Abstract
Background and aims:
Diabetes mellitus is a coronary heart disease (CHD) risk-equivalent for the outcome of peripheral vascular disease. The impact of diabetes with comorbid risk factors on the outcome of peripheral vascular disease remains unexplored.
Methods:
We performed a cross-sectional analysis of participants in Lifeline Vascular Screening Inc. age 40–90 who were screened for peripheral vascular disease, defined as lower extremity peripheral artery disease (PAD, ABI <0.9) and/or carotid artery stenosis (CAS, internal CAS ≥50%). CHD was defined as prior myocardial infarction or revascularization. Risk factors included hypertension, hyperlipidemia, smoking, obesity, sedentary lifestyle and family history of cardiovascular disease.
Results:
Among 3,517,804 participants, PAD and CAS was identified in 4.4% and 3.7%, respectively. Diabetes was identified in 376,528 participants, 324,680 (86%) of whom did not have CHD. Among diabetic participants without CHD, prevalence of PAD increased with 1–2 (4.3%), 3–4 (7.3%), and ≥5 (12.0%) comorbid risk factors (p trend < 0.0001). The pattern was similar for CAS (3.7%, 6.2%, 8.8%, p trend < 0.0001). Compared to participants without diabetes, those with diabetes and 1–2, 3–4 and ≥5 risk factors had increasing odds of PAD and CAS after adjustment for age, sex and race/ethnicity (1.0, 95% CI 0.98–1.06; 1.8, 95% CI 1.8–1.89; 3.5, 95% CI 3.43–3.64, respectively, p trend < 0.0001). By comparison, in nondiabetic participants, CHD increased odds of PAD and CAS by 2-fold (2.06, 95% CI 2.02—2.1; 2.19, 95% CI 2.15–2.23 respectively).
Conclusions:
Diabetes, particularly with comorbid risk factors, confers increased odds of PAD and CAS, even in the absence of CHD. Counseling regarding screening and prevention for peripheral vascular disease among individuals with diabetes and multiple risk factors may be useful.
Keywords: Peripheral vascular disease, Peripheral arterial disease, Carotid artery stenosis, Diabetes mellitus, Coronary heart disease
1. Introduction
Diabetes mellitus (diabetes) is a strong risk factor for the development atherosclerotic coronary and peripheral arterial disease [1]. The association between diabetes and coronary heart disease (CHD) is well established; less is known about the effect of diabetes on other arterial beds. Peripheral vascular disease, which includes disease of the carotid, aorta, and lower extremity arterial beds, is primarily caused by atherosclerosis and is therefore influenced by the presence of diabetes [2]. Peripheral vascular disease and diabetes are both increasingly prevalent conditions, affecting an estimated 200 and 360 million people worldwide, respectively [3,4]. Despite the high prevalence and well established morbidity of both of these conditions, use of guideline-directed medical therapy remains suboptimal in patients diagnosed with peripheral vascular disease and diabetes, especially in individuals without a history of coronary heart disease [3,5].
Diabetes is considered a CHD-risk equivalent (i.e. an individual having a 10-year risk >20%) [1]. This determination is supported by evidence that CHD event rates in diabetic individuals without known CHD were as high as those in nondiabetic individuals with prior CHD [6–9]. Subsequent analyses demonstrated that it was only in the presence of multiple cardiovascular disease risk factors that diabetes conferred risk of CHD similar to the risk experienced by those with prior CHD events [10]. Recent analysis demonstrated that diabetes is also a CHD-risk equivalent for the outcome of peripheral vascular disease [11]. However, whether the relationship between diabetes and peripheral vascular disease varies by presence of cardiovascular disease risk factors has not been evaluated. Using a cross-sectional, observational study design, we aimed to compare the relative impact of diabetes and comorbid risk factors versus coronary heart disease on odds of peripheral vascular disease. Describing this relationship may guide screening and improve adherence to guideline directed medical management for individuals with peripheral vascular disease and diabetes.
2. Patients and methods
2.1. Study population
Data was provided by Life Line Research Group (LLS, Independence, Ohio) for research purposes. The dataset contained screening results collected between 2003 and 2008 from 3,696,778 mostly self-referred adults in all 50 US states. Before undergoing the screening procedure, individuals completed a questionnaire that included information on demographics, smoking, exercise, cardiovascular risk factors, medical comorbidities, and family history of atherosclerosis and vascular disease [11,12]. For the purposes of this study, peripheral vascular disease is defined as lower extremity periphery artery disease (PAD) or carotid artery stenosis (CAS). Analysis was performed on a subgroup of participants who were between 40 and 90 years old with no prior diagnosis of PAD or CAS.
2.2. Definition of peripheral vascular disease
Presence of PAD and CAS was ascertained using non-invasive, validated, sensitive and specific methods: the ankle brachial index (ABI) and carotid duplex [13], respectively, as previously described [14]. Systolic blood pressure was measured in both arms (brachial arteries) and both ankles (posterior tibial arteries). Left and right ABIs were calculated by dividing the ankle systolic blood pressure by the highest brachial pressure. If posterior tibial Doppler signal was inaudible, the dorsalis pedis artery signal was measured [13,14]. Participants with ABI greater than 1.4 were excluded to avoid falsely elevated values secondary to arterial calcification. Carotid artery stenosis was detected using carotid artery duplex and defined as CAS >50% (internal carotid artery peak systolic velocity > 125 cm/s).
2.3. Definition of diabetes and other variables of interest
Diabetes mellitus (diabetes) was defined as a self-reported physician diagnosis or use of glucose lowering agents. Diabetes severity was stratified by [1] no use of medications (e.g. diet controlled) [2], use of oral glucose-lowering agents only, or [3] use of insulin with or without the use of oral glucose-lowering agents. Coronary heart disease (CHD) was defined as prior myocardial infarction or prior coronary revascularization (coronary artery bypass, angioplasty, or percutaneous coronary intervention). Hypertension and hypercholesterolemia were defined as self-reported physician diagnosis or medication use. Smoking was defined as use of at least 100 cigarettes during their lifetime; current and former smokers were defined separately. Participants who reported vigorous exercise in their leisure time at least once per week were considered active, all others were sedentary. Family history of cardiovascular disease included self-reported first-degree relative with history of heart attack, stroke, or procedure to increase blood flow to the legs prior to the age of 60. Race and ethnicity were self-reported.
2.4. Statistical analysis
Each individual was assigned a unique identifier, and the investigators had access only to de-identified data. Demographics and prevalence of cardiovascular disease risk factors were reported among participants with diabetes.
In diabetic participants without CHD, adjusted odds ratios and 95% confidence intervals were calculated for individual cardiovascular disease risk factors with logistic regression adjusted for age, sex and race/ethnicity using participants without the risk factor as a referent. Cardiovascular disease risk factors included in this analysis were hypertension, severe obesity, sedentary lifestyle, former smoker, current smoker, hyperlipidemia, and family history of cardiovascular disease. Current smoking was given a value of 2 risk factors [15]. Each cardiovascular disease risk factor with a significantly elevated adjusted odds ratio was included in determining a participant's risk factor profile for either PAD or CAS. Profiles were defined by presence of diabetes and 1–2, 3–4 or ≥5 cardiovascular disease risk factors. Number of participants in each profile were reported in the supplement.
Prevalence and 95% confidence intervals of PAD and CAS were reported by gender, prior CHD status, diabetes status, and cardiovascular disease risk factor profile. Chi-squared and Cochran-Armitage trend tests were used to compare prevalence between risk factor profiles. Odds ratios and 95% confidence intervals were calculated with logistic regression adjusted for age and sex using the patient population without diabetes as a referent.
3. Results
Among 3,517,804 eligible participants, 376,528 had diabetes and 242,900 had CHD. Among participants with diabetes, those with CHD were older, more likely to be male, to be current smokers, to require insulin for glycemic control, and to have a greater risk factor burden than diabetic participants without CHD (Table 1). Seventy-five percent of diabetic participants reported a diagnosis of hypertension, and 69% reported taking medications to lower blood pressure. Seventy-three percent of diabetic participants reported a diagnosis of hyperlipidemia, and 57% reported taking medications to lower cholesterol (Table 1).
Table 1.
Characteristics of participants with diabetes.
| All participants with diabetesa (n = 376,528) | CHDb (n = 51,848) | No CHD (n = 324,680) | |
|---|---|---|---|
| Demographics | |||
| Age(±SD) | 66.35(9.41) | 69.87(8.64) | 65.79(9.4) |
| Male (%) | 40.5 | 58.73 | 37.58 |
| Race(%) | |||
| Black | 5.59 | 4.02 | 5.84 |
| Hispanic | 3.44 | 2.56 | 3.58 |
| Asian | 2.78 | 2.13 | 2.88 |
| Native American | 3.55 | 4.22 | 3.45 |
| Other | 0.8 | 0.8 | 0.8 |
| Medical history | |||
| Hypertensionc(%) | 74.58 | 82.89 | 73.25 |
| No medications | 5.70 | 4.37 | 5.92 |
| Medications | 68.8 | 78.5 | 67.3 |
| Smoking(%) | |||
| Current | 25.81 | 29.68 | 25.19 |
| Former | 26.27 | 38.27 | 49.47 |
| Never | 47.92 | 32.05 | 25.34 |
| High cholesterold(%) | 73.04 | 85.4 | 71.07 |
| No medications | 16.0 | 9.71 | 17.0 |
| Medications | 57.1 | 75.7 | 54.1 |
| Obesitye(%) | 48.0 | 46.1 | 48.3 |
| Sedentaryf(%) | 54.28 | 55.07 | 54.15 |
| Family history of CVDg(%) | 27.38 | 45.18 | 24.55 |
| Diabetes medications(%) | |||
| Diet controlled | 21.99 | 16.87 | 22.81 |
| Oral medication | 59.43 | 59.24 | 59.46 |
| Insulin | 18.58 | 23.89 | 17.73 |
Diabetes: diabetes mellitus defined as self-reported physician diagnosis or use of glucose lowering agents.
CHD: coronary heart disease defined as prior myocardial infarction or prior coronary revascularization (coronary artery bypass, angioplasty, or percutaneous coronary intervention).
Hypertension: defined as self-reported physician diagnosis or medication use.
High cholesterol: defined as self-reported physician diagnosis or medication use.
Obesity: BMI >30 kg/m2.
Sedentary: did not report vigorous exercise in their leisure time at least once per week.
Family history of cardiovascular disease (CVD): defined as self-reported first-degree relative with history of heart attack, stroke or procedure or surgery to increased blood flow to the legs prior to the age.
PAD and CAS were identified in 4.4% and 3.7% of participants, respectively. In participants without diabetes or CHD, the prevalence of PAD and CAS was 3.8% and 3.0%. PAD prevalence for participants with diabetes on no medications was 6.5% (95% CI 6.4–6.7), on oral medications was 8.5% (8.3–8.6), and on insulin was 11.4% (11.2–11.7). Results were similar for CAS; prevalence was 5.5% (5.4–5.7) in participants with diabetes on no medication, 7.0% (6.9–7.1) in participants on oral medications, and 8.5% (8.2–8.7) in participants with insulin-treated diabetes.
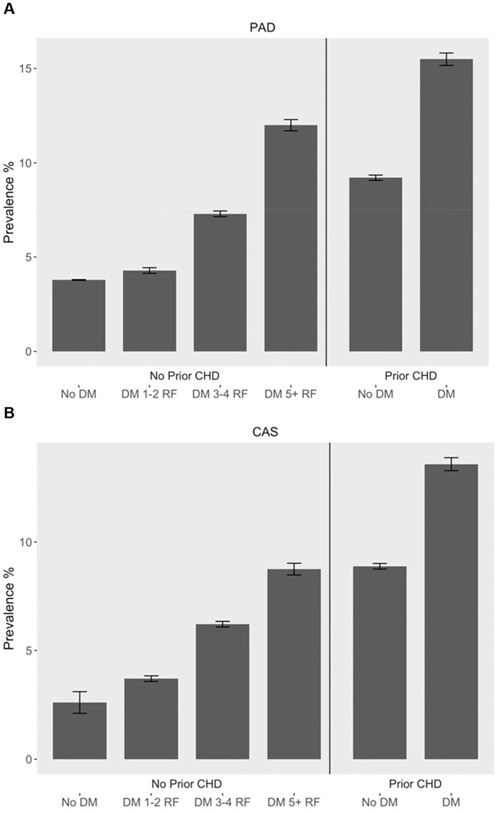
Fig. 1A and B presents the prevalence of lower extremity PAD and CAS by risk factor profile. Absolute counts of participants within each risk factor profile are listed in the supplementary appendix (Supplemental Tables 2 and 3). In diabetic participants without CHD, prevalence of PAD increased with 1–2 (4.3%), 3–4 (7.3%), and ≥5 (12.0%) comorbid risk factors (p trend < 0.0001). The pattern was similar for CAS (3.7%, 6.2%, and 8.8%, p trend < 0.0001). These results were consistent between male and female participants (Supplemental Table 1). Prevalence of PAD was significantly higher in diabetic participants with more than 5 risk factors (12%), compared to nondiabetic participants with CHD (9.2%, p < 0.0001).
Fig. 1.
Prevalence (%) of peripheral artery disease and carotid artery stenosis by risk factor profile. (A) Peripheral artery disease prevalence (%) by risk factor profile, (B) carotid artery stenosis prevalence (%) by risk factor profile.
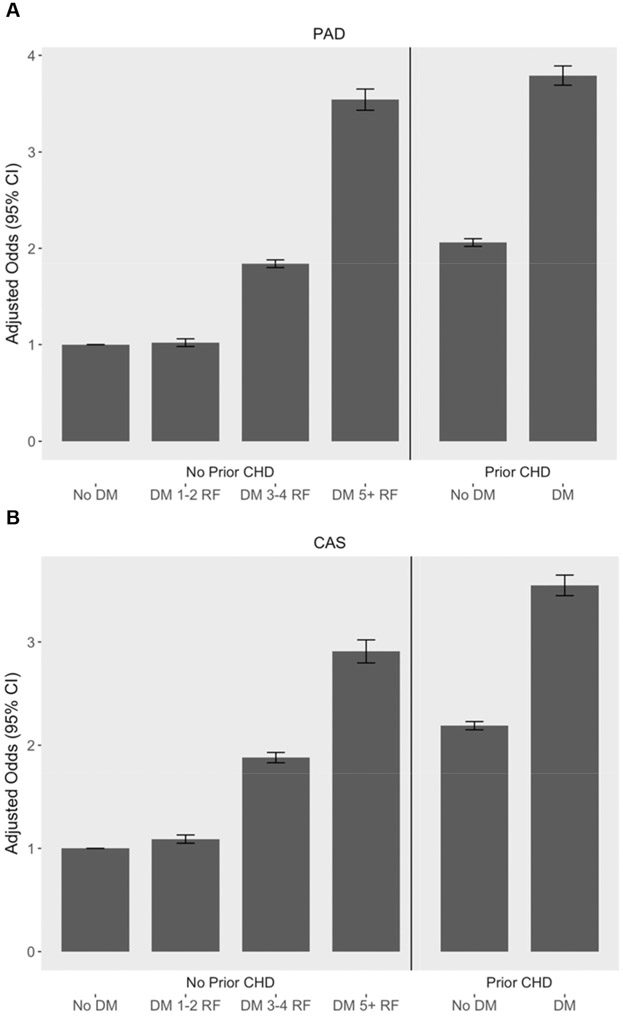
Table 2 presents age, sex and race/ethnicity adjusted odds ratios of individual risk factors in participants with diabetes. All risk factors except severe obesity significantly increased odds of PAD and CAS. In diabetic participants without CHD, age and sex adjusted odds of PAD and CAS increased with 1–2 additional risk factors, nearly doubled with 3–4 risk factors, and approximately tripled with 5 or more risk factors. In comparison, participants with CHD alone had a more than 2-fold increased odds of PAD and CAS, and those with CHD and diabetes together had a more than 3-fold increased odds of PAD and CAS (Fig. 2).
Table 2.
Age, sex and race/ethnicity adjusted odds ratios for PAD and CAS in participants with diabetes.
| Odds ratio (95% CI) |
||
|---|---|---|
| Peripheral arterial disease | Carotid artery stenosis | |
| Hypertensive | 1.47 (1.43,1.52) | 1.58 (1.53,1.64) |
| Obese (≥30) | 1.17 (1.14,1.20) | 0.99 (0.96,1.02) |
| Severely obese (≥40) | 2.44 (2.34,2.55) | 0.91 (0.86,0.96) |
| Sedentary | 1.67 (1.62,1.71) | 1.25 (1.21,1.29) |
| Former smoker | 1.77 (1.71,1.82) | 1.66 (1.61,1.72) |
| Current smoker | 2.2 (2.14,2.27) | 1.84 (1.78,1.91) |
| Hyperlipidemia | 1.16 (1.13,1.19) | 1.5 (1.45,1.55) |
| Family history of CVD | 1.32 (1.28,1.35) | 1.3 (1.26,1.34) |
| Requires insulin | 1.48 (1.44,1.52) | 1.32 (1.27,1.36) |
Fig. 2.
Adjusted odds ratios of peripheral artery disease and carotid artery stenosis by risk factor profile. (A) Odds and 95% confidence intervals of peripheral artery disease adjusted for age, sex and race/ethnicity by risk factor profile. (B) Odds and 95% confidence intervals of carotid artery stenosis adjusted for age, sex and race/ethnicity by risk factor profile.
To examine the relative impact of smoking versus metabolic risk factors on odds of peripheral vascular disease in diabetes, we calculated age, sex and race adjusted odds of PAD and CAS according to clusters of risk factors. Supplemental Table 5 shows that the cluster of metabolic risk factors including hypertension, severe obesity, hyperlipidemia and sedentary lifestyle conferred the highest odds of PAD (aOR 2.56 95% CI 2.41–2.7). Odds of PAD were higher among participants with the cluster of metabolic risk factors than among participants who currently smoke, had histories of hypertension, hyperlipidemia and a family history of CVD (aOR 1.94 95% CI 1.81–2.06). This pattern was not consistent in participants with diabetes and CAS. Odds of CAS were higher in patients who smoked, either formerly or currently, compared with participants with the metabolic risk factor cluster. The metabolic risk factor cluster did not increase odds of CAS relative to participants without diabetes or CHD (aOR 1.03 95% CI 0.94–1.13) (Supplemental Table 5).
4. Discussion
In this analysis of 3.5 million participants, we found that individuals with diabetes and multiple comorbid cardiovascular disease risk factors had a three-fold increased odds of peripheral vascular disease, even in the absence of CHD. In these high-risk participants, peripheral vascular disease burden was comparable to – or greater than – the burden of peripheral vascular disease among nondiabetic participants with known CHD. All risk factors except severe obesity significantly increased odds of PAD and CAS. Current smoking was associated with the highest odds of both PAD (2.2, 95% CI 2.14–2.27) and CAS (1.84, 95%CI 1.78–1.91). While more than 50% of participants with diabetes had three or more comorbid cardiovascular disease risk factors, those with fewer risk factors had minimally increased odds of peripheral vascular disease relative to participants without diabetes. Our results were consistent with other large cross-sectional analyses demonstrating a two-fold or greater odds of peripheral vascular disease in participants with diabetes in high risk populations relative to healthy counterparts [16,17]. However, to our knowledge, this is the first study to examine the impact of diabetes and cardiovascular risk factor burden on non-coronary vascular disease risk.
Diabetes is a clear risk factor for cardiovascular disease. However, whether diabetes without other cardiovascular risk factors (e.g. hypertension, obesity, smokers or hyperlipidemia) should be considered an independent risk factor for cardiovascular disease remains controversial. Some of the strongest evidence supporting diabetes as a CHD risk-equivalent comes from a Finnish cohort study comparing 1373 nondiabetic participants with history of CHD and 1059 diabetic participants without CHD. Hazard ratios for CHD-related death during an 18-year follow up period were equivalent between the groups, even among subgroups with a shorter duration of diabetes and without comorbid metabolic syndrome [9].
However, other studies that evaluated low-risk individuals with diabetes found that CHD risk was not greatly elevated. For example, in the United Kingdom Prospective Diabetes Study, younger participants with diabetes and lower BMIs had less than 10–15% 10-year risk of CHD, whereas those with higher BMIs (>120% of ideal body weight) had 20% risk or greater [18]. Similarly, diabetic participants in the Strong Heart Study with 1–2 risk factors had minimally increased risk of CHD compared with nondiabetics with CHD [10]. Our results indicate that participants with diabetes and two or fewer risk factors had minimally increased odds of peripheral vascular disease relative to nondiabetic participants with CHD, suggesting that diabetes alone is not an independent risk factor for peripheral vascular disease. However, we identified a very small group of diabetics without any comorbid risk factors (Supplemental Tables 2 and 3), suggesting that this phenotype may be uncommon among the general population of adults with diabetes.
Similar to patients with diabetes, those with peripheral vascular disease are at high risk for major adverse cardiovascular events [19,20], making risk factor modification and medical therapy to prevent cardiovascular events paramount. According to national surveys of ambulatory care practices, adherence to guideline-directed medical therapy in patients with PAD lags behind that of CHD, whereas a co-diagnosis of CHD increases the odds of proper PAD treatment [3]. Adherence to CHD risk factor modification has also been found to be suboptimal in patients with diabetes [5]. We found high rates of peripheral vascular disease among participants with diabetes and multiple cardiovascular disease risk factors but without prevalent CHD, revealing a potentially underdiagnosed and undertreated group of patients. It may be especially important to identify these high-risk patients given recent evidence that anti-platelet and anticoagulation therapy may provide significant reductions in rates of cardiac, cerebral and lower extremity vascular disease events [21].
We found highly consistent trends between PAD and CAS among participants grouped by risk factor profile. Despite shared risk factor profiles, screening guidelines for PAD and CAS differ significantly with regard to diabetes. Current American College of Cardiology/American Heart Association guidelines state that screening for PAD by ankle-brachial index is reasonable (level of evidence BNR) among asymptomatic individuals if they are over age 50 with diabetes plus one additional cardiovascular disease risk factor, or if they have known atherosclerosis in another vascular bed [2]. The broad screening criteria are considered justifiable given the non-invasive nature of ABI testing and the high prevalence of abnormal resting ABIs in asymptomatic high risk patients [2].
In contrast, recommendations for CAS screening is less clear. Joint society guidelines from 2011 recommend against screening for asymptomatic carotid artery stenosis [20]. These societies note that screening for CAS may be reasonable among asymptomatic patients with known PAD, CHD, abdominal aortic aneurysm, or two or more cardiovascular disease risk factors. However, they do not include diabetes as one of these risk factors [20]. Our findings demonstrate that diabetes is as strong a risk factor for CAS as for PAD, particularly in the setting of multiple comorbid cardiovascular disease risk factors, thus supporting concordance between guidelines for the management of PAD and CAS. Limited CAS screening is justified by low prevalence of CAS in the general population and potential harm of subsequent invasive interventions. However, given the above reported high prevalence of CAS in high-risk individuals with diabetes, a discussion about screening may be warranted.
4.1. Limitations and strengths
The limitations of this study include those inherent to cross-sectional observational analyses. There are several additional limitations. First, the study population is a self-referred group who paid out of pocket for their screening tests, which may limit the generalizability of our findings. Prevalence of PAD tends to be higher in lower income settings [23]. Therefore, assuming the population paying for screening represents a higher socioeconomic sample, our results may underestimate the burder of PAD. However, prevalence of PAD and CAS in the dataset were similar to other cohorts suggesting good external validity of our dataset [12,24].
Second, our population is subject to misclassification bias, as diabetes, hypertension, hyperlipidemia and CHD were self-reported. However, prevalence of these conditions in the Lifeline screening population was similar to that in the general US population, again demonstrating good external validity of our dataset [12]. Additionally, error associated with under-reporting is likely to bias the results towards the null hypothesis.
The dataset used for this analysis does not include information on diabetes duration or percent glycated hemoglobin of participants with diabetes. Nonetheless, type of medical therapy (no medication, oral glucose lowering, and insulin use) may be used as a surrogate for diabetes severity [25]. Additionally, there is no information on complications of diabetes, specifically kidney disease with albuminuria, which has been shown to be a strong predictor of PAD [26].
The definition of smoking used in the current study was chosen based on NHANES surveys, which define any smoking as 100 cig-arettes or more during a lifetime. In prior analysis, this definition has shown to significantly impact prevalence of PAD [27]. The cut off of 100 cigarettes, which may include participants with minimal vascular damage from smoking, may attenuate the relationship between smoking and vascular disease; however, this would tend to bias our results toward the null hypothesis. Furthermore, relative to never smokers, our data shows that current and former smokers had increased odds of PAD and CAS. This suggests that even at a low threshold to define smoking, the risk factor is significant.
In addition to the above limitations there are several limitations in the measurement of ABI used in our study. PAD was diagnosed using ABI measurements from the posterior tibial artery; dorsalis pedis was only used when Doppler from the posterior tibial was inaudible. Traditionally ABI is calculated using the higher of these two measurements [28]. However, a recent study compared traditional method of using the higher of the two ankle pressure measurements to an alternative method of using the lower of the 2 ankle artery pressures, and both methods had similar predictive value for all-cause cardiovascular mortality [28]. Finally, patients with an ABI >1.4 were excluded because of arterial wall calcification, and the toe brachial index was not available to confirm the presence of occlusive PAD in participants with a high ABI. Although the estimated prevalence of an ABI >1.4 in the general population is relatively low [31], this finding is often associated with diabetes and end stage renal disease [32,33]. Generalizing our results to patients with end stage renal disease should be avoided, as traditional ABIs may be insensitive in this subgroup [33]. Despite these limitations, the current study represents the largest evaluation to date of the relationship between peripheral vascular disease and diabetes, stratified by risk factor profiles. The dataset was strengthened by inclusion of relatively large numbers of women across a broad age range.
4.2. Conclusion
In this analysis of 3.5 million US participants, diabetic individuals without coronary heart disease with multiple cardiovascular risk factors face increased odds of both PAD and CAS. These individuals would benefit from conversations regarding screening to determine their optimal medical therapy just as an individual with a prior diagnosis of coronary heart disease.
Supplementary Material
Footnotes
Conflicts of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.atherosclerosis.2018.04.026.
References
- [1].Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report, Circulation 106 (25) (2002) 3143–3421. [PubMed] [Google Scholar]
- [2].Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. , 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the american College of cardiology/american heart association task force on clinical practice guidelines, J. Am. Coll. Cardiol 69 (11) (2017) e71–e126. [DOI] [PubMed] [Google Scholar]
- [3].Berger JS, Ladapo JA, Underuse of prevention and lifestyle counseling in patients with peripheral artery disease, J. Am. Coll. Cardiol 69 (18) (2017) 2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whiting DR, Guariguata L, Weil C, Shaw J, IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030, Diabetes Research and Clinical Practice 94 (3) (2011) 311–321. [DOI] [PubMed] [Google Scholar]
- [5].Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW, Achievement of goals in U.S. diabetes care, 1999-2010, N. Engl. J. Med 368 (17) (2013) 1613–1624. [DOI] [PubMed] [Google Scholar]
- [6].Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group, BMJ 317 (7160) (1998) 703–713. [PMC free article] [PubMed] [Google Scholar]
- [7].Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, et al. , Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry, Circulation 102 (9) (2000) 1014–1019. [DOI] [PubMed] [Google Scholar]
- [8].Heart Outcomes Prevention Evaluation Study I, Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, et al. , Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients, N. Engl. J. Med 342 (3) (2000) 145–153. [DOI] [PubMed] [Google Scholar]
- [9].Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M, Type 2 diabetes as a “coronary heart disease equivalent”. An 18-year prospective population-based study in Finnish subjects, Diabetes Care 28 (12) (2005) 2901–2907. [DOI] [PubMed] [Google Scholar]
- [10].Howard BV, Best LG, Galloway JM, Howard WJ, Jones K, Lee ET, et al. , Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors, Diabetes Care 29 (2) (2006) 391–397. [DOI] [PubMed] [Google Scholar]
- [11].Newman JD, Rockman CB, Kosiborod M, Guo Y, Zhong H, Weintraub HS, et al. , Diabetes mellitus is a coronary heart disease risk equivalent for peripheral vascular disease, Am. Heart J 184 (2017) 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shah B, Rockman CB, Guo Y, Chesner J, Schwartzbard AZ, Weintraub HS, et al. , Diabetes and vascular disease in different arterial territories, Diabetes Care 37 (6) (2013) 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Criqui MH, Alberts MJ, Fowkes FG, Hirsch AT, O'Gara PT, Olin JW, et al. , Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? Circulation 118 (25) (2008) 2830–2836. [DOI] [PubMed] [Google Scholar]
- [14].Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. , Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects, J. Am. Coll. Cardiol 61 (16) (2013) 1736–1743. [DOI] [PubMed] [Google Scholar]
- [15].Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. , ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the american association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the american association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; TransAtlantic inter-society consensus; and vascular disease foundation, Circulation 113 (11) (2006) e463–e654. [DOI] [PubMed] [Google Scholar]
- [16].Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. , Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey, Diabetes Care 27 (7) (2004) 1591–1597. [DOI] [PubMed] [Google Scholar]
- [17].Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, et al. , Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men, Jama 308 (16) (2012) 1660–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34), UK prospective diabetes study (UKPDS) group, Lancet 352 (9131) (1998) 854–865. [PubMed] [Google Scholar]
- [19].Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. , Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis, Jama 304 (12) (2010) 1350–1357. [DOI] [PubMed] [Google Scholar]
- [20].Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. , 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease, Stroke 42 (8) (2011) e464–e540. [DOI] [PubMed] [Google Scholar]
- [21].Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. , Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial, Lancet 391 (10117) (2018) 219–229. [DOI] [PubMed] [Google Scholar]
- [23].Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. , Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis, Lancet 382 (9901) (2013) 1329–1340. [DOI] [PubMed] [Google Scholar]
- [24].Stein RA, Rockman CB, Guo Y, Adelman MA, Riles T, Hiatt WR, et al. , Association between physical activity and peripheral artery disease and carotid artery stenosis in a self-referred population of 3 million adults, Arterioscler. Thromb. Vasc. Biol 35 (1) (2015) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. , Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization, Am. J. Manag. Care 14 (1) (2008) 15–23. [PMC free article] [PubMed] [Google Scholar]
- [26].Matsushita K, Ballew SH, Coresh J, Arima H, Arnlov J, Cirillo M, et al. , Measures of chronic kidney disease and risk of incident peripheral artery disease: a collaborative meta-analysis of individual participant data, Lancet Diabetes Endocrinol. 5 (9) (2017) 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Selvin E, Erlinger TP, Prevalence of and risk factors for peripheral arterial disease in the United States. Results from the national health and nutrition examination survey, 1999–2000, Circulation 110 (6) (2004) 738–743. [DOI] [PubMed] [Google Scholar]
- [28].Nead KT, Cooke JP, Olin JW, Leeper NJ, Alternative ankle-brachial index method identifies additional at-risk individuals, J. Am. Coll. Cardiol 62 (6) (2013) 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Resnick HE, Foster GL, Prevalence of elevated ankle-brachial index in the United States 1999 to 2002, Am. J. Med 118 (6) (2005) 676–679. [DOI] [PubMed] [Google Scholar]
- [32].Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC Jr., et al. , Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA), J. Vasc. Surg 45 (2) (2007) 319–327. [DOI] [PubMed] [Google Scholar]
- [33].O'Hare A, Johansen K, Lower-extremity peripheral arterial disease among patients with end-stage renal disease, J. Am. Soc. Nephrol 12 (12) (2001) 2838–2847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.