Abstract
Objective:
The aim of this study was to determine the clinical outcome, microbiological outcome and safety profile of CSE-1034, a novel combination of Ceftriaxone, Sulbactam and EDTA in patients with complicated urinary tract infections (cUTI).
Materials and Methods:
This was a randomized, controlled, open-labeled Phase-3 trial with the primary objective of assessing the efficacy and safety of CSE-1034 versus Ceftriaxone for the empirical treatment of cUTI. Adult cUTI patients were randomized to receive either intravenous dose of CSE-1034 or Ceftriaxone. The primary end point was composite cure rate (clinical response and bacterial eradication) in mMITT population at test of cure (TOC) visit. Secondary measures included verification of primary endpoint across other visits in different population sets, safety of patients and treatment duration.
Results:
Overall, 204 patients were enrolled in the study and received one of the two treatments. At primary endpoint (TOC visit), the composite cure rate was much higher in CSE-1034 treatment arm compared to Ceftriaxone arm i.e. 97% (68/70) vs 83% (58/71) (treatment difference 12.6%; 95% CI: 5.9% to 26.4%). The adverse events (AEs) rates reported in two treatment arms were 21% in CSE-1034 and 36% in Ceftriaxone groups. Additionally, the treatment duration in CSE-1034 arm was significantly less (P < 0.05).
Conclusions:
CSE-1034 3 g every 24 h showed a high favorable clinical and bacteriological response, and 95% CI around the treatment difference prove the superiority of CSE-1034 vs. Ceftriaxone for the treatment of cUTI. Therefore, CSE-1034 provides an effective alternative in the treatment of patients with cUTI.
Keywords: Ceftriaxone, CSE-1034, cUTI, anti-microbial resistance
INTRODUCTION
Urinary tract infections (UTIs) are one of the most prevalent infectious diseases, accounting for around 150 million cases, worldwide.[1] They can be classified into two types, namely, uncomplicated and complicated. A cUTI is a urinary infection occurring in patients with predisposed structural or functional abnormality of genitourinary tract including urine retention, renal failure or transplant, excessive antibiotic consumption or increased introduction of indwelling devices or instruments.[2,3] The pathogenic strains isolated from cUTI cases are reported more resistant to antimicrobials than uncomplicated ones. The recurrence and acquisition of infections through urological interventions are the main factors that contribute to high prevalence of resistance in cUTIs.[3,4]
In most cases, the empirical therapy is started for cUTI before the culture results are obtained. The first line of treatment recommended for UTI include trimethoprim, cephalexin, amoxycillin-clavulanate or nitrofurantoin.[5] Moreover, IDSA has recommended fluoroquinolones, cephalosporins and other β-lactams with or without β-lactamase inhibitors as the second choice of therapy in case first line of treatment is not appropriate.[5] However, antimicrobial resistance (AMR) to urinary tract pathogens is reported to increase worldwide, especially to commonly used antimicrobials. Other than limiting available options, AMR has important clinical implications as well, resulting in increased healthcare costs, morbidity and mortality rates.
As a result, the pharmaceutical industry and medical researchers have shifted their focus on finding alternatives like bacteriophage, antibacterial herbs, and most importantly Antibiotic Resistance Breakers (ARBs). An ARB is typically a non-antibiotic moiety that does not have any antibacterial activity of its own, but in combination with an antibiotic (or a combination), it helps to break resistance mechanisms, thereby enhancing the overall anti-microbial activity of the combination.[6] The original concept of ARBs dates back to early 1970s when the first class of beta-lactamase inhibitors were discovered, however, it has received much recognition only in the recent literature to describe compounds that have not been previously used for treating infections. One of the first drug combinations that incorporates an ARB to break resistance mechanisms is CSE-1034, approved by the DCGI in 2011. CSE-1034 is a novel combination of Ceftriaxone (a beta-lactam antibiotic), Sulbactam (a beta-lactamase inhibitor) and Disodium EDTA (an Antibiotic Resistance Breaker). Various in-vitro and in-vivo studies have reported enhanced activity of CSE-1034 against multi-drug resistant (MDR) pathogens.[7,8,9]
The present study was undertaken to evaluate the clinical efficacy and safety of CSE-1034 in comparison to Ceftriaxone for the treatment of cUTIs.
MATERIALS AND METHODS
Study design
This open-label, multi-centered, randomized study was conducted in nine Indian hospitals between March 2010 and August 2010. The protocol was designed in compliance with the Declarations of Helsinki and ethics committee approvals were obtained from each participating hospital. Informed consent was obtained before enrolment of any patient in the trial.
Study population
Patients aged between 18-65 years with evidence of UTI, diagnosed based on signs, symptoms and laboratory examinations, requiring intravenous (IV) therapy for more than three days were enrolled in the study. For the assessment of cUTI, patients were required to have at least two of the following signs and symptoms: fever (>38°C), dysuria, costovertebral-angle tenderness or supra-pubic tenderness, flank pain, increased urinary frequency and urgency, pyuria and bacterial colony count of ≥105 CFU/ml.
Patients excluded from the study included a) Subjects with a history of previous allergy to β-lactam antibiotics b) Subjects with clinically significant cardiovascular, renal, hepatic, gastrointestinal, neurological, psychiatric, respiratory, other severely immune-compromised, hematological or malignant disease which may interfere with the assessment c) Subjects who had participated in a new drug trial in previous six months d) Pregnant or lactating women.
Prior to dosing, urine samples were collected and tested to identify the causative pathogen and measure the bacterial colony count.
Drug administration
Patients were randomized at each site to receive IV injections of either 3.0 g of CSE-1034 or 2.0 g of Ceftriaxone every 24 h daily for 3-10 days.
Assessment and monitoring
Demographics, baseline characteristics, drug dosage, treatment duration and concomitant medications were recorded for all subjects who participated in the study.
Clinical and microbiological outcomes were assessed in different population sets across the three study visits (End of treatment [EOT], Test of cure [TOC] and Late follow up [LFU]).
Clinical assessment for improvement in the signs and symptoms was performed throughout the treatment regimen. Clinical responses were categorized as “cure”, “failure” or “improved”. The clinical/microbiological response was termed as indeterminate when an assessment could not be done due to unavailability of study data.
Bacteriological responses were evaluated on the basis of persistence or eradication of baseline pathogens in cultures of collected specimens from the subjects. The responses were categorized as ‘eradication’ or ‘failure’. Microbiological eradication was defined as urine cultures demonstrating growth <104 CFUs/mL of the baseline uropathogen/s, and the patient was not bacteremic (if the patient was bacteremic at baseline, the bacteremia has resolved). Composite cure was defined as both clinical cure and microbiological eradication of all baseline pathogens.
Routine hematology, biochemistry, urinalysis profiles and urine cultures were carried out at the beginning and at different visits to evaluate clinical progress.
AEs associated with the treatment were also recorded. AEs were defined as any untoward medical occurrence taking place during or after treatment with the drug. AEs whether treatment related or not were decided by the physicians. The seriousness of AEs was determined as per the ICH-E2D guidelines and the data were compiled according to the ICH Medical Dictionary for Regulatory Activities.
Endpoints
The primary endpoint was proportion of patients with both clinical cure and microbiological eradication (composite cure) at TOC visit in mMITT population.
Secondary endpoints included verification of primary endpoint at EOT and LFU visits, clinical cure in CE and microbiological eradication in ME populations across EOT, TOC, LFU visits, safety of patients and treatment duration.
It also included assessment of duration of therapy and safety in terms of adverse events.
Data analysis
Statistical analyses were performed using the SAS software, version 9.2 (SAS Institute, Cary, N.C., USA). Fisher's exact test was used to check the statistical significance. P values were two-tailed and a value of < 0.05 was considered as statistically significant. 95% confidence intervals were calculated using the unstratified method of Miettinen and Nurminen.
RESULTS
Demographic and baseline characteristics
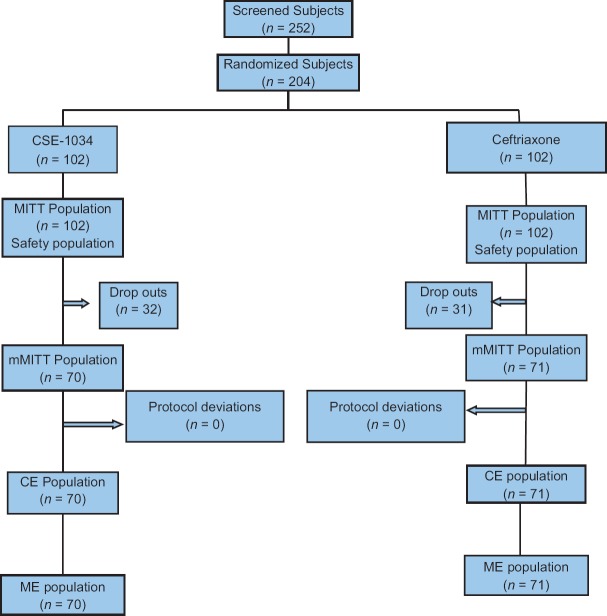
A total of 252 patients were screened, of which, 204 (81%) eligible patients were randomized to one of the two study groups. MITT population was defined as subjects who received atleast one dose of study medication and the population was used for safety analysis. Of the randomized subjects, 102 (50%) patients were randomized to CSE-1034 and 102 (50%) to Ceftriaxone treatment arm [Figure 1] and comprised the MITT population. mMITT population (n = 141; 69%) included all patients with a confirmed cUTI diagnosis and with growth of one or no more than two Gram-negative uropathogens of at least 105 CFU/mL in urine culture. The Clinically evaluable (CE) population comprised of 70 (69%) in CSE-1034 group and 71 (70%) in Ceftriaxone group. The CE population included mMITT patients who received therapy for ≥48hrs, with ≥80% of the scheduled drug administered or received therapy < 48h before discontinuing treatment due to an adverse event (AE), had no protocol deviations that would affect the assessment of efficacy. The microbiologically evaluable (ME) population also comprised of 141 (69%) in total with 70 patients in CSE-1034 group and 71 in Ceftriaxone group. The ME population included patients in the CE population who had a microbiological outcome response at TOC and LFU visits and had no protocol deviations [Figure 1].
Figure 1.
A schematic representation of study populations in two treatment arms
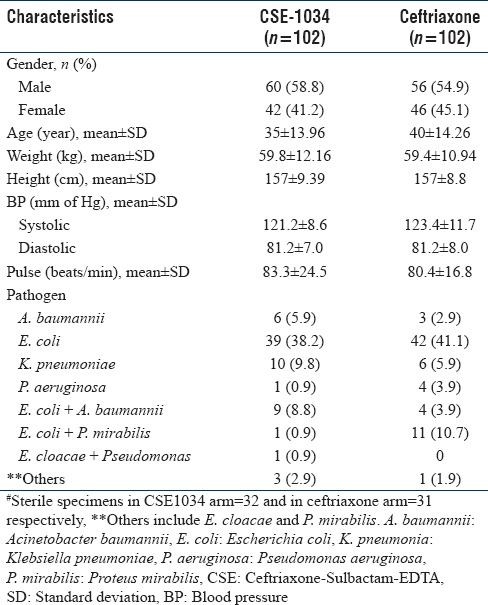
No significant difference in demographics and baseline medical characteristics between the two groups were observed with males predominant in both the arms. 114 (55.9%) patients had a monomicrobial infection in urine sample as the most frequently isolated one, whereas 26 (12.7%) had bi-microbial infections. No significant difference in the incidence and distribution pattern of pathogens isolated was observed in the two treatment arms. E. coli was the most predominant pathogen (39 (38.2%) vs. 42 (41.1%) in CSE-1034 and Ceftriaxone group), followed by K. pneumoniae and A. baumanii. For details, refer to [Table 1].
Table 1.
Demographic, baseline and microbiological characteristics of all study subjects in the two treatment arms (n=204)
In vitro antimicrobial susceptibility assay has shown that all the isolates detected at baseline in two arms were susceptible to CSE-1034 and Ceftriaxone respectively.
Composite cure rate
Overall comparison of the two treatment arms has shown that the clinical efficacy was significantly higher in CSE-1034 group compared to Ceftriaxone group. For the primary end-point, the composite cure rate at TOC was much higher in CSE-1034 treatment arm compared to Ceftriaxone arm i.e. 97% (68/70) vs 83% (58/71) (treatment difference 14.0%; 95% CI: 5.9% to 26.4%) [Table 2 and Figure 2].
Table 2.
Clinical cure rates, microbiological cure rates and composite clinical cure rates in two treatment arms (n=141)
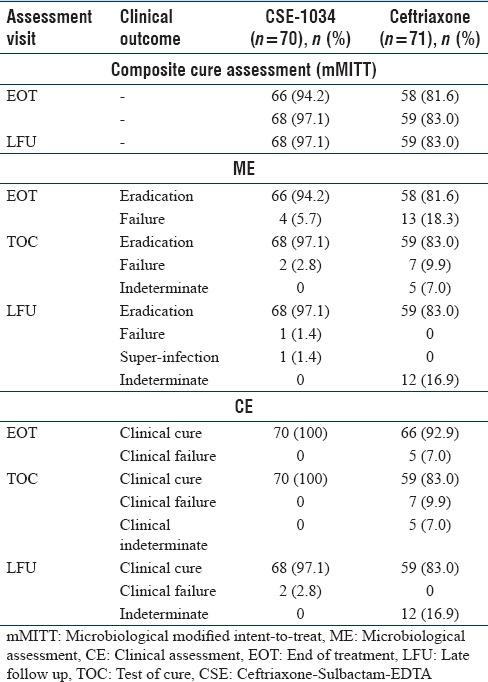
Figure 2.
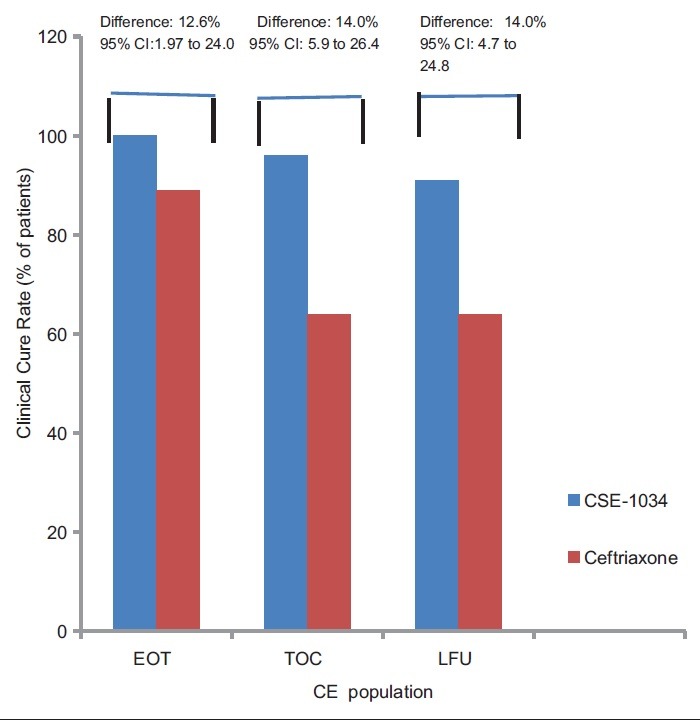
Composite cure rate at different visits in CSE-1034 and Ceftriaxone groups (n=141)
These results were consistent at both the EOT visit (94% and 82% for CSE-1034 and Ceftriaxone arm respectively: treatment difference: 12.6%; 95% CI, 1.97% to 24.0%) and LFU visit (97% and 83%; treatment difference 14.0%; 95% CI, 4.7% to 24.8%) [Table 2].
2 subjects in CSE-1034 arm and none of the subjects in Ceftriaxone arm had discordant outcomes in terms of microbiological cure and clinical failure at TOC visit. The microbiological and clinical outcome were concordant across all other visits.
Clinical efficacy assessment
The clinical cure rate observed in CSE-1034 arm was 100% (70/70) compared to 93% (66/71) in Ceftriaxone arm in CE population at EOT visit (treatment difference 7.01%; 95% CI: 2.99% to 17.3%) [Figure 3].
Figure 3.
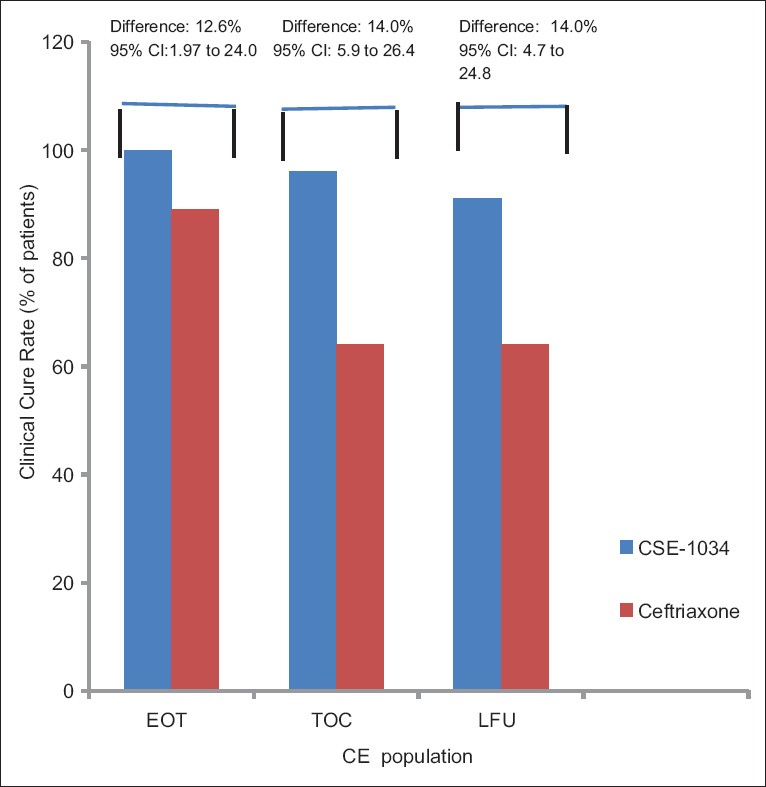
Clinical cure rate for CE populations at different visits in CSE-1034 and Ceftriaxone groups (n=141)
At TOC visit, the clinical failure rate in CE population increased to 9.9% (7) in Ceftriaxone arm whereas no failure was reported in CSE-1034 group. At LFU visit, two clinical failures were reported in CSE-1034 treatment arm and the clinical failure rate increased to 17% (12) in other treatment arm [Table 2]. The two failures in CSE-1034 at LFU visits were clinical relapse in 1 patient and super-infection in other patient.
Microbiological eradication assessment
At EOT visit, the overall eradication rate in ME population was 94% (66/70) in CSE-1034 group which was significantly higher compared to 82% (58/71) in the Ceftriaxone group (treatment difference 12.6%; 95% CI, 1.9% to 24.0%). 4 subjects were declared as bacteriological failure in CSE-1034 and 12 in Ceftriaxone groups, respectively [Table 2 and Figure 4]. As these 4 failure patients in CSE-1034 arm were reported to have microbiological persistence but symptomatic resolution, so technically can be classified to have asymptomatic bacteriuria after treatment with study drug.
Figure 4.
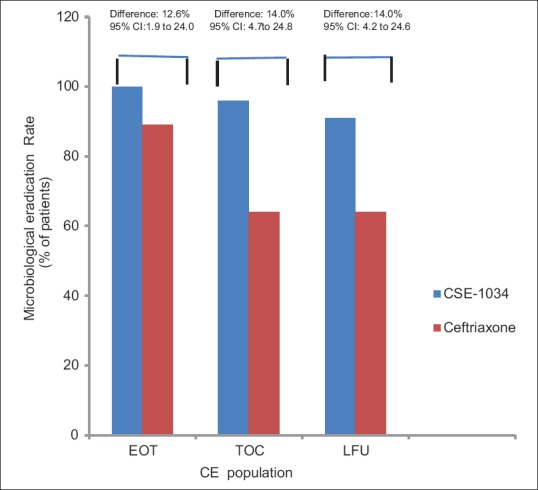
Microbiological eradication rate for ME populations at different visits in CSE-1034 and Ceftriaxone groups (n=141)
At TOC visit, the microbiological eradication rate increased to 97% (68/70) in CSE-1034 arm and 83% (59/71) in Ceftriaxone arm. At LFU visit, one patient reported as microbiological failure at TOC visit in CSE-1034 was reported cured whereas one of the patients contracted superinfection. Thus, the microbiological eradication rate remained similar to TOC visit in both the treatment arms [Table 2]. One subject in CSE-1034 arm who contracted super-infection was reported to have E. coli at the baseline and was super-infected with A. baumannii at LFU visit.
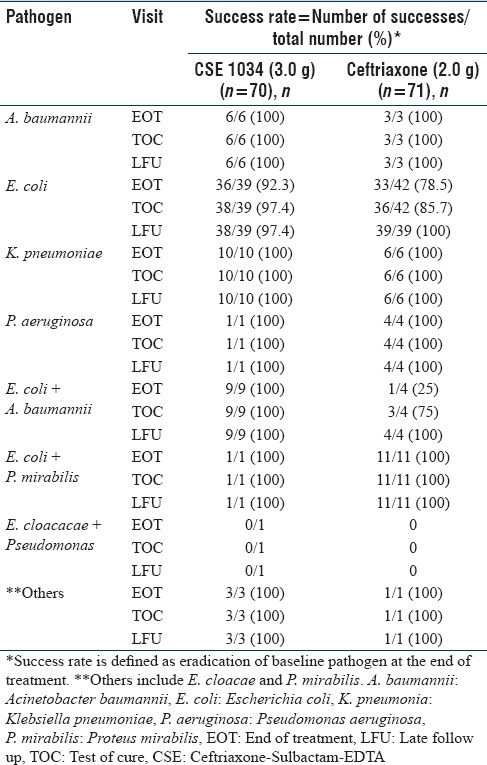
Microbiological outcome as per pathogen
Table 3 presents bacteriological eradication rate in ME analysis sets of two treatment arms in detail. The per-pathogen cure rates for the most common pathogen E. coli at TOC visit in ME population were 99% for the CSE-1034 arm and 91.5% for the Ceftriaxone arm (treatment difference: 7.5%; 95% CI, 0.7% to 25.8%). 100% of K. pneumoniae infections were eradicated at TOC visit in both the treatment arms.
Table 3.
Pathogenwise microbiological outcome at different visits for microbiological assessment population
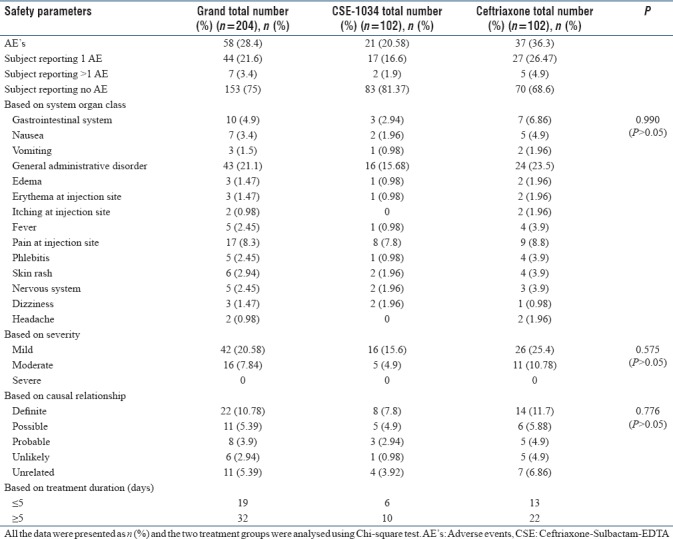
Safety analysis
Overall, a total of 51 (25%) subjects reported 58 AEs in both the groups. The proportion of AEs was significantly higher in Ceftriaxone treatment arm compared to CSE-1034 arm (Ceftriaxone treatment arm: 37; CSE-1034 treatment arm: 21). The common AEs were general administration disorders (18.6%) including pain at site, skin rash, phlebitis and fever followed by gastro-intestinal disorders (4%) including nausea and vomiting. All of the reported AEs were mild and moderate in intensity [Table 4]. No severe adverse reaction was observed during the entire trial course in both the treatment groups.
Table 4.
Detailed description of adverse events in the two treatment arms (n=204)
Treatment duration
Among 141 evaluated subjects, the treatment duration was ≤5 days for 51 (72.9%) subjects in CSE-1034 arm compared to 36 (51%) subjects in Ceftriaxone arm. Duration of treatment was >5 days for 19 (27%) subjects under CSE-1034 treatment compared to 35 (49%) under Ceftriaxone treatment. The difference in the proportion was found to be statistically significant at 5% level of significance (P < 0.05).
DISCUSSION
Over the past two decades, the sharp rise in AMR among urinary tract pathogens especially against commonly used antimicrobials including Ceftriaxone is reported worldwide. The worldwide AMR pattern assessment by various studies in common bacterial uropathogens has shown a resistance rate of 45-70% to various antibiotics normally prescribed for UTI including Cotrimoxazole, Cefotaxime and Ceftriaxone.[10,11] Assessment of AMR pattern of E. coli isolates in one of the tertiary care hospitals in India reported high rates of resistance in Ampicillin (88.4%), Amoxicillin-Clavulanic acid (74.4%), Norfloxacin (74.2%), Cefuroxime (72.2%), Ceftriaxone (71.4%) and Co-trimoxazole (64.2%).[12] This phase III study was carried out to evaluate the clinical efficacy, bacterial efficacy and safety of CSE-1034 in cUTIpatients and market it as alternate for treating cUTI patients. Ceftriaxone (2 g/24 h), the comparator chosen in this study, is a conventional drug which is used to treat various bacterial infections including cUTI.[13]
This multi-centre, open-label, randomized clinical trial demonstrated CSE-1034 as an effective treatment for patients with cUTI. Moreover, the clinical response rate and microbiological eradication rate observed with CSE-1034 clearly indicate that the administered dosing regimen of CSE-1034 was therapeutically superior to Ceftriaxone for the treatment of cUTI patients.
The drugs studied in this clinical trial were evaluated on the basis of clinical and bacteriological response. The study population and design and the endpoints of treatment were strictly as per recommendations of health authority guidelines. The subjects with no pathogen isolation at baseline were not included in the efficacy analysis. However, these subjects were evaluated for safety purposes. Both CE and ME populations were assessed at EOT, TOC and LFU visits based on improvement in clinical symptoms (CE population) and pathogen eradication (ME population). Comparably, high rates of microbiological and clinical cure rates were observed in subjects treated with CSE-1034 compared to Ceftriaxone. At the primary endpoint (TOC visit), a large treatment difference was observed in favor of CSE-1034 in both ME (14%) and CE (16.9%) population. These results were consistent at both EOT and LFU visits.
Baseline pathogens were typical of cUTI with E. coli as the most common uropathogen detected in both treatment arms and isolated from around 60% of the study population. Other baseline pathogens isolated included K. pneumoniae, followed by A. baumannii. Mixed pathogens were isolated at baseline from around 18% of the subjects. Overall, the pathogens belonging to Enterobacteriaceae accounted for 84.4% of total cases and non-Enterobacteriaceae family accounted for 15.6% cases. Consistent with our results, various studies in the past have documented E. coli as the major uropathogen isolated from UTI patients.[14,15,16]
In a comparison of the two treatment arms at pathogen level, the cure rate for E. coli was substantially higher with CSE-1034 than Ceftriaxone. The clinical response rates observed in subjects treated with CSE-1034 are consistent with the prior studies in which the same molecule has been evaluated against various bacterial infections.[17,18,19,20] Results of a PMS study conducted after this trial evaluating the efficacy and safety of CSE-1034 in patients with different bacterial infections reported complete cure in 87% subjects with cUTI and clinical improvement in 13% of the subjects treated with CSE-1034 in adult age group.[21]
In compliance with our studies, a prospective, randomized, double-blind multi-center study comparing Ertapenem versus Ceftriaxone for treatment of cUTIs in adults has reported that bacteriological response was achieved in 93% of the patients in Ceftriaxone group. A similar kind of comparison in a combined analysis of two randomized, double-blind, multi-center trials has also reported favorable microbiological response in 91% of the subjects who received Ceftriaxone. Moreover, analyzing drug susceptibility and treatment response of common UTI pathogens in children, Chen PC et al.[22] have reported the sensitivity rates for various antibiotics as Cefmetazole (90%), Ceftriaxone (85%), Gentamicin (77%) and Ampicillin (20%), and the overall response rate of UTI caused by E. coli to first-line antibiotics such as first-generation Cephalosporins and/or Gentamicin as 78% which is in accordance with our results.
Safety analysis has shown favorable safety records of study drugs in both the treatment arms. The patients receiving CSE-1034 reported 21 AEs and the patients in Ceftriaxone treatment arm reported 37 AEs in total. The type of AEs reported in two treatment groups were also similar and no significant relation was reported between the kind of AEs and any treatment group. Importantly, all AEs reported were related to Ceftriaxone drug and none of them was of a new kind. Analysing the clinical data, it was observed that AEs reported had an association with treatment duration. The number of AEs reported in patients with decreased treatment duration was less. Thus, it can be suggested that less number of AEs reported in CSE-1034 treatment arm could be possibly related to a lower treatment duration compared to other treatment group. However, before concluding, it becomes imperative to mention that there were certain limitations associated with this study. One of the main limitations was the open-label nature of the trial, although this limitation was partly offset by the randomization of the patient by investigators through a software to avoid the possible bias. Also, there wasn't a pre-specified hypothesis at test in this trial so it needs to be highlighted that the post-hoc analysis done may have led to bias in statistical significance. One more limitations of this trial is the comparator being Ceftriaxone, which was supposedly susceptible in most patients, so the added advantage of Sulbactam+EDTA in the combination becomes less relevant.
In conclusion, all these results support that CSE-1034 is a valuable option for the treatment of cUTI cases because of several associated advantages. First, CSE-1034 was proven to have better clinical and bacteriological efficacy. Secondly no serious AE was reported in subjects who received CSE-1034. Moreover, the statistically significant lower treatment duration of CSE-1034 is clinically relevant and the use of CSE-1034 in clinical practice will promote better antibiotic stewardship. Although a critical step forward towards fighting cUTI, further well designed studies against standard-of-care drugs need to be conducted to establish the true efficacy of CSE-1034.
Funding
There was no funding received.
Ethical approval
The ethical approval was duly taken.
Is your submission a randomized controlled trial?
Yes, this is randomized controlled trial with CTRI no. CTRI/2010/091/00174.
Financial support and sponsorship
Nil.
Conflicts of interest
Manu Chaudhary, Shiekh Gazalla Ayub and Mohd Amin Mir are the employees of Venus Remedies, Panchkula. All other authors declare that they have no conflict of interest.
Acknowledgements
Dr. Deepak Bhambe (Narendra Prakash Health Care Center, New Delhi), Dr. Ajay Bhagawant Dande (Dande Diabetes and Health Care Center), Dr Sandeep Kumar Gupta (M. V. Hospital and Research Center, UP), Dr Shriram V. Kulkarni (Kharghar Diabetes and Heart Care Center, Mumbai), Dr Satyaprakash Yadav (Pushpanjali Hospital, Haryana), Dr Yogesh Kumar Yadav (Sewayatan Hospital, Jaipur), Dr Sapna Verma (Ramakrishnan Medical Center Mother and Child Clinic, New Delhi), Dr Ashish Makkar (Makkar Medical Center, Delhi).
REFERENCES
- 1.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–84. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levison ME, Kaye D. Treatment of complicated urinary tract infections with an emphasis on drug-resistant gram-negative uropathogens. Curr Infect Dis Rep. 2013;15:109–15. doi: 10.1007/s11908-013-0315-7. [DOI] [PubMed] [Google Scholar]
- 3.Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Finazzi Agrò E. Nosocomial urinary tract infections: A review. Urologia. 2014;81:222–27. doi: 10.5301/uro.5000092. [DOI] [PubMed] [Google Scholar]
- 4.Pallett A, Hand K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2010;65:iii25–iii33. doi: 10.1093/jac/dkq298. [DOI] [PubMed] [Google Scholar]
- 5.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 6.Gill EE, Franco OL, Hancock REW. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem Biol Drug Des. 2015;85:56–78. doi: 10.1111/cbdd.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shameem M, Mir MA. Management of Pneumonia and Blood Stream Infections with New Antibiotic Adjuvant Entity (Ceftriaxone+Sulbactam+Disodium Edetate)- A Novel Way to Spare Carbapenems. J Clin Diagn Res JCDR. 2016;10:LC23–LC27. doi: 10.7860/JCDR/2016/20904.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia P. Alternative empiric therapy to carbapenems in management of drug resistant gram negative pathogens: A new way to spare carbapenems. Res J Infect Dis. 2015;3:2. [Google Scholar]
- 9.Verma S. A retrospective study to evaluate the efficacy of a new antibiotic adjuvant entity (β-lactam/β-lactamase inhibitor/adjuvant disodium edetate combination) for management of sepsis. Res J Infect Dis. 2015;3:3. [Google Scholar]
- 10.Mamuye Y. Antibiotic Resistance Patterns of Common Gram-negative Uropathogens in St. Paul's Hospital Millennium Medical College. Ethiop J Health Sci. 2016;26:93–100. doi: 10.4314/ejhs.v26i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Australian Group for Antimicrobial Resistance (AGAR)-ASA: Australian Society of Antimicrobials. no date [Google Scholar]
- 12.Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res. 2014;139:945–48. [PMC free article] [PubMed] [Google Scholar]
- 13.Tratselas A, Simitsopoulou M, Giannakopoulou A, Dori I, Saoulidis S, Kollios K, et al. Effect of ceftriaxone on the outcome of murine pyelonephritis caused by extended-spectrum-β-lactamase-producing Escherichia coli. Antimicrob Agents Chemother. 2014;58:7102–11. doi: 10.1128/AAC.03974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koçak M, Büyükkaragöz B, Çelebi Tayfur A, Çaltik A, Köksoy AY, Çizmeci Z, et al. Causative pathogens and antibiotic resistance in children hospitalized for urinary tract infection. Pediatr Int Off J Jpn Pediatr Soc. 2016;58:467–71. doi: 10.1111/ped.12842. [DOI] [PubMed] [Google Scholar]
- 15.Hamdan HZ, Kubbara E, Adam AM, Hassan OS, Suliman SO, Adam I. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Ann Clin Microbiol Antimicrob. 2015;14:26. doi: 10.1186/s12941-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nzalie RN, Gonsu HK, Koulla-Shiro S. Bacterial Etiology and Antibiotic Resistance Profile of Community-Acquired Urinary Tract Infections in a Cameroonian City. Int J Microbiol. 2016;2016:3240268. doi: 10.1155/2016/3240268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary M, Ayub SG, Mir MA. Post-marketing safety and efficacy evaluation of a novel drug CSE-1034: A drug-use analysis in paediatric patients with hospital- acquired pneumonia. J Clin Diagn Res. 2018;12:25–8. [Google Scholar]
- 18.Kumar M, Chaudhary S, Makkar DK, Garg N, Chugh S. Comprative antimicrobial efficacy evaluation of a new product elores against meropenem on gram negative isolates. Asian J Pharm Clin Res. 2015;8:251–54. [Google Scholar]
- 19.Chaudhary M, Payasi GA and A. Advancing in the direction of right solutions: Treating multidrug-resistant pneumonia. Contemp Top Pneumonia. 2017:126–41. [Google Scholar]
- 20.Chaudhary M, Ayub SG, Mir MA. Comparative efficacy and safety analysis of CSE-1034: An open labeled phase III study in community acquired pneumonia. J Infect Public Health. 2018;11:691–7. doi: 10.1016/j.jiph.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhary M, Mir MA, Ayub SG. Protocol 06 Group. Safety and efficacy of a novel drug elores (ceftriaxone+sulbactam+disodium edetate) in the management of multi-drug resistant bacterial infections in tertiary care centers: A post-marketing surveillance study. Braz J Infect Dis Off Publ Braz Soc Infect Dis. 2017;21:408–17. doi: 10.1016/j.bjid.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen PC, Chang LY, Lu CY, Shao PL, Tsai IJ, Tsau YK, et al. Drug susceptibility and treatment response of common urinary tract infection pathogens in children. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2014;47:478–83. doi: 10.1016/j.jmii.2013.07.011. [DOI] [PubMed] [Google Scholar]