Abstract
Photosynthetic species of the dinoflagellate genus Cochlodinium such as C. polykrikoides, one of the most harmful bloom-forming dinoflagellates, have been extensively investigated. Little is known about the heterotrophic forms of Cochlodinium, such as its type species, Cochlodinium strangulatum. This is an uncommon, large (~200 μm long), solitary, and phagotrophic species, with numerous refractile bodies, a central nucleus enclosed in a distinct perinuclear capsule, and a cell surface with fine longitudinal striae and a circular apical groove. The morphology of C. polykrikoides and allied species is different from the generic type. It is a bloom-forming species with single, two or four-celled chains, small cell size (25–40 μm long) with elongated chloroplasts arranged longitudinally and in parallel, anterior nucleus, eye-spot in the anterior dorsal side, and a cell surface smooth with U-shaped apical groove. Phylogenetic analysis based on LSU rDNA sequences revealed that C. strangulatum and C. polykrikoides/C. fulvescens formed two distally related, independent lineages. Based on morphological and phylogenetic analyses, the diagnosis of Cochlodinium is emended and C. miniatum is proposed as synonym of C. strangulatum. The new genus Margalefidinium gen. nov., and new combinations for C. catenatum, C. citron, C. flavum, C. fulvescens and C. polykrikoides are proposed.
Keywords: HABs, harmful algal blooms, molecular phylogenetics, red tide, toxic Dinoflagellata, unarmoured dinoflagellate
1. Introduction
The unarmored dinoflagellate genus Cochlodinium F. Schütt was established by Schütt (1896) for the forms previously described as Gymnodinium F. Stein without ocelloid in which the cingulum had a length of 1.5 turns or more around the cell (Schütt, 1895, 1896; Kofoid and Swezy, 1921). The type species was C. strangulatum (F. Schütt) F. Schütt (=Gymnodinium strangulatum F. Schütt). The genus Cochlodinium currently comprises about 40 species, most of which are heterotrophic (Kofoid and Swezy, 1921; Gómez, 2012). Some of the photosynthetic species, especially Cochlodinium polykrikoides Margalef, have been extensively studied as they are responsible for harmful algal blooms (e.g., Matsuoka et al., 2008; Richlen et al., 2010; Kudela and Gobler, 2012).
To date, the available molecular phylogenetic data for these groups are restricted to the photosynthetic forms of Cochlodinium. Phylogenies generated using these data revealed that Cochlodinium is polyphyletic and should be divided into at least three different genera. In these analyses, C. polykrikoides Margalef and C. fulvescens M. Iwataki, H. Kawami & Matsuoka branched together (Iwataki et al., 2007, 2008; Reñé et al., 2013a). The bloom-forming photosynthetic species Cochlodinium geminatum (F. Schütt) F. Schütt branched within the Gymnodinium clade, and consequently was transferred into Polykrikos Buetschli (Qiu et al., 2013). Two photosynthetic species, Cochlodinium convolutum Kofoid & Swezy and C. helix (C.H.G. Pouchet) Lemmermann, grouped in the clade comprising Ceratoperidinium Margalef that also included the species formerly known as Gyrodinium falcatum Kofoid & Swezy (Reñé et al., 2013b, 2015).
Historically the gymnodinioid dinoflagellates have been classified based on morphological features such as the cingular displacement or torsion of the cingulum (Kofoid and Swezy, 1921). More recent taxonomic reevaluation of gymnodinioid dinoflagellates based on molecular sequences and ultrastructure revealed that other features such as the shape of the apical groove or acrobase were stable characters and could distinguish the genera (Daugbjerg et al., 2000). The apical grooves of C. polykrikoides and C. geminatum are U- and horseshoe-shaped, respectively (Iwataki et al., 2010; Qiu et al., 2013). The species Cochlodinium helix, C. convolutum, Ceratoperidinium falcatum (Kofoid & Swezy) Reñé & de Salas and allied taxa showed a circular apical groove with its two ends in contact with the sulcus (Takayama, 1998; Reñé et al., 2013b). Takayama (1998) provided scanning electron micrographs of C. strangulatum. The apex was truncated or slightly emarginated with a circular apical groove connected to the sulcus and the cell surface covered with fine striae (Takayama, 1998).
Classification of Cochlodinium species has been challenging due to the absence of molecular data from the heterotrophic species, and especially from the type species, C. strangulatum. Consequently, any taxonomical innovation and genus transfer should be avoided until the phylogenetic position of the type species is available. This study illustrates the morphology of C. strangulatum and provides the first molecular data based on the LSU rRNA gene sequences. This study also provides additional observations of C. polykrikoides collected from the type locality.
2. Materials and methods
2.1. Sampling, isolation and light microscopy
Cells were collected from October 2007 to September 2008 from the Mediterranean Sea by slowly filtering surface seawater taken from the pier of the Station Marine d’Endoume at Marseille, France (43° 16′ 48.05″ N, 5° 20′ 56.22″ E, bottom depth 3 m). Sieves of 20, 40, and 60-μm mesh size were used to collect planktonic organisms from water volumes ranging between 10 and 100 L, depending on particle concentration. The plankton concentrate was scanned in settling chambers at ×l00 magnification with an inverted microscope (Nikon Eclipse TE200; Nikon Inc., Tokyo, Japan). Cells were photographed alive at ×200 or ×400 magnifications with a Nikon Coolpix E995 digital camera. Additional samples were collected using the same method from October 2008 to August 2009 from surface waters (depth of 2 m) of the port of Banyuls-sur-Mer, France (42° 28′ 50″ N, 3° 08′ 09″ E). The concentrated sample was examined in Utermöhl chambers with an inverted epifluorescence microscope (Olympus IX51; Olympus Inc., Tokyo, Japan) and photographed with an Olympus DP71 digital camera. Sampling continued from September 2009 to February 2010 in the Bay of Villefranche-sur-Mer, France. At this location, sampling was performed at the long-term monitoring site Point B (43° 41′ 10″ N, 7° 19′ 00″ E, water column depth ~80 m). Water column samples (0–80 m) were obtained using phytoplankton net tows (53 μm mesh size, 54 cm diameter, 280 cm length). Samples were prepared according to the same procedure described above and cells were observed with an inverted microscope (Olympus IX51, Olympus Inc.) and photographed with an Olympus DP71 digital camera. Sampling continued from May 2012 to February 2013 in the port of Valencia, Spain (39° 27′ 38.13″ N, 0° 19′ 21.29″ W, water column depth of 4 m). Cells were obtained using a phytoplankton net (20 μm mesh size). Samples were prepared according to the same procedure described above and cells were observed with an inverted microscope (Nikon Eclipse T2000; Nikon Inc.) and photographed with an Olympus DP71 digital camera.
In the South Atlantic Ocean, sampling continued after March 2013 in the São Sebastião Channel (23° 50′ 4.05″ S, 45° 24′ 28.82″ W), and from December 2013 to December 2015 off Ubatuba (23° 31′ 27.80″ S, 45° 04′ 59.48″ W). Cells collected from Brazilian waters were obtained using phytoplankton net tows (20 μm mesh size) in surface waters. The living concentrated samples were examined in Utermöhl chambers at magnification of ×200 with inverted microscopes [Diaphot-300 (Nikon Inc.) at São Sebastião, and Eclipse TS-100 (Nikon Inc.) and Olympus IX73 (Olympus Inc.) at Ubatuba], and photographed with a digital camera (Cyber-shot DSC-W300; Sony, Tokyo, Japan) mounted on the microscope’s eyepiece.
Cells of C. strangulatum from Brazil were isolated for incubation experiments with the aim of observing intraspecific morphological variability. Cells were isolated using a micropipette and placed in 12-well tissue culture plate with 0.2 μm-filtered seawater collected that day from the same locality, and supplemented with aliquots of cultures of diatoms. The culture plates were placed in an incubator used for microalgae culturing, at 23°C, 100 μmol photons m2 s−1 from cool-white tubes and photoperiod 12:12 L:D.
Cells of Cochlodinium polykrikoides were collected from the type locality, Phosphorescent Bay, located about 3.2 km east of La Parguera, southwest coast of Puerto Rico (17° 58′ 30″ N, 67° 01′ 10″ W). This bioluminescent bay is irregular in shape, having three inlets or arms oriented north of the main central body, and a narrow (~150 m wide) and shallow outlet that connects with the ocean. The bloom of C. polykrikoides was located in the inner part of the northwestern inlet. As the plankton net immediately clogged, the samples were collected and transported with buckets. The live samples were examined in a composite settling chamber with an inverted microscope (3030 Accu-scope, Commack, NY, USA) and photographed with a digital camera. Live cells of C. polykrikoides were also examined with an Olympus IX41 epifluorescence microscope. In order to determinate the position and shape of the nucleus, the cells were stained with DAPI (4′,6′ diamino-2-phenylindole, Sigma, St. Louis, MO, USA) and examined with the same microscope.
For scanning electron microscopy, samples of C. polykrikoides were fixed immediately after collection with glutaraldehyde (5% final concentration) and kept cold until analysis. Fixed cells were filtered onto a 0.8 μm size Nuclepore membrane filter, washed with distilled water, fixed with osmium, dehydrated with a graded series of ethanol (30%, 50%, 70%, 90%, 95%, 99%, 100%) and critical-point-dried with CO2. Filters were mounted on stubs, sputter-coated with gold and viewed under a Phillips XL30 (FEI Company, Hillsboro, OR, USA) scanning electron microscope.
For molecular studies, the cells of C. strangulatum were micropipetted individually with a fine capillary into a clean chamber and washed several times in a series of drops of 0.2 μm-filtered and sterilized seawater. One to four cells C. strangulatum were placed in a 0.2 mL tube filled with absolute ethanol. The samples were kept at room temperature and in darkness until the molecular analysis could be performed.
2.2. DNA extraction, PCR amplification of large subunit rRNA gene (LSU rDNA) and sequencing.
Prior to polymerase chain reaction (PCR), the sample tube was centrifuged, and ethanol was evaporated by placing the tube overnight in a desiccator at room temperature. Genomic DNA was extracted using Chelex (InstaGene™ Matrix; Bio-Rad, Hercules, California, USA) following protocols adapted from Richlen and Barber (2005). Extractions were carried out using 75 μL of Chelex solution. Samples were vortexed for approximately 5 s and briefly spun in a small benchtop microcentrifuge for 10-15 s. Samples were incubated at 56 °C for 30 minutes, followed by incubation at 95 °C for 20 minutes. Following incubation, samples were again vortexed, and then stored at −20 °C. The D1-D2 domains of the large subunit ribosomal RNA gene (LSU rDNA were amplified using primers D1R and D2C (Scholin et al., 1994). PCR amplifications were performed in a 25 μL reaction volume containing 1 μL of template DNA (supernate from each Chelex extraction), 1 × PCR Buffer (500 mM KCL and 100 mMTris–HCl, pH 8.3), 2 mM MgCl2, 0.8 mM dNTPs, 0.5 mM of each primer, and 0.5 U of AmpliTaq DNA Polymerase (Applied Biosystems Inc., Foster City, CA, USA). Hot start PCR amplifications were performed in an Eppendorf Mastercycler Nexus thermal cycler (Eppendorf, Hamburg, Germany) with the following cycling conditions: initial denaturation (94°C/4 min); 35 cycles of denaturation (94°C/45 s), annealing (55°C/1 min), and extension (72°C/1 min); final extension (72°C/10 min). PCR products were visualized on a 1% agarose gel stained with GelRed (Biotium, Hayward, California USA). Positive PCR products were cloned into vector pCR 2.1 using a TOPO TA cloning kit (Invitrogen, Carlsbad, CA, USA). Clones were screened for inserts by PCR amplification with plasmid primers M13F and M13R, and positive clones from each PCR amplicon were purified using the Qiaquick PCR purification kit (Qiagen, Hilden, Germany), and sequenced in both the forward and reverse direction (Eurofins MWG Operon, Ebersberg, Germany). Sequence reads were aligned and assembled in Geneious Pro 8.1 (Biomatters, Auckland, New Zealand). The newly generated consensus sequences were deposited in DDBJ/EMBL/GenBank under accession numbers KY468922–KY468925.
2.3. Phylogenetic analyses
The DNA sequences of Cochlodinium strangulatum were analyzed using Basic Local Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi ) against databases in GenBank. A Matrix comprising the LSU rDNA sequences was assembled from most similar sequences identified using BLAST. Available D1-D2 LSU rDNA sequences of Cochlodinium spp. and other dinokaryotic dinoflagellates were aligned with ClustalW (Thompson et al., 1994) using default parameters (Larkin et al., 2007), and refined using MUSCLE (Edgar, 2004), as implemented in Geneious Pro 8.1. This alignment was subsequently inspected and edited by eye. The final alignment included 66 sequences and 678 positions.
Phylogenetic trees were constructed using maximum likelihood (ML) analysis and Bayesian inference. For these analyses, Modeltest V. 3.7 (Posada and Crandall, 1998) was used to select the most appropriate model of nucleotide substitution. ML analysis was carried out using PhyML (Guindon et al., 2010), with the general time reversible + gamma (GTR) substitution model, and 500 bootstrap replications. Bayesian inference was performed using MrBayes 3.2.6 (Ronquist and Huelsenbeck, 2003), again with a GTR model. Posterior probabilities were estimated using four Markov chain Monte Carlo chains, which ran for 2,000,000 generations. Trees were sampled every 400 generations following a burn-in period of 100,000 generations, after which log-likelihood values stabilized. Bayesian posterior probabilities (BPP) were calculated for each clade. For both analyses, Perkinsus marinus (GenBank number AY876319) was used as an outgroup.
3. Results
3.1. Observations of Cochlodinium strangulatum
Individuals of C. strangulatum were occasionally observed at sampling stations in the coastal Mediterranean Sea (Fig. 1) and the South Atlantic Ocean (Fig. 2). No clear temporal pattern was found due to the paucity of records. The occurrence tended to be more frequent during the winter season, and was associated with proliferations of diatoms. The species was only observed as solitary cells, not as chained colonies (Figs. 1–2). The cells were among the largest in the gymnodinioid dinoflagellates (150–200 μm in length). They showed a variable shape, characteristic of unarmored dinoflagellates, and especially of phagotrophic species able to ingest large prey. The shape of the episome was more variable than the hyposome in cells under the stress of the capture and observation (Fig. 1A–E), and in general, stressed cells or those that recently ingested prey showed more globular shapes. Later, during incubation experiments, these globular cells changed to a spindle-shaped morphology in which the antapex was more or less pointed, and the end of the apex was brunt or truncate. Unfortunately, the cells under laboratory conditions did not survive beyond four days. In the recently collected stressed cells, the episome was almost flat (Fig. 1A), subtriangular (Fig. 1B) or hemispherical (Fig. 1C). The most typical morphology was an ellipsoidal episome with a round apex oriented toward the right side in ventral view (Fig. 1F–G, 2J–K) or triangular with a round apex (Fig. 2A–I) or the end of the episome was flat (Fig. 2Q, U–W). The apical groove was circular, encircled the apex and connected with the anterior extension of the sulcus (Figs 1F, 2E, H–L, T–W). The anterior extension of the sulcus extended from the proximal end of the cingulum towards the left side to meet the apical groove. The cingulum was deeply constricted, descending with a left-spiral course and turning 1.5–1.8 times around cell (Figs 1D–J, M–S, 2A–D, F, I, M–N, Q–S). The anterior part of the sulcus extended along the episome as a wide loop, ending near the apex on the dextrodorsal surface. The posterior part of the sulcus descended to the left side in ventral view, turning obliquely in the middle of the cell and straight near the antapex (Figs 1F–J, M–S, 2A–D, F, I, Q–T). The most typical of morphology corresponded to cells with hyposome being almost triangular with a more or less pointed antapex (Figs 1G, I–J, O–Q, 2B, I, R). The antapex was truncate or round in cells with a globular shape that apparently recently ingested a large prey (Fig. 1C), with a large vacuole in the hyposome (Fig. 2P), or in dividing cells (Figs 1L, 2M–N). The nucleus was spheroidal, large (~55 μm in diameter), and located slightly posterior to the central part of the body (Figs 1G, M, 2B–D, I–J, Q, T). The nucleus was more anteriorly displaced in the cells with a large posterior vacuole (Fig. 2P). The nucleus was the typical dinokaryon and showed two clearly differentiated regions - a distinct perinuclear capsule located outside and an inner portion at some distance with the nuclear envelope (Fig. 2U). In some cells, the perinuclear capsule showed an irregularly undulated surface (Fig. 2U). The nucleus often remained after cell lysis (Fig. 1M). The cell surface was minutely striate with about 8–9 equidistant striae in 10 μm in the middle of the cell and more densely distributed in other areas (Figs 1F, J, 2S–T). The surface of the cingulum was covered by coarser striae, about three striae in 10 μm (Fig. 1F). Additionally, some cells were superficially covered by a type of granule about 1.5 μm in diameter with a darker color (Figs 1N, O, 2G). The cells showed a yellowish coloration and numerous spherical refractile bodies or putative oil droplets of 10–20 μm in diameter (Figs 1A–C, K, 2C, J, O, Q, V). Cells were phagotrophic and a large vacuole in the posterior half of the cell with dark brown pigmentation was eventually observed (Fig. 2P). Although the mechanism of predation was not directly observed, it is probable that prey are ingested by engulfing.
Fig. 1.
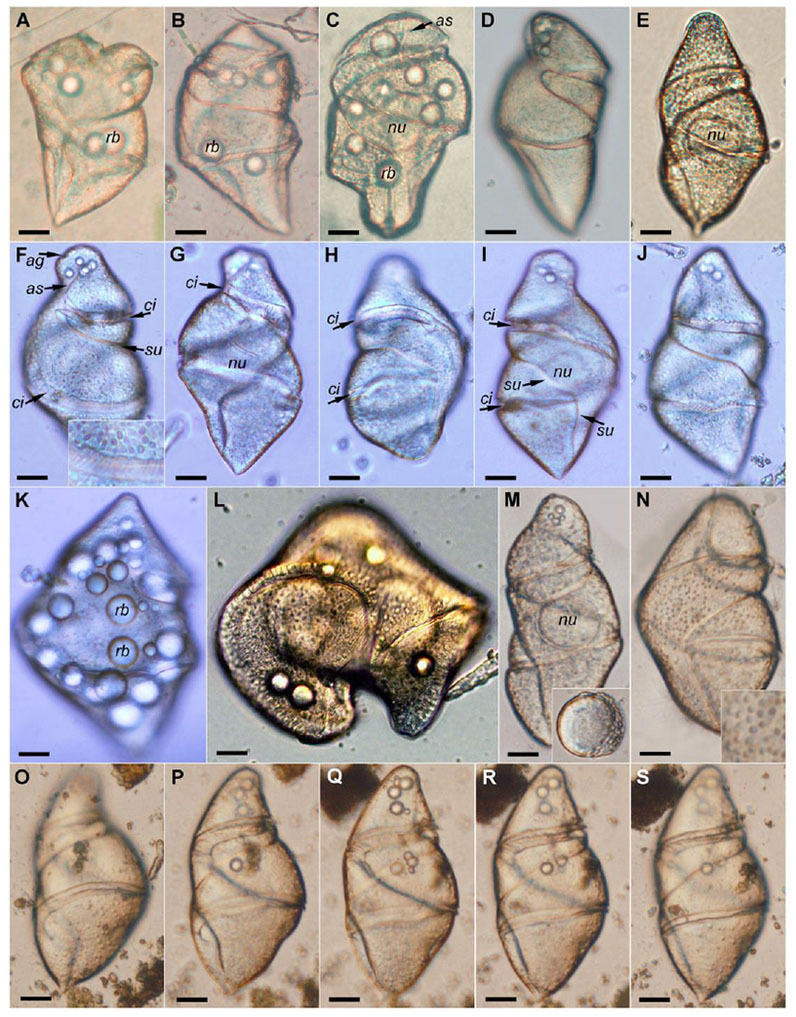
Light micrographs of Cochlodinium strangulatum from the Mediterranean Sea. (A–C) Cells from Marseille. (D–K) Cells from Banyuls-sur-Mer. (L) Cells from Villefranche-sur-Mer. (M–S) Cells from Valencia. (F–J) Cell in different views and focus level. (F–G) Ventral view. (F) Front focus. The inset shows the striae in the cingulum. (G) Rear (deeper) focus. (H–I) Dorsal view. (H) Front focus. (I) Rear focus. (J) Sinistro-lateral view. (K) Another cells with numerous refractile bodies. (L) Dividing cell. (M) The inset shows the nucleus after the cell lysis. (N) The inset shows the dark granules in the cell surface. (O–S) Another cell in different focus levels. Abbreviations: ag = apical groove; as = anterior sulcus; ci = cingulum; nu = nucleus; rb = refractile body; su = sulcus. Scale bar = 20 μm.
Fig. 2.
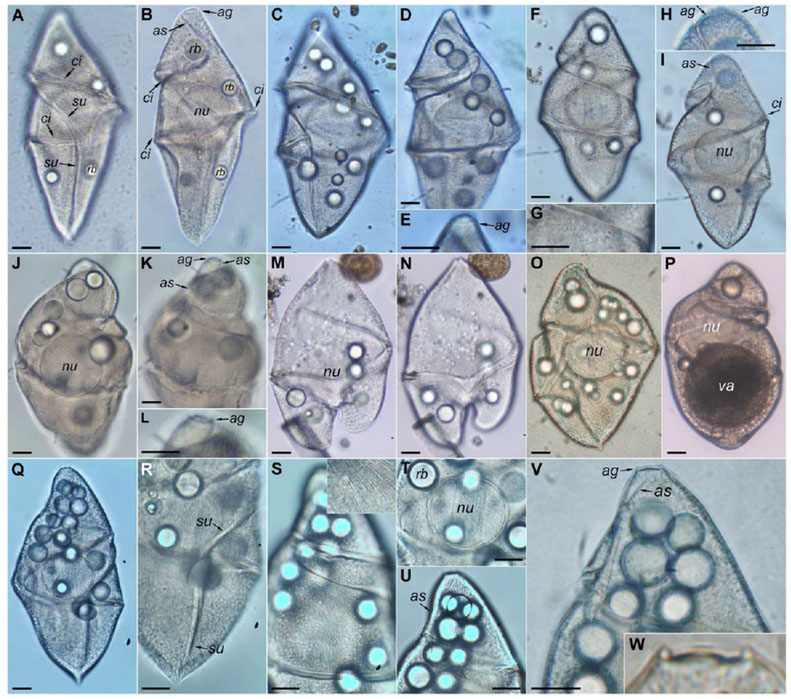
Light micrographs of Cochlodinium strangulatum from the South Atlantic Ocean (São Sebastião Channel and off Ubatuba). (A–B) Cell in dorsal view. Isolate FG#10 (accession numbers KY468923–4). (A) Front focus. (B) Rear (deeper) focus. (C–E) Another cell in dorsal view. Isolate FG#12 (accession numbers KY468922, KY468925). (C) Front focus. (D–E) Rear focus. (F–I) Another cell in sinistro-lateral view. (F) Frontal view. (G) Detail of the cell surface. Note the granules along the cell surface. (H) Detail of the apex. (I) Rear focus. (J–L) Another cell in ventral view. (J) Rear focus. (K–L) Front focus. (M–N) Dividing cell. (O) Another cell. (P) Another cell. Note the large vacuole. (Q–W) Another cell in sinistro-ventral view. (Q) Rear focus. (R) Detail of the posterior sulcus. (S) The inset shows the fine surface striae. (T) Nucleus and refractile bodies. Note double-layered contour of the nucleus. (U) Apex. (V–W) Detail of the apex. Abbreviation: ag = apical groove; as = anterior sulcus; ci = cingulum; nu = nucleus; rb = refractile body; su = sulcus; va = vacuole. Scale bar = 20 μm.
Cells were observed in the earlier stages of the division (Figs 1L, 2M–N), while pairs of daughter cells still joined after the recent division were not observed. The live cells did not show active swimming, but were slowly displaced onto the bottom of the settling chamber (see Video S1 as supplementary material, https://youtu.be/U-JzngXFgxc). During incubation experiments, the cells were resistant to manipulation and were not easily lysed compared to other unarmored dinoflagellates. They were sensitive and clearly were lysed due to the environmental conditions in the dense plankton concentrates, as cells tended to appear only during the first hours of observations and were absent in subsequent examinations of the samples. The cells that were isolated and placed in filtered seawater with aliquots of diatoms as prey survived up to four days.
3.2. Observations of Cochlodinium polykrikoides
The species C. polykrikoides formed a dense bloom with a golden brownish color in the northern inlet of Phosphorescent Bay, Puerto Rico (see video S1 as supplementary material, https://youtu.be/U-JzngXFgxc ). Collection with 20- or 40-μm pore size plankton net failed because the net clogged due to a mucilaginous substance. Non-concentrated samples were observed about one hour after the collection. Less than 10% of the cells appeared as four-celled chains (Fig. 3A), while most of the cells appeared as single or two-celled chains (Fig. 3B–S, see video S1 as supplementary material, https://youtu.be/U-JzngXFgxc ). On one occasion, the microscopic observations were carried out directly on site. In that case, the percentage of cells forming four-celled chains was higher. This demonstrates that the cell chains decomposed during transport and laboratory manipulation. The chained cells were actively swimming and when they stopped, the chained cells began to separate. A cytoplasmic connection appeared between the right side of the antapex of one cell and the left side of the apex of other cell and finally the chain separated (Fig. 3C). The non-swimming single cells then acquired a more elongated shape and secreted a mucus-like substance, after which they rapidly lysed.
Fig. 3.
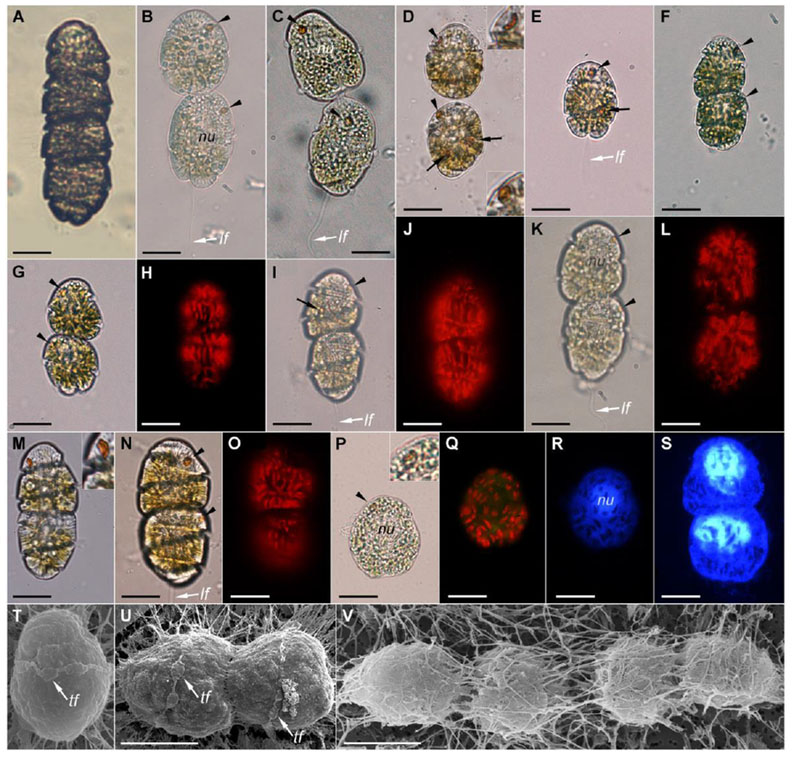
Light (A–S) and scanning electron (T–V) micrographs of Cochlodinium polykrikoides from Phosphorescent Bay, Puerto Rico. The arrowheads point the eyespot. (A) Four-celled chain. (B–C) Cells before lysis. (C) Note the cytoplasm bridge between the two cells. (D) The arrows point the reddish-orange corpuscles, tentatively a prey. The insets show the different shape of the eyespot. Note the hyaline membrane around the cells. (E) Single cell. (F–O) Two-celled chains. (G) Note the notched antapex. (U, J, L, O). Autofluorescence of chloroplasts. (P) The inset shows the eyespot surrounded by a chloroplast. (R–S) Nucleus stained with DAPI. (T–V) The cell surface was masked by a filamentous structure that emerged from the cells. Abbreviations: lf = longitudinal flagellum; nu = nucleus; tf = transversal flagellum. Scale bar = 20 μm
The cells of C. polykrikoides were ellipsoidal and 25–40 μm in length. Cells larger than 40 μm were not observed. The apical groove or the anterior extension of the sulcus was not visible in the observations by light microscopy. The cingulum encircled the cell about 1.8–2.0 turns. A posterior sulcal extension was positioned immediately beneath the cingulum (Fig. 3G, I). A reddish orange pigmented body was visible on the anterior dorsal side (Fig. 3B–F). That structure is named an eyespot or stigma in the literature. The shape of the eyespot depended of the angle of view. It was usually globular (Fig. 3B–C) and in some cases was lenticular (Fig. 3D, M). The eyespot was orange to red in color, usually kidney-shaped, and its concave side faced the dorsal surface of the cell (Fig. 3D). Some cells showed other orange corpuscles of different shapes and position (Fig. 3D–E, I). They may tentatively correspond to ingested prey such as Cryptophyta. Chloroplasts were rod-like shape and aligned longitudinally in parallel (Fig. 3F–O). The chloroplasts were randomly distributed in stressed cells before lysis (Fig. 3P–Q). The nucleus was almost spherical and anterior (Fig. 3R–S). Samples immediately fixed with glutaraldehyde at the collection site were examined by scanning electron microscopy (Fig. 3T–V). The transversal flagellum was observed in some cells (Fig. 3T–U). Other morphological features were not observed because the cell surface was covered by filamentous substance that emerged from the cells (Fig. 3V).
Molecular phylogeny
LSU rDNA (D1-D2) sequences were obtained from two samples of Cochlodinium strangulatum from the surface waters in the middle of the São Sebastião Channel isolated on 23 August 2013 (isolate #FG10, Fig. 2A–B) and 29 November 2013 (isolate #FG12, Fig. 2C–E). The phylogenetic position of C. strangulatum was examined using a dataset comprising a variety of dinoflagellate LSU rDNA sequences, focusing on available sequences of Cochlodinium spp. and other unarmoured dinoflagellates (Fig. 4). Sequences of C. strangulatum constituted a novel clade that branched as a sister group to Gyrodinium spp. The new sequences were grouped within the large lineage comprising Gymnodiniales, Peridiniales, Dinophysales and Prorocentrales. While these major clades were generally supported by high bootstrap values, the relationships among them were poorly resolved due to low bootstrap support of deeper branches, making it difficult to infer its affinity with known dinoflagellates. The species currently classified as Cochlodinium branched into three distantly related clades with high bootstrap support: the first for the type species, the second comprising Cochlodinium polykrikoides and allied species, and third clade for Cochlodinium cf. convolutum (KF245460), Cochlodinium cf. helix (KF245459) and Ceratoperidinium spp. (Fig. 4).
Fig. 4.
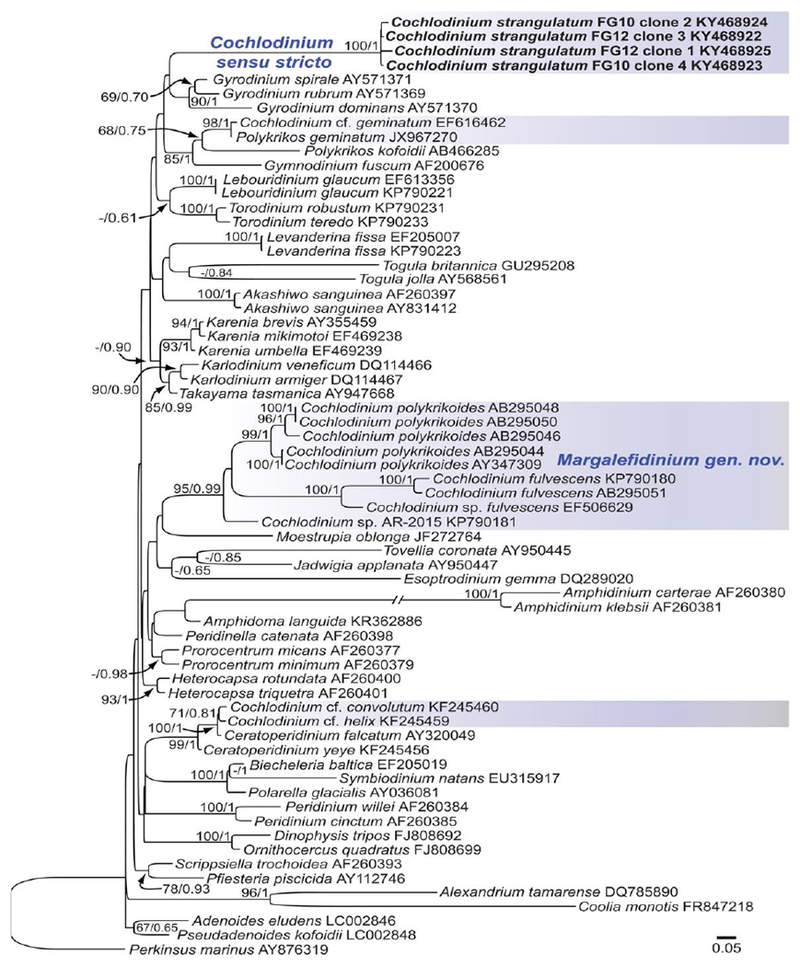
Maximum-likelihood phylogenetic tree of selected species based on 678 positions of the D1–D2 domains of the LSU rRNA gene. Supports at internal nodes are Bayesian posterior probabilities (BPP) and bootstrap support values obtained after 500 replicates. Only bootstrap values >50 and BPP >0.5 are shown. Perkinsus marinus was used as outgroup. New sequences are highlighted in bold. The clades containing sequences of Cochlodinium are highlighted in blue shaded boxes. The scale bar represents the number of substitutions for a unit branch length.
3.3. Taxonomical considerations
Numerous studies of the morphology of Cochlodinium polykrikoides confirmed that this species was not related to the type species of Cochlodinium. Molecular and morphological data do not support the placement of C. polykrikoides and allied species (C. fulvensens and C. catenatum) within the genus Cochlodinium, or any other known dinoflagellate genus (Table 1, Fig. 4). Therefore, this study emends the diagnosis of the genus Cochlodinium that is restricted to species with characteristics of its type, C. strangulatum. A new genus name is proposed here for C. polykrikoides and allied species.
Table 1.
Distribution of selected morphological characters in Gyrodinium spirale, Cochlodinium strangulatum, Margalefidinium spp, and Cochlodinium convolutum.
| Gyrodinium spirale | Cochlodinium strangulatum | Margalefidinium spp. | Cochlodinium convolutum | |
|---|---|---|---|---|
| Cell length | <150 μm | 120–200 μm | <60 μm | <80 μm |
| Cingulum turns | ~1 times | 1.5–1.8 times | ~1.5–2 times | ~1.5 times |
| Sulcus | deep | deep | shallow | deep |
| Chloroplast | absent | absent | rod-like/granulate | reticulate |
| Eyespot | absent | absent | dorsal episome | absent |
| Nucleus position | central | central | anterior | central |
| Nucleus shape | spherical, encapsulated | spherical, encapsulated | spherical | roundly rectangular |
| Cell surface | coarse striae | fine striae | smooth | smooth |
| Apical groove | elliptical bisected | circular | U-slhaped | circular |
| Cell-chain | Single-cell | Single-cell | 1–16 celled-chain | 1–2 celled-chain |
| Hyaline cyst | no | no | rare | common |
| Harmful | no | no | very harmful | less harmful |
Cochlodinium F. Schütt emend. F. Gómez, Richlen & D.M. Anderson
Emended diagnosis:
Unarmored free-living heterotrophic dinoflagellate. Cells are solitary, not forming chained colonies. The deeply constricted cingulum has a descending left-spiral course, turning 1.5–2 times around cell. The sulcus invades the episome as a wide loop, ending near the apex on the dextro-dorsal surface. The apical groove is circular, encircling the apex and connecting with the anterior sulcus. The surface is covered by longitudinal equidistant fine striae. The nucleus is enclosed in a distinct perinuclear capsule.
Type species:
Cochlodinium strangulatum (F. Schütt) F. Schütt (Schütt, 1896; pp. 5, fig. 7).
Basionym:
Gymnodinium strangulatum F. Schütt (Schütt, 1895; pp. 164, plate 22, fig. 72).
Neotype:
Fig. 5.
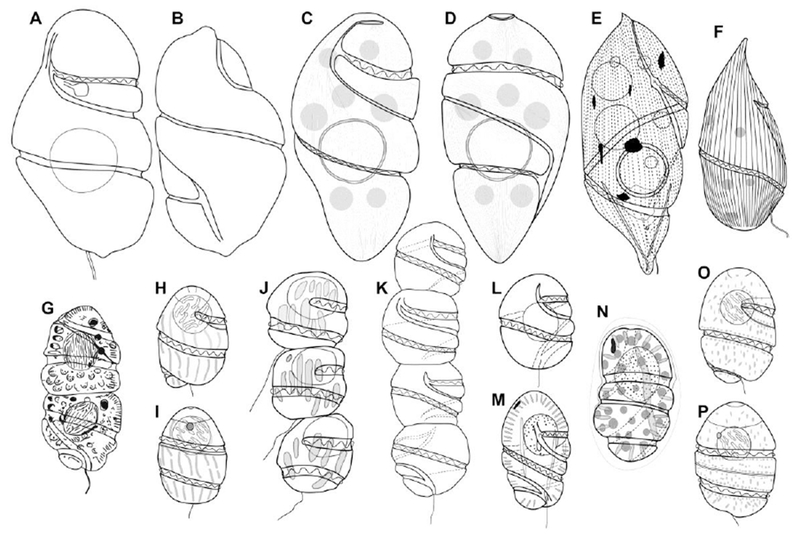
Line drawings of Cochlodinium spp., Margalefidinium spp. and Plectodinium nucleovolvatum. (A–B) Ventral and dorsal view of Gymnodinium strangulatum, redrawn from Schütt (1895). (C–D) Ventral and dorsal view of C. strangulatum, redrawn and modified from Takayama (1998). (E) Cochlodinium miniatum, redrawn from Kofoid and Swezy (1921). (F) Plectodinium nucleovolvatum, redrawn from Biecheler (1934). (G) Margalefidinium polykrikoides, redrawn from Margalef (1961). (H–I) Ventral and dorsal view of Margalefidinium polykrikoides, redrawn and modified from Iwataki et al. (2007). (J) Margalefidinium catenatum, redrawn from Okamura (1916). (K) Cochlodinium catenatum, redrawn from Kofoid and Swezy (1921, plate 9, fig. 105). (L) Cochlodinium catenatum, redrawn from Kofoid and Swezy (1921, fig. GG14). (M) Margalefidinium citron, redrawn from Kofoid and Swezy (1921). (N) Margalefidinium flavam, redrawn upside down from Kofoid (1931). (O–P) Ventral and dorsal view of Margalefidinium fulvescens, redrawn and modified from Iwataki et al. (2007).
Heterotypic synonyms:
Cochlodinium miniatum Kofoid & Swezy, Plectodinium miniatum (Kofoid & Swezy) F.J.R. Taylor, non Plectodinium nucleovolvatum Biecheler.
Other species that are considered to belong to Cochlodinium:
Cochlodinium atromaculatum Kofoid & Swezy
Cochlodinium constrictum (F. Schütt) Lemmermann
Cochlodinium cereum Kofoid & Swezy
Margalefidinium F. Gómez, Richlen & D.M. Anderson, gen. nov. (Fig. 3)
Diagnosis:
Unarmored, free-living dinoflagellates, solitary or forming cell chains. Cells subspherical to ellipsoidal, of medium size (25–60 μm in length). The cingulum encircles the cell about twice, and a narrow sulcus encircles the cell approximately once. The apical groove is U-shaped and connected to the anterior sulcal extension on the dorsal side of the episome. The cell surface is smooth, lacking ridges or striae. Chloroplasts contain peridinin as major carotenoid. A reddish-orange pigmented body or eyespot is located in the episome. The dinokaryotic nucleus lacks the perinuclear capsule.
Etymology:
In honor of Ramón Margalef who described C. polykrikoides. The suffix ‘– dinium’ meaning ‘vortex’ is commonly applied to dinoflagellates. The gender is neuter.
Type species:
Margalefidinium polykrikoides (Margalef) F. Gómez, Richlen & D.M. Anderson, gen. & comb. nov., hic designatus.
Basionym:
Cochlodinium polykrikoides Margalef (1961, pp. 76, 78, fig. 27m).
Heterotypic synonym:
Cochlodinium heterolobatum E.S. Silva.
Epitype:
Other species:
Margalefidinium catenatum (Okamura) F. Gómez, Richlen & D.M. Anderson, comb. nov.
Basionym:
Cochlodinium catenatum Okamura (1916, pp. 41, figs. 1–3). Non Cochlodinium catenatum Okamura sensu Kofoid & Swezy (1921).
Margalefidinium citron (Kofoid & Swezy) F. Gómez, Richlen & D.M. Anderson, comb. nov.
Basionym:
Cochlodinium citron Kofoid & Swezy (1921, pp. 358, pl. 7, fig. 79, text-fig. HH, 12).
Margalefidinium flavum (Kofoid) F. Gómez, Richlen & D.M. Anderson, comb. nov.
Basionym:
Cochlodinium flavum Kofoid (1931, pp. 26–27, plate 2, fig. 13).
Margalefidinium fulvescens (M. Iwataki, H. Kawami & Matsuoka) F. Gómez, Richlen & D.M. Anderson, comb. nov.
Basionym:
Cochlodinium fulvescens M. Iwataki, H. Kawami & Matsuoka (2007, pp. 235, fig. 9).
4. Discussion
4.1. Why did Cochlodinium strangulatum disappear?
The species C. polykrikoides is the most investigated species of Cochlodinium because it forms major blooms that can be harmful, and has been dispersing globally in recent years (Richlen et al., 2010; Kudela and Gobler, 2012). A genus is defined by its type species, and it was evident that C. polykrikoides and allied species are distantly related to C. strangulatum. The lack of information on C. strangulatum delayed reclassification. The Cochlodinium type species was described in one of the earliest dinoflagellate monographs (Fig. 5A–B, Schütt, 1895). That publication was easily accessible, and consequently the original description was further reproduced in the monographs of Kofoid and Swezy (1921) and Schiller (1933). Schütt (1895) did not report the type locality; rather, his specimens were collected from the Atlantic Ocean or more likely from samples collected from the Gulf of Naples.
The species Cochlodinium strangulatum is one of the largest gymnodinioid dinoflagellates (~200 μm long). Phytoplankton studies have been numerous in Naples or other Mediterranean marine stations over the last century. Despite the intensity of these activities, C. strangulatum is reported in the Mediterranean Sea in only two species lists: a Ph.D. about microbes in the Ligurian Sea (Lins da Silva, 1991), and a doubtful phytoplankton study (due to the excess of listed species) along the Libyan coasts (Skolka et al., 1986).
Why did Cochlodinium strangulatum virtually “disappear” from plankton records after its description? The answer relates to fixation artifacts and mistaken assignments due to similarities to large Gyrodinium cells. The species Cochlodinium strangulatum is an uncommon but nevertheless widespread species. This study reveals that C. strangulatum can be found in the waters near the French marine stations at Banyuls-sur-Mer, Marseille and Villefranche-sur-Mer, which have been sites of phytoplankton studies for over a century. In fact, C. strangulatum can be found in any coastal area in warm seas when examining freshly collected live samples. Most of the past studies and monitoring programs are based on fixed material, and C. strangulatum is difficult to recognize in these samples, as is common with unarmored dinoflagellates. After the original illustration by Schütt (1895), further original illustrations are restricted to a poorly detailed line drawing in Wood (1968), and the scanning electron microscopy pictures in an unpublished Ph.D. (Fig. 5C–D; Takayama, 1998). When Kofoid and Swezy (1921) and Schiller (1933) published their monographs, information about C. strangulatum was restricted to that reported in the Schütt’s description. Kofoid and Swezy reproduced the Schütt’s description and clearly stated that the cell surface was striate. Kofoid and Swezy (1921), Schiller (1933) and Wood (1968) illustrated C. strangulatum with a smooth surface. This inconsistency could contribute to this taxon’s absence in the literature.
Kofoid and Swezy (1921, pp. 383) reported for C. strangulatum: “This is one of the largest species in the genus, being exceeded in size by only one species, C. miniatum. It shares with this species the peculiarity of having a perinuclear, hyaline zone, as well as a striate surface”. Kofoid and Swezy (1921, pp. 383) described the new species C. miniatum from the observation of a single specimen, consequently ignoring intraspecific variability (Fig. 5E). The only differences between the C. strangulatum and C. miniatum were reddish corpuscles and a more pointed cell apex. This study revealed that the apex of C. strangulatum varied from rounded to slightly pointed (Figs 1, 2). Kofoid and Swezy (1921, pp. 383) also split C. miniatum from C. strangulatum based on the coloration of corpuscles. This is a poor diagnostic character in these phagotrohic species where the color of accumulation bodies depends of the prey. Kofoid and Swezy (1921, pp. 344) also reported “ Two species only, C. miniatum and C. strangulatum, present a perinuclear membrane of the type occasionally found in GyrodiniumT. Although the pointed apex is uncommon in C. strangulatum, the single specimen described as the new species C. miniatum fits within the intraspecific variability of C. strangulatum. Kofoid and Swezy (1921) apparently did not observe C. strangulatum, but they described it as C. miniatum, with the same cell size and shape, the distinctive perinuclear capsule and the fine surface striation that they omitted in the illustration of C. strangulatum. The species C. miniatum (Fig. 5E) is here considered a synonym of C. strangulatum (Fig. 5A-D). Similar examples can be found in the morphotypes of Balechina pachydermata (Kofoid & Swezy) Loeblich & A.R. Loeblich, which Kofoid and Swezy (1921) described as several separate species (Gómez et al., 2015). Taylor (1980) reported that Kofoid and Swezy (1921) illustrated C. miniatum upside down, with incorrect flagellar details. Taylor (1980) transferred C. miniatum into the genus Plectodinium Biecheler based on a supposed resemblance with the type, Plectodinium nucleovolvatum Biecheler (=? Gyrodinium rhabdomante Balech) (Fig. 5F). According to the illustration by Biecheler (1934), P. nucleovolvatum strongly resembled Gyrodinium spirale (Bergh) Kofoid & Swezy. Sournia (1986) considered Plectodinium as a synonym of Gyrodinium Kofoid & Swezy. Given its cell size, shape, the more coarse striation, P. nucleovolvatum (Fig. 5F) is quite different from C. miniatum (Fig. 5E). Plectodinium as well as Cochlodinium strangulatum have disappeared from the literature and are omitted in the dinoflagellate guides (Steidinger and Tangen, 1997).
Cochlodinium strangulatum was illustrated in the literature with a smooth surface (Kofoid and Swezy, 1921; Schiller, 1933; Wood, 1968). Observations of cells of C. strangulatum with surface striation could be mistaken for other large unarmored dinoflagellates with surface markings. In the molecular phylogeny, C. strangulatum branched as a sister group of Gyrodinium spp. (Fig. 4). Bootstrap values were too low to confirm the phylogenetic relationship between these genera. Both genera exhibit ecological and morphological similarities (Table 1), and C. strangulatum may be mistaken for large cells of Gyrodinium spirale. Both species show high cell shape plasticity, especially in stressed cells or after prey ingestion, as typical in heterotrophic unarmored dinoflagellates able to engulf large prey. Both species are similar in diagnostic characters such as longitudinal striae, the central nucleus surrounded by a capsule, and the refractile bodies and vacuoles (Hansen and Daugbjerg, 2004). These characteristics are also present in other unarmored phagotrophic dinoflagellates such as Cucumeridinium F. Gómez, P. Lopez-Garcia, H. Takayama & D. Moreira and Lebouridinium F. Gómez, H. Takayama, D. Moreira & P. Lopez-Garcia (Gómez et al., 2015, 2016). Only species such as Gyrodinium spirale reached cell sizes similar to that of C. strangulatum. Although the size of G. spirale is typically about 100 μm, cells up to 200 μm long have been reported in the literature (Schiller, 1933). The surface striae of C. strangulatum are finer than in G. spirale. Cells of G. spirale have also been represented with fine striation (Wulff, 1916). Consequently, in some observations C. strangulatum was mistaken for G. spirale, especially from preserved material. From the observation of live material, however, it is easy to distinguish these genera. In addition to the differences in the turns of the cingulum and the surface striae (Table 1), the coloration is yellow-brownish and ash-grey for C. strangulatum and G. spirale, respectively. The apex of Gyrodinium is pointed (Hansen and Daugbjerg, 2004), while usually flattened in C. strangulatum (Figs 1–2). The species G. spirale is an active swimmer, while C. strangulatum moves slowly (Video S1, https://youtu.be/U-JzngXFgxc ).
4.2. Older descriptions and the enigma of Cochlodinium catenatum
Unarmored dinoflagellates such as C. polykrikoides are very delicate. It is difficult to observe cells in good condition, as the chain readily decomposes, the cell shape becomes distorted, and cells frequently lyse during transport and manipulation. Despite numerous studies, morphological characters such as the apical groove have not been reported until recently due to the cell delicacy (Iwataki et al., 2010). The observations of cell morphology and behavior in this study are similar to those described by Margalef (1961) (Figs 3, 5G, Video S1 as supplementary material, https://youtu.be/U-JzngXFgxc). Margalef stated that he was unable to measure the precise cell size due to the lack of calibration for the microscope, and provided a tentative length of 50 μm. The cells of C. polykrikoides in the type locality were smaller than 40 μm long (Fig. 3). The species was described in 1961, relatively late given that C. polykrikoides is a bloom-forming species with a cosmopolitan distribution (Kudela and Gobler, 2012; Reñe et al., 2013a). Consequently, it is possible that this taxon appeared in the literature before 1961.
Matsuoka et al. (2008) discussed the relationship between C. polykrikoides and C. catenatum from Yokohama Harbor (Bay of Tokyo) that Okamura described in 1916 (Fig. 5J). Matsuoka et al. (2008) concluded that C. polykrikoides and C. catenatum are independent species. The bloom of C. polykrikoides examined by Margalef (1961) is a natural phenomenon that still persists because environmental conditions of the type locality remain unaltered (Video S1, https://youtu.be/U-JzngXFgxc_). Fortunately, this facilitates morphological and molecular studies of C. polykrikoides. In contrast, C. catenatum has not been investigated by modern methods because blooms of this species have not been reported in recent decades. The species C. polykrikoides was reported from the South to the North of Japan (Shimada et al., 2016), and as a common blooming species in Japan and Korea (Jeong et al., 2004). This could be interpreted as C. polykrikoides having replaced and occupied the ecological niche of C. catenatum, or that they are in fact the same species.
The observations by Okamura (1916) were based on stressed cells, in which their morphology was distorted before cytolysis. In the opinion of the first author, C. polykrikoides and C. catenatum are synonyms, with the latter having priority. The molecular phylogenies showed that the sequences of C. polykrikoides separate into several subclades (Iwataki et al., 2010; Reñe et al., 2013a). If the split of both species is maintained, the members of the clade dominant in Japan and Korea could be assigned to C. catenatum, while the subclade with the isolates from Puerto Rico and North America could be assigned to C. polykrikoides. The split between the subclades of C. polykrikoides did not follow a clear geographical pattern. The subclade with sequences of C. polykrikoides from Puerto Rico and North America also includes isolates from Malaysia (Iwataki et al., 2010; Richlen et al., 2010; Reñe et al., 2013a).
In addition to the controversy of C. catenatum as senior synonym of C. polykrikoides, Kofoid and Swezy (1921) added more confusion when they reported C. catenatum as a heterotrophic species, without eyespot and with a central nucleus (Fig. 5K–L). Based on these features, Matsuoka et al. (2008) concluded that C. catenatum in Okamura (1906) and in Kofoid and Swezy (1921) were independent species. Kofoid and Swezy illustrated C. catenatum with a line drawing of a single cell with a central spherical nucleus (figure GG14 of their publication) and with a color illustration of a 4-celled chain without details on the nuclei (figure 105 of their publication). Kofoid and Swezy did not report the eyespot, possibly because this organelle is not visible in some views, or perhaps these authors mistook it for a small food vacuole. Margalefidinium polykrikoides is a mixotrophic species (Jeong et al., 2004), and in this study showed that in addition to the eyespot, some cells showed dispersed reddish bodies that may correspond to food vacuoles (Fig. 3D-E). As Kofoid and Swezy observed only one chain, it cannot be discarded that the eyespot went unnoticed or was mistaken for small food vacuoles. The omission of the eyespot is not restricted to older descriptions. For example, Reñe et al. (2015) reported Cochlodinium sp. AR-2015 (accession number KP890181) that branched as a separate species within the clade of Margalefidinium (Fig. 4). Based on their micrograph, that species does not possess an eyespot (Reñe et al., 2015; their figure 3b)
Kofoid and Swezy’s figure 105 showed a 4-celled chain with yellow-greenish pigmentation that suggested the presence of chloroplasts. In the same plates, Kofoid and Swezy illustrated the heterotrophic species (i.e., Warnowia spp.) as colorless. The free-living chain-forming dinoflagellates are photosynthetic species [Gymnodinium catenatum H.W. Graham, Polykrikos geminatum (Schütt) D. Qiu & S. Lin, Alexandrium spp.], thus the heterotrophy of C. catenatum sensu Kofoid and Swezy is anomalous. Kofoid and Swezy (1921, pp. 356) reported in the section describing synonymy “Okamura has described minute linear or dotlike chromatophores, yellowish brown in color, in the forms he observed. There were not present in the individuals found at La Jolla and may possibly have been food bodies or oil droplets. The two forms correspond so closely in other respects that is seems inadvisable to separate them”. Kofoid and Swezy (1921) suggested that Okamura (1916) mistook the chloroplasts for food vacuoles or oil droplets.
Another difference between C. catenatum in Okamura and in Kofoid and Swezy is the position of the nucleus (Fig. 5J, L). The nucleus is spherical and anterior in species of Margalefidinium as illustrated by Okamura (1906). Other species currently under Cochlodinium (C. convolutum, C. helix) possesses a central nucleus, but it is more or less rectangular rather than rounded in shape (Table 1; Matsuoka et al., 2008; Reñé et al., 2013a,b). In contrast, figure GG14 in Kofoid and Swezy (1921) illustrated a spherical nucleus in a central position (Fig. 5L). Their single cell of C. catenatum showed an elongated shape (Fig. 5L) compared with cells in the chain (Fig. 5K). This a common feature observed before cell lysis (Fig. 3). This study also revealed that in some distorted cells of Margalefidinium polykrikoides prior to lysis, the nucleus was displaced to a central position (Fig. 3B–C). Consequently, the single cell shown in their figure GG14 may be a moribund distorted cell, with the nucleus artificially displaced.
All these features suggest that C. catenatum sensu Kofoid and Swezy is a species of Margalefidinium, and probably the same species described by Okamura (1916). The older descriptions and iconotypes are always subject to different interpretations (Fig. 5J–L). Consequently, this study follows the current accepted synonymy of these species as reported in Matsuoka et al. (2008) – i.e., to accept that C. polykrikoides and C. catenatum are not synonyms, to accept that C. catenatum has disappeared and its blooms in Japan have been replaced by those of C. polykrikoides, and to accept that the four-celled chain shown in Kofoid and Swezy (1921) does not belong to the genus Margalefidinium because it corresponds to a heterotrophic species, without eyespot and with a central nucleus. These topics cannot be resolved here, and this study only assigns a new generic name for C. polykrikoides and allied species.
4.3. Other members of Margalefidinium
Kofoid and Swezy (1921) described numerous species of the genus Cochlodinium, many of which were never again reported in the literature. Their descriptions were based on the observation of single or few cells, often moribund before cytolysis. Some taxa may correspond to chain-forming species, but the chains likely decomposed due to handling and manipulation, and consequently they were described as single-celled species. In some cases, it is not clear whether the cells were photosynthetic or not, because the chloroplasts or even the eyespot were mistaken for food vacuoles or oil droplets. This study defined the genus Margalefidinium for photosynthetic cells with an eyespot and an anterior spherical nucleus. The position of the nucleus is not always a stable diagnostic character, as it can vary even within the same strain of a dinoflagellate (Jeong et al., 2012). The presence and type of eyespot are more stable diagnostic characters. A highly elaborated eyespot is characteristic of the warnowiid dinoflagellates that form a monophyletic group (Gómez et al., 2009; Reñe et al., 2013b, 2015), and the type of eyespot is a stable diagnostic character used to differentiate among different clades of woloszynskioid dinoflagellates (Daugbjerg et al., 2014). At present, this study can only transfer into Margalefidinium the photosynthetic species of Cochlodinium with an eyespot and an anterior nucleus. These diagnostic characters could have been misidentified or unnoticed in original descriptions of other Cochlodinium species.
Kofoid and Swezy (1921) described Cochlodinium citron as a photosynthetic species with an anterior spherical nucleus, and the line drawings showed an eyespot in the periphery of the episome (Fig. 5M, Kofoid and Swezy’s text figure HH12). Margalefidinium citron was described with a cell length of 35–49 μm. It is larger than most of the cells of M. polykrikoides (which did not exceed 40 μm), and similar to M. fulvescens, which ranged between 37–57 μm (Iwataki et al., 2007), or Cochlodinium sp. AR-2015 (49 μm long) in Reñe et al. (2015, their figure 3b). Kofoid and Swezy (1921) reported Margalefidinium citron as a common species from off La Jolla, California. This species name has disappeared from the literature, while currently Margalefidinium fulvescens is a blooming species in Californian waters (Howard et al., 2012; Gárate-Lizárraga, 2014).
Kofoid (1931) described Cochlodinium flavum based on the observation of a single specimen collected from Mutsu Bay, northern Japan, in a seawater temperature of 25 °C (Fig. 5N). Kofoid described it as “radially arranged rhabdosomes, a crescentic reddish body; numerous discoidal, yellow chromatophores, peripherally located. Dimension: –Length, 32 μm; transdiameter, 20 μm. This specimen had a red granule of spherical form in the epicone”. The rhabdosomes for Kofoid correspond to elongated chloroplasts as in Torodinium Kofoid & Swezy (Gómez et al., 2016). Kofoid (1931) was unclear in the position of the eyespot because in the species diagnosis it is reported in the episome, while in the colored illustration the crescentic reddish body was in the hyposome (Kofoid, 1931; his plate II, fig. 13). The stressed specimen of C. flavum was surrounded by a hyaline layer (Fig. 5N). The formation of a hyaline cyst is more common in Cochlodinium species in the Ceratoperidinium clade (i.e., C. convolutum), but Margalefidinium polykrikoides also forms hyaline cysts (Kim et al., 2002; Matsuoka et al., 2008). The “crescentic reddish body” or the “red granule of spherical form” unequivocally corresponded to the eyespot that is a diagnostic character of Margalefidinium. Other features such the cell shape, chloroplasts, and nucleus corresponded to Margalefidinium as well. The species C. flavum is a member of Margalefidinium if the Kofoid’s illustration is upside down and the original species diagnosis is considered (Fig. 5N). The occurrence as a single specimen, probably from a decomposed chain, the formation of hyaline membrane, and the cell elongation suggests that the cell was stressed and beginning the cytolysis. The cell length of 32 μm is in the range of M. polykrikoides, also known from northern Japan (Shimada et al., 2016), and also of Margalefidinium fulvescens that was described from Japan (Iwataki et al., 2007).
5. Conclusions
The type species of Cochlodinium, C. strangulatum, is widespread in warm waters. Despite being one of the largest gymnodinioid dinoflagellates (200 μm long), it disappeared from the scientific literature because of fixation artifacts, and because it was probably mistaken for large cells of Gyrodinium. The early illustrations of C. strangulatum with a smooth cell surface (Kofoid and Swezy, 1921; Schiller, 1933) also contribute to the misidentifications.
The species Cochlodinium strangulatum is ecologically and morphologically similar to large cells of Gyrodinium spirale. Both taxa have similar cell shapes, with a central nucleus surrounded by a capsule, vacuoles and refractile bodies, and a cell surface with longitudinal striae that are finer in C. strangulatum. Despite low phylogenetic support, this study cannot discard that these genera derived from a common ancestor.
In the LSU rDNA phylogeny, the sequences of species currently classified as Cochlodinium branch into at least three distantly related clades.
The harmful species Cochlodinium polykrikoides and C. fulvescens, and an unidentified species branch together and are distantly related to C. strangulatum or any other known dinoflagellate genus.
The species Cochlodinium polykrikoides and C. fulvescens, and other photosynthetic species with an eyespot in the episome and an anterior nucleus (C. catenatum, C. citron, C. flavum) are placed in Margalefidinium gen. nov.
The species Cochlodinium catenatum as described by Okamura (1916) or by Kofoid and Swezy (1921), C. citron, and C. flavum could be senior synonyms of C. polykrikoides or C. fulvescens.
A third clade of Cochlodinium is comprised of C. convolutum and C. helix. They branch with species of Ceratoperidinium spp., and need to be placed under Ceratoperidinium or other generic name.
Supplementary Material
Video S1. Morphology and behavior of Cochlodinium strangulatum and M. polykrikoides, https://youtu.be/U-JzngXFgxc
Highlights.
The type species of Cochlodinium, C. strangulatum, is widespread, but was mistaken for large cells of Gyrodinium.
First molecular data of a Cochlodinium heterotrophic species, the generic type Cochlodinium strangulatum.
The morphology and molecular phylogeny of Cochlodinium polykrikoides is distantly related to the generic type.
New genus, Margalefidinium gen. nov., and combinations for Cochlodinium polykrikoides and allied species.
Acknowledgments
F.G. was supported by the Brazilian Conselho Nacional de Desenvolvimento Cientifíco e Tecnológico [grant number BJT 370646/2013–14], Support for M.L.R. and D.M.A. was provided through the Woods Hole Center for Oceans and Human Health, National Science Foundation [grant number OCE–1314642] and National Institute of Environmental Health Sciences [grant number 1–P01–ES021923–01]. This is a contribution to the ANR Biodiversity program [ANR BDIV 07 004–02 ‘Aquaparadox’]. We thank E. Otero-Morales and B.M. Soler for the lab facilities and hospitality extended during the sampling in Puerto Rico, and T. Sehein for assistance with laboratory analyses.
References
- Biecheler B, 1934. Sur une Dinoflagellé a capsule périnucleaire, Plectodinium, n. gen. nucleovolvatum, n. sp. et sur les relations des Peridiniens avec les Radiolaires. C. R Hebd. Séanc. Acad. Sci. Paris 198 (4), 404–406. [Google Scholar]
- Daugbjerg N, Hansen G, Larsen J, Moestrup Ø, 2000. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 39 (4), 302–317. [Google Scholar]
- Daugbjerg N, Andreasen T, Happel E, Pandeirada MS, Hansen G, Craveiro SC, Calado AJ, Moestrup Ø, 2014. Studies on woloszynskioid dinoflagellates VII. Description of Borghiella andersenii sp. nov.: light and electron microscopy and phylogeny based on LSU rDNA. Eur. J. Phycol 49 (4), 436–449. [Google Scholar]
- Edgar RC, 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32 (5), 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate-Lizárraga I, López-Cortes DJ, Bustillos-Guzmán JJ, Hernández-Sandoval F, 2004. Blooms of Cochlodinium polykrikoides (Gymnodiniaceae) in the Gulf of California, Mexico. Rev. Biol. Trop. 52 (1), 51–58. [PubMed] [Google Scholar]
- Gárate-Lizárraga I, 2014. Occurrence of Cochlodinium fulvescens (Gymnodiniales: Dinophyceae) in the southwestern Gulf of California. Rev. Biol. Mar. Oceanogr 49 (1), 123–127. [Google Scholar]
- Gómez F, 2012. A checklist and classification of living dinoflagellates (Dinoflagellata, Alveolata). CICIMAR Océanides 27 (1), 65–140. [Google Scholar]
- Gómez F, López-García P, Moreira D, 2009. Molecular phylogeny of the ocelloid-bearing dinoflagellates Erythropsidinium and Warnowia (Warnowiaceae, Dinophyceae). J. Eukaryot. Microbiol 56 (5), 440–445. [DOI] [PubMed] [Google Scholar]
- Gómez F, López-García P, Takayama H, Moreira D, 2015. Balechina and the new genus Cucumeridinium gen. nov. (Dinophyceae), unarmoured dinoflagellates with cell coverings. J. Phycol 51 (6), 1088–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez F, Takayama H, Moreira D, López-García P, 2016. Unarmoured dinoflagellates with small hyposome: Torodinium and Lebouridinium gen. nov. for Katodinium glaucum (Gymnodiniales, Dinophyceae). Eur. J. Phycol 51 (2), 226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O, 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol 59 (3), 307–321. [DOI] [PubMed] [Google Scholar]
- Hansen G, Daugbjerg N, 2004. Ultrastructure of Gyrodinium spirale, the type species of Gyrodinium (Dinophyceae), including a phylogeny of G. dominans, G. rubrum and G. spirale deduced from partial LSU rDNA sequences. Protist 155 (3), 271–294. [DOI] [PubMed] [Google Scholar]
- Howard MD, Jones AC, Schnetzer A, Countway PD, Tomas CR, Kudela RM, Hayashi K, Chia P, Caron DA, 2012. Quantitative real-time polymerase chain reaction for Cochlodinium fulvescens (Dinophyceae), a harmful dinoflagellate from California coastal waters. J. Phycol 48 (2), 384–393. [DOI] [PubMed] [Google Scholar]
- Iwataki M, Kawami H, Matsuoka K, 2007. Cochlodinium fulvescens sp. nov. (Gymnodiniales, Dinophyceae), a new chain-forming unarmored dinoflagellate from Asian coasts. Phycol. Res 55 (3), 231–239. [Google Scholar]
- Iwataki M, Kawami H, Mizushima K, Mikulski CM, Doucette GJ, Relox JR Jr, Anton A, Fukuyo Y, Matsuoka K, 2008. Phylogenetic relationships in the harmful dinoflagellate Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) inferred from LSU rDNA sequences. Harmful Algae 7 (3), 271–277. [Google Scholar]
- Iwataki M, Hansen G, Moestrup Ø, Matsuoka K, 2010. Ultrastructure of the harmful unarmored dinoflagellate Cochlodinium polykrikoides (Dinophyceae) with reference to the apical groove and flagellar apparatus. J. Eukaryot. Microbiol 57 (4), 308–321. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Yoo YD, Kim JS, Kim TH, Kim JH, Kang NS, Yih W, 2004. Mixotrophy in the phototrophic harmful alga Cochlodinium polykrikoides (Dinophycean): Prey Species, the effects of prey concentration, and grazing impact. J. Eukaryot. Microbiol 51 (5), 563–569. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Jang SH, Kang NS, Yoo YD, Kim MJ, Lee KH, Yoon EY, Potvin E, Hwang YJ, Kim JI, Seong KA, 2012. Molecular characterization and morphology of the photosynthetic dinoflagellate Bysmatrum caponii from two solar saltons in western Korea. Ocean Sci. J 47 (1), 1–18. [Google Scholar]
- Kim C-H, Cho H-J, Shin J-B, Moon C-H, Matsuoka K, 2002. Regeneration from hyaline cysts of Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae), a red tide organism along the Korean coast. Phycologia 41 (6), 667–669. [Google Scholar]
- Kofoid CA, Swezy O, 1921. The free-living unarmored Dinoflagellata. Mem. Univ. California 5, 1–564. [Google Scholar]
- Kofoid CA, 1931. Report on the Biological Survey of Mutsu Bay. 18. Protozoan Fauna of Mutsu Bay. Subclass Dinoflagellata; Tribe Gymnodininoidae. Sci. Rep. Tohoku Imp. Univ., 4th ser., Biol. 6, 1–43. [Google Scholar]
- Kudela RM, Gobler C, 2012. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14 (1), 71–86. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG, 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23 (21), 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lins da Silva NM, 1991. Étude de la répartition spatio-temporelle des peuplements microbiens planctoniques en Mer Ligure (Méditerranée Occidental). Ph.D. Thesis, Université de P. et M. Curie, Paris. [Google Scholar]
- Margalef R, 1961. Hidrografia y fitoplancton de un area marina de la costa meridional de Puerto Rico. Invest. Pesq 18 (1), 33–96. [Google Scholar]
- Matsuoka K, Iwataki M, Kawami H, 2008. Morphology and taxonomy of chain-forming species of the genus Cochlodinium (Dinophyceae). Harmful Algae 7 (3), 261–270. [Google Scholar]
- Okamura K, 1916. Akashio ni Tsuite (On red-tides). Suisan Koushu Sikenjo Kenkyu Hokoku 12, 26–41. [Google Scholar]
- Qiu D, Huang L, Liu S, Zhang H, Lin S, 2013. Apical groove type and molecular phylogeny suggests reclassification of Cochlodinium geminatum as Polykrikos geminatum. PLoS ONE 8 (8), e71346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D, Crandall KA, 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14 (9), 817–818. [DOI] [PubMed] [Google Scholar]
- Reñé A, Garcés E, Camp J, 2013a. Phylogenetic relationships of Cochlodinium polykrikoides Margalef (Gymnodiniales, Dinophyceae) from the Mediterranean Sea and the implications of its global biogeography. Harmful Algae 25 (1), 39–46. [Google Scholar]
- Reñé A, de Salas M, Camp J, Balagué V, Garcés E, 2013b. A new clade, based on LSU rDNA sequences, of unarmoured dinoflagellates. Protist 164 (5), 673–685. [DOI] [PubMed] [Google Scholar]
- Reñé A, Camp J, Garcés E, 2015. Diversity and phylogeny of Gymnodiniales (Dinophyceae) from the NW Mediterranean Sea revealed by a morphological and molecular approach. Protist 166 (2), 234–263. [DOI] [PubMed] [Google Scholar]
- Richlen ML, Barber PH, 2005. A technique for the rapid extraction of microalgal DNA from single live and preserved cells. Mol. Ecol. Notes 5 (3), 688–691. [Google Scholar]
- Richlen ML, Morton SL, Jamali EA, Rajan A, Anderson DM, 2010. The catastrophic 2008-2009 red tide in the Arabian Gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9 (2), 163–172. [Google Scholar]
- Ronquist F, Huelsenbeck JP, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 (12), 1572–1574. [DOI] [PubMed] [Google Scholar]
- Schiller J, 1933. Dinoflagellatae (Peridineae) in monographischer Behandlung In: Kolkwitz R (Ed.), Rabenhorst’s Kryptogamen Flora Von Deutschland, Österreich und der Schweiz. Lieferung 3. Akademische Verlag., Leipzig, pp. 433–617. [Google Scholar]
- Scholin CA, Herzog M, Sogin M, Anderson DM, 1994. Identification of group specific and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae): II. Sequence analysis of a fragment of the LSU ribosomal RNA gene. J. Phycol 30 (6), 999–1011. [Google Scholar]
- Schütt F, 1895. Die Peridineen der Plankton-Expedition. I. Theil Studien Über die Zellen der Peridineen. Ergebnisse der Plankton-Expedition der Humboldt-Stiftung, IV. M.a.A. Lipsius & Tischler, Kiel, Leipzig. [Google Scholar]
- Schütt F, 1896. Peridiniales In: Engler A, Prantl K (Eds), Die Natürlichen Pflanzenfamilien. I. Teil. Abt. 1b. W. Engelmann, Leipzig, pp. 1–30. [Google Scholar]
- Shimada H, Sakamoto S, Yamaguchi M, Imai I, 2016. First record of two warm-water HAB species Chattonella marina (Raphidophyceae) and Cochlodinium polykrikoides (Dinophyceae) on the west coast of Hokkaido, northern Japan in summer 2014. Reg. Stud. Mar. Sci 7, 111–117. [Google Scholar]
- Skolka VH, Bodeanu N, Roban A, 1986. Contributions to the systematic study of Mediterranean phytoplankton along the Libyan coasts. Cercetari Marine/Recherche Marine, Constanta 19, 23–54. [Google Scholar]
- Sournia A, 1986. Atlas du phytoplancton marin Volume I: Cyanophycées, Dictyochophycées, Dinophycées, Raphidophycées. CNRS, Paris. [Google Scholar]
- Steidinger KA, Tangen K, 1997. Dinoflagellates In: Tomas CR (Ed.), Identifying Marine Phytoplankton. Academic Press, San Diego, pp. 387–584. [Google Scholar]
- Takayama H, 1998. Morphological and taxonomical studies of the free-living unarmored dinoflagellates occurring in the Seto Island Sea and adjacent waters. Ph.D. Thesis. University of Tokyo, Tokyo. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 (22), 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PS, Metzqer DA, Higuchi R, 1991. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10 (4), 506–512. [PubMed] [Google Scholar]
- Wood EJF, 1968. Dinoflagellates of the Caribbean Sea and adjacent areas. University of Miami Press, Coral Gables, Florida. [Google Scholar]
- Wulff A, 1916. Über das Kleinplankton der Barentssee. Wiss. Meeresunters. Helgoland NF 13 (1), 95–125. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Morphology and behavior of Cochlodinium strangulatum and M. polykrikoides, https://youtu.be/U-JzngXFgxc


