-effect profile (Naber and Lambert, 2004).
Remarkably, UNC9994 was reported not only to be completely devoid of agonist activity in assays of D2R-mediated Gi/o protein activation but was also found to lack the ability to antagonize dopamine-induced inhibition of adenylate cyclase activity at D2R (Urs et al., 2016). It was thus suggested that UNC9994 might be able to interact only with arrestin-coupled- but not G protein-coupled D2R, making it a truly “arrestin-selective” ligand, which could theoretically allow for a mild side-effect profile as outlined above. However, the lack of antagonistic effects of UNC9994 on D2R-mediated G protein signaling has been described only on the basis of cAMP accumulation experiments, which lacked temporal resolution (GloSensor, a cAMP assay; Urs et al., 2016). Furthermore, while UNC9994 is a relatively low-potency ligand with an EC50 of 0.45 to 1 µM in some arrestin recruitment assays (Allen et al., 2011), only concentrations up to 1 µM of UNC9994 were tested in the GloSensor experiments when competing against dopamine (Urs et al., 2016).
While a completely signaling pathway-selective D2R ligand would be of great utility to the field of neuropharmacology, purportedly arrestin-selective ligands for other receptors, such the angiotensin-I receptor agonist, SII, have recently been shown to be partial agonists in G protein activation assays (Grundmann et al., 2018), underscoring the need for independent validation. To further investigate the interaction (or lack thereof) of UNC9994 with G protein-dependent signaling, we examined the activity of this ligand toward D2R- and D3R-mediated activation of GIRK channels, a sensitive and time-resolved assay that has previously allowed us to study weak partial D2R agonists (Sahlholm et al., 2011).
Methods
Molecular Biology
Human GIRK1 (Kir3.1) and GIRK4 (Kir3.4) cDNA (provided by Dr. Terence Hebert, University of Montreal, Canada) and Regulators of G protein Signaling (RGS)4 (from the Missouri cDNA Resource Center; www.cdna.org) were in pCDNA3 (Invitrogen). cDNA encoding the human dopamine D2L (long isoform of D2R) and D3 receptors and human beta-arrestin2 was in pXOOM (from Dr. Søren-Peter Olesen, University of Copenhagen, Denmark), and the catalytic subunit of PTX (PTX-S1; from Dr. Eitan Reuveny, Weizmann Institute of Science, Israel) was in pGEM-HE. For in vitro transcription, plasmids were linearized (GIRK 1/4, NotI; RGS4, D2LR and D3R, XhoI; PTX-S1, NheI) and transcribed in vitro using the T7 mMessage mMachine kit (Ambion, Austin, TX). cRNA concentration and purity were determined using a spectrophotometer.
Oocyte Isolation and Injection
Oocytes were surgically isolated from female African clawed toads, Xenopus laevis, and injected with cRNA as previously described (Sahlholm et al., 2011). The surgical procedures had been approved by the Swedish National Board for Laboratory Animals and the local ethics committee, Stockholms Norra Djurförsöksetiska nämnd. One nanogram of each GIRK subunit cRNA, 0.2 ng of dopamine D2L or D3 receptor cRNA, and, when used, 40 nfg of RGS4, 5.6 ng of beta-arrestin2, and 3 ng of PTX-S1 cRNA were injected per oocyte. RGS proteins are GTPase-activating proteins expressed in native tissues, which speed up the G protein cycle such that GIRK channel activity more closely follows receptor occupancy by agonist (Dascal and Kahanovitch, 2015).
Receptor Ligands
Dopamine was purchased from Sigma-Aldrich (St. Louis, MO) and UNC9994 and aripiprazole were from Axon MedChem BV (Groeningen, The Netherlands). Dopamine was dissolved in recording buffer, whereas UNC9994 was dissolved in DMSO. (-)-3PPP was a gift from Astra Zeneca (Södertälje, Sweden). Ligands were diluted in the recording solution to obtain the desired concentrations. The maximum final concentration of DMSO used in any experiment did not exceed 0.1% v/v.
Electrophysiology
The electrophysiological experiments were performed at room temperature (20–22°C) 5 to 7 days after cRNA injection using a 2-electrode voltage-clamp setup (CA-1 amplifier, Dagan, Minneapolis, MN) as previously described (Sahlholm et al., 2011). Data were acquired at 134 Hz using pCLAMP 8 (Molecular Devices) software. A high-potassium solution (in mM: 64 NaCl, 25 KCl, 0.8 MgCl2, 0.4 CaCl2, 15 HEPES, 1 ascorbic acid, adjusted to pH 7.4), giving a K+ reversal potential of about -40 mV, was used for GIRK current recording. Ascorbic acid was present to prevent the oxidation of dopamine. Single -80 mV pulses were applied to study GIRK current responses to D2R activity. Ligands were added to the 20-µL recording chamber by superfusion at 1.5 mL/min using a computer-controlled, pressure-driven perfusion system (SmartSquirt, AutoMate Scientific, Berkeley, CA). When evaluating UNC9994 in the agonist mode, 3 to 4 increasing concentrations of the ligand were applied at 35- or 50-second intervals to oocytes expressing D2R or D3R, respectively. The UNC9994-evoked current response was determined by subtracting the basal (agonist-independent) current from the experimental record. The UCN9994-induced current responses were subsequently normalized to the mean response to 1 µM dopamine in 4 different oocytes (expressing D2R or D3R, respectively, together with RGS4 and GIRK1/4), which had not previously been exposed to UNC. In the antagonist mode, 100 nM dopamine was first applied to provide a baseline response, followed by 3 to 4 applications of increasing concentrations of UNC9994 at 50-second intervals in the continued presence of dopamine. For each oocyte, the current amplitude at the end of each antagonist application interval was normalized to the control response to 100 nM dopamine obtained at the start of the protocol. For dopamine concentration-response data, 4 to 5 increasing concentrations of dopamine were applied at 25-second intervals, ending with a response-saturating concentration (100 µM) of dopamine (supplementary Figure 1).
Data Analysis
Variable slope sigmoidal concentration-response curves were fitted to the concentration-response data using GraphPad (Prism Software). The following equation was fitted to agonist data:
where Y is the response as a fraction of 1, top is the maximal response of the agonist expressed as a fraction of 1, X is the logarithm of ligand concentration, and n is the Hill slope. When analyzing dopamine concentration-response data, top was set to 1.
For fitting antagonist data, the fitted equation was:
where bottom is the maximal response inhibition evoked by the antagonist.
Results
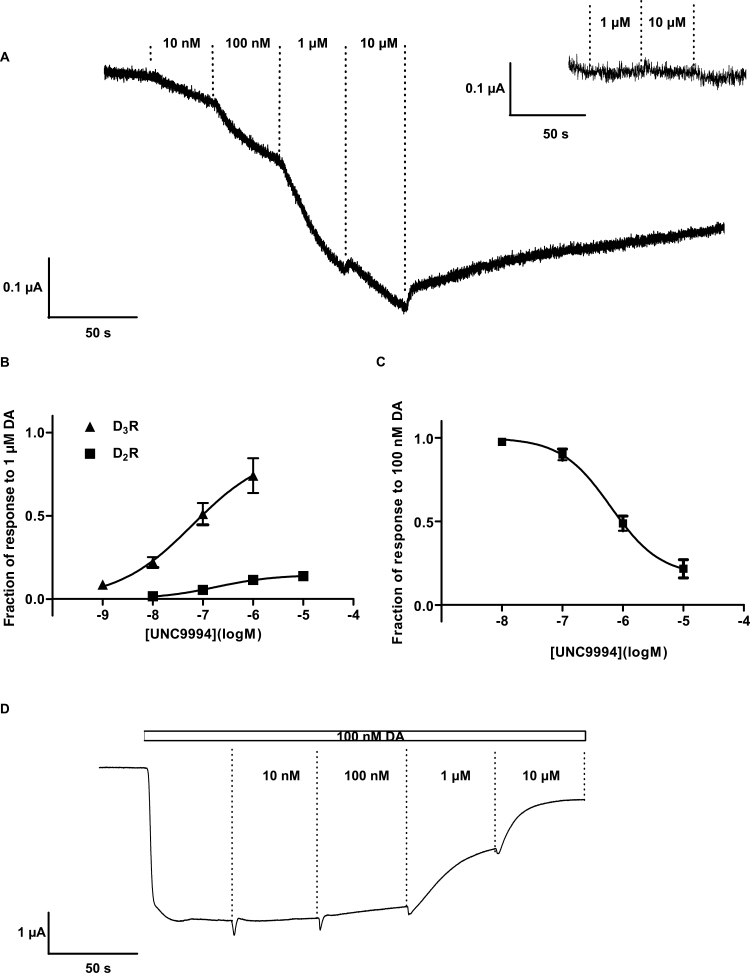
First, UNC9994 was applied alone to Xenopus oocytes expressing D2R together with RGS4 and GIRK1/4 channels. The ligand evoked small but clearly detectable inward currents when applied at concentrations between 10 nM and 10 µM (Figure 1A). Such currents were not observed when UNC9994 was applied to oocytes injected with RNA encoding D2R but not GIRK1/4, nor in oocytes expressing GIRK channels alone (not shown), and were abolished by coexpression of the catalytic subunit of pertussis toxin (PTX-S1; Figure 1A, inset), which inactivates inhibitory Gi/o proteins by ADP ribosylation (Vivaudou et al., 1997). The pEC50 for UNC9994-induced current activation was 6.73±0.40 (EC50 185 nM; comparable with the Ki of 79 nM of this compound at D2R, as reported by Allen et al., 2011). The top of the UNC9994 concentration-response curve was 14.5±2.8% of the mean response to 1 µM dopamine (which evokes a maximal response; see supplementary Figure 1), indicating weak partial agonism of UNC9994 at GIRK activation elicited via D2R (Figure 1B). The similarly submaximal responses elicited by 10 µM of the known partial D2R agonists, (-)-3-PPP and aripiprazole, which evoked 27.7±7.5 and 9.2±2.3% of the mean response to 1 µM dopamine, respectively (supplementary Figure 2), suggest that the receptor reserve under these conditions was low or absent.
Figure 1.
Actions of UNC9994 on G protein-dependent G protein-coupled inward rectifier (GIRK) signaling elicited via dopamine D2 receptor (D2R) and D3R. (A) Representative current trace recorded in an oocyte coexpressing D2R with Regulators of G protein Signaling (RGS)4 and GIRK1/4 channels, demonstrating GIRK channel activation upon application of increasing concentrations of UNC9994, as indicated. Inset shows the absence of response to 1 and 10 µM UNC9994 in an oocyte coexpressing D2R with RGS4, GIRK1/4, and the catalytic subunit of PTX. (B) Concentration-response curves for GIRK activation by UNC9994 in oocytes expressing D2R or D3R, as indicated, together with RGS4 and GIRK1/4 channels, n=3 to 5 per data point. The mean current amplitude elicited by each concentration of UNC9994 was normalized to the mean amplitude elicited by 1 µM dopamine in separate cells (n=4 for both D2R and D3R). (C) Concentration-response curve for the inhibition of dopamine-induced GIRK activation by UNC9994. Data were obtained from experiments such as that shown in D, n=4 to 6 for each data point. (D) Representative current trace recorded in an oocyte coexpressing D2R with RGS4 and GIRK1/4 channels, showing the inhibition of the GIRK response to 100 nM dopamine by increasing concentrations of UNC9994, as indicated.
When applied to oocytes expressing D3R together with RGS4 and GIRK1/4 channels, the efficacy of UNC9994 was greater than at D2R (Figure 1B), evoking 89.1±24.3% of the maximal response to 1 µM dopamine. In agreement with radioligand competition data reported by Allen et al. (2011), the functional potency of UNC9994 was higher at the D3R that at the D2R, with a pEC50 of 6.21±0.55 (EC50 62.1 nM). PTX-S1 coexpression prevented UNC9994-induced GIRK activation also in D3R-expression oocytes (data not shown).
When applied to oocytes expressing D2R together with RGS4 and GIRK1/4 channels in the presence of 100 nM dopamine, UNC9994 inhibited the dopamine-induced response with an IC50 of 630 nM (Figure 1C, D). Assuming a Kd of dopamine of 33 nM based on its EC50 value for GIRK channel activation (supplementary Figure 1), the Ki of UNC9994 was estimated as 155 nM, again comparable with the Ki reported by Allen et al. (2011).
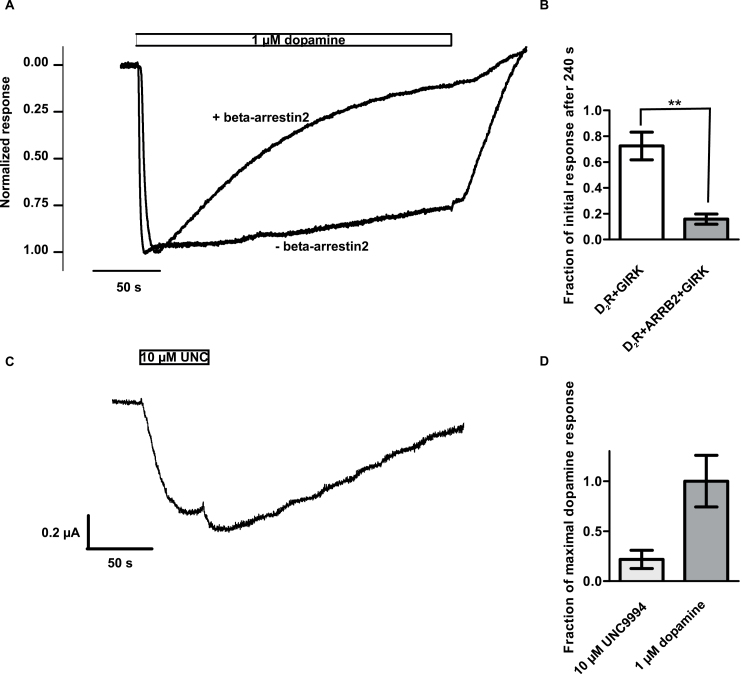
Finally, we evaluated the effects of UNC9994 in oocytes expressing D2R together with GIRK1/4 and beta-arrestin2. The expression levels of beta-arrestin2 were sufficiently high to induce prominent desensitization of the response to 1 µM dopamine compared with control cells, which had not been injected with RNA encoding beta-arrestin2 (Figure 2A, B). A total of 10 µM UNC9994 was found to induce GIRK activation also under these conditions, which was 21.7±9.1% of the mean response to 1 µM dopamine (Figure 2C, D).
Figure 2.
UNC9994 acts as a partial agonist at G protein-coupled inward rectifier (GIRK) activation also in the presence of beta-arrestin2. (A) Representative current traces recorded from oocytes coexpressing dopamine D2 receptor (D2R) with GIRK1/4 channels with or without beta-arrestin2, as indicated, demonstrating the impact of beta-arrestin2 coexpression on the time course of the current response to 1 µM dopamine. (B) Ratio between the peak inward current response and the response amplitude at the end of the 240-second application of dopamine. **P<.01, Student’s t test, n=5 for each group of oocytes. (C) Representative current trace showing GIRK channel activation elicited upon application of 10 µM UNC9994 (UNC) in an oocyte coexpressing D2R with GIRK1/4 channels and beta-arrestin2. (D) Average response to 10 µM UNC9994 as a fraction of the average response to 1 µM dopamine in oocytes coexpressing D2R with GIRK1/4 channels and beta-arrestin2. n=6 for UNC9994; n=5 for dopamine.
Discussion
UNC9994 has previously been described as an arrestin-biased ligand incapable of activating G protein-dependent signaling downstream of D2R (Allen et al., 2011) and, even more intriguingly, unable to antagonize dopamine-evoked adenylate cyclase inhibition via this receptor (Urs et al., 2016). It should be emphasized that the present study does not address the relative efficacies of G protein- vs arrestin-dependent signaling elicited by UNC9994. Rather, our results challenge previous reports of a complete lack of interaction of UNC9994 with G protein-dependent signaling, as the present findings strongly suggest that this compound acts as an agonist at GIRK channel activation at D2R and D3R. GIRK channels are typically activated by Gβγ subunits released from Gi/o proteins (Dascal and Kahanovitch, 2015). In accordance with a Gi/o-dependent process, GIRK activation by UNC9994 was blocked by coexpression of the catalytic subunit of PTX (Vivaudou et al., 1997). Furthermore, we showed that at the D2R, where the efficacy of UNC9994 is low, this ligand antagonizes the near-maximal GIRK response induced by 100 nM dopamine. Xenopus oocytes do not endogenously express functional arrestins (Kovoor et al., 1997); however, we found UNC9994 to elicit GIRK activation also in oocytes expressing human beta-arrestin2.
The reasons for the discrepancy between the present work and earlier published findings by Allen et al. (2011) and Urs et al. (2016) are unclear at present, but may, in part, reflect functional selectivity for GIRK channel activation over adenylate cyclase inhibition. Such selectivity has been reported previously for the antipsychotic aripiprazole and its analogue, SV-III-130s, which are both structurally related to UNC9994. Opposite to UNC9994, aripiprazole and SV-III-130s were found to be partial agonists in adenylate cyclase inhibition assays while acting as neutral antagonists when D2R-mediated GIRK channel activation was examined (Urban et al., 2007; Luedtke et al., 2012). Here, however, aripiprazole was found to be a partial agonist at GIRK activation, with an efficacy similar to that of UNC9994. Interestingly, aripiprazole and UNC9994 were shown to have similar efficacy also at beta-arrestin signaling (Urs et al., 2017), which could suggest that, when considering GIRK activation as the readout of Gi/o activity, there is little difference in arrestin/G protein bias between the 2 compounds. However, bias is context dependent and should be investigated in the same cellular background and at the same time-point following agonist addition (Klein Herenbrink et al., 2016; Onfroy et al., 2017). Measures of biased agonism, as well as antagonism, have been reported to be strongly influenced by the stoichiometry of receptor, G protein, and effector molecules (Onfroy et al., 2017), and these parameters likely differed between our study and those of Urs et al. (2016) and Allen et al. (2011), which may have contributed to the divergent results. For example, a large receptor reserve in the experiments by Urs et al. (2016) may have precluded the detection of antagonism at 1 µM UNC9994, which was the highest concentration tested. In addition, the lack of temporal resolution of the adenylate cyclase assay might have prevented observation of a modulatory effect of UNC9994 (see Klein Herenbrink et al., 2016, for an investigation of the effect of incubation time on measures of ligand efficacy).
Nevertheless, the absence of antagonism of G protein-dependent signaling reported by Urs et al. (2016) stands in sharp contrast to the present data, and our findings may thus warrant reinterpretation of some of the findings reported from studies where UNC9994 was used as an arrestin-specific ligand (e.g., Scarduzio et al., 2017; Pack et al., 2018). The abolition of antipsychotic-like activity of this ligand in beta-arrestin2 knockout animals certainly suggests an important contribution of the arrestin pathway to such activity (Allen et al., 2011); however, it cannot be excluded that G protein-dependent signaling also plays some role. This is particularly relevant considering that arrestin signaling was suggested to be low or absent in striatal medium spiny neurons (Urs et al., 2016), yet A2AR-expressing neurons, which are mainly of this type, appear to play a role in the ability of UNC9994 to counteract amphetamine- and PCP-induced hyperlocomotion (Sahlholm et al., 2018; Urs et al., 2016). Furthermore, UNC9994 has higher affinity for D3R than for D2R (Allen et al., 2011), and the G protein-dependent agonist activity of UNC9994 at D3R revealed in the present study may confer procognitive properties similar to those described for the similarly D3R-preferring antipsychotic, cariprazine (Zimnisky et al., 2013; Németh et al., 2017).
In summary, our present findings suggest that UNC9994 is able to induce G protein-dependent GIRK activation via D2R- and D3R, acting as a weak partial agonist at the former while being more efficacious at the latter receptor. In addition, contrasting with previous findings examining adenylate cyclase activity, UNC9994 inhibits the G protein-dependent GIRK response to dopamine via D2R.
Funding
This work was supported by research grants from Stiftelsen Lars Hiertas Minne (FO2016-0434 to K.S.), Åhlén-stiftelsen (no grant number, to K.S.), Magnus Bergvalls stiftelse (2016-01878 and 2017-02452 to K.S.), Karolinska Institutet Funds (2016fobi47610 to K.S.), and the Swedish Research Council (15083 to P.Å. and 21785-01-4 to J.N.).
Supplementary Material
Acknowledgments
K.S. is a recipient of postdoctoral fellowships from the Swedish Brain Foundation (PS2015-0030) and the Swedish Society of Medicine (SLS-687851). (-)-3PPP was a gift from Astra Zeneca (Södertälje, Sweden).
Statement of Interest
None.
References
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, Jensen NH, Che X, Bai X, Frye SV, Wetsel WC, Caron MG, Javitch JA, Roth BL, Jin J(2011)Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 108:18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang M, Paspalas CD(2015)Dopamine’s actions in primate prefrontal cortex: challenges for treating cognitive disorders. Pharmacol Rev 67:681–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Girgis RR, Gray DL, Mailman RB(2017)Novel dopamine therapeutics for cognitive deficits in schizophrenia. Biol Psychiatry 81:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR(2011)The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG(2005)An akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122:261–273. [DOI] [PubMed] [Google Scholar]
- Dascal N, Kahanovitch U(2015)The roles of gβγ and gα in gating and regulation of GIRK channels. Int Rev Neurobiol 123:27–85. [DOI] [PubMed] [Google Scholar]
- Freyberg Z, Ferrando SJ, Javitch JA(2010)Roles of the akt/GSK-3 and wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M, et al. (2018)Lack of beta-arrestin signaling in the absence of active G proteins. Nat Commun 9:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Herenbrink C, Sykes DA, Donthamsetti P, Canals M, Coudrat T, Shonberg J, Scammells PJ, Capuano B, Sexton PM, Charlton SJ, Javitch JA, Christopoulos A, Lane JR(2016)The role of kinetic context in apparent biased agonism at gpcrs. Nat Commun 7:10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klewe IV, Nielsen SM, Tarpø L, Urizar E, Dipace C, Javitch JA, Gether U, Egebjerg J, Christensen KV(2008)Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 54:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Nappey V, Kieffer BL, Chavkin C(1997)Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in xenopus oocytes. J Biol Chem 272:27605–27611. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mishra Y, Wang Q, Griffin SA, Bell-Horner C, Taylor M, Vangveravong S, Dillon GH, Huang RQ, Reichert DE, Mach RH(2012)Comparison of the binding and functional properties of two structurally different D2 dopamine receptor subtype selective compounds. ACS Chem Neurosci 3:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel B, Jaiteh M, Zeifman A, Randakova A, Möller D, Hübner H, Gmeiner P, Carlsson J(2017)Structure-guided screening for functionally selective D2 dopamine receptor ligands from a virtual chemical library. ACS Chem Biol 12:2652–2661. [DOI] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG(2008)Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A 105:13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorvy JD, Butler KV, Kelly B, Rechsteiner K, Karpiak J, Betz RM, Kormos BL, Shoichet BK, Dror RO, Jin J, Roth BL(2018)Structure-inspired design of β-arrestin-biased ligands for aminergic gpcrs. Nat Chem Biol 14:126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA(2012)Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry 17:1206–1227. [DOI] [PubMed] [Google Scholar]
- Naber D, Lambert M(2004)Aripiprazole: a new atypical antipsychotic with a different pharmacological mechanism. Prog Neuropsychopharmacol Biol Psychiatry 28:1213–1219. [DOI] [PubMed] [Google Scholar]
- Németh G, Laszlovszky I, Czobor P, Szalai E, Szatmári B, Harsányi J, Barabássy Á, Debelle M, Durgam S, Bitter I, Marder S, Fleischhacker WW(2017)Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double-blind, controlled trial. Lancet 389:1103–1113. [DOI] [PubMed] [Google Scholar]
- Onfroy L, Galandrin S, Pontier SM, Seguelas MH, N’Guyen D, Sénard JM, Galés C(2017)G protein stoichiometry dictates biased agonism through distinct receptor-G protein partitioning. Sci Rep 7:7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack TF, Orlen MI, Ray C, Peterson SM, Caron MG(2018)The dopamine D2 receptor can directly recruit and activate GRK2 without G protein activation. J Biol Chem 293:6161–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlholm K, Barchad-Avitzur O, Marcellino D, Gómez-Soler M, Fuxe K, Ciruela F, Arhem P(2011)Agonist-specific voltage sensitivity at the dopamine D2S receptor–molecular determinants and relevance to therapeutic ligands. Neuropharmacology 61:937–949. [DOI] [PubMed] [Google Scholar]
- Sahlholm K, Ielacqua GD, Xu J, Jones LA, Schlegel F, Mach RH, Rudin M, Schroeter A(2017)The role of beta-arrestin2 in shaping fmri BOLD responses to dopaminergic stimulation. Psychopharmacology (Berl) 234:2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlholm K, Gómez-Soler M, Valle-León M, López-Cano M, Taura JJ, Ciruela F, Fernández-Dueñas V(2018)Antipsychotic-like efficacy of dopamine D2 receptor-biased ligands is dependent on adenosine A2A receptor expression. Mol Neurobiol 55:4952–4958. [DOI] [PubMed] [Google Scholar]
- Scarduzio M, Zimmerman CN, Jaunarajs KL, Wang Q, Standaert DG, McMahon LL(2017)Strength of cholinergic tone dictates the polarity of dopamine D2 receptor modulation of striatal cholinergic interneuron excitability in DYT1 dystonia. Exp Neurol 295:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivaudou M, Chan KW, Sui JL, Jan LY, Reuveny E, Logothetis DE(1997)Probing the G-protein regulation of GIRK1 and GIRK4, the two subunits of the kach channel, using functional homomeric mutants. J Biol Chem 272:31553–31560. [DOI] [PubMed] [Google Scholar]
- Urban JD, Vargas GA, von Zastrow M, Mailman RB(2007)Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology 32:67–77. [DOI] [PubMed] [Google Scholar]
- Urs NM, Gee SM, Pack TF, McCorvy JD, Evron T, Snyder JC, Yang X, Rodriguiz RM, Borrelli E, Wetsel WC, Jin J, Roth BL, O’Donnell P, Caron MG(2016)Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci U S A 113:E8178–E8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Peterson SM, Caron MG(2017)New concepts in dopamine D2 receptor biased signaling and implications for schizophrenia therapy. Biol Psychiatry 81:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimnisky R, Chang G, Gyertyán I, Kiss B, Adham N, Schmauss C(2013)Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl) 226:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.