Abstract
Background:
Community acquired urinary tract infections (CA-UTI) caused by extended spectrum beta lactamase (ESBL) producing organisms is on the rise throughout the world. There are known risk factors such as age <1 year, children on uroprophylaxis, recurrent UTI, recent antibiotic usage etc which can predict the occurrence of these ESBL producers.
Objectives:
To correlate known risk factors with occurrence of ESBL UTI and antibiotic susceptibility of uropathogens isolated.
Materials and Methods:
Clinical and microbiological data were collected from 100 children 1month to 12 years with CA-UTI. The risk factors were compared in the ESBL and non-ESBL group and analysed.
Results and Conclusion:
The antimicrobial sensitivity pattern in our study showed increased resistance to cephalosporins, cotrimoxazole, fluoroquinolones and amoxicillin-clavulanic acid which are frequently used in the treatment of UTI, with most isolates being sensitive to aminoglycosides, piperacillin-tazobactam and carbapenems. Statistical analysis did not identify any significant risk factor that predisposes to ESBL UTI.
Keywords: Extended-spectrum beta-lactamase, risk factors, urinary tract infections
INTRODUCTION
Extended-spectrum beta-lactamases (ESBL)-producing organisms present an ever-growing burden in both hospital and community settings. The ESBL confer resistance to penicillins, cephalosporins, and monobactams and are inhibited by beta-lactam inhibitors.[1]
Age <1 year, children on uroprophylaxis, recent antibiotic usage, recurrent urinary tract infections (UTI), male gender, etcetera have been reported as risk factors for UTI with ESBL-producing organisms,[2] recognition of which will predict ESBL- or non-ESBL-producing pathogen and guide in appropriate antibiotic use. Urinary infection with ESBL-producing organisms is increasing at an alarming rate,[2] and very few studies have been done to determine the contributing factors. We studied risk factors for ESBL in relation to UTI in Indian children.
METHODS
This was a retrospective cross-sectional study carried out for 12 months between May 2013 and April 2014 in a pediatric tertiary care hospital from a single pediatric unit. Ours is a 220-bedded hospital with about 13,000 admissions per year, of which around 1000 children have UTI. The study protocol was approved by the Institutional Ethics Committee. Informed consent was obtained from the parents of all children included in the study. The study population consisted of 100 consecutive children admitted to the hospital due to community-acquired UTI (CA-UTI) caused by Gram-negative organisms. All children from 1 month to 12 years with a diagnosis of UTI and having a positive urine culture with colony count of >105 CFU/ml of midstream urine or >104 for samples collected by a catheter within 48 h of admission were included in the study and were followed up for 3 months. Some of them were diagnosed to have UTI after hospitalization for fever of undetermined origin. Exclusion criteria were age <1 month and >12 years, urine collected more than 48 h after admission, colony count less than that mentioned above. Clinical details retrieved from medical records were age, gender, symptoms, risk factors such as age <1 year, uroprophylaxis, recent antibiotics, or hospitalization in the past 3 months. Only one positive culture per patient was included in the study and repeated positive cultures from the same patient were excluded from the analysis.
Those who were diagnosed to have ESBL UTI were considered as cases and those who had non-ESBL UTI were considered as controls.
CA isolates were defined as those detected at hospital admission or within the first 48 h culture collection from a patient not admitted to the hospital previously while samples originating from patients hospitalized for 48 h or more were considered nosocomial and excluded noting the difference between the time of admission and time of sample collection. In the presence of any potential growth, antibiotic sensitivity testing was done by Modified Kirby–Bauer disc diffusion method according to the Clinical and Laboratory Standards Institute guidelines.
The ESBL detection was done by double disc synergy test testing the strain against ceftazidime and ceftazidime/clavulanic acid. The synergy was determined between a disc of amoxicillin-clavulanate (20 μg/10 μg) and a 30 μg disc of each third-generation cephalosporin test antibiotic placed at a distance of 20 mm from center to center on a Mueller-Hinton agar plate swabbed with the test isolate. A clear extension of the edge of the inhibition zone of cephalosporin toward the amoxicillin-clavulanate disc was interpreted as positive for ESBL production. Escherichia coli ATCC 25,922 was used as ESBL-negative strain.
Statistical analysis
We analyzed the presence of known risk factors[2] which might have a role in predisposing or having a cause and effect relationship with ESBL E. coli UTI in children. Chi-square test and contingency quotient were performed on all the above variables. If the calculated P < 0.05, it was considered as a statistically significant relationship between the two groups.
We used univariate logistic regression to find out the relationship between the dependent variable and independent variables. There was no statistically significant relationship between the dependent variable and the individual independent variables.
RESULTS
Table 1 captures the demographic data including risk factors. Of the 100 Gram-negative isolates, E. coli was isolated in maximum number (80%) followed by Klebsiella pneumoniae in 12%. The other organisms isolated included Pseudomonas aeruginosa (2%), Klebsiella oxytoca (2%), Proteus mirabilis (2%), Morganella morganii (1%), and Citrobacter (1%) [Table 2].
Table 1.
The demographic and clinical data captured in the study and the associated risk factors
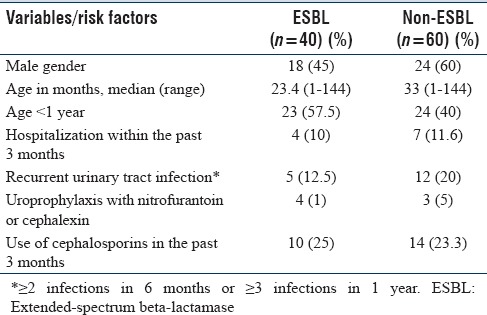
Table 2.
Laboratory and imaging data
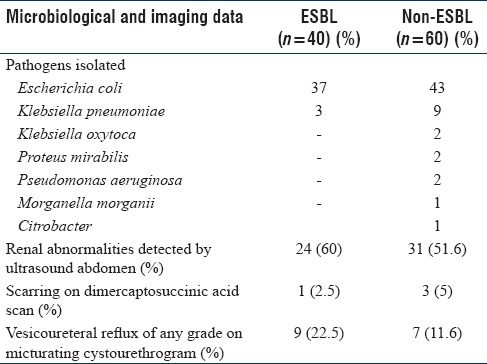
ESBL constituted 40% of all isolates. The proportions of ESBL producers among E. coli and Klebsiella were 46.25% and 25%, respectively.
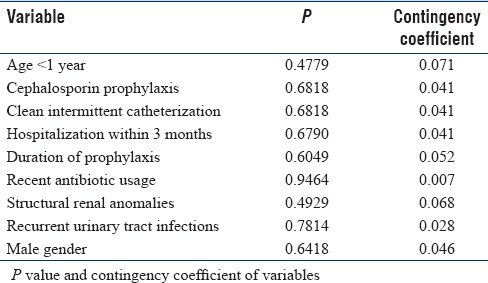
We found that none of the variables had any significant correlation and none of them had a cause and effect relationship with the presence of ESBL pathogen [Table 3].
Table 3.
Analysis of risk factors
When we analyzed the antimicrobial sensitivity pattern of these isolates, there was increasing rates of resistance to third-generation cephalosporins (for ceftriaxone and cefotaxime, 68% and cefuroxime, 90%). Resistance to amoxicillin-clavulanic acid was 90%, trimethoprim-sulfamethoxazole 72%, and fluoroquinolones 66%. Sensitivity to some antibiotics was high (carbapenem 93%, cefoperazone-sulbactam 87%, amikacin 86%, piperacillin-tazobactam 84%, and nitrofurantoin 76%).
Even though in vitro resistance to third-generation cephalosporins was observed in large numbers among the ESBL group, approximately one-third (32.5%) of cases among the ESBL group have responded to therapy and hence were treated with the same. Remaining ESBL producers were successfully treated with piperacillin-tazobactam for 5–7 days until these children became afebrile and were able to take orally and discharged on intramuscular amikacin to complete the total duration of therapy for 14 days. UTI due to non-ESBL producers was managed with third-generation cephalosporins (cefotaxime) initially followed by oral cefixime.
DISCUSSION
Worldwide, even from the community settings, the prevalence of ESBL pathogens has been steadily increasing.[3] In our series, E. coli and Klebsiella were the most common uropathogens, similar to the data from other studies in India.[4] Our study identified 40% of uropathogens as ESBL producers and with earlier reports in India ranging from 26.9% to 48.3%.[5,6,7] Eshwarappa et al. in a study from South India observed adults with UTI showed ESBL positivity rate of 52%.[8] It is, therefore, important to know the local epidemiology of the isolates and their antibiogram to choose appropriate empiric antibiotics.
With the knowledge of the risk factors that can predispose to ESBL producers, we can avoid the unnecessary delay in the initiation of appropriate antibiotics and thereby prevent complications. In our study, we compared the known risk factors between the two groups and found that there were no risk factors to predict the presence of ESBL pathogen. This is in contrast to the earlier observations by Kizilca et al.,[2] who reported age <1 year, high UTI recurrence rate, long duration of prophylaxis, use of cephalosporin for prophylaxis, hospitalization within previous 3 months, and clean intermittent catheterization to be significant risk factors predicting presence of ESBL producers.
We analyzed the antibiogram of the isolates and the antimicrobial sensitivity pattern was similar to the findings in an earlier report from India.[9] Majority (68%) of the isolates in our series were resistant to third-generation cephalosporins, probably because of other type of beta-lactamases that confer resistance to extended-spectrum cephalosporins such as plasmid-mediated AmpC cephalosporinases (AmpC) in Enterobacteriaceae and plasmid-based bla AmpC in E. coli and Klebsiella.[10] AmpC-producing bacteria are not generally inhibited by beta-lactamase inhibitors, are nonsusceptible to cephamycin, and do not confer resistance to cefepime. Among these isolates that are resistant to third-generation cephalosporins, 58.8% (40/68) of the isolates were ESBL producers while the remaining were other beta-lactamase producers. Of the ESBL producers (40%) identified, nearly one-third of ESBL isolates – 13/40 (32.5%) – were responsive in vivo to third-generation cephalosporins. A study from Serbia in 2012 observed that the clinical response to ceftriaxone was same in both the ESBL-positive E. coli and ESBL-negative E. coli, and it was concluded that in vitro resistance of ESBL E. coli to ceftriaxone determined by standard methods is not sufficiently predictive of its in vivo sensitivity.[10] This may be due to the fact that the drug is concentrated in urine while susceptibility testing is mostly based on blood concentration determinations. Urinary concentrations of antimicrobial agents enable bactericidal levels to be achieved despite apparent in vitro resistance. Therefore, ceftriaxone may be used as an empiric therapy for acute pyelonephritis in children and the same may be continued if there is a clinical response even if the isolates were reported as ESBL producers. Successful treatment was defined as resolution of fever, sterile urine cultures at <72 h, and decreasing trend in leukocytes, C-reactive protein, and urine white blood cell within 5–7 days.
CONCLUSION
This study showed high resistance among uropathogens to third-generation cephalosporins, trimethoprim-sulfamethoxazole, amoxicillin-clavulanic acid, and fluoroquinolones which are commonly used in the empiric treatment of UTI. Sensitivity to aminoglycosides, piperacillin-tazobactam, and carbapenems was good which may be used in empiric treatment, especially in high-risk cases. We have concluded that there are no risk factors that can predict the development of ESBL UTI.
Children with UTI due to ESBL organisms clinically responding to cephalosporins may be treated with the same class of agents.
Strength
Our study negates the argument that risk factors such as recent antibiotic use, uroprophylaxis, and male age predispose to UTI with ESBL organisms.
Limitations
Small sample size of 100 patients
It is not a community-based study in the strictest sense as those admitted to the hospital with CA-UTI were studied.
Implications for future research
Genetic predisposition for infection with ESBL-producing organisms may be there, which need to be explored.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lukac PJ, Bonomo RA, Logan LK. Extended-spectrum ß-lactamase-producing Enterobacteriaceae in children: Old foe, emerging threat. Clin Infect Dis. 2015;60:1389–97. doi: 10.1093/cid/civ020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kizilca O, Siraneci R, Yilmaz A, Hatipoglu N, Ozturk E, Kiyak A, et al. Risk factors for community-acquired urinary tract infection caused by ESBL-producing bacteria in children. Pediatr Int. 2012;54:858–62. doi: 10.1111/j.1442-200X.2012.03709.x. [DOI] [PubMed] [Google Scholar]
- 3.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taneja N, Chatterjee SS, Singh M, Singh S, Sharma M. Pediatric urinary tract infections in a tertiary care center from north India. Indian J Med Res. 2010;131:101–5. [PubMed] [Google Scholar]
- 5.Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: A multicenter study. J Infect Dev Ctries. 2008;2:354–8. doi: 10.3855/jidc.196. [DOI] [PubMed] [Google Scholar]
- 6.Taneja N, Rao P, Arora J, Dogra A. Occurrence of ESBL and Amp-C beta-lactamases and susceptibility to newer antimicrobial agents in complicated UTI. Indian J Med Res. 2008;127:85–8. [PubMed] [Google Scholar]
- 7.Tankhiwale SS, Jalgaonkar SV, Ahamad S, Hassani U. Evaluation of extended spectrum beta lactamase in urinary isolates. Indian J Med Res. 2004;120:553–6. [PubMed] [Google Scholar]
- 8.Eshwarappa M, Dosegowda R, Aprameya IV, Khan MW, Kumar PS, Kempegowda P. Clinico-microbiological profile of urinary tract infection in south India. Indian J Nephrol. 2011;21:30–6. doi: 10.4103/0971-4065.75226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res. 2014;139:945–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Peco-Antic A, Paripovic D, Buljugic S, Spasojevic-Dimitrijeva B, Cvetkovic M, Laban-Nestorovic S, et al. In vivo susceptibility of ESBL producing Escherichia coli to ceftriaxone in children with acute pyelonephritis. Srp Arh Celok Lek. 2012;140:321–5. [PubMed] [Google Scholar]


