Abstract
Rationale: Recent studies confirmed that osteoarthritis (OA) is associated with systemic inflammation. Adipose-derived stromal cells (ASCs) could become the most promising cell-based therapy in OA, based not only on their differentiation capacities and trophic and paracrine effects on the existing cartilage, but also on their immunomodulatory properties. Here, we wanted to determine the biological effect of autologous ASC intra-articular (IA) injection.
Method: To this aim, we monitored the profile of immune cells in fresh peripheral blood after IA injection of autologous ASCs in the knee of 18 patients with severe OA (ADIPOA phase I study). Specifically, we used 8-color flow cytometry antibody panels to characterize the frequencies of innate and adaptive immune cell subsets (monocytes, dendritic cells, regulatory T cells and B cells) in blood samples at baseline (before injection) and one week, one month and three months after ASC injection.
Results: We found that the percentage of CD4+CD25highCD127lowFOXP3+ regulatory T cells was significantly increased at 1 month after ASC injection, and this effect persisted for at least 3 months. Moreover, CD24highCD38high transitional B cells also were increased, whereas the percentage of classical CD14+ monocytes was decreased, at 3 months after ASC injection. These results suggest a global switch toward regulatory immune cells following IA injection of ASCs, underscoring the safety of ASC-based therapy. We did not find any correlation between the scores for the Visual Analogic Scale for pain, the Western Ontario and McMaster Universities Osteoarthritis Index (pain subscale and total score) at baseline and the immune cell profile changes, but this could be due to the small number of analyzed patients.
Conclusion: ASCs may drive an immediate local response by releasing paracrine factors and cytokines, and our results suggest that ASCs could also initiate a cascade resulting in a long-lasting systemic immune modulation.
Keywords: osteoarthritis, adipose-derived stromal cells, mesenchymal stromal cells, immune monitoring, multiparametric flow cytometry, immunomodulation
Introduction
Osteoarthritis (OA), the most prevalent form of arthritis, affects up to 15% of the adult population. This joint disease is characterized by degradation of the articular cartilage, synovitis and osteoclast activation in the subchondral bone 1. Chronic, low-grade inflammation contributes to the symptoms and to disease progression. Indeed, recent studies suggest that OA is not only a local disease of the joint(s), and that is also associated with systemic disorders, such as inflammation, metabolic dysregulation and obesity 2,3. For instance, the knee cartilage volume is negatively correlated with the concentration of circulating inflammatory cytokines, such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α) and C-reactive protein (CRP). Additionally, some inflammatory mediators (chemokines, and cytokines, such as IL-1β and alarmins) affect chondrocyte viability and enhance chondrocyte hypertrophy. A moderate increase of CRP and of some inflammatory mediators (PGE2, IL-1, IL-6) could play a key role in the development of complications in patients with OA, such as cardiovascular damage 4. Indeed, patients with OA have a higher overall mortality rate compared with the general population, mainly due to increased cardiovascular events 5. For all these reasons, OA should be considered not only as a local articular disease, but as a systemic disease.
Mesenchymal stromal or stem cells (MSCs) are multipotent cells that can be isolated from a variety of adult and neonatal tissues, such as bone marrow (BM-MSCs), fat tissue (adipose stem cells, ASCs), placenta and umbilical cord. MSC cell-based therapy in OA is justified by their differentiation capacities and also their paracrine and immunosuppressive properties. MSCs can regulate immune responses and are used to alleviate immune disorders 6. In vitro and in vivo preclinical experiments suggest that MSCs can regulate the activity of many immune cell types, such as T cells, B cells, dendritic cells (DC), macrophages, neutrophils, and natural killer cells 7-11. In clinical settings, MSC-based therapies have been successfully used to reverse graft-versus-host disease (GvHD) in patients receiving allogeneic bone marrow transplantation 12-15. More recently, it has been reported that MSCs can suppress inflammation and reduce tissue damage through the induction of regulatory T (Treg) cells in patients with autoimmune diseases, such as systemic lupus erythematosus 16,17 and Crohn disease 18-20.
At the beginning, it was thought that MSC beneficial effects were mainly explained by their engraftment and tissue regeneration; however, it is now widely accepted that the main MSC therapeutic effects are mediated primarily through the short-term secretion of trophic factors that reduce inflammation and modulate immune cells. Despite a large body of in vitro experimental studies on MSC effects on immune cells, little is known about the biological mechanisms underlying MSC-mediated inhibition of the immune response in vivo.
The goal of this study was to determine the impact of MSC-based therapy on the circulating immune cell profile. To this aim, we used multiparametric flow cytometry to prospectively quantify the different immune cells in fresh whole blood samples collected before and at different time points after therapy. Blood samples were from the 18 patients with severe OA who took part in the ADIPOA Phase I clinical trial. These patients received one intra-articular (IA) injection of autologous ASCs and were then followed for 6 months. This ASC-based therapy showed a good safety profile with preliminary evidence of efficacy on OA-related symptoms 21.
Materials and Methods
Patients and healthy controls
The ADIPOA1 study design was previously described 22. Briefly, it was a phase I, prospective, bi-center (Montpellier University Hospital Center, France, and Orthopedic Department of Würzburg University, Germany), single-arm, open-label study conducted between March 2012 and April 2014. Eighteen consecutive patients with symptomatic, severe knee OA were enrolled. The study was performed without a control arm and involved three different groups (6 patients/each) who received one single IA injection of autologous ASCs at low (2 x 106 cells), medium (10 x 106 cells), or high dose (50 x 106 cells). Autologous ASCs were prepared by a single Good manufacturing practice-certified facility (Etablissement Français du Sang Midi-Pyrénées, France) as described elsewhere 23. Peripheral blood samples were collected during the scheduled visits at baseline (D0), and then one week (D7), one month (M1) and three months (M3) after ASC injection. Pain, stiffness and physical functions before/after treatment were monitored with different scales during the trial, among which the Western Ontario and McMaster University Arthritis Index (WOMAC) 24 and a Visual analog scale (VAS) for pain. The WOMAC total score and pain sub-score and the VAS score were used in the present study for the correlation analyses.
Patients were classified in responders (n=4) and non-responders (n=12) to the ASC-based treatment using the responder criteria index of the OsteoArthritis Research Society International)/ Outcome Measures in Rheumatology (OARSI-OMERACT) 25. Fresh peripheral blood was obtained from healthy age and sex matched volunteers with no history or current pain due to OA (n=6).
Reagents
The FITC-conjugated anti-CD64 (10.1), phycoerythrin (PE)-conjugated anti-CD33 (WM53), PerCP-Cy5.5-conjugated anti-HLA-DR (L243), PE-CyTM7-conjugated anti-CD56 (B159), PE-CyTM7-conjugated anti-CD14 (M5E2), allophycocyanine (APC)-conjugated anti-CD32 (FL18.26), APC-H7-conjugated anti-CD4 (RPA-T4), APC-H7-conjugated anti-CD16 (3G8), V450-conjugated anti-CD3 (UCHT1), V500-conjugated anti-CD45 (HI30), PerCP-CyTM5.5- conjugated anti-CD123 (7G3), PE-CyTM7-conjugated anti-CD45RO (UCHL1), APC-H7- conjugated anti-HLA-DR (L243), V450-conjugated anti-CD11c (B-ly6), V500-conjugated anti-CD4 (RPA-T4), FITC-conjugated anti-CD24 (ML5), PE-conjugated anti-IgD (IA6-2), PerCP-Cy5.5-conjugated anti-CD138 (MI15), PE-CyTM7-conjugated anti-CD38 (HIT2), APC-conjugated anti-CD27 (M-T271), APC-H7-conjugated anti-CD20 (L27), V450-conjugated anti-CD5 (L17F12), AmCyan-conjugated anti-CD19 (SJ25C1), FITC-conjugated anti-CD62L (DREG-56), PE-conjugated anti-CD127 (HIL-7R-M21), PerCP-CyTM5.5-conjugated anti-CD25 (M-A251), V450-conjugated anti-CD4 (RPA-T4), APC-conjugated anti-CD152 (BNI3) antibodies and FACS Lysing solution were purchased from BD Biosciences. The PE-conjugated anti-CD314 (BAT221), PE-conjugated anti-CD1c (AD5-8E7) and APC-conjugated anti-CD303 (AC144) antibodies were purchased from Miltenyi Biotech. The FITC-conjugated anti-human lineage cocktail 1 (lin1) (CD3 clone SK7, CD14 clone Mop9, CD16 clone 3G8, CD19 clone SJ25C1, CD20 clone L27, CD56 clone NCAM16.2) was from Beckmann Coulter and the PE-Cyanine7-conjugated anti-FOXP3 (PCH101) antibody was from eBioscience.
Flow Cytometric Analysis
Samples were incubated with antibodies within 20 hours and more precisely within 4 hours for Montpellier's patients and 20 hours for Würzburg's patients, after blood collection. The characterization of monocytes, DCs, T and B cells were performed on whole blood cells and freshly prepared peripheral blood mononuclear cells (PBMC) were used for the identification of FOXP3+ Treg cells. All the frequencies analyzed in our study were found to be stable over the 20 hours since no significant variation was observed between patients from the 2 clinical centers. Cells in fresh EDTA-treated whole blood samples were labeled using three 8-color flow cytometry panels of conjugated antibodies to quantify the different subsets of monocytes (CD64, CD33, CD314, HLA-DR, CD56, CD14, CD32, CD4, CD16, CD3, CD45), DCs (lin1, CD1c, CD123, CD45RO, CD303, HLA-DR, CD11c, CD4) and B cells (CD24, IgD, CD138, CD38, CD27, CD20, CD5, CD19). All fluorochrome-conjugated antibodies were used according to the manufacturer's recommendations. Following incubation with antibodies, red blood cells were lysed and cells were washed twice (PBS containing 2% fetal calf serum and 0.1% sodium azide) prior to acquisition on a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). In parallel, PBMCs were isolated through a Ficoll Hypaque gradient. Then, 0.5 million of PBMCs were incubated with antibodies against cell surface markers (CD62L, CD127, CD25, HLA-DR, CD4, CD45) for the characterization of Treg cells. Intracellular CD152 and FOXP3 staining was performed after cell fixation and permeabilization using the FoxP3 Staining Buffer Set (eBioscience). Application settings were used to standardize the flow cytometer during the clinical trial. All data acquired with one cytometry panel were analyzed by the same analyst using the BD FACSDiva software. Representative dot plots are shown using the FlowJo software (TreeStar).
Statistical analysis
A general description of the population under study was performed using frequencies for qualitative variables and means with standard deviation and minimal and maximal values for quantitative variables. Analyses of the quantitative changes in the immune cell populations according to the responder criteria index of the OARSI-OMERACT was analyzed using the Mann Whitney test or one-way ANOVA, as applicable. Unilinear correlations were used to examine the link between clinical symptoms (WOMAC and VAS pain scores) and immune status at baseline, and also to correlate changes in clinical symptoms and immune subpopulations during the follow-up. Pearson correlation coefficients were obtained assuming a Gaussian distribution of the data. Responders and non-responders were compared using the Student's t test. All FACS analysis data are presented as the mean ± standard error of the mean. The effect of ASC injection on the various immune cell subsets was analyzed using Wilcoxon matched-pairs test and the significance level was set at 5% for all tests. Analyses were performed with Prism version 6.0c (GraphPad Software Inc., La Jolla, CA, USA).
Results
Changes in Peripheral Innate Immune Cells after ASC Injection in the Knee
As the handling of blood samples has a large impact on cytometry data and based on the experience of other consortia, such as EuroFlow 26, Human Immunology Project 27, and Milieu Interieur 28, cytometry experiment was performed using fresh whole blood samples to avoid variability induced by freeze/thaw cycles, especially in monocyte and DC populations.
For DC characterization, cells were plotted according to their size and granularity followed by doublet exclusion. Lineage-negative cells were first selected, and then CD4+HLA-DR+ double positive cells were gated to detect the two major DC subsets: the myeloid subset (mDC: HLA-DR++CD11c++CD1c+CD123low) and the plasmacytoid subset (pDC: HLA-DR++CD11clowCD123high) (Figures 1A-C). The phenotype of both populations was confirmed by labeling with anti-CD303 and -CD45RO and -HLA-DR antibodies (Figures 1D-F). The percentage of the mDC (52.2 ± 2.1% at day 0 and 49.8 ± 2.2% at 3 months) and pDC subsets (35.2 ± 2.12% at day 0 and 36.3 ± 2.7% at 3 months) was not affected by ASC injection (Figures 1G, 1H). These results emphasized the reproducibility of our experimental procedure for immune cell quantification and suggest that no alteration of major DC subsets could be monitored following autologous ASCs injection.
Figure 1.
Circulating DC subsets are not affected by ASC injection in the knee. Gating strategy and representative dot plots to identify CD4+HLA-DR+ cells (A), CD123+ plasmacytoid DCs (pDCs) (B), and CD1c+ myeloid DCs (mDCs) (C). Histograms showing the expression levels of HLA-DR (D), CD303 (E) and CD45RO (F) in pDCs (red) and mDCs (blue). Percentage of CD123+ pDCs (G) and CD1c+ mDCs (H) within the HLA-DR+ CD4+ cell population at day 0 (D0, circles), day 7 (D7, squares), 1 month (M1, triangles) and 3 months (M3, inverted triangles) after injection of different amounts of ASCs (red = 2x106 cells, green= 10x106 cells, blue 50x106 cells) in individual patients with severe OA.
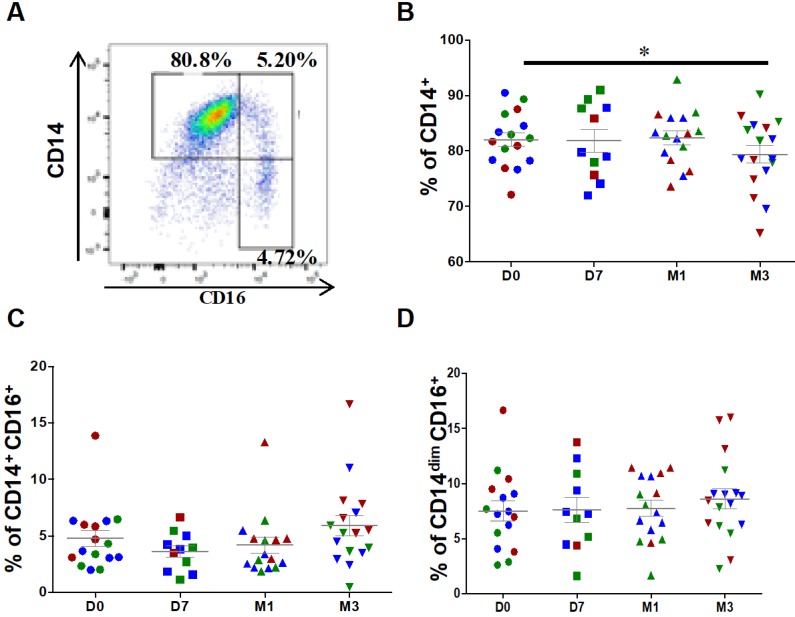
For monocyte analysis, debris and doublets were excluded followed by selection of CD45+ leucocytes. Contaminating neutrophils, granulocytes and T cells were excluded from CD45+ monocytic cells. At baseline (before injection), classical (CD14++CD16-), intermediate (CD14+CD16+) and patrolling (also known as non-classical) monocytes (CD14dimCD16+) represented, on average, 82.1 ± 1.2%, 4.8 ± 0.7% and 7.5 ± 0.8%, respectively, of all monocytes (Figure 2A). After ASC injection, the percentage of classical monocytes was significantly reduced (82.1 ± 1.2% at day 0 versus 79.3 ± 1.6% at 3 months, P=0.0335), but not that of intermediate (4.8 ± 0.7% at day 0 versus 5.9 ± 0.9% at 3 months, P=0.0654) and patrolling monocytes (7.5 ± 0.9% at day 0 versus 8.6 ± 0.9% at 3 months, P=0.1167) (Figures 2B-D). This result suggests that ASC injection slightly modulates the distribution of monocyte subsets with a higher impact on classical monocyte.
Figure 2.
Changes in the percentages of classical and intermediate monocytes after injection of ASCs. (A) Representative image of a dot plot to identify the three monocytes subsets with the corresponding percentage. Quantification of CD14+ classical monocytes (B), CD14+CD16+ intermediate monocytes (C), and CD14dimCD16+ patrolling monocytes (D) at day 0 (D0, circles), day 7 (D7, squares), 1 month (M1, triangles) and 3 months (M3, inverted triangles) after injection of different amounts of ASCs (red = 2x106 cells, green= 10x106 cells, blue 50x106 cells) in individual patients with severe OA; *p<0.05.
The classification of monocytes in three groups has shifted attention to the mobilization of non-classical monocytes during inflammatory diseases and on their inflammatory characteristics, particularly the secretion of important inflammatory cytokines (i.e., TNF-α), and supports a more significant role for intermediate monocytes in inflammation 29. In agreement, in our 18 patients, the percentage of classical monocytes was inversely correlated with CRP level (r = -0.39; 95% CI -0.59; -0.14; p=0.0029). Conversely, the percentages of intermediate monocytes (r = 0.46; 95% CI 0.22; 0.64; p=0.0003), and to a lower extent, of patrolling monocytes (r = 0.50; 95% CI -0.01; 0.80; p=0.049) were positively correlated with CRP levels.
Changes in Peripheral Adaptive Immune Cells after ASC Injection in the Knee
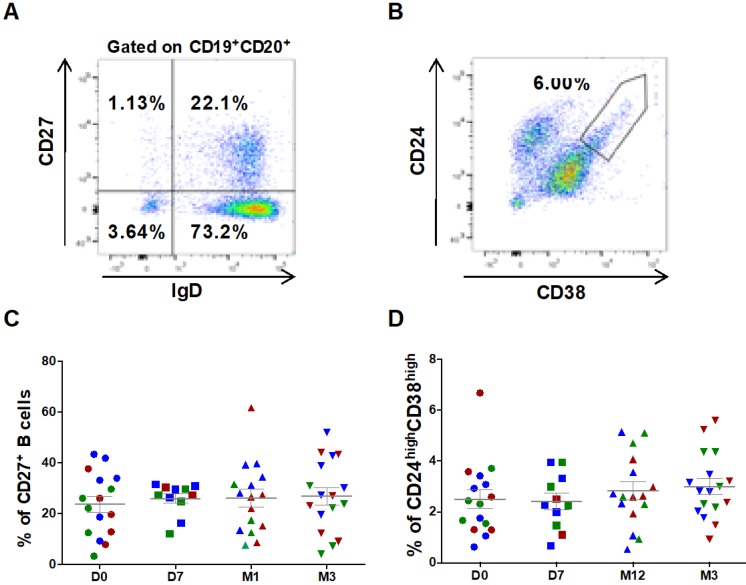
B cell characterization was performed using whole blood cell samples. Cells were plotted according to their size and granularity followed by doublets exclusion. Within the CD19+CD20+ B cell population, CD27 and IgD were used to identify switched (CD27+IgD-) and un-switched memory B cells (CD27+IgD+) (Figure 3A). ASC injection did not significantly change the percentage of CD27+ (23.6 ± 3.1% at day 0 versus 26.9 ± 3.4% at 3 months, Figure 3C), CD27+ IgD- switched (16.1±2.6% at day 0 versus 18.1±3.1% at 3 months, not significant) and CD27+IgD+ un-switched memory B cells. Similarly, within the subset of CD27- cells, no variation in the percentage of naïve B cells (CD27-IgD+) was observed (90.5 ± 1.5 % at day 0 versus 90.5 ± 2.0% at 3 months, not significant). Conversely, transitional B cells (CD24highCD38high, Figure 3B), slightly increased after ASC injection (2.5 ± 0.4% at day 0 versus 3.0 ± 0.3% at 3 months, not significant) (Figure 3D).
Figure 3.
Circulating B cell subsets are not modified by ASC injection in the knee. Representative dot plots to identify switched and un-switched CD27+ memory B cells (A), and CD24highCD38high transitional B cells (B). The percentage of CD27+ memory B cells (C) and CD24highCD38high transitional B cells (D) at day 0 (D0, circles), day 7 (D7, squares), 1 month (M1, triangles) and 3 months (M3, inverted triangles) after injection of different amounts of ASCs (red = 2x106 cells, green= 10x106 cells, blue 50x106 cells) in individual patients with severe OA.
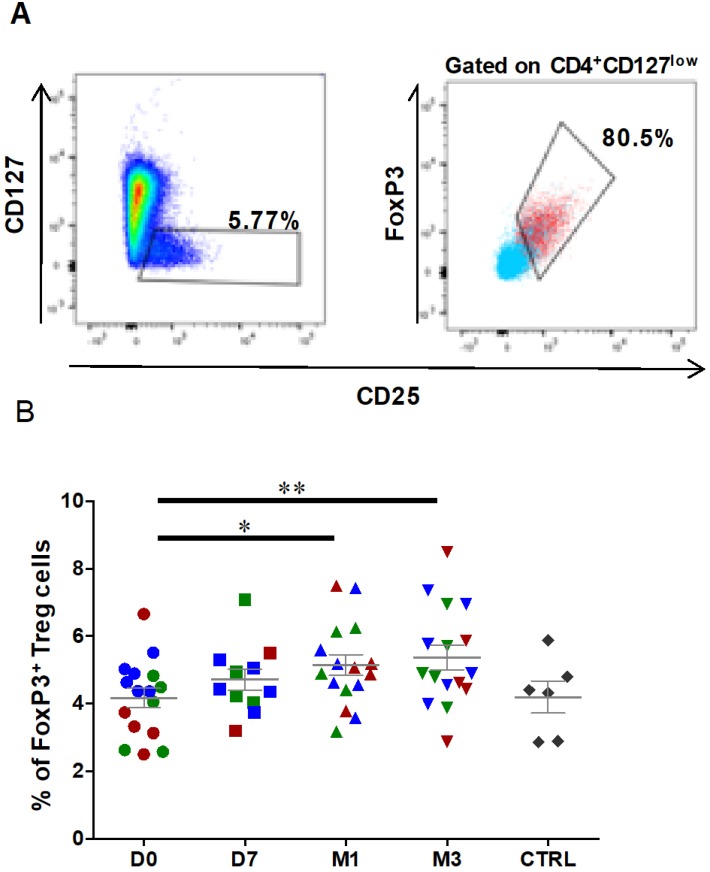
Treg characterization was performed using freshly prepared PBMCs. First, lymphocytes were gated according to their size and granularity followed by doublet exclusion. Then, CD45+ lymphocytes were selected based on CD4 expression, and Treg cells were defined as the FOXP3+ subset among all CD25highCD127low cells within the CD4+ population (Figures 4A). The percentage of CD25highCD127lowFOXP3+ Treg cells was significantly increased at 1 month after ASC injection (4.2 ± 0.3% at day 0 to 5.1 ± 0.3% at 1 month, P=0.0151), and this effect was still observed at 3 months post-treatment (5.4 ± 0.4% at 3 months, P=0.0054) (Figure 4B). Analysis of the fraction of CD25highCD127lowFOXP3+ Treg cells in healthy controls (4.2±0.5% for controls, n=6) did not show any significant difference with OA patients. Therefore, Treg cells are significantly increased in OA patients after one single ASC injection in the knee, and this effect persists at least for 3 months.
Figure 4.
The percentage of circulating regulatory T cells is increased after ASC injection in the knee. Representative dot plots to identify CD127lowCD25+ cells (left), and CD25+FOXP3+ cells (right) within the CD4+ cell population. Gated CD4+ cells are in blue, and gated CD127lowCD25+ cells in red. (B) Frequencies of Treg cells (CD127lowCD25+ FOXP3+) within the CD4+ population at day 0 (D0, circles), day 7 (D7, squares), 1 month (M1, triangles) and 3 months (M3, inverted triangles) after injection of different amounts of ASCs (red = 2x106 cells, green= 10x106 cells, blue 50x106 cells) in individual patients with severe OA. The percentage of Treg cells in age- and sex-matched healthy controls (CTRL) are represented in grey; *p<0.05 and **p<0.01.
Correlations between Clinical Responses and Frequencies of Immune Cells Subsets
During the ADIPOA1 trial, the different ASC concentrations all led to symptom decrease; however, only patients in the lowest dose group reported a significant improvement in pain and physical functions compared with baseline, up to 6 month after ASC injection 22. To examine whether pain and physical function, were correlated with a specific immune cell signature at baseline, multiple regression analyses were performed using the baseline scores of the VAS pain, WOMAC pain subscale, WOMAC total score, and the baseline values for the Treg, monocyte, and B cell populations (Table 1). No significant correlation between clinical and cellular data was obtained. Similarly, no correlation was found between changes in the pain (VAS) and functional (WOMAC) scores and changes in the different cell subsets between baseline and month 3 post-injection (Table 2). Moreover, analysis of the quantitative changes in the immune cell populations according to the responder criteria index of OARSI-OMERACT revealed no association between the clinical response at month 3 post-ASC injection and Treg changes. Finally, analysis of the relative changes in the immune subsets in function of the number of injected ASCs (low, medium and high dose) also did not highlight any difference (data not shown).
Table 1.
Correlation between clinical features and immune cell subsets at baseline
| Correlation (r) | 95% CI | p | |
|---|---|---|---|
| WOMAC Total | |||
| vs. FOXP3+ | 0.32 | -0.23; 0.71 | 0.24 |
| vs. Tregs | -0.05 | -0.54; 0.47 | 0.85 |
| vs. Classical monocytes | 0.06 | -0.46; 0.55 | 0.82 |
| vs. Intermediate monocytes | -0.39 | -0.74; 0.13 | 0.12 |
| vs. Transitional B cells | 0.19 | -0.35; 0.64 | 0.19 |
| WOMAC Pain | |||
| vs. FOXP3+ | 0.25 | -0.30; 0.67 | 0.35 |
| vs. Tregs | -0.29 | -0.70; 0.26 | 0.28 |
| vs. Classical monocytes | 0.03 | -0.49; 0.53 | 0.92 |
| vs. Intermediate monocytes | -0.29 | -0.68; 0.24 | 0.26 |
| vs. Transitional B cells | 0.43 | -0.08; 0.76 | 0.09 |
| VAS Pain | |||
| vs. foxp3+ | 0.16 | -0.37; 0.62 | 0.54 |
| vs. Tregs | -0.09 | -0.57; 0.43 | 0.73 |
| vs. Classical monocytes | 0.01 | -0.50; 0.51 | 0.97 |
| vs. Intermediate monocytes | -0.39 | -0.74; 0.13 | 0.13 |
| vs. Transitional B cells | 0.19 | -0.35; 0.64 | 0.48 |
VAS: visual analog scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Table 2.
Correlation between absolute changes in clinical features and immune cell subsets between baseline (D0) and 3 months (M3) post-injection
| Correlation (r) | 95% CI | p | |
|---|---|---|---|
| ∆ WOMAC Total (M3-D0) | |||
| vs. ∆ FOXP3+ | 0.33 | -0.21; 0.72 | 0.21 |
| vs. ∆ Tregs | 0.48 | -0.04; 0.80 | 0.06 |
| vs. ∆ Classical monocytes | 0.07 | -0.46; 0.55 | 0.81 |
| vs. ∆ Intermediate monocytes | -0.01 | -0.51; 0.50 | 0.98 |
| vs. ∆ Transitional B cells | -0.25 | -0.67; 0.23 | 0.35 |
| ∆ WOMAC Pain (M3-D0) | |||
| vs. ∆ FOXP3+ | 0.21 | -0.33; 0.65 | 0.44 |
| vs. ∆ Tregs | 0.36 | -0.18; 0.73 | 0.17 |
| vs. ∆ Classical monocytes | 0.27 | -0.27; 0.69 | 0.30 |
| vs. ∆ Intermediate monocytes | -0.16 | -0.62; 0.38 | 0.55 |
| vs. ∆ Transitional B cells | -0.16 | -0.62; 0.38 | 0.55 |
| ∆ VAS Pain (M3-D0) | |||
| vs. ∆ FOXP3+ | 0.05 | -0.47; 0.55 | 0.85 |
| vs. ∆ Tregs | 0.21 | -0.34; 0.65 | 0.44 |
| vs. ∆ Classical monocytes | -0.02 | -0.52; 0.49 | 0.95 |
| vs. ∆ Intermediate monocytes | -0.33 | -0.70; 0.18 | 0.18 |
| vs. ∆ Transitional B cells | -0.07 | -0.54; 0.44 | 0.79 |
∆: absolute change between baseline and month 3; VAS: visual analog scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Discussion
Our study on the immune monitoring of 18 patients with severe OA after local treatment by autologous ASC injection in the knee found slight but significant changes in circulating immune cells, suggesting a systemic effect that contributed to the good tolerance of this MSC-based therapy. Specifically, the significant increase in the fraction of Treg and the significant reduction of the percentage of classical CD14+ monocytes at 3 months post-IA injection suggest a long-lasting immune modulation, and a global switch toward a more regulatory immune cell profile. These significant changes are also associated with a slight increase in the transitional B cells population which show regulatory capacity 30. No such modification of the frequencies of these 3 populations of interest were monitored within 1 month in untreated OA patients, suggesting that the observed significant increase in Treg and significant decrease in classical monocytes are not due to the normal evolution of the disease (personal unpublished data).
It is thought that ASCs act as coordinators of the immune system by providing a tolerogenic environment for both the innate and the adaptive immune response 34. Emerging evidence has demonstrated the impact of ASCs on key cell types involved in the continuum between innate and adaptive immunity, and in modulating inflammation in acute organ injury, such as acute GvHD, organ transplantation, diabetes, multiple sclerosis and Crohn's disease. However, only few studies have monitored immune cell changes by flow cytometry after MSC-based therapy, particularly local treatment. BM-MSCs strongly inhibit the in vivo activation of T lymphocytes that are particularly involved in organ rejection. For example, BM-MSCs decrease the Th1 response in patients with GvHD 35 or autoimmune diseases, such as systemic lupus erythematosus (SLE). In patients with severe and treatment-refractory SLE, umbilical cord MSC transplantation resulted in a significantly increase of CD4+FOXP3+ Treg cells at month 3 and 6 after infusion and in a strong decrease in the secretion of autoantibodies, especially against dsDNA, suggesting a possible impact on B cell function 16. Dander at al. confirmed these results in patients with GvHD who received multiple infusions of BM-MSCs 37. They found that the T cell fraction was not significantly affected by BM-MSC infusion at different time points over 6 months. Conversely, Treg cell percentage was increased and concomitantly the Th1/Treg and Th17/Treg ratios were decreased, particularly in patients with a better response. These ratios were significantly higher in non-responders before and after BM-MSC infusion. Similar MSC-mediated effects on Th17 cell function and differentiation were also reported following injection of third-party MSCs after allogeneic hematopoietic stem cell transplantation 36. A recent study compared the percentage of CD4+CD25+FOXP3+ Tregs after MSC injection in patients with GvHD and found a significant increase from week 8 post-injection and at least up to month 36 38. Conversely, they did not observe any change in CD19+ B cells. Similarly, monitoring of circulating T lymphocytes after local MSC injection in patients with fistula in inflammatory bowel disease showed that the percentage of CD4+CD25brightFOXP3+ cells was significantly increased after the second injection (1 month) and persisted for 12 months 18. Altogether these data and our results indicate that the circulating Treg cell fraction is increased upon systemic or local MSC injection with a higher Treg increase following systemic injection.
Moreover, the absence of systemic immune cell depletion, which is observed with anti-inflammatory drugs and Janus kinase inhibitors (a new emerging therapy) 31,32, consolidates the safety of autologous ASCs. A recent systematic literature analysis of the reported adverse events upon IA injection of culture-expanded stem cells 33 indicated that there is no compelling argument against its use in human patients.
Does the observed slight but significant increase in Treg frequency is physiologically relevant? To discuss this point, it is interesting to analyze the variation of Treg frequencies in autoimmune settings. Indeed, break of tolerance in autoimmune diseases have been often associated with a decrease either in number or function of Treg cells. In murine model of autoimmune diseases, increased Treg cell number lead to protection of mice. For instance, injection of anti-CD3 antibodies in NOD mice protect mice from diabetes with an increase in Treg cells from 10 to 14% in the pancreatic and mesentheric LN representing a 1.4-fold increase 39. In human, in newly diagnosed immune-mediated diabetes as well as long-standing type 1 diabetic patients, the mean percentage of the CD4+CD25+Treg cells was significantly reduced to 2.6 ± 0.23% and 3.7 ± 0.69%, respectively, compared with 6.9 ± 0.4% and 6.3 ± 0.48% in the normal control and type 2 diabetic groups, respectively 40. These results suggest that less than a 2-fold decrease in Treg cell frequency may have important consequences on the immune homeostasis. In rheumatoid arthritis (RA), results concerning the decreased number of peripheral Treg cells are very controversial because of the heterogeneity of the definition of Treg cells (reviewed in 41). Recent studies showed that the frequencies of Treg cells were significantly lower in both active RA patients (2.89 ± 0.17%) and inactive RA patients (3.21 ± 0.21%) than those in healthy controls (5.83 ± 0.39%) 42. Another study found that the proportion of Treg cells was significantly lower in RA (3.8 ± 1.0%) and Behcet's Disease (3.3 ± 0.5%) compared to heathy controls (5.0 ± 0.5%) with a specific significant decrease in activated Treg 43. Furthermore, in RA, clinical remission following biotherapy treatment using Infliximab has been associated with an increase in Treg number from 2% to 3% at 6 weeks representing a 1.5-fold increase 44. All these results suggest that slight variation in Treg number, even less than 2-fold, may have important consequences on immune homeostasis suggesting that the 1.3-fold increase in Treg cell number, observed in our study, may be physiologically relevant.
Concerning the results obtained for monocytes and B cells, it should be noted that both cell types have shown high intra-assay variability (up to 20% in some cases) among longitudinal studies and among centers 45. Several studies have previously reported an impact of MSCs on B cell behavior in vitro, and recently, Peng et al found an increase in the percentage of IL-10-secreting CD5+ B cells in patients with GvHD upon MSC infusion 46. The clinical improvement after MSC infusion was accompanied by a 1.5-fold increase in CD5+ B cells underscoring that thigh variation of such B cell population may have physiological impact. Concerning the variation in the monocyte subsets, it may be informative to follow the frequencies of such populations in several clinical situations at base line and after treatment. Similar slight but significant frequencies alterations of the various monocyte subsets have been demonstrated 47,48. Decreased proportions of circulating monocytes to leucocytes was recently observed in OA compared with controls mainly due to a decrease in classical monocytes and a higher number of circulating lymphocytes 49. Moreover, changes in monocyte frequencies, phenotype and function occur in the advanced-age that correlate with chronic diseases 50. However, we still do not know if these slight but measurable changes predispose individuals to age-related chronic inflammatory disease.
Finally, we did not find in our study any correlation between baseline clinical features and immune cell profile changes post-treatment. This might be due to the low number of patients that did not allow to do a correlation between the clinical response and the different ASC doses. Our results suggest that the increased immune tolerance after ASC injection is mainly an indication of a good ASC safety profile. Thus, we did not observe any association between increasing Treg cells frequency and a better clinical response although it might be expected by the anti-inflammatory action mediated by ASCs. The present results on the immune monitoring of the 18 patients included in the ADIPOA phase I study need to be consolidated in a large clinical trial to evaluate the immunological impact of MSC-based therapy with long term monitoring, including a placebo-treated group. In the ongoing ADIPOA phase IIb clinical trial (randomized, placebo-controlled; n=150 patients) with two ASC doses, ASC efficacy in OA is evaluated using standardized and established outcome scores. Immune-monitoring of this phase IIb clinical trial, as well as in other immune disorders will allow deciphering MSC long-term biological and immunological impact in various clinical settings.
In conclusion, the present study reveals a global switch of the immune response toward immune homeostasis, following local ASC injection. It is acknowledged that ASCs drive the immediate response by releasing paracrine factors and cytokines, and might also initiate a cascade resulting in a long-term effect on the immune homeostasis. More data on the in vivo immune cell profile changes upon ASC injection could help to improve our understanding of stromal cell mechanisms of action and to better design future clinical studies.
Acknowledgments
We are grateful to Leila Houhou, Jasmin Baumann, Anne Cadene and Michelle Moya for their expert assistance during the clinical trial. We thank Karin Tarte, Cedric Ménard and Joelle Dulong from the SITI Lab/ECELLFRANCE immunomonitoring platform for the graphical abstract and for their helpful discussions and collaborative work.
Ethics statement
The study protocol was approved by the local Ethics Committees of both institutions (Comité de Protection des Personnes of Montpellier, ref UF8606-120203 and Ethik-Kommission bei der Medizinischen of Würzburg) and by the national competent authorities (ref TC301; EudraCT N°: 2011-000183-10). All patients have been informed and a written consent has been obtained for all patients and healthy volunteers.
Author contributions
CJ, YMP, RF and PLP designed the study. YMP, RF, OP, UN and PLP designed and organized the database. JQ, NA, NE, EDL and MC conducted experiments and acquired data. JQ, NA, NE, MC, YMP and FE analyzed data. YMP and PLP wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by research funding from the European Union 7th FWP under grant agreement 241719 (ADIPOA), from the ANR infrastructure project (N° 11-INBS-0005 “ECELLFRANCE: Development of a national adult mesenchymal stem cell-based therapy platform”) and from institutional fundings.
Abbreviations
- ASC
Adipose-derived stromal cells
- CRP
C-reactive protein
- IA
intra-articular
- IL
interleukin
- OA
osteoarthritis
- MSC
Mesenchymal stromal or stem cells
- BM-MSC
Bone marrow-derived MSC
- TNF
tumor necrosis factor
- WOMAC
Western Ontario and McMaster University Arthritis Index and VAS: Visual analog scale.
References
- 1.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 2.Cicuttini FM, Wluka AE. Osteoarthritis: is oa a mechanical or systemic disease? Nat Rev Rheumatol. 2014;10:515–6. doi: 10.1038/nrrheum.2014.114. [DOI] [PubMed] [Google Scholar]
- 3.Pers Y-M, Ruiz M, Noel D, Jorgensen C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: state of the art and perspectives. Osteoarthritis Cartilage. 2015;23:2027–35. doi: 10.1016/j.joca.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A. et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 5.Gkretsi V, Simopoulou T, Tsezou A. Lipid metabolism and osteoarthritis: lessons from atherosclerosis. Prog Lipid Res. 2011;50:133–40. doi: 10.1016/j.plipres.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Tyndall A. Mesenchymal stem cell treatments in rheumatology: a glass half full? Nat Rev Rheumatol. 2014;10(2):117–24. doi: 10.1038/nrrheum.2013.166. [DOI] [PubMed] [Google Scholar]
- 7.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E. et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–29. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 8.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M. et al. Interferon-gamma-dependent inhibition of b cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776–86. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 9.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin e2. Blood. 2008;111:1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 10.Djouad F, Charbonnier L-M, Bouffi C, Louis-Plence P, Bony C, Apparailly F. et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–32. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 11.Luz-Crawford P, Kurte M, Bravo-Alegria J, Contreras R, Nova-Lamperti E, Tejedor G. et al. Mesenchymal stem cells generate a cd4+cd25+foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F. et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G. et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17:534–41. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J. et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–11. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase 2 study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 16.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H. et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–75. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 17.Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D. et al. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317–22. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 18.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C. et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising crohn's disease. Gut. 2011;60:788–98. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 19.Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW. et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal crohn's disease: results of a phase 1 study. Gut. 2010;59:1662–69. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 20.Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory t cells by human mesenchymal stem cells. Blood. 2011;117:4826–35. doi: 10.1182/blood-2010-12-324038. [DOI] [PubMed] [Google Scholar]
- 21.Pers Y-M, Jorgensen C. Adipose derived stem cells for regenerative therapy in osteoarticular diseases. Horm Mol Biol Clin Investig. 2016;28(3):113–20. doi: 10.1515/hmbci-2016-0010. [DOI] [PubMed] [Google Scholar]
- 22.Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F. et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase 1 dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56. doi: 10.5966/sctm.2015-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bura A, Planat-Benard V, Bourin P, Silvestre J-S, Gross F, Grolleau J-L. et al. Phase 1 trial: the use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–57. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of womac: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 25.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M. et al. Omeract-oarsi initiative: osteoarthritis research society international set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389–99. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.van Dongen JJ, Orfao A. EuroFlow: resetting leukemia and lymphoma immunophenotyping basis for companion diagnostics and personalized medicine. Leukemia. 2012;26:1899–907. doi: 10.1038/leu.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan M, Beitz B, Rouilly V, Libri V, Urrutia A, Duffy D. et al. Semi-automated and standardized cytometric procedures for multi-panel and multi-parametric whole blood immunophenotyping. Clin Immunol. 2015;157:261–76. doi: 10.1016/j.clim.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5. doi: 10.1186/s40169-014-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR. et al. cd19(+)cd24(hi)cd38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winthrop KL. The emerging safety profile of jak inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234–43. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 33.Peeters CM, Leijs MJ, Reijman M, van Osch GJ, Bos PK. Safety of intra-articular cell-therapy with culture-expanded stem cells in humans: a systematic literature review. Osteoarthritis Cartilage. 2013;21:1465–73. doi: 10.1016/j.joca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 34.Najar M, Raicevic G, Fayyad-Kazan H, Bron D, Toungouz M, Lagneaux L. Mesenchymal stromal cells and immunomodulation: a gathering of regulatory immune cells. Cytotherapy. 2016;18:160–71. doi: 10.1016/j.jcyt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M. et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 36.Jitschin R, Mougiakakos D, Von Bahr L, Volkl S, Moll G, Ringden O. et al. Alterations in the cellular immune compartment of patients treated with third-party mesenchymal stromal cells following allogeneic hematopoietic stem cell transplantation. Stem Cells. 2013;31:1715–25. doi: 10.1002/stem.1386. [DOI] [PubMed] [Google Scholar]
- 37.Dander E, Lucchini G, Vinci P, Introna M, Masciocchi F, Perseghin P. et al. Mesenchymal stromal cells for the treatment of graft-versus-host disease: understanding the in vivo biological effect through patient immune monitoring. Leukemia. 2012;26:1681–84. doi: 10.1038/leu.2011.384. [DOI] [PubMed] [Google Scholar]
- 38.Zhao K, Lou R, Huang F, Peng Y, Jiang Z, Huang K. et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus-host disease after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21:97–104. doi: 10.1016/j.bbmt.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Belghith M, Bluestone JA, Barriot S, Megret J, Bach J-F, Chatenoud L. Tgf-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to cd3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 40.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K. et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–40. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita T, Shima Y, Wing JB, Sakaguchi S, Ogata A, Kumanogoh A. The proportion of regulatory t cells in patients with rheumatoid arthritis: a meta-analysis. PLoS One. 2016;11:e0162306. doi: 10.1371/journal.pone.0162306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao S, Hao B, Yang XF, Chen WQ. Decreased cd200r expression on monocyte-derived macrophages correlates with th17/treg imbalance and disease activity in rheumatoid arthritis patients. Inflamm Res. 2014;63:441–50. doi: 10.1007/s00011-014-0716-6. [DOI] [PubMed] [Google Scholar]
- 43.Kim J-R, Chae J-N, Kim S-H, Ha J-S. Subpopulations of regulatory t cells in rheumatoid arthritis, systemic lupus erythematosus, and behcet's disease. J Korean Med Sci. 2012;27:1009–13. doi: 10.3346/jkms.2012.27.9.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA. et al. Compromised function of regulatory t cells in rheumatoid arthritis and reversal by anti-tnf alpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streitz M, Miloud T, Kapinsky M, Reed MR, Magari R, Geissler EK. et al. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the one study. Transplant Res. 2013;2:17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L. et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of cd5+ regulatory b cells producing interleukin 10. Leukemia. 2015;29:636–46. doi: 10.1038/leu.2014.225. [DOI] [PubMed] [Google Scholar]
- 47.Tsukamoto M, Seta N, Yoshimoto K, Suzuki K, Yamaoka K, Takeuchi T. Cd14(bright)cd16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res Ther. 2017;19:28. doi: 10.1186/s13075-016-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U. The cd14(bright)cd16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the th17 cell population. Arthritis Rheum. 2012;64:671–77. doi: 10.1002/art.33418. [DOI] [PubMed] [Google Scholar]
- 49.Loukov D, Karampatos S, Maly MR, Bowdish DME. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, bmi and pain. Osteoarthritis Cartilage. 2018;26:255–63. doi: 10.1016/j.joca.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 50.Verschoor CP, Johnstone J, Millar J, Parsons R, Lelic A, Loeb M. et al. Alterations to the frequency and function of peripheral blood monocytes and associations with chronic disease in the advanced-age, frail elderly. PLoS One. 2014;9:e104522. doi: 10.1371/journal.pone.0104522. [DOI] [PMC free article] [PubMed] [Google Scholar]