Abstract
We report the case of a middle aged man, 43-pack-years active smoker, who upon radiographic screening for lung cancer found to have a non-spiculated lung nodule concerning for malignancy. Histologic evaluation of this lesion turned out to be a nodule due to Histoplasma capsulatum.
Keywords: Histoplasma capsulatum, histoplasmosis, pulmonary nodule
We report the case of a 58-year-old veteran who is an active smoker and had smoked one pack of cigarettes daily for 43 years and underwent a low-dose computed tomography (CT) scan for lung cancer screening. A nonspiculated nodule in the left upper lobe measuring 1.0 cm × 0.8 cm with a central air bronchogram was appreciated [Figure 1a and b]. There was no mediastinal, axillary, or hilar lymphadenopathy. A video-assisted thoracoscopic wedge resection of the left upper lobe was performed. Upon gross inspection of the specimen, a firm, oval, round, well-circumscribed nodule measuring 0.8 cm × 0.6 cm was identified. A histologic evaluation showed a caseating granuloma [Figure 2a]. An acid–fast stain was negative. A Gomori methenamine-silver nitrate stain showed numerous organisms measuring 2–4 μm [Figure 2b]. Serological tests for Coccidioides, Blastomyces, and Histoplasma antibodies were all negative. An interferon-gamma release assay was negative for Mycobacterium tuberculosis.
Figure 1.
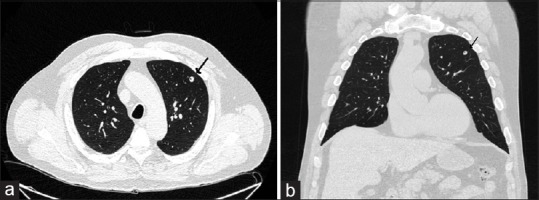
(a) Computed tomography scan showing pulmonary nodule (arrow). (b) Computed tomography scan, coronal view, showing pulmonary nodule with air bronchogram (arrow)
Figure 2.
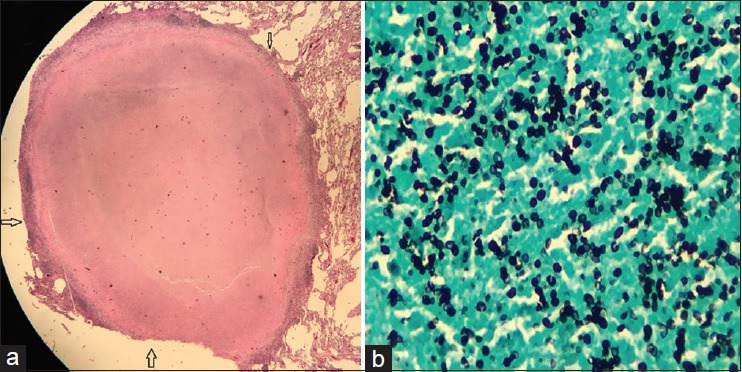
(a) A caseating granuloma (outlined by arrows) (H and E, ×10). (b) Gomori methenamine-silver stain showing numerous organisms (in black color) measuring 2–4 μm (×40)
Immunohistochemical staining confirmed the organisms to be Histoplasma capsulatum [Figure 3]. A polymerase chain reaction performed on tissue also confirmed the presence of H. capsulatum organisms. Histoplasmosis is endemic in the United States along the areas surrounding the Ohio and Mississippi River valleys.[1] Our Veteran, although a native of Long Island, had his military training in rural Ohio. The most common cause for histoplasmosis in nonendemic areas is prior exposure to endemic areas through travel or residence.[2] The organism exists as a mold in the environment and infection occurs through inhalation.[3] Symptomatic infection is rare, but clinical manifestations can range from acute pneumonia with erythema nodosum or erythema multiforme to progressive respiratory disease and to disseminated disease as seen in immune compromised individuals.[3] Asymptomatic exposures are often identified based on abnormal radiographic imaging of lungs, like CT scans, showing enlarged mediastinal or hilar lymph nodes or pulmonary nodules that can be calcified.[4] Greendyke and Emerson reported the occurrence of fibrocaseous granulomas due to histoplasmosis in 20 surgically resected solitary pulmonary nodules from 1951 to 1957 among patients in Rochester, New York.[5] In a Brazilian series of pulmonary histoplasmosis, 15 patients presented with solitary lesions mimicking primary neoplasia or metastasis; 7 of the 15 got tested for serum antibodies to H. capsulatum; only one patient tested positive.[6] Our patient tested negative for serum Histoplasma antibody and Histoplasma galactomannan urinary antigen. Negative blood and urine tests denote that either the infection is inactive or fungal burden is low.[4] Serum antibody titers decline following a self-limited infection, reaching low or undetectable levels in 2–5 years.[7] The Infectious Diseases Society of America guidelines recommend no antifungal treatment for histoplasmosis with solitary pulmonary nodule in asymptomatic patients.[8] Our patient recovered well after the surgery and no antifungal treatment was given.
Figure 3.
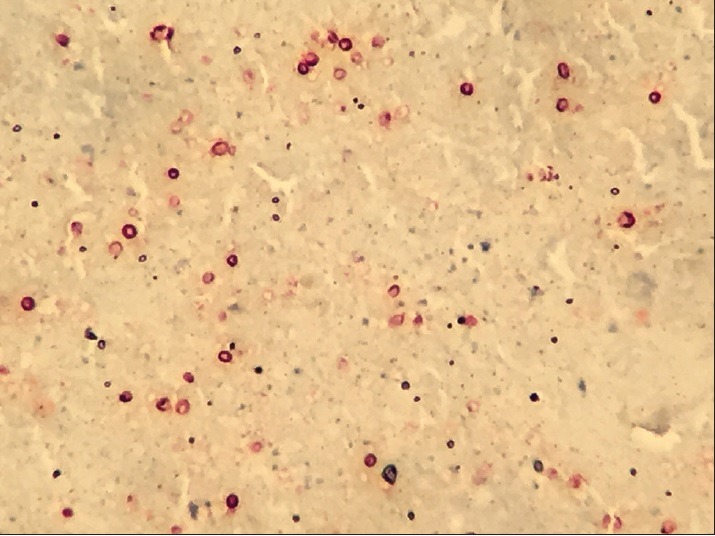
Special immunohistochemical stain reactive for Histoplasma organisms (in maroon color) (×40)
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Benedict K, Mody RK. Epidemiology of histoplasmosis outbreaks, United States, 1938-2013. Emerg Infect Dis. 2016;22:370–8. doi: 10.3201/eid2203.151117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wheat LJ. Histoplasmosis: A review for clinicians from non-endemic areas. Mycoses. 2006;49:274–82. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 3.Kauffman CA. Histoplasmosis: A clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–32. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hage CA, Wheat LJ, Loyd J, Allen SD, Blue D, Knox KS. Pulmonary histoplasmosis. Semin Respir Crit Care Med. 2008;29:151–65. doi: 10.1055/s-2008-1063854. [DOI] [PubMed] [Google Scholar]
- 5.Greendyke RM, Emerson GL. Occurrence of histoplasma in solitary pulmonary nodules in a non-endemic area. Am J Clin Pathol. 1958;29:36–42. doi: 10.1093/ajcp/29.1.36. [DOI] [PubMed] [Google Scholar]
- 6.Dall Bello AG, Severo CB, Guazzelli LS, Oliveira FM, Hochhegger B, Severo LC. Histoplasmosis mimicking primary lung cancer or pulmonary metastases. J Bras Pneumol. 2013;39:63–8. doi: 10.1590/S1806-37132013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheat LJ, Kauffman CA. Histoplasmosis. Infect Dis Clin North Am. 2003;17:1–19, vii. doi: 10.1016/s0891-5520(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 8.Wheat LJ, Freifeld AG, Kleiman MB, Baddley JW, McKinsey DS, Loyd JE, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America. Clin Infect Dis. 2007;45:807–25. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]


