Abstract
Purpose
To compare common carotid artery (CCA) wall thickness measured manually by using US and semiautomatically by using MRI, and to examine their associations with incident coronary heart disease and stroke.
Materials and Methods
This prospective study enrolled 698 participants without a history of clinical cardiovascular disease (CVD) from the Multi-Ethnic Study of Atherosclerosis (MESA) from July 2000 to December 2013 (mean age, 63 years; range, 45 to 84 years; same for men and women). All participants provided written informed consent. CCA wall thickness was measured with US as well as both noncontrast proton-density–weighted and intravenous gadolinium-enhanced MRI. Cox proportional hazards models were used to assess the associations between wall thickness measurements by using US and MRI with CVD outcomes.
Results
The adjusted hazard ratios for coronary heart disease, stroke, and CVD associated with per standard deviation increase in intima-media thickness were 1.10, 1.08, and 1.14, respectively. The corresponding associations for mean wall thickness measured with proton-density–weighted MRI were 1.32, 1.48, and 1.37, and for mean wall thickness measured with gadolinium-enhanced MRI were 1.27, 1.58, and 1.38. When included simultaneously in the same model, MRI wall thickness, but not intima-media thickness, remained associated with outcomes.
Conclusion
For individuals without known cardiovascular disease at baseline, wall thickness measurements by using MRI were more consistently associated with incident cardiovascular disease, particularly stroke, than were intima-media thickness by using US.
© RSNA, 2018
Summary
A prospective study of individuals without known clinical cardiovascular disease at baseline found that carotid wall thickness measured semiautomatically with MRI was more consistently associated with incident cardiovascular disease, particularly stroke, than was intima-media thickness measured manually with US.
Implications for Patient Care
■ Carotid wall thickness measured semiautomatically by using MRI was a more precise risk marker for coronary heart disease and stroke than was intima-media thickness measured manually by using US.
■ MRI wall thickness measurement with gadolinium-based contrast enhancement was more strongly associated with stroke compared with noncontrast MRI measurement (hazard ratios, 1.58 vs 1.48).
Introduction
Carotid intima-media thickness (IMT) measured manually with B-mode US is the most widely used imaging method to assess the level of early carotid atherosclerosis because it is inexpensive, noninvasive, involves no ionizing radiation, requires no contrast agent (at least as is traditionally performed), and is readily repeatable (1). However, recent meta-analyses suggest that its clinical usefulness may be limited because it does not improve cardiovascular disease (CVD) risk prediction beyond traditional risk factors in the general population (2–4). A meta-analysis using individual data from 14 population-based cohorts (45 828 participants) reported that for every 0.1-mm increase in IMT, the hazard ratio of first-time myocardial infarction or stroke was 1.09 (95% confidence interval: 1.07, 1.12), with minor improvement in prediction when added to the Framingham risk score (increase of 0.002 in the C statistic) (3). Vessel wall MRI has emerged as a promising noninvasive technique for imaging the arterial wall that could provide insight into CVD risk (5). MRI can be used to image the entire wall circumference, in contrast to US measurements of IMT that are usually based solely on views of the far wall of the carotid artery (5,6). Additionally, MRI includes adventitia in the measurement of wall thickness, which might be important for defining vascular inflammation because it is the source of vasa vasorum (5,7).
Given these inherent differences in wall thickness measurements between US and MRI, we hypothesized that the associations of wall thickness with CVD risk factors and with incident CVD events may differ between modalities. Previous analyses of the Multi-Ethnic Study of Atherosclerosis (MESA) have found positive associations between baseline maximum carotid IMT and incident CVD events, with hazard ratios for CVD associated with each standard deviation increment in IMT ranging from 1.2 to 1.3 (8,9). The purpose of our current MESA analysis was therefore to determine whether the associations of wall thickness measurements with traditional CVD risk factors and with incident CVD events were different when measured by using MRI compared with US.
Materials and Methods
Study Design and Population
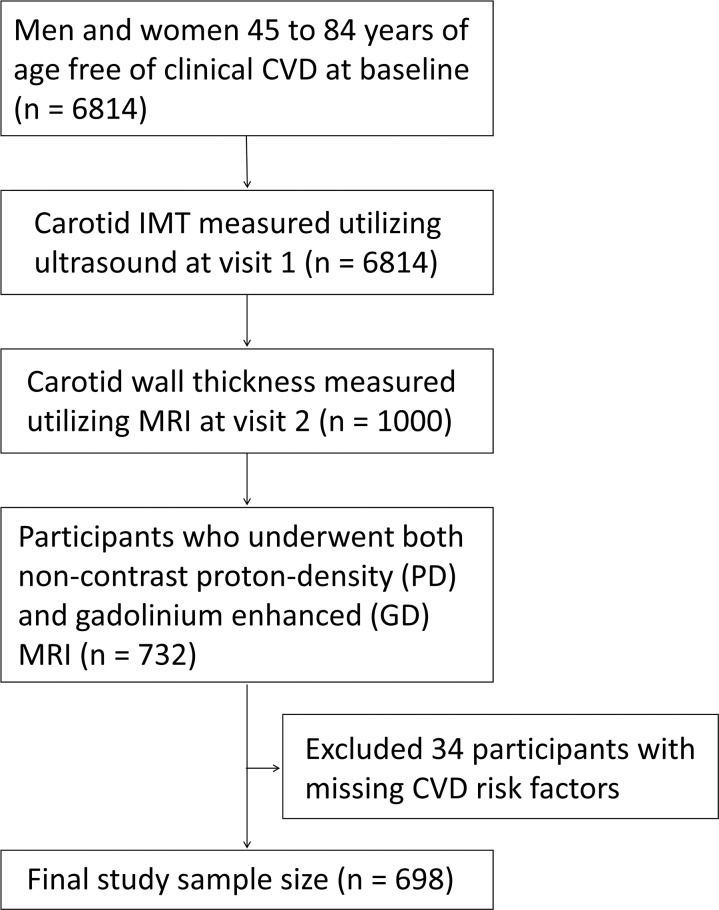
MESA is a multicenter prospective cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors that influence its progression (10). All centers obtained approval from their respective institutional review boards, and all participants provided written informed consent. All study procedures were conducted in a Health Insurance Portability and Accountability Act–compliant manner. At baseline (visit 1, July 2000 to August 2002), 6814 men and women aged 45 to 84 years free of clinical CVD were recruited from six U.S. communities (Baltimore, Md; Chicago, Ill; Forsyth County, NC; Los Angeles County, Calif; northern Manhattan, NY; and St. Paul, Minn) (10) (Fig 1). A full list of participating MESA investigators and institutions is available online (https://www.mesa-nhlbi.org). At visit 1, carotid US was performed in all participants for IMT measurements (11,12). At visit 2 (September 2002 to February 2004), we used stratified sampling according to IMT status at visit 1 to enroll approximately 1000 participants for carotid MRI, including 600 participants from all six centers at or above the top 15th percentile of carotid IMT, and 400 participants from the Baltimore and Los Angeles centers below the top 15th percentile (11). The median interval between IMT and MRI measurements was 1.7 years (interquartile range, 1.4–1.9 years; overall range, 1.2–2.6 years). Our current analysis was restricted to 732 participants who underwent both noncontrast proton-density–weighted and intravenous gadolinium-enhanced MRI. We excluded 34 participants with missing CVD risk factors. Our final study sample size was 698 (387 men and 311 women).
Figure 1:
Study flow diagram. CVD = cardiovascular disease, IMT = intima-media thickness.
Clinical Data Collection
MESA participants underwent a comprehensive medical history and cardiovascular examination by centrally trained clinical teams during each standardized clinical visit. Detailed methods regarding clinical data collection are available in Appendix E1 (online).
Carotid IMT
US measurement of carotid IMT has been described previously (12). J.F.P. (with 29 years of experience in performing carotid IMT measurements) designed the acquisition protocol and supervised the acquisition of the IMT data. Carotid image acquisitions were performed by 18 sonographers at six different geographic locations. The sonographers were trained centrally during a one-and-a-half-day curriculum that included didactic sessions on the physics of US imaging, the pathophysiology of carotid disease, the technical aspects of carotid artery IMT evaluations, overall US machine operation (knobology), reader workstation operation, and measurement processes. Machine settings were standardized and all sonographers performed practice images under supervision (J.F.P.). On return to the clinic sites, the sonographers had to submit five practice images that were reviewed for protocol adherence and image quality, after which they were certified to perform the carotid studies. Reader performance reports were generated on a monthly basis. Direct communication by phone was instigated in cases of poor imaging compliance. The supervising physician routinely performed periodic reviews on a monthly to bimonthly interval.
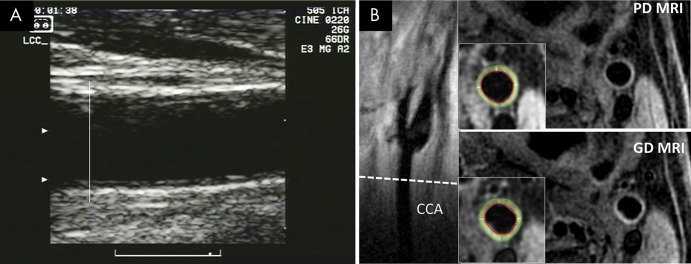
The six clinic US machines were Logiq 700 devices (GE Healthcare, Milwaukee, Wis) equipped with the same linear array probe (M12 L). Imaging frequency was set at 13 MHz and two contiguous focal zones were placed in the common carotid artery (CCA) lumen just above the common carotid far wall. The image frame rate was 31–32 frames per second (Fig E1 [online]). Short video loops were acquired on videotapes and sent to the US reading center (Tufts Medical Center, Boston Mass). Videotapes were reviewed and end-diastolic images (smallest artery diameter) were digitized by using a frame grabber. Far wall CCA IMT measurements were performed on both sides of the neck below the bulb, starting at the point at which the outer wall (adventitia) of the artery begins to diverge (Fig E1 [online]). A continuous tracing was made manually of the far wall lumen-intima and media-adventitia interfaces. The paired tracings were then processed by using a proprietary measurement algorithm to calculate the mean distance between both interfaces as well as the maximum value. We then calculated the mean of the mean right and left IMT values, as well as the mean of the maximum right and left IMT values (12). An example US image is provided in Figure 2, A.
Figure 2:
Images show common carotid artery (CCA) wall thickness assessment in a 62-year-old man by using, A, US and, B, MRI. Far wall intima-media thickness was measured below carotid bulb, starting at point where outer wall of artery begins to diverge (A). Corresponding MR images (B) show location of CCA section (dashed line) on long-axis view of left carotid bifurcation, with noncontrast proton-density (PD)–weighted and intravenous gadolinium-enhanced (GD) MR images obtained at this location. Insets show inner and outer boundaries of segmented vessel walls, with white radial lines delineating 12 sectors over which wall thickness was computed.
Blinded replicate images were acquired in 150 participants and both sets of images were read blindly by the same reader; intraclass correlation coefficient was 0.91 for CCA IMT (13). Interreader and intrareader reproducibility was assessed on the image sets of 78 and 41 participants, respectively. The intraclass correlation coefficient was 0.79 for interreader reproducibility and 0.92 for intrareader reproducibility.
Carotid MRI
The carotid MRI protocol has been described previously (11,14). The principal investigator (B.A.W., with 14 years of cardiovascular MRI experience) supervised the process. B.A.W. traveled to each site to train the MRI technologists, who were then certified centrally by the MRI reading center based on volunteer examinations. Image quality and protocol adherence were monitored centrally throughout the study with immediate feedback provided to individual technologists when protocol deviations were identified. MRI examinations were performed at six sites with 1.5-T MRI units (three sites used CV/i scanners [GE Healthcare], one site used a Quantum version mobile scanner or an Avanto platform [Siemens Health Care, Erlangen, Germany], and two sites used a Sonata platform [Siemens Health Care]) by using bilateral dedicated carotid coils. A transverse MRI section (acquired resolution, 2 mm × 0.54 mm × 0.54 mm) was obtained through the distal CCA on the side judged to have the thicker carotid wall (to reduce measurement time) centered 15 mm below the flow divider of the carotid bifurcation with proton-density weighting (repetition time, 2 R-R intervals; echo time, 5 msec) to measure wall thickness with proton-density–weighted MRI. Flow suppression was achieved by using a double inversion-recovery fast spin-echo sequence with peripheral pulse gating, and fat saturation was applied. The acquisition was repeated following intravenous injection of 0.1 mmol/kg gadodiamide (Omniscan; GE Healthcare) to measure wall thickness with gadolinium-enhanced MRI. Image analysis was conducted at the Johns Hopkins MRI core laboratory (Baltimore, Md) (14). Two trained analysts contoured the outer (adventitial) wall and lumen for each CCA image by using semiautomated software (VesselMass; Leiden University, Leiden, the Netherlands), and all contours were subsequently checked for accuracy by B.A.W. The contoured vessel wall was divided into 12 radial segments by using an automated feature of the software, and mean and maximum segmental wall thickness measurements were recorded. MRI analysts were unaware of prior IMT US measurements. An example MR image is provided in Figure 2, B.
Repeated MRI or readings were not performed in MESA; however, B.A.W. performed a reliability study by using 1.5-T units and similar methods in participants in the Atherosclerosis Risk in Communities Study (15). By randomly reassigning images for interpretation by the same (n = 53) or different (n = 111) reader, the reliability estimates based on repeated MRI were 0.89 for maximum wall thickness of left CCA and 0.42 for maximum wall thickness of right CCA. Image variability includes errors due to the reader and variations in image acquisition. Repeat images were generally as reliable as repeat readings of the same image, suggesting that overall reliability was primarily related to reader variability, and that the error due to the MRI acquisition was small (15).
Follow-up and Cardiovascular Events
MESA event ascertainment has been described previously (16). In brief, participants were followed for incident cardiovascular events during standardized MESA visits or by telephone interviewer every 9–12 months to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, and deaths. MESA-defined coronary heart disease includes myocardial infarction, resuscitated cardiac arrest, definite or probable angina, and coronary heart disease death. MESA-defined CVD includes coronary heart disease, stroke, and other atherosclerotic or CVD death. Participants were followed through December 2013 until they had a first incident event, or were censored at death or last follow-up.
Statistical Analyses
We used seemingly unrelated regression to calculate and compare the cross-sectional associations between CVD risk factors and wall thickness measurements by using US and MRI (17). Seemingly unrelated regression is a generalization of a linear regression model that consists of several regression equations, each having its own dependent variable and potentially different sets of explanatory variables (17). Because carotid US was performed at visit 1 and MRI was performed at visit 2, we used CVD risk factors obtained at visit 1 for analyzing IMT data, and risk factors collected at visit 2 for analyzing MRI data. IMT and MRI wall thickness measurements were standardized (with mean of 0 and standard deviation of 1), and all models were adjusted for age, race, and sex.
We used Cox proportional hazards models to calculate hazard ratios for each CVD outcome that was associated with per standard deviation difference in IMT or MRI wall thickness measures. In the IMT analysis, participants were followed for incident CVD from visit 1 through December 2013 (median follow-up, 12.3 years; 97, 70, and 30 participants with incident CVD, coronary heart disease, and stroke, respectively). In the MRI analysis, follow-up started from visit 2 through December 2013 (median follow-up, 10.7 years; 87, 64, and 26 participants with incident CVD, coronary heart disease, and stroke, respectively). Participants who developed incident CVD events between visit 1 and 2 were excluded when evaluating the association between MRI wall thickness and CVD outcomes (10 participants with CVD, six participants with coronary heart disease, and four participants with stroke). To examine the association of US IMT and MRI wall thickness with outcomes, we first modeled IMT and MRI wall thickness separately by themselves. Two models with progressive degrees of adjustment were used. The initial model was adjusted for age, race, and sex. The second model was further adjusted for body mass index, smoking, total and high-density lipoprotein cholesterol, triglycerides, diabetes, hypertension, and C-reactive protein. Second, we included US IMT and MRI wall thickness measures simultaneously in the same model, and used bootstrap with 1500 iterations to estimate the difference between the log-hazard ratios of IMT and MRI wall thickness measures.
Additionally, we performed several sensitivity analyses. First, we repeated all analyses by using the mean of the maximum right and left IMT values in place of the mean of the mean right and left IMT. Second, because US measurement of IMT was performed on both sides of the neck, whereas MRI could be obtained from either side (the CCA with the thicker wall was targeted), we restricted the analysis to using only IMT measurements performed on the same side as the MRI. Third, we further adjusted for use of lipid-lowering medications, as well as systolic and diastolic blood pressure in the Cox model. Finally, because the follow-up duration for IMT was slightly longer than for MRI, we repeated the analysis for IMT by restricting the follow-up time of IMT to the same as that for MRI. All analyses were performed by using Stata (version 14; StataCorp, College Station, Tex). A two-sided P value < .05 was considered to indicate statistical significance.
Results
The average age of our study participants at visit 1 was 63.4 years (men, 63.2 years; women, 63.7 years; range, 45 to 84 years; same for men and women) (Table 1). Men comprised 55.4% of all participants and 40.1% were white. The mean IMT, wall thickness measured with proton-density–weighted MRI, and wall thickness measured with gadolinium-enhanced MRI were 0.78 mm, 0.98 mm, and 0.99 mm (Table 2). The correlations between IMT and MRI wall thickness measures ranged from 0.38 to 0.42, whereas the correlations between different MRI measures ranged from 0.80 to 0.93 (Table 3). The associations between demographic and CVD risk factors with wall thickness measurements did not generally differ by mode of measurement (US vs MRI), except that Chinese-American participants appeared to have lower MRI-measured thickness (P < .05) but similar IMT compared with white participants (P = .36), age was more strongly associated with IMT (P < .001), and C-reactive protein was more strongly associated with MRI-measured wall thickness (P < .5) (Table 4). Additionally, wall thickness measured with both IMT and MRI was higher in men, but the association was stronger with MRI-measured wall thickness (Table 3).
Table 1:
Baseline Characteristics of Participants
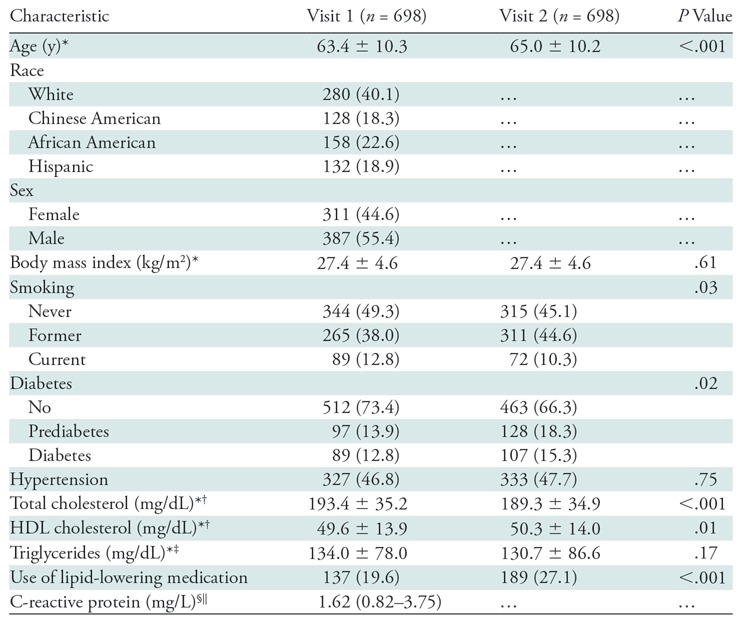
Note.—Unless otherwise specified, data are number of participants, with percentages in parentheses. HDL = high-density lipoprotein.
*Data are means ± standard deviations.
†To convert to SI units (millimoles per liter), multiply by 0.0259.
‡To convert to SI units (millimoles per liter), multiply by 0.0113.
§Data are medians, with interquartile ranges in parentheses.
||To convert to SI units (nanomoles per liter), multiply by 9.524.
Table 2:
CCA Wall Thickness Measured by Using US and MRI

Note.—CCA = common carotid artery, GDWT = wall thickness measured with gadolinium-enhanced MRI, IMT = intima-media thickness, PDWT = wall thickness measured with proton-density–weighted MRI.
*Measured with US.
†Measured with MRI.
Table 3:
Spearman Correlation between CCA Wall Thickness Measured by Using US and MRI

Note.—CCA = common carotid artery, GDWT = wall thickness measured with gadolinium-enhanced MRI, IMT = intima-media thickness, PDWT = wall thickness measured with proton-density–weighted MRI.
*Measured with US.
†Measured with MRI.
Table 4:
Age-, Race-, and Sex-Adjusted Differences in CCA Wall Thickness Measured by Using US and MRI Associated with Each Cardiovascular Risk Factor

Note.—Data are standardized intima-media thickness (IMT) and MRI wall thickness measurements (with mean of 0 and standard deviation [SD] of 1). CCA = common carotid artery, GDWT = wall thickness measured with gadolinium-enhanced MRI, HDL = high-density lipoprotein, PDWT = wall thickness measured with proton-density–weighted MRI.
*P values were obtained from seemingly unrelated regression comparing the difference in IMT associated with each cardiovascular risk factor versus the difference in MRI wall thickness associated with each cardiovascular risk factor.
†P < .001.
‡P < .05.
§P < .01.
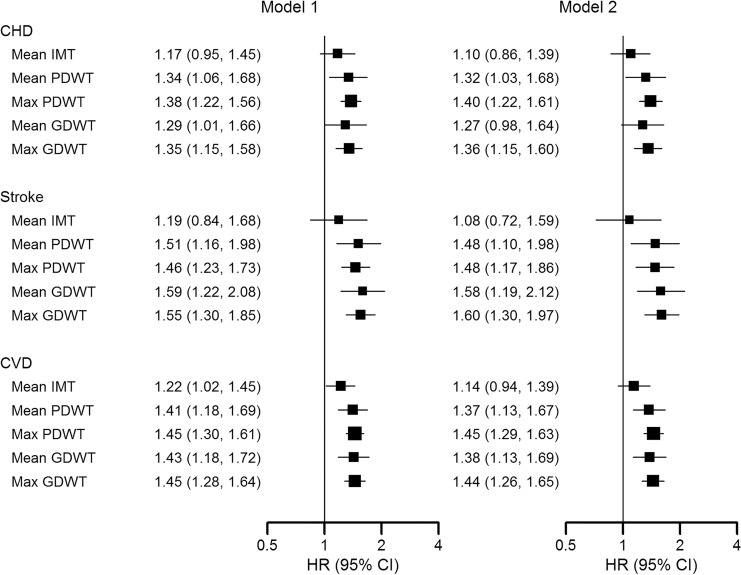
The risk factor–adjusted hazard ratios for coronary heart disease, stroke, and CVD associated with 1 standard deviation increase in IMT were 1.10 (95% CI: 0.86, 1.39), 1.08 (95% CI: 0.72, 1.59), and 1.14 (95% CI: 0.94, 1.39), respectively (Fig 3). The corresponding hazard ratios were 1.32 (95% CI: 1.03, 1.68), 1.48 (95% CI: 1.10, 1.98), and 1.37 (95% CI: 1.13, 1.67) for mean wall thickness measured with proton-density–weighted MRI, and 1.27 (95% CI: 0.98, 1.64), 1.58 (95% CI: 1.19, 2.12), and 1.38 (95% CI: 1.13, 1.69) for mean wall thickness measured with gadolinium-enhanced MRI. Sensitivity analyses further adjusting for lipid-lowering medications, systolic and diastolic blood pressure, or restricting the follow-up duration of IMT to the same as that for MRI found consistent results as the main analysis (data not shown). Additionally, by using the mean of the maximum right and left IMT in place of the mean of the mean right and left IMT resulted in similar hazard ratios of 1.21 (95% CI: 0.99, 1.47), 0.99 (95% CI: 0.68, 1.43), and 1.18 (95% CI: 1.00, 1.40) for coronary heart disease, stroke, and CVD, respectively.
Figure 3:
Image shows hazard ratios (HRs) for incident cardiovascular disease (CVD) events associated with common carotid artery wall thickness measured by using US and MRI. HRs for incident CVD associated with 1 standard deviation increase in intima-media thickness (IMT) or MRI wall thickness measurements were derived from Cox proportional hazards models. Model 1 was adjusted for age, race, and sex. Model 2 was further adjusted for body mass index, smoking, total and high-density lipoprotein cholesterol, triglycerides, diabetes, hypertension, and C-reactive protein. CHD = coronary heart disease, CI = confidence interval, GDWT = wall thickness measured with gadolinium-enhanced MRI, PDWT = wall thickness measured with proton-density–weighted MRI.
When including IMT and MRI wall thickness simultaneously in the same model, wall thickness measurements by using MRI, but not IMT, remained associated with outcomes after adjusting for traditional CVD risk factors (Fig 4). When only using IMT measurements obtained from the same side as the MRI (n = 618; 353 from the right side and 265 from the left side), the correlations between IMT and MRI wall thickness ranged from 0.33 to 0.36 (Tables E1 and E2 [online]). The associations of IMT and MRI wall thickness with CVD outcomes were similar to those found in our overall study population (Tables E3 and E4 [online]).
Figure 4:
Image shows hazard ratios (HRs) for incident cardiovascular disease (CVD) events associated with common carotid artery wall thickness measured by using US and MRI, including intima-media thickness (IMT) and MRI wall thickness measurements simultaneously in same model. HRs for incident CVD events associated with 1 standard deviation increase in IMT or MRI wall thickness measurements were derived from Cox proportional hazards models. All analyses included IMT and MRI wall thickness measurements simultaneously in same model, and were adjusted for age, race, sex, body mass index, smoking, total and high-density lipoprotein cholesterol, triglycerides, diabetes, hypertension, and C-reactive protein. CHD = coronary heart disease, CI = confidence interval, GDWT = wall thickness measured with gadolinium-enhanced MRI, PDWT = wall thickness measured with proton-density–weighted MRI.
Discussion
In our prospective cohort of participants without known clinical CVD, the associations between traditional CVD risk factors (except for age, race, sex, and C-reactive protein) and wall thickness measures by using MRI or IMT measured by using US were generally similar. MRI measures of wall thickness, however, were more consistently associated with incident CVD events, particularly stroke, compared with US measures of IMT. Additionally, the associations with stroke appeared to be slightly stronger with intravenous gadolinium-enhanced MRI compared with noncontrast MRI. These findings suggest that wall thickness measurement by using MRI may be a more precise risk marker for coronary heart disease and stroke than IMT measurement by using US.
Our study found a moderate correlation between US and MRI measures of wall thickness, consistent with a previous study from MESA that reported a correlation coefficient of 0.50 between maximum IMT and MRI mean wall thickness (11). Several earlier reports found correlations between wall thickness measurements by using US and MRI ranging from 0.72 to 0.93, although all studies were based on fewer than 50 participants (18–20). Because US and MRI were performed during different study visits in MESA (median time interval, 1.7 years), we expect that the correlation between MRI and US measures in our study might be weaker than in studies with concurrent measurements. Additionally, although MRI wall thickness measurements were obtained from either the right or left side of the neck, whereas US was performed on both sides in MESA, we found similar correlations when using only IMT obtained from the same side as evaluated at MRI.
IMT has been consistently associated with coronary artery disease and stroke in previous studies, but its clinical usefulness in adding predictive risk discrimination beyond traditional risk factors identified by the Framingham risk score may be limited, to our knowledge (2–4). MRI has more recently emerged as an alternative noninvasive modality to image the arterial wall with improved characterization of plaque features (21). MRI measures of plaque burden and plaque characteristics (including lipid core, fibrous cap thickness, and intraplaque hemorrhage) are associated with the presence and severity of coronary artery disease in small cross-sectional studies (22,23), and with incident CVD events in prospective cohort studies (11,24).
Few studies, to our knowledge, have examined the associations between MRI measures of carotid wall thickness and CVD outcomes, and it is unknown if the association with incident CVD events may differ for wall thickness measured by using MRI and US. In a study of 154 patients with an asymptomatic 50%–79% carotid stenosis, maximum wall thickness measured with MRI was statistically significantly associated with subsequent ipsilateral cerebrovascular events in the unadjusted analysis. Because of the limited number of events, the authors did not perform multivariable analysis (24). Our study showed for the first time, to our knowledge, that MRI measures of wall thickness were more strongly associated with incident CVD events, particularly stroke, compared with US measures of IMT, suggesting that MRI may be clinically more relevant in the prediction of future CVD events.
Several mechanisms may explain the stronger association between MRI measures of wall thickness and CVD events. Measurements of wall thickness by using MRI and US differ on wall boundary detection. IMT measurements by using US include the sum of the intima and media, whereas wall thickness measurements by using MRI also include adventitia in addition to intima and media (6,7). This is supported by our results that wall thickness measurements by using MRI were on average greater than were IMT by using US. The adventitia is important for defining vascular inflammation because it is the source of vasa vasorum that proliferate into the arterial wall with intimal thickening (25). Adventitial vasa vasorum may provide insight into the progression of atherosclerosis to symptomatic disease (26,27). However, it is also possible that MRI techniques may exaggerate thickness estimates due to partial volume averaging related to its lower spatial resolution (28).
Furthermore, MRI with contrast enhancement can improve accuracy of wall area measurements (29,30). This has been attributed to the considerable enhancement of the adventitia by using gadolinium, likely a result of the vascularity of the adventitia from the vasa vasorum. Indeed, our study found stronger associations of stroke with wall thickness measured with gadolinium-enhanced MRI compared with IMT and wall thickness measured with noncontrast proton-density–weighted MRI, suggesting that intravenous gadolinium administration might enable even earlier detection of atherosclerosis formation. Several potential safety issues related to the use of intravenous gadolinium-based contrast agents also need to be considered, including the risk of nephrogenic systemic fibrosis in patients with renal dysfunction (31), allergic reactions (32), and the recent growing concerns about the accumulation of free gadolinium in the body, although currently there are no reported adverse effects of gadolinium deposition, to our knowledge (33).
It is worth pointing out that our analysis was focused on wall thickness measurements by using US and MRI, rather than plaque assessment. In our study, IMT measurements were made below the bulb, starting at the point at which the outer wall of the artery begins to diverge. MRI wall thickness measurements were made 15 mm below the flow divider, which was typically further from the bulb (where plaque tends to form) compared with IMT measurements. Furthermore, the MRI was a 2-mm section that covered a more limited segment of the vessel and did not extend to the bulb. Therefore, the inclusion of plaque is unlikely to explain the stronger associations between MRI wall thickness measurements and incident CVD events.
Carotid wall thickness measurements by using MRI are more reproducible than measurements by using US (19,34,35). With recent increases in field strength of MRI units used in clinical practice, one can achieve higher spatial resolution and expect further improvement in reliability estimates, as well as in our ability to detect early disease with small structural changes (36,37). However, the clinical applicability of carotid MRI must be considered in light of several potential limitations, including relatively high cost and somewhat more limited availability, longer imaging times, and potential safety concerns of exposure to the magnetic field (5,38). Our study suggests that MRI wall thickness measurements may be a better marker for predicting CVD events, but its implementation in clinical practice needs to be considered in the context of cost-effectiveness analyses and future clinical trials. The higher cost of MRI compared with US may limit its use as a first-line screening test for asymptomatic atherosclerosis, and MRI may better serve as a second step in multimodality screening (5,39). Further research is needed to determine the cost-effectiveness of MRI for asymptomatic individuals.
Our study had some limitations. Despite a relatively large sample size, few incident CVD events occurred during follow-up, limiting our ability to produce stable estimates for subgroup analyses or to categorize wall thickness measures into quartiles to study the dose-response relationship. Because of the small number of events, our study may lack the statistical power to detect a significant association between IMT and CVD outcomes. The sampling strategy for MRI (600 participants from the top 15th percentile of carotid IMT and 400 participants from the rest) was expected to increase correlations of carotid measures with each other. Because US and MRI were performed on different study visits, there might be some progression of disease from the baseline visit (US examination) to visit 2 (MRI examination), making it easier for MRI to reveal atherosclerosis that progressed faster and had a stronger association with subsequent events. However, the time interval between IMT and MRI was relatively short (median, 1.7 years; interquartile range, 1.4–1.9 years; overall range, 1.2–2.6 years) to have substantial progression in wall thickness, and all associations were adjusted by age. A previous analysis of the MESA study found that the mean IMT rate of change ± standard deviation was 0.01 ± 0.05 mm per year (interquartile range, 0.00004–0.0264 mm per year), which is very small compared with the absolute difference between IMT and MRI wall thickness (40). Moreover, the levels of CVD risk factors were similar between visits 1 and 2, and the lipid profile even appeared to be improved by visit 2, potentially due to the higher prevalence of lipid-lowering medication use. These observations suggest that different acquisition times for the MRI and US examinations, and MRI revealing disease progression are unlikely to explain the stronger event associations with MRI observed in our study. Finally, our analyses were based on US and MRI examinations performed from 2000 to 2004; newer US and MRI technologies may achieve higher spatial resolutions with better soft tissue characterization and further improve the ability to delineate carotid wall structures and thickness.
In our prospective study of individuals without known clinical cardiovascular disease at baseline, wall thickness measured semiautomatically by using MRI was more consistently associated with incident cardiovascular disease, particularly stroke, than was IMT measured manually by using US. Future studies are needed to confirm our findings in other study populations with more events and to elucidate the underlying mechanism.
APPENDIX
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions.
Supported by contracts R01HL069905, R01HL105930, HHSN268201500003I, and N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advancing Translational Sciences (UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420).
Disclosures of Conflicts of Interest: Y.Z. disclosed no relevant relationships. E.G. disclosed no relevant relationships. S.M. disclosed no relevant relationships. B.C.A. disclosed no relevant relationships. J.F.P. disclosed no relevant relationships. Y.Q. disclosed no relevant relationships. A.S.G. disclosed no relevant relationships. D.M.H. disclosed no relevant relationships. A.R.S. disclosed no relevant relationships. D.A.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is currently Editor of Radiology. Other relationships: disclosed no relevant relationships. B.A.W. disclosed no relevant relationships.
Abbreviations:
- CCA
- common carotid artery
- CI
- confidence interval
- CVD
- cardiovascular disease
- IMT
- intima-media thickness
- MESA
- Multi-Ethnic Study of Atherosclerosis
References
- 1.O’Leary DH, Bots ML. Imaging of atherosclerosis: carotid intima-media thickness. Eur Heart J 2010;31(14):1682–1689. [DOI] [PubMed] [Google Scholar]
- 2.van den Oord SC, Sijbrands EJ, ten Kate GL, et al. Carotid intima-media thickness for cardiovascular risk assessment: systematic review and meta-analysis. Atherosclerosis 2013;228(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3.Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 2012;308(8):796–803. [DOI] [PubMed] [Google Scholar]
- 4.Helfand M, Buckley DI, Freeman M, et al. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151(7):496–507. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Guallar E, Qiao Y, Wasserman BA. Is carotid intima-media thickness as predictive as other noninvasive techniques for the detection of coronary artery disease? Arterioscler Thromb Vasc Biol 2014;34(7):1341–1345. [DOI] [PubMed] [Google Scholar]
- 6.Martin AJ, Gotlieb AI, Henkelman RM. High-resolution MR imaging of human arteries. J Magn Reson Imaging 1995;5(1):93–100. [DOI] [PubMed] [Google Scholar]
- 7.Qiao Y, Steinman DA, Etesami M, Martinez-Marquese A, Lakatta EG, Wasserman BA. Impact of T2 decay on carotid artery wall thickness measurements. J Magn Reson Imaging 2013;37(6):1493–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med 2008;168(12):1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2013;2(2):e000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 11.Zavodni AE, Wasserman BA, McClelland RL, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014;271(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polak JF, Post WS, Carr JJ, Szklo M, O’Leary DH. Associations of common carotid intima-media thickness with coronary heart disease risk factors and events vary with distance from the carotid bulb. J Am Soc Echocardiogr 2014;27(9):991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polak JF, Szklo M, O’Leary DH. Carotid intima-media thickness score, positive coronary artery calcium score, and incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6(1):e004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman BA, Sharrett AR, Lai S, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the Multi-Ethnic Study of Atherosclerosis (MESA). Stroke 2008;39(2):329–335. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman BA, Astor BC, Sharrett AR, Swingen C, Catellier D. MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. J Magn Reson Imaging 2010;31(2):406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52(25):2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc 1962;57(298):348–368. [Google Scholar]
- 18.Mani V, Aguiar SH, Itskovich VV, et al. Carotid black blood MRI burden of atherosclerotic disease assessment correlates with ultrasound intima-media thickness. J Cardiovasc Magn Reson 2006;8(3):529–534. [DOI] [PubMed] [Google Scholar]
- 19.Underhill HR, Kerwin WS, Hatsukami TS, Yuan C. Automated measurement of mean wall thickness in the common carotid artery by MRI: a comparison to intima-media thickness by B-mode ultrasound. J Magn Reson Imaging 2006;24(2):379–387. [DOI] [PubMed] [Google Scholar]
- 20.Boussel L, Serusclat A, Skilton MR, et al. The reliability of high resolution MRI in the measurement of early stage carotid wall thickening. J Cardiovasc Magn Reson 2007;9(5):771–776. [DOI] [PubMed] [Google Scholar]
- 21.Underhill HR, Hatsukami TS, Fayad ZA, Fuster V, Yuan C. MRI of carotid atherosclerosis: clinical implications and future directions. Nat Rev Cardiol 2010;7(3):165–173. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Zhao Q, Chu B, et al. Prevalence of compositional features in subclinical carotid atherosclerosis determined by high-resolution magnetic resonance imaging in Chinese patients with coronary artery disease. Stroke 2010;41(6):1157–1162. [DOI] [PubMed] [Google Scholar]
- 23.Underhill HR, Yuan C, Terry JG, et al. Differences in carotid arterial morphology and composition between individuals with and without obstructive coronary artery disease: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2008;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI—initial results. Stroke 2006;37(3):818–823. [DOI] [PubMed] [Google Scholar]
- 25.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries—a possible role in the pathophysiology of atherosclerosis. N Engl J Med 1984;310(3):175–177. [DOI] [PubMed] [Google Scholar]
- 26.Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004;110(18):2843–2850. [DOI] [PubMed] [Google Scholar]
- 27.Qiao Y, Etesami M, Astor BC, Zeiler SR, Trout HH, 3rd, Wasserman BA. Carotid plaque neovascularization and hemorrhage detected by MR imaging are associated with recent cerebrovascular ischemic events. AJNR Am J Neuroradiol 2012;33(4):755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med 2008;60(5):1020–1028. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Cai J, Luo Y, et al. Measurement of carotid wall volume and maximum area with contrast-enhanced 3D MR imaging: initial observations. Radiology 2003;228(1):200–205. [DOI] [PubMed] [Google Scholar]
- 30.Phan BA, Chu B, Kerwin WS, et al. Effect of contrast enhancement on the measurement of carotid arterial lumen and wall volume using MRI. J Magn Reson Imaging 2006;23(4):481–485. [DOI] [PubMed] [Google Scholar]
- 31.Deo A, Fogel M, Cowper SE. Nephrogenic systemic fibrosis: a population study examining the relationship of disease development to gadolinium exposure. Clin J Am Soc Nephrol 2007;2(2):264–267. [DOI] [PubMed] [Google Scholar]
- 32.ACR Committee on Drugs and Contrast Media . ACR manual on contrast media: version 10.2. https://www.acr.org/Quality-Safety/Resources/Contrast-Manual. Accessed May 2, 2017.
- 33.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270(3):834–841. [DOI] [PubMed] [Google Scholar]
- 34.Duivenvoorden R, de Groot E, Elsen BM, et al. In vivo quantification of carotid artery wall dimensions: 3.0-Tesla MRI versus B-mode ultrasound imaging. Circ Cardiovasc Imaging 2009;2(3):235–242. [DOI] [PubMed] [Google Scholar]
- 35.Harloff A, Zech T, Frydrychowicz A, et al. Carotid intima-media thickness and distensibility measured by MRI at 3 T versus high-resolution ultrasound. Eur Radiol 2009;19(6):1470–1479. [DOI] [PubMed] [Google Scholar]
- 36.Yarnykh VL, Terashima M, Hayes CE, et al. Multicontrast black-blood MRI of carotid arteries: comparison between 1.5 and 3 tesla magnetic field strengths. J Magn Reson Imaging 2006;23(5):691–698. [DOI] [PubMed] [Google Scholar]
- 37.Alizadeh Dehnavi R, Doornbos J, Tamsma JT, et al. Assessment of the carotid artery by MRI at 3T: a study on reproducibility. J Magn Reson Imaging 2007;25(5):1035–1043. [DOI] [PubMed] [Google Scholar]
- 38.Mark DB, Shaw LJ, Lauer MS, O’Malley PG, Heidenreich P. 34th Bethesda Conference: Task force #5—is atherosclerosis imaging cost effective? J Am Coll Cardiol 2003;41(11):1906–1917. [DOI] [PubMed] [Google Scholar]
- 39.Degnan AJ, Young VE, Gillard JH. Advances in noninvasive imaging for evaluating clinical risk and guiding therapy in carotid atherosclerosis. Expert Rev Cardiovasc Ther 2012;10(1):37–53. [DOI] [PubMed] [Google Scholar]
- 40.Polak JF, Pencina MJ, O’Leary DH, D’Agostino RB. Common carotid artery intima-media thickness progression as a predictor of stroke in Multi-Ethnic Study of Atherosclerosis. Stroke 2011;42(11):3017–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.