Abstract
Background:
Patients undergoing cardiovascular (CV) procedures often have suboptimal CV risk factor control and may benefit from strategies targeting healthy lifestyle behaviors and education. Implementation of prevention strategies may be particularly effective at this point of heightened motivation.
Methods:
A prospective, randomized, pilot study was conducted in 400 patients undergoing a nonurgent CV procedure (cardiac catheterization ± revascularization) to evaluate the impact of different prevention strategies. Patients were randomized in a 1:1:1 fashion to usual care (UC; group A, n = 134), in-hospital CV prevention consult (PC; group B, n = 130), or PC plus behavioral intervention program (telephone-based motivational interviewing and optional tailored text messages) (group C, n = 133). The primary end point was the Δ change in non–high-density lipoprotein cholesterol (non–HDL-C) from baseline to 6 month.
Results:
The mean age was 64.6 ± 10.8 years, 23.7% were female, and 31.5% were nonwhite. After 6 months, the absolute difference in non–HDL-C for all participants was −19.8 mg/dL (95% CI −24.1 to −15.6, P < .001). There were no between-group differences in the primary end point for the combined PC groups (B and C) versus UC, with a Δ adjusted between group difference of −5.5 mg/dL (95% CI −13.1 to 2.1, P = .16). Patients in the PC groups were more likely to be on high-intensity statins at 6 months (52.9% vs 38.1%, P = .01). After excluding participants with baseline non–HDL-C <100 mg/dL (initial exclusion criterion), Δ non–HDL-C and Δ low-density lipoprotein cholesterol were improved in the PC groups compared to UC (non–HDL-C −8.13 mg/dL [−16.00 to −0.27], P = .04; low-density lipoprotein cholesterol −7.87mg/dL [−15.10 to −0.64], P = .03).
Conclusions:
Although non–HDL-C reduction at 6 months following a nonurgent CV procedure was not significant in the overall cohort, an increased uptake in high-potency statins may translate into improved long-term health outcomes and cost reductions.
The benefit of risk factor optimization and guideline-directed medical therapy (GDMT) for secondary prevention of cardiovascular disease (CVD) is well established.1-3 However, risk factor control and guideline adherence remain suboptimal, even for patients with established CVD.3-8 This discordance may be due to patient factors including insufficient patient knowledge of CV risk factors and medical therapy,9 medication nonadherence,10,11 and insufficient lifestyle change,3 or provider factors such as prescription of suboptimal pharmacologic therapy and inadequate time dedicated to patient education.4 With evidence that GDMT, such as use of high-potency statins, improves outcomes and reduces cost, reducing the medical and economic burden of CVD,5,12-16 we sought to assess prevention-based strategies that could potentially improve risk factor control.
In the setting of a cardiovascular procedure, patients appear to be at a heightened point of motivation, and studies have shown that interventions targeted at education and motivation during this time can be effective at improving health behaviors.17,18 It is this concept that led to the development of an inpatient prevention-focused cardiology service at our institution designed to target cardiovascular risk with a single comprehensive consult by a cardiovascular prevention specialist. With these consultations, lifestyle and medical therapy recommendations are made for optimization of cardiovascular risk factors (eg, physical inactivity, diet, lipids, hypertension, and diabetes). In conjunction with this inpatient consult, behavior change strategies aimed to effect motivation and self-efficacy (confidence) with an outpatient motivational interviewing (MINT) component18 could potentially serve to make the recommendations of the initial prevention consultation more durable.
In the past decade, strategies using technology-based products such as text messaging have also been demonstrated to help patients achieve lifestyle change outside the clinic setting.19,20 Coupled with inpatient prevention consults and MINT, these technology-based strategies could help patients achieve sustainable changes in risk factors and optimal medication regimens. The combined use of these programs aims to bridge the inpatient and outpatient experiences targeting patient education, motivation, self-efficacy, and patient engagement. The Investigation of Motivational Interviewing and Prevention Consults to Achieve Cardiovascular Targets (IMPACT) trial aimed to assess various prevention interventions on the outcome of non–HDL-C at 6 months in patients undergoing cardiovascular procedures.
Methods
Design and participants
A complete description of the design of IMPACT has been published previously.21 Briefly, the IMPACT trial (ClinicalTrials.gov: NCT01642355) was a randomized, controlled, and outcome-blinded pilot study conducted at the New York University School of Medicine. Subjects planned for nonurgent, percutaneous coronary or peripheral interventions were screened for eligibility prior to the procedure. Subjects meeting inclusion and exclusion criteria were approached by the research coordinator or by a member of the clinical research team, and informed consent was obtained. Following informed consent, subjects were randomized in a 1:1:1 manner to group A: usual Care (UC; care team-based education and educational material), group B: UC plus targeted cardiovascular prevention consult, or group C: UC plus targeted cardiovascular prevention consult plus a behavioral intervention program (telephone-based MINT and optional tailored text messages). A texting component was optional for those who elected to participate (see study intervention). The study was approved by the NYU Langone Institutional Review Board on May 3, 2012.
Subjects were at least 21 years of age and proficient in English undergoing a cardiac catheterization with or without cardiovascular procedure. Subjects were excluded if they were unable to walk, had a projected life expectancy less than 1 year, non–high-density lipoprotein cholesterol (non–HDL-C) <100 mg/dL, or inability to provide written informed consent. Because of slower-than-projected enrollment, the inclusion criteria were broadened in December 2013 to include subjects scheduled for a percutaneous cardiovascular intervention or diagnostic catheterization either with established cardiovascular disease or with a cardiovascular risk of >7.5% using the 2013 American College of Cardiology/American Heart Association pooled cohort risk assessment tool even if they did not receive a CV intervention. Subjects were also identified and enrolled before cholesterol measures were available; therefore, initial exclusion criteria of non–HDL-C >00 was removed.1
Study intervention
Usual care (group A)
Subjects in group A received UC following their cardiovascular procedure with education provided by the clinical team (patient’s physician, physician assistants and/or nursing staff). Discharge medications were reviewed by the clinical care team, and new prescriptions were given for any medication changes made.
Prevention consult (group B)
Subjects in group B received UC as well as a dedicated PC by a cardiovascular prevention fellow and attending. The consult included a full history and physical with dedicated teaching about heart disease and the importance of lifestyle behaviors and risk factors. Depending on the subject’s response to a brief lifestyle survey,21 there was individualized discussion about diet, physical activity, smoking cessation (if appropriate), and medication adherence. Changes in medication regimen for the control of hypertension, dyslipidemia, and diabetes were suggested based on the most recent guideline recommendations at the time of the consult.2,22-28 Subjects were given a card with their body mass index (BMI), low-density lipoprotein cholesterol (LDL-C), hemoglobin (Hb)A1C, and blood pressure as well as their goals for each risk factor. A consult letter was sent to the patients' referring physician with recommendations for medication and risk factor optimization.
Prevention consult + behavioral intervention (group C)
Subjects in group C received UC, a dedicated PC, and an outpatient behavioral program. The behavioral program included 9 telephone-based MINT sessions with tailored daily text messages (if they opted to receive text messaging) over a 6-month period. The individual MINT sessions were conducted by a Health Educator trained by a member of the MINT network.29 The goal of the sessions was to help subjects focus on problem solving, goal setting, and prevention of relapse with regard to each of the recommended lifestyle changes. The sessions focused on individual needs to tailor intervention strategies to the subject’s personal context including social support, specific behavior change goals, problem solving, and maintaining motivation during challenging situations.30 Each session lasted up to 30 minutes and was conducted with the aid of a standardized counseling script. To ensure adherence to MINT principles, sessions were audiotaped using the Motivational Interviewing Treatment Integrity scale.31 Prior to beginning the MINT sessions, subjects randomized to this group completed a pretailoring questionnaire that was used to individualize the content of the text messages they received throughout the study period. The content of the personalized messages was driven by the psychosocial and cognitive determinants of the recommended lifestyle behaviors (ie, self-efficacy, intrinsic motivation)32. If they agreed to texting, group C participants received a daily personalized text message as well as one assessment message to evaluate progress toward the behavioral goals they set during the MINT sessions. To appreciate the effect of the motivational aspect of the texts above the assessment texts, subjects randomized to group A and B also received a weekly assessment message which was not tailored to address motivation or self-efficacy.33
The study events schedule is listed in the supplementary appendix.
Outcomes
The primary prespecified objective was to test whether a targeted cardiovascular prevention consult (combined prevention consult groups: B + C) at the time of a cardiovascular procedure reduces the primary outcome of non–HDL-C at 6 months compared with usual care (group A). A secondary objective was to test whether a targeted cardiovascular prevention consult plus a behavioral intervention program (group C) reduces the primary outcome at 6 months compared with UC. The 2 prevention groups were combined for the primary analysis to assess the benefit of the prevention consult. A secondary analysis evaluated the additional benefit of an outpatient behavioral component.
Other objectives include investigating the effect of these different preventive strategies on other well-established healthy behaviors and cardiovascular risk factors, including physical activity, diet, smoking cessation, metabolic health (eg, weight, BMI, HbA1C, glucose, lipid profile), medication adherence, quality of life, and guideline-based medication use assessed by validated surveys listed in published design paper.20
Sample size considerations for primary end point
Non–HDL-C is an important target in subjects with dyslipidemia. Several reports have demonstrated than non–HDL-C is more closely related to adverse cardiovascular events than LDL-C.34 Sample size was calculated based on a goal 10% reduction in non–HDL-C with a prevention consult (groups B and C) compared to UC (group A). In preliminary analyses from our group, the mean non–HDL-C was 140 ± 35 mg/dL. A sample size of 400 subjects with a 20% attrition rate would provide 80% power to reject the null hypothesis at the 5% significance level. This sample size is sufficient to detect a 12-mg/dL difference between groups. For analyses comparing each group (A vs B, A vs C, and B vs C), we would be able to detect a 13-mg/dL between group difference.
Statistical analysis
Baseline demographic and clinical characteristics of all randomized participants were summarized using descriptive statistics, and baseline imbalance was tested between groups. For the outcome analyses, linear regression was used with the Δ change for each outcome from baseline to 6 months (eg, Δ non–HDL-C= non–HDL-C6 months — non–HDL-Cbaseline) as the dependent variable. Treatment group assignment was used as the primary covariate in the unadjusted analysis. Treatment group and selected baseline characteristics were used as covariates in the adjusted analysis. The candidate baseline characteristics included in the adjusted model were sex, age, ethnicity, education status, prior myocardial infarction, prior coronary artery disease, baseline statin, or fibrates.35 Stepwise variable selection was performed, and P value threshold .2 was used to select the significant baseline characteristics in the adjusted model. Adjustment for significant predictors of the outcome enables the analysis to more specifically compare patients of similar risk and thereby increases the statistical power.35 Testing for interactions between the treatment assignment and prespecified baseline characteristics was also performed to assess consistency in the primary results.
Analyses were repeated for the primary and major secondary end points after excluding subjects who were enrolled with a non–HDL-C <100 mg/dL at baseline (original exclusion criteria). Analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). All comparisons were 2-sided with a 5% level of significance.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Funding
This study is funded by research grants from Mr and Mrs Henry Arnhold and from the Marc and Ruti Bell Program in Vascular Biology at New York University School of Medicine. Funders had no role in the design and conduct of the study.
Results
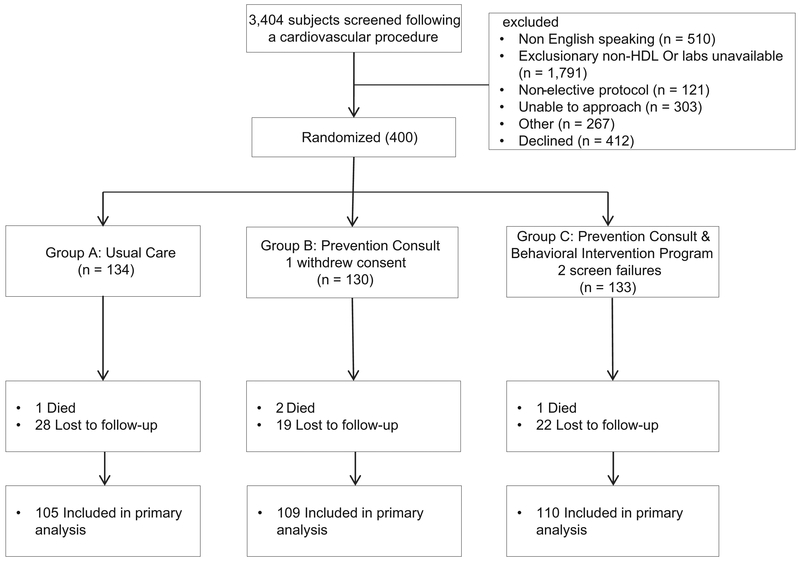
Between May 2012 and August 2015, 400 patients were enrolled and randomized (Figure 1); however, there were 2 screen failures and 1 patient withdrew consent. At the end of the study, 4 patients died and 76 (19%) were lost to follow-up, and therefore, 80 patients did not have follow-up data for non–HDL-C at 6 months. Dropout rates did not differ significantly between assignment groups (P = .34). Mean age was 64.6 ± 10.8 years, 24% were women, 68% were white, mean non–HDL-C level was 134.4±34.7 mg/dL, mean systolic blood pressure was 130.7 ± 17.3 mm Hg, and mean BMI was 29.7 ± 5.1 kg/m2. Baseline characteristics were similar between the assignment groups (Table I). Participants in group C completed a median of 5 of the 9 counseling sessions (range 0-9, interquartile range 0-9 sessions).
Figure 1.
Flow of participants through trial.
Table I.
Subject baseline characteristics: prevention groups versus usual Care
| Overall |
Usual care |
Prevention consult |
||
|---|---|---|---|---|
| N = 397 | n = 134 | n = 263 | P value | |
| Age | 64.56 (10.76) | 63.44 (11.28) | 65.13 (10.47) | .15 |
| Female sex | 94 (23.68%) | 30 (22.39%) | 64 (24.33%) | .67 |
| Race | .75 | |||
| White | 272 (68.51%) | 89 (66.42%) | 183 (69.58%) | |
| Black | 56 (14.11%) | 22 (16.42%) | 34 (12.93%) | |
| Asian | 21 (5.29%) | 8 (5.97%) | 13 (4.94%) | |
| Other | 48 (12.09%) | 15 (11.19%) | 33 (12.55%) | |
| Ethnicity | .86 | |||
| Hispanic | 34 (8.56%) | 11 (8.21%) | 23 (8.75%) | |
| Non-Hispanic | 363 (91.44%) | 123 (91.79%) | 240 (91.25%) | |
| BMI | 29.69 (5.1) | 30.04 (5.24) | 29.51(5.03) | .33 |
| Education | .91 | |||
| >High school graduate | 226 (58.40%) | 76 (58.02%) | 150 (58.59%) | |
| ≤High school graduate | 161 (41.60.%) | 55 (41.98%) | 106 (41.41%) | |
| Current smoker | 51 (12.85%) | 18 (13.43%) | 33 (12.55%) | .80 |
| Hypertension | 315 (79.35%) | 105 (78.36%) | 210 (79.85%) | .73 |
| Hyperlipidemia | 346 (87.15%) | 115 (85.82%) | 231 (87.83%) | .57 |
| Diabetes | 126 (31.74%) | 43 (32.09%) | 83 (31.56%) | .91 |
| Family history (any) | 168 (42.53%) | 54 (40.91%) | 114 (43.35%) | .64 |
| Prior angioplasty or stent | 154 (38.79%) | 53 (39.55%) | 101 (43%) | .82 |
| Depression | 58 (14.91%) | 19 (14.39%) | 39 (15.18%) | .84 |
| Stress work/home | .40 | |||
| At least some | 286 (73.33%) | 101 (75.94%) | 185 (71.98%) | |
| [Never] | 104 (26.67%) | 32 (24.06%) | 72 (28.02%) | |
| Financial stress | .99 | |||
| At least moderate | 150(38.66%) | 51(38.64%) | 99(38.67%) | |
| Little or none | 238(61.34%) | 81(61.36%) | 157(61.33%) | |
| Medications | .2120 | |||
| Aspirin | 319 (80.35%) | 103 (76.87%) | 216 (82.13%) | |
| Clopidogrel | 146 (36.78%) | 51 (38.06%) | 95 (36.12%) | .7049 |
| ACE inhibitors | 107 (26.95%) | 31 (23.13%) | 76 (28.90%) | .2211 |
| Angiotensin-receptor blockers | 109 (27.46%) | 37 (27.61%) | 72 (27.38%) | .9603 |
| Any statin | 279 (70.28%) | 88 (65.67%) | 191(72.62%) | .15 |
| High intensity | 103 (25.94%) | 29(21.64%) | 74 (28.14%) | .16 |
| Ezetimibe | 21 (5.29%) | 7 (5.22%) | 14 (5.32%) | .9667 |
| Fish oil | 51 (12.85%) | 9 (6.72%) | 42 (15.97%) | .0092 |
| Fibrates | 21 (5.29%) | 4 (2.99%) | 17 (6.46%) | .14 |
| Metformin | 76 (19.14%) | 26 (19.40%) | 50 (19.01%) | .9253 |
| PCI performed | 376 (94.71%) | 126 (94.03%) | 250 (95.06%) | .67 |
Family history defined as a first-degree relative with coronary artery disease; male <55 years old and female <65 years old; Ns reflect the number of patients per group assignment at baseline. ACE, angiotensin-converting enzyme; PCI, percutaneous coronary intervention
In comparing the baseline characteristics for the groups for which there were follow-up data at 6 months and those for whom there were not (Supplemental Table IV), differences were noted in age (P = .04), education (P = .02), and aspirin use (P = .01). Baseline high-potency statin use and any statin use were not different between these groups (P = .11 and .72, respectively).
Primary outcome
Non–HDL-C improved significantly within the UC strata (−18.4 mg/dL [−25.9 to −11.0], P < .001) and PC strata (−20.5 mg/dL [−25.7 to −15.3], P < .001) from baseline to 6 months. There was no significant difference in the adjusted analysis of the primary outcome (change in non–HDL-C) between the combined PC groups and UC group (−2.1 mg/dL [−11.2 to 7.0], P = .65) (Table II). Six baseline characteristics (baseline non-HDL, baseline fibrate, baseline statin, education status, and history of heart failure or myocardial infarction) were identified as significantly associated with the primary end point of Δ non–HDL-C. After adjusting for these covariates, PC was associated with a nonsignificant reduction in the primary end point versus UC (−5.4 mg/dL [−13.0 to 2.1], P = .16).
Table II.
Primary and secondary end points (risk factors): prevention groups versus usual care
| Usual care (n = 134) |
Prevention consult (n = 263) |
Prevention consult vs usual care |
||||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Mean difference in outcome |
P value | Mean difference in outcome |
P value | Unadjusted difference between groups |
P value | Adjusted difference between groups |
P value |
| Δ Non–HDL-C (mg/dL) | −18.4 (−25.9 to −11.0) | <.0001 | −20.5 (−25.7 to −15.3) | <.0001 | −2.1 (−11.2 to 7.0) | .65 | −5.5 (−13.1 to 2.1) | .16 |
| Δ LDL-C (mg/dL) | −15.0 (−21.8 to −8.3) | <.0001 | −17.34 (−22.0 to −12.7) | <.0001 | −2.3 (−10.5 to 5.9) | .58 | −5.6 (−12.4 to 1.2) | .11 |
| Δ Total cholesterol | −14.7 (−22.3 to −7.0) | .0002 | −18.8 (−24.2 to −13.5) | <.0001 | −4.2 (−13.5 to 5.2) | .38 | −7.4 (−15.3 to 0.5) | .07 |
| Δ Triglycerides | −23.7 (−43.4 to −4.1) | .01 | −17.4 (−31.1 to −3.7) | .02 | 6.31 (−17.6 to 30.2) | .6 | 0.2 (−17.7 to 18.1) | .98 |
| Δ HbA1C | −0.16 (−0.3 to 0.01) | .06 | 0.01 (−0.1 to 0.13) | .88 | 0.17 (−0.04 to 0.38) | .1 | 0.18 (−0.03 to 0.38) | .09 |
| Δ Weight (kg) | −0.9 (−2.0 to 0.2) | .09 | −1.1 (−1.8 to −0.3) | .01 | −0.14 (−1.5 to 1.21) | .84 | −0.31 (−1.68 to 1.06) | .66 |
| Δ BMI (kg/m2) | −0.4 (−0.7 to 0.02) | .06 | −0.37 (−0.63 to −0.1) | .01 | −0.01 (−0.47 to 0.46) | .98 | −0.05 (−0.51 to 0.42) | .85 |
| Δ SBP (mm Hg) | −3.0 (−6.9 to 0.9) | .12 | −3.3 (−6.02 to −0.63) | .02 | −0.33 (−5.1 to 4.4) | .89 | −2.97 (−6.92 to 0.98) | .14 |
| Δ DBP (mm Hg) | 0.21 (−2.4 to 2.8) | .88 | 0.06 (−1.8 to 1.9) | .95 | −0.15 (−3.4 to 3.1) | .93 | −1.5 (−4.02 to 1.02) | .24 |
Subgroup analysis of the primary outcome
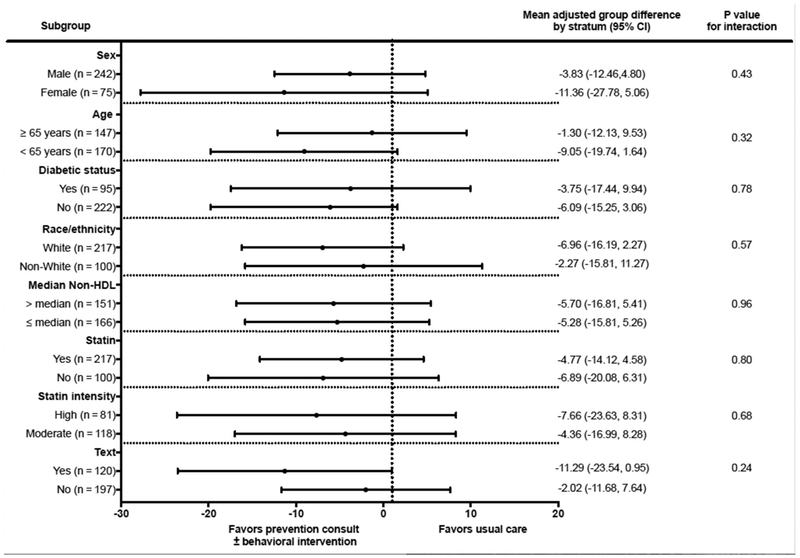
Figure 2 shows the results of prespecified subgroup analysis of the primary outcome (non–HDL-C). There was no statistically significant heterogeneity in the treatment effects; yet, there was an indication that active interventions were more effective at lowering non–HDL-C among individuals who received text messages, with a nonsignificant reduction of −11.52 ml/dL (−26.4 to 3.36), P = .1 after adjusting for predictors.
Figure 2.
Primary outcome among subgroups. N reflects the number in each subgroup for total of 317 available at the end of the trial.
Secondary objectives
Other risk factors
LDL-C decreased significantly in the UC (−15.0 mg/dL [−21.8 to −8.3], P < .001) and PC (−17.3 mg/dL [−22.0 to −12.7], P < .001) groups from baseline to 6 months, but the between-group difference was not significant (−2.3 mg/dL [−10.5 to 5.9], P = .58). After multivariable adjustment, the reduction in LDL-C between PC and UC failed to meet statistical significance (−5.7 mg/dL [−12.4 to 1.1], P = .1).
Although BMI significantly improved in the PC group (−0.37 kg/m2 [−0.63 to −0.1], P = .01) with no difference in the UC (P = .06), the adjusted between-group difference was not statistically significant (−0.05 kg/m2 [−0.51 to 0.42], P = .85). Systolic blood pressure improved in the prevention consult (P = .02) but not in the usual care (P = .12) group, with no statistically significant adjusted between-group difference (−2.97 mm Hg [−6.92 to 0.98], P = .14) (Table II).
Lifestyle factors
There were no significant differences between the groups in end points related to physical activity, saturated fat intake, quality of life, or medication adherence. Importantly, physical activity, saturated fat intake, and medication adherence each improved significantly from baseline to 6 months in all groups (Table III). Baseline smoking prevalence was 13% and 12.6% in UC and PC groups, respectively, and improved to 9.9% and 11.5%, respectively, at 6 months, with no statistically significant difference between groups (unadjusted OR 1.19 [0.52-2.69], P = .68; adjusted OR 1.32 [0.56-3.11], P = .52).
Table III.
Secondary end points (lifestyle factors): prevention groups versus usual care
| Usual care (n = 134) |
Prevention consult (n = 263) |
PC vs UC |
PC vs UC |
|||||
|---|---|---|---|---|---|---|---|---|
| Outcomes | Mean difference in outcome |
P value | Mean difference in outcome |
P value | Unadjusted difference between groups |
P value | Adjusted difference between groups |
P value |
| Δ Quality of life | 0.003 (−0.02 to 0.03) | .84 | −0.01 (−0.03 to 0.01) | .19 | −0.01 (−0.05 to 0.02) | .37 | −0.01 (−0.04 to 0.02) | .36 |
| Δ Yale physical activity | 8.8 (3.7-14.0) | .003 | 6.9 (3.3-10.5) | .0001 | −1.96 (−8.2 to 4.3) | .54 | 0.1 (−5.48 to 5.67) | .97 |
| Δ Saturated fat intake | −3.2 (−4.18 to −2.1) | <.0001 | −3.3 (−4.02 to −2.59) | <.0001 | −0.16 (−1.4 to 1.1) | .8 | −0.47 (−1.58 to 0.63) | .4 |
| Δ Fruit & vegetable Intake | −0.03 (−0.38 to 0.32) | .86 | −0.02 (−0.26 to 0.22) | .89 | 0.01 (−0.41 to 0.44) | .95 | −0.1 (−0.49 to 0.28) | .6 |
| Δ Medication adherence | −0.24 (−0.44 to −0.04) | .0084 | −0.45 (−0.58 to −0.31) | <.0001 | −0.21 (−0.45 to 0.04) | .1 | −0.17 (−0.68 to 0.35) | .52 |
| Δ Patient health questionnaire | −0.64 (−1.2 to −0.1) | .03 | −0.66(−1.03 to −0.29) | .0005 | −0.02 (−0.68 to 0.64) | .95 | 0.18 (−0.37 to 0.72) | .52 |
Quality of life measured by EQ5D survey; physical activity measured by Part 2 of Yale Physical Activity Questionnaire; saturated fat measured by NWLRC Fat Intake Survey; fruit and vegetable intake measured by Fruit and Vegetable Intake Screener; medication adherence defined by Morisky Scale; patient health defined by Personal Health Assessment Questionnaire. Ns reflect the number of patients assigned to each group at baseline. The N for each specific variable can be found in supplemental table.
Medication changes
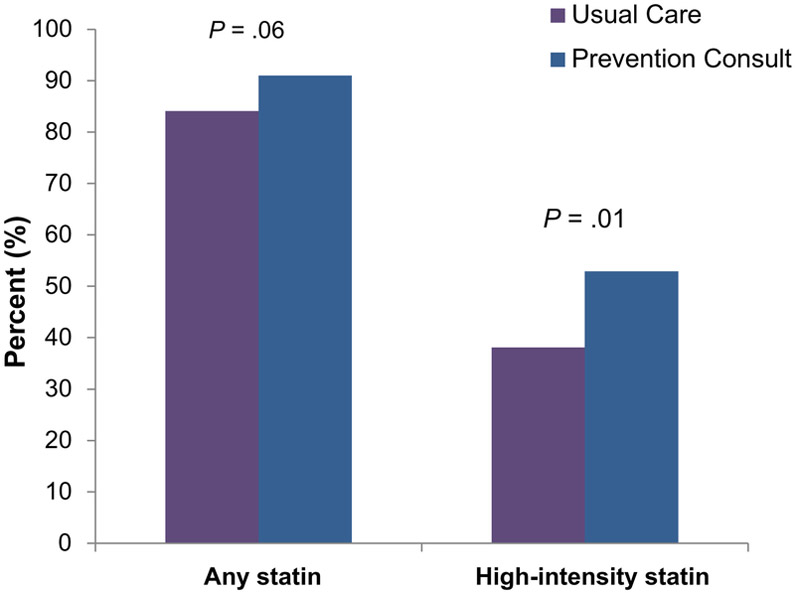
At enrollment, statins were used in 70% of all subjects, with a nonsignificant difference between PC and UC groups in the number of patients on statin, 73% versus 66% (P = .15), and on high-potency statin, 28% versus 22% (P = .16), respectively. At 6 months, 91% of PC subjects received a statin compared with 84% in the UC groups (P = .06) (Figure 3), and 53% in PC received a high-potency statin versus 38.1% in UC (P = .01) (Figure 3). A difference in high-intensity statin use was also noted at hospital discharge (50.6% vs 32.8%). However, assessing the increase in statin and high-potency statin use from baseline to 6 months (because there was a nonsignificant baseline difference in statin use between groups) revealed no between-group differences (20% vs 27%, P = .17). The number of patients who were no longer taking statins at 6 months despite statin use at baseline was greater in the UC group (7% vs PC group 2% [5/221], P = .04), which may account for the overall differences in patients on statins at 6 months. Use of ezetimibe, fish oil, niacin, and fibrates did not differ between groups at 6 months.
Figure 3.
Use of statins and high-intensity statins at 6 months. The x-axis reflects statin and high-potency statin categories; the y-axis reflects percent of patients taking each.
Sensitivity analysis
Results were consistent with the prespecified assessment of PC-alone versus UC (group A) and PC plus behavioral intervention (group C) versus UC (group A; Supplemental Table I). In adjusted analyses, the PC plus behavioral intervention group appeared to have a greater reduction in systolic blood pressure (P = .04), diastolic blood pressure (P = .09), and LDL-C (P = .05) than usual care.
To facilitate our slower-than-expected enrollment, we identified and approached individuals before their laboratory data were available. Thus, 53 individuals were enrolled with non–HDL-C <100 mg/dL. After excluding these 53 subjects and adjusting for predictors, there was a significant difference in the primary outcome (change in non–HDL-C) between the PC group versus UC group (−8.13 mg/dL [−16.00 to −0.27], P = . 04). Reduction in LDL-C was noted to be significant between groups after exclusion of these subjects (−7.87mg/dL [−15.10 to −0.64], P = .03).
Discussion
A cardiovascular PC at the time of cardiovascular intervention did not improve the primary outcome of reduction of non–HDL-C at 6 months compared with usual care. The reductions in non–HDL-C (18.0 mg/dL) and LDL-C (15.0 mg/dL) from baseline to 6 months in all study participants were large and both clinically and statistically significant, but there were no between-group differences. It is noteworthy that improvement in the primary outcome (non–HDL-C) neared significance after an adjusted analysis excluding subjects with a very low non–HDL-C (<100 mg/dL), which likely reflects enrolling too many patients at goal for CV risk factors and a limited sample size. Among patients randomized to the PC group, there was a trend toward an increase in any statin use and a significant increase in the use of high-intensity statins.
Previous studies suggest that targeted risk factor control and lifestyle intervention may improve overall health and promote positive behavioral changes, such as smoking cessation. A PC aims to improve cardiovascular risk factors and behavioral patterns through increasing patient education and motivation, GDMT use, and medication adherence. To our knowledge, no other study has evaluated the impact of a single PC.36 Our study noted a trend toward better overall risk factors and lifestyle behaviors in the PC group, but this was not statistically significant. Although the 1-time PC may have been valuable in patient and provider education, it may have been inadequate to empower patients to make long-term behavioral change. The outpatient behavioral intervention was intended to serve that purpose; however, the low uptake of sessions (median 5/9 sessions) may explain the lack of a difference. The study was not designed to detect a clinically relevant difference in cardiovascular outcomes.
The IMPACT trial was designed as a pilot study to investigate varied prevention strategies post cardiac procedures. Based on the lessons learned from this trial, both from individual patient feedback and high rates of patients lost to follow-up, individualized strategies may be a more effective option for a future trial. Patient preference with respect to the frequency of telephone-based motivational calls, texting, and returning for follow-up was variable in this study. A future clinical trial with larger numbers of subjects might assess treatment arms tailored to patient preference and needs, knowing that prespecified options may not be ideal for all.
A clinically important difference was noted in the secondary outcome of guideline-recommended high-intensity statins. High-intensity statins were more commonly used in the PC group than UC (53% vs 38%, P = .01) and more commonly prescribed at discharge in the groups receiving a prevention consult (P = 0.001 51% vs. 33%), which highlights the discharge period as a pivotal time to impact GDMT implementation. However, the current study was too small and too short to evaluate the clinical effect of this finding. It is also important to point out that >50% of all subjects undergoing a CV procedure are not on a high-intensity statin at discharge or at 6 months. Clearly, more work is needed in the area of cardiovascular prevention.
In evaluating the effect of the inpatient prevention consults, it should be noted that recommendations were sent to the referring outpatient physician without a protocol to put GDMT changes into effect prior to discharge. Knowing the importance of the discharge period as a time of heightened motivation for the patient and the ability of inpatient care to guide outpatient care, this process may have been more effective if GDMT changes were put into effect immediately at discharge with timely communication with the referring physician. It is also important to mention that as the inpatient prevention consult service went into effect and recommendations were given to referring physicians and teams over time, there may have been an unintended impact on referring physician practice behaviors for the UC group.
The study had several limitations. First, the strict inclusion criteria may limit generalizability. Second, although the preventive cardiology consult was intended to be uniform so that it could be implemented as a future intervention, there is likely some variability based on physician practice style among the attending preventive cardiologists. Third, the study was likely underpowered because of the improvement in CV risk factors observed in the usual care groups. Fourth, because ofthe nature of the PC, it was not possible to blind participants, which may have influenced self-reported outcomes. Fifth, although subjects were recommended to have their blood collected in the fasting state, it was unclear how many adhered to those recommendations. However, the use of non-HDL in this study should be impacted less by a fasting or nonfasting state. Finally, because of a slower-than-expected enrollment, we approached and enrolled lower-risk subjects and those without baseline laboratory results available. In fact, after excluding subjects with non–HDL-C values (<100 mg/dL), the primary end point neared significance in the adjusted subgroup. These limitations might have biased the results toward the null. Nonetheless, this was the first full-scale randomized controlled trial to evaluate the impact of a single PC on cardiovascular risk factors and lifestyle in patients undergoing a cardiovascular intervention. The study had a sample size statistically powered to identify change in the primary outcome, and it had a lower-than-expected attrition rate.
Conclusions
A 1-time targeted PC at the time of cardiovascular intervention was not more effective in lowering non–HDL-C than usual care in this trial. Both groups had large significant improvements in risk factors and behavioral health (eg, non–HDL-C, LDL-C, physical activity, saturated fat intake). In a subgroup of patients with baseline non–HDL-C greater than 100, there was a statistically significant change in non–HDL-C from baseline to 6 months between groups after multivariable adjustment. A PC resulted in an increase in the use of statins and high-intensity statins, although these findings may be due to chance and should be confirmed in future studies. Whether this intervention is cost-effective and leads to improvement in clinically important events requires further study.
Ns reflect the number of patients assigned to each group at baseline. For non–HDL, there were 104 subjects in UC and 213 in PC at 6 months. Ns for other variables depended on data available at follow-up which were similar and can be found in Supplemental Table IV. SBP, systolic blood pressure; DBP, diastolic blood pressure.
Supplementary Material
Acknowledgements
We gratefully acknowledge the voluntary participation of all study subjects. We wish to acknowledge Mobile Health Interventions for providing the texting services for our study subjects. We also wish to acknowledge the hard work of research coordinator Farah Noorani.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahj.2017.12.019.
Relationship with industry: none.
Clinical Trial Unique Identifier: NCT01642355.
References
- 1.Stone NJ, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013;134(4):1–34. [Google Scholar]
- 2.Eckel RH, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2960–84. [DOI] [PubMed] [Google Scholar]
- 3.Tully L, et al. Suboptimal risk factor control in patients undergoing elective coronary or peripheral percutaneous intervention. Am Heart J 2014;168(3):310–6. e3. [DOI] [PubMed] [Google Scholar]
- 4.Borden WB, et al. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 2011;305(18):1882–9. [DOI] [PubMed] [Google Scholar]
- 5.Cannon CP. Cardiovascular disease and modifiable cardiometabolic risk factors. Clin Cornerstone 2007;8(3):11–28. [DOI] [PubMed] [Google Scholar]
- 6.Farkouh ME, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol 2013;61(15):1607–15. [DOI] [PubMed] [Google Scholar]
- 7.Fihn SD, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64(18):1929–49. [DOI] [PubMed] [Google Scholar]
- 8.Karalis DG, et al. Achieving optimal lipid goals in patients with coronary artery disease. Am J Cardiol 2011;107(6):886–90. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, et al. Awareness, perception, and knowledge of heart disease risk and prevention among women in the United States. American Heart Association Women's Heart Disease and Stroke Campaign Task Force. Arch Fam Med 2000;9(6):506–15. [DOI] [PubMed] [Google Scholar]
- 10.Rosenson RS, et al. Underutilization of high-intensity statin therapy after hospitalization for coronary heart disease. J Am Coll Cardiol 2015;65(3):270–7. [DOI] [PubMed] [Google Scholar]
- 11.Bansilal S, et al. Assessing the impact of medication adherence on long-term cardiovascular outcomes. J Am Coll Cardiol 2016;68(8):789–801. [DOI] [PubMed] [Google Scholar]
- 12.Roger VL, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123(4):e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Writing Group M, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2016;133(4):e38–360. [DOI] [PubMed] [Google Scholar]
- 14.De Smedt D, et al. Cost-effectiveness of optimizing prevention in patients with coronary heart disease: the EUROASPIRE III health economics project. Eur Heart J 2012;33(22):2865–72. [DOI] [PubMed] [Google Scholar]
- 15.Ladapo JA, et al. Projected cost-effectiveness of smoking cessation interventions in patients hospitalized with myocardial infarction. Arch Intern Med 2011;171(1): 39–45. [DOI] [PubMed] [Google Scholar]
- 16.Laing ST. High-intensity statins: guideline expectations and clinical application. JAMA 2017;317(24):2543–4. [DOI] [PubMed] [Google Scholar]
- 17.Mosca L, et al. A novel family-based intervention trial to improve heart health: FIT Heart: results of a randomized controlled trial. Circ Cardiovasc Qual Outcomes 2008;1(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundahl B, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns 2013;93(2):157–68. [DOI] [PubMed] [Google Scholar]
- 19.Afshin A, et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc 2016;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow CK, et al. Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314(12):1255–63. [DOI] [PubMed] [Google Scholar]
- 21.Gianos E, et al. Rationale and design of the Investigation of Motivational Interviewing and Prevention Consults to Achieve Cardiovascular Targets (IMPACT) trial. Am Heart J 2015;170(3):430–7. e9. [DOI] [PubMed] [Google Scholar]
- 22.Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA 2001;285(19):2486–97. [DOI] [PubMed] [Google Scholar]
- 23.Chobanian AV, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560–72. [DOI] [PubMed] [Google Scholar]
- 24.Garber AJ, et al. American Association of Clinical Endocrinologists' comprehensive diabetes management algorithm 2013 consensus statement—executive summary. Endocr Pract 2013;19(3):536–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol 2004;44(3): 720–32. [DOI] [PubMed] [Google Scholar]
- 26.Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan. Endocr Pract 2011;17(Suppl 2):1–53. [DOI] [PubMed] [Google Scholar]
- 27.James PA, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8).JAMA 2014;311(5):507–20. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein AH, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114(1):82–96. [DOI] [PubMed] [Google Scholar]
- 29.MINT: interviewing network of trainers. , http://www.motivationalinterviewing.org/2013 [Last accessed on January 28, 2015].
- 30.Resnicow K, et al. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol 2002;21(5):444–51. [PubMed] [Google Scholar]
- 31.Moyers TB, et al. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat 2005;28(1):19–26. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh AR, et al. Psychosocial predictors of fruit and vegetable consumption in adults a review of the literature. Am J Prev Med 2008;34(6):535–43. [DOI] [PubMed] [Google Scholar]
- 33.Lindquist R, et al. Design of control-group conditions in clinical trials of behavioral interventions. J Nurs Scholarsh 2007;39(3):214–21. [DOI] [PubMed] [Google Scholar]
- 34.Boekholdt SM, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA 2012;307(12):1302–9. [DOI] [PubMed] [Google Scholar]
- 35.Pocock SJ, et al. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med 2002;21(19): 2917–30. [DOI] [PubMed] [Google Scholar]
- 36.Auer R, et al. Efficacy of in-hospital multidimensional interventions of secondary prevention after acute coronary syndrome: a systematic review and meta-analysis. Circulation 2008;117(24):3109–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.