Abstract
Background:
Bloodstream infection (BSI) due to carbapenem-resistant enterobacteriaceae (CRE) is the leading cause of morbidity and mortality in patients with hematological malignancy. These patients receive chemotherapy during treatment, which lead to severe mucositis of gastrointestinal tract and myelosuppression. It was hypothesized that the gut colonizer translocate into the blood circulation causing BSI. Colonization rate with CRE among these patients in India is unknown.
Aim:
This study aims to determine the carriage rate of CRE in cancer patients.
Setting and Design:
A prospective study was conducted in a tertiary care hospital of India.
Materials and Methods:
Rectal swab of 93 patients were collected and processed as per the Center for Disease Control and Prevention protocol for detection of CRE. The isolate CREs were identified by standard phenotypic tests and confirmed for carbapenem resistance by disk diffusion test using carbapenem disk (imipenem, meropenem, doripenem, and ertapenem), Carba-NP test and modified Hodge test. Resistant to any of the carbapenem disc is considered as CRE.
Results:
A total of 86 isolates were detected from 93 patients. Seventy-six isolates were identified as CRE, and 10 isolates were Gram-positive cocci and other Gram-negative bacilli. Acute myeloid leukemia was the most common clinical presentation followed by acute lymphoid leukemia. Thirty-nine out of 93 patients were on chemotherapy. Sixty-seven out of 76 isolates of CRE were observed positive for carbapenemase production by Carba-NP test.
Conclusion:
This study highlights very high rate of CRE carriage among the hematological malignancy patients; who are highly vulnerable to infection. This confirms the need of infection control prevention activities among the hematological malignancy patients.
Keywords: Carba-NP test, carbapenem-resistant enterobacteriaceae, haematological malignancy, modified Hodge test
INTRODUCTION
The emergence of carbapenem-resistant enterobacteriaceae (CRE) appeared as a major threat worldwide.[1] This becomes more critical in patients with hematological malignancies. The cancer patients develop severe mucositis during chemotherapy. As a result of which, gut colonizers may translocate into blood causing bloodstream infection (BSI).[2,3] Infection that occurs due to granulocytopenia in the postchemotherapy period is the predominant cause of morbidity and mortality in these patients. Other patients sharing common places in the health-care settings are also at risk due to the likely chance of acquisition of infection due to CRE. Rectal carriage surveillance is important. The patients with CRE colonization should be identified accurately and isolated for adequate preventive measures for infection control practices. There is paucity of data available on the prevalence of CRE in India.[4,5,6] To date, only one study is available exhibiting the prevalence of the colonization rate of multidrug-resistant organism and CRE among pediatric cancer patients. Hence, we carried out a prospective study to determine the incidence of rectal carriage of CRE in patients with hematological malignancy.
MATERIALS AND METHODS
The study was conducted in the Department of Microbiology, All India Institute of Medical Sciences (AIIMS), New Delhi with collaboration of the Department of Medical Oncology and Hematology with 30 and 11, beds, respectively. It was carried out over a period of 1 year (2016–2017). The ethical clearance for the study was obtained from Institute Ethics Committee of AIIMS, New Delhi. Patients clinically diagnosed with hematological malignancy and admitted for chemotherapy to either of the department were included in the study. Patients with fever and neutropenia due to underlying condition other than hematological malignancy are excluded from the study. Patient's demographic data along with the risk factors associated with the malignancy conditions such as neutropenia, previous hospital stay, previous antibiotic therapy, history of chemotherapy, steroid therapy, and comorbid conditions were collected. Rectal/perianal swabs were collected from these patients on the day of admission and processed for the isolation of CRE following the Center for Disease Control and Prevention (CDC) protocol.[7] The isolates were phenotypically identified as per the standard operative protocol using biochemical test and tested for carbapenem resistance by disk diffusion using Kirby-Bauer method on Muller-Hinton agar (MHA). Carbapenem resistance was tested using all four carbapenem disks (imipenem [10 μg], meropenem [10 μg], ertapenem [10 μg], and doripenem [10 μg]) (HiMedia, Mumbai). Interpretation of the result was made as per the Clinical and Laboratory Standards of Institute (CLSI) 2016 guideline.[8] CRE had been defined as an isolate resistant to any of the carbapenem disks. All the CRE isolates were further tested for carbapenemase-producing CRE (CP-CRE) using modified Hodge test (MHT) and RAPIDEC ® Carba-NP test (BioMeriux, France). The MHT test was performed as per standard protocol of CLSI 2014.[9] The indicator strain Escherichia coli ATCC 25922 strain was used for lawn culture. Klebsiella pneumoniae ATCC® BAA-1705 and ATCC® BAA-1706 were used as positive control and negative control, respectively. The indicator strain was first made up to 0.5 McFarland standard suspension and diluted into 1:10 dilution with normal saline. The suspension was inoculated on the MHA agar plate and allowed to dry for 3–10 min. Meropenem (10 μg) or ertapenem disk (10 μg) was put in the center of the plate. Heavy inoculum of the test strain, positive control and negative control were inoculated from the edge of the disk up to 20–25 mm in straight lines. The plates were incubated overnight at 37°C. Enhanced growth of the indicator strain toward the antibiotic disk was considered as positive for carbapenemase production [Figure 1]. Carba-NP test was performed and interpreted as per the manufacturer instruction [Figure 2].
Figure 1.
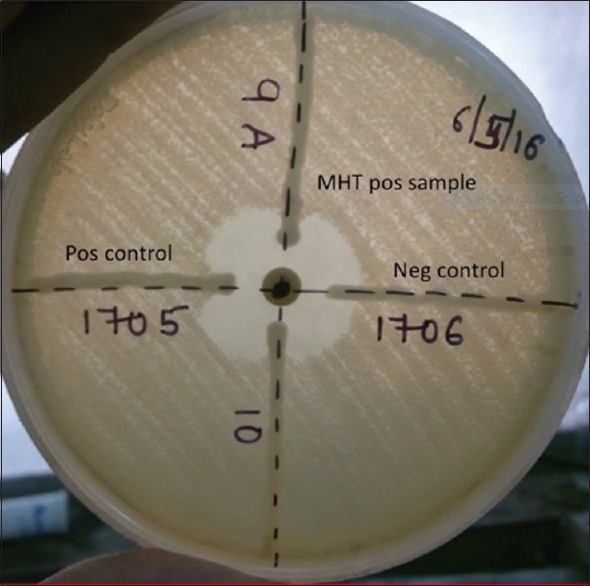
Modified Hodge test showing production of carbapenemase enzyme
Figure 2.
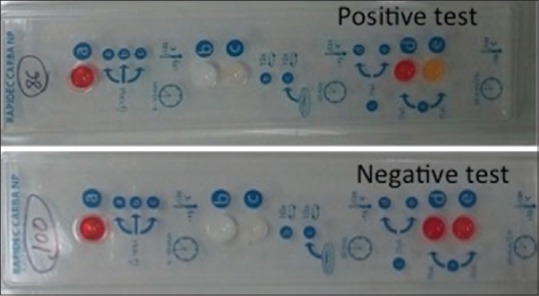
Carba-NP test showing production of carbapenemase enzyme
Statistical analysis
Chi-square test is applied to compare the risk factors between CRE and non-CRE group of patients (P = 0.01). Kappa test is used to compare the percentage of agreement between Carba-NP test and MHT for detection of carbapenemase. Kappa test is also used to determine the inter-rater reliability between the individual carbapenem disk with Carba-NP and MHT test.
RESULTS
Rectal swabs were collected from 93 patients diagnosed with hematological malignancy and processed following CDC protocol. Sixty-eight patients out of 93 (73.1%) were confirmed to have CRE as colonizers in their gut following CDC protocol. A total of 76 isolates of CRE were identified from 68 patients (60 patients with single CRE isolate and eight patients with 2 CRE isolates). Among the rest 25 patients, Gram-positive cocci were found in six patients, Acinetobacter spp. was in three patients, and Pseudomonas spp. was found in one patient without any carriage of CRE in their gut. No growth was observed among rest of the sixteen patients.
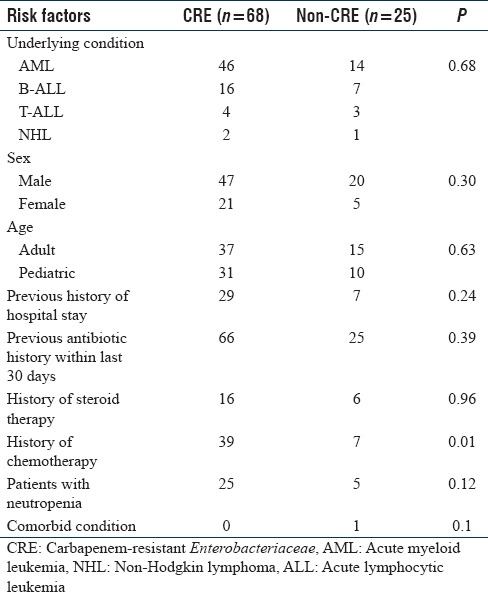
On comparing the demographic data, male was the predominant group (72.1%, [67/93]) followed by female (27.9%, [26/93]) [Table 1]. Acute myeloid leukemia was the predominant underlying condition (64.5%, [60/93]) followed by others. Thirty-six patients out 93 had a history of previous hospital stay and 91 patients had a history of intake of antibiotic prophylaxis within the past 30 days of admission. Fever was present among 12% (11/93) of patients during the hospital stay. Proportions between the CRE and non-CRE were compared using Chi-square test. Thirty-nine patients with CRE received chemotherapy during the disease. Thirty out of 93 patients (33%) were neutropenic among which 25 were CRE and five were non-CRE. Comorbidity was observed only in one patient in the nonCRE category. No significant difference between the sex or age group of patients was observed. Among the various risk factors, history of chemotherapy observed to have significant association among CRE cases (P = 0.01).
Table 1.
Comparison of various risk factors between the carbapenem-resistant Enterobacteriaceae and noncarbapenem-resistant Enterobacteriaceae group of patients
E. coli was the most common CRE isolate (82.8%, [63/76]), followed by Klebsiella spp. (9.2%, [7/76]) and Enterobacter spp. (7.8%, [6/76]). When we compare the four carbapenem disk susceptibility, most of the isolates (71/76) of CRE were observed resistant to ertapenem disk followed by doripenem, meropenem, and imipenem disk [Table 2]. On comparison for the carbapenemase production by the CRE isolates by Carba-NP test and MHT, Carba-NP test showed more positive result than MHT [Table 3]. Carba-NP was tested for all (76) the CRE isolates whereas MHT was carried out on 71 CRE isolates only due to loss of strains. However, the kappa between this test was observed to be 9% only. This indicates a poor agreement of result between Carba-NP and MHT. Nine CRE isolates negative by Carba-NP, and MHT observed resistant by ertapenem. The level of agreement individual carbapenem disk, i.e., doripenem, meropenem, and imipenem with Carba-NP test was observed to be 25.1%, 25.9%, and 8%, respectively, and with MHT was 25.4%, 31%, and 24.7%, respectively.
Table 2.
Comparison of different carbapenem disc for detection of carbapenem-resistant Enterobacteriaceae
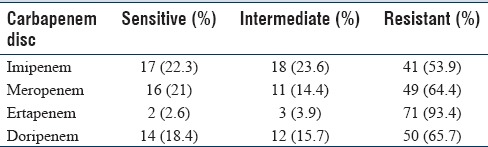
Table 3.
Comparison of two phenotypic methods for detection of carbapenemase producing carbapenem-resistant Enterobacteriaceae

DISCUSSION
BSI in cancer patients is the major cause of mortality, especially during the phase of neutropenia. These infections are often due to the translocation of the endogenous colonizing bacteria, which subsequently cause BSI. Thacker et al. showed association of solid tumor more frequently with colonization of ESBL and CRE than hematological malignancy.[10] The current study showed very high incidence (73%) of CRE colonization among the hematological malignancy patients. The most important risk factor associated with condition is a previous history of antibiotic intake within the past 3 months, previous hospital admission, etc., The earlier study from various hospitals of India showed a varied incidence of CRE ranges from 1.8% to 51% in different patient population.[6] The first Indian study among the pediatric cancer patients had reported the prevalence of CRE colonization as 20.2%.[10] In the current study, the incidence of CRE has been observed much higher which should be considered seriously. The CRE rates all over India are quite high and increasing day-by-day. Hence, strict preventive measures should be taken to control and prevent of further dissemination. Acquisition of multidrug-resistant or carbapenem-resistant organism is multifactorial. Irrational use of antibiotic either misuse or overuse has been a definite factor for acquiring resistance. More ever, the increased use of drugs and antibiotics in agriculture, animals, and contaminated water sources also proven to cause acquiring these resistant genes.[10] Many of the studies had been shown a high incidence of resistant isolates from cow dung.[11,12] The use of antibiotics in soap and gels also plays a role in the acquisition of resistant isolates.[13] Majority of the patients in the current study had a history of antibiotic intake in the previous 3 months, which might be the reason of higher incidence of CRE in these patients.
Unlike the reports from the developed countries, E. coli remains the predominant isolates among the CRE in the current study followed by Klebsiella spp.[14] Accurate detection of CRE is very important for the implementation of infection control practices. Molecular method is considered as the gold standard for detection of CRE, but high cost has limited its use. Hence, phenotypic tests are mostly used in most of the laboratory for confirmation of CRE. Among the carbapenem disk, resistant to ertapenem was observed in 98.6%. All the isolates found positive by Carba-NP test were observed resistant by ertapenem disk. However, the nine strains observed resistant by ertapenem disk but negative or indeterminate by Carba-NP test may be due to the presence of different carbapenemase gene or due to the presence of noncarbapenemase mechanisms such as loss of porin channels, mutation of the efflux pumps, or ampC production.[15,16] As per the CLSI 2016, sensitivity and specificity of Carba-NP test vary with the presence of different carbapenemase gene. The sensitivity was observed to be >90% in KPC, NDM-1, IMP, VIM SME, and SMS carbapenemase. However, it may be as low as 11% in OXA-48 carbapenemases. There are several evidences regarding the effective infection prevention and control practices for patients with hematopoietic malignancy.[17] Strict adherence to five movements of hand hygiene prescribed by the WHO is the most important practice to be followed up. Apart from that, standard barrier precaution, infection-specific isolation, rooms with more than 12 air changes/hour and air quality control through high-efficiency particulate air filtration and administration of prophylactic antibiotic during any intervention may significantly reduce the mortality rates due to infection among these patients.
CONCLUSION
Thus, the study highlights a significant high rate of CRE carriage among one of the most vulnerable group of patients. Thus, urgent need of infection control and preventive measures among the hematological malignancy patients thereby further decrease morbidity and mortality due to CRE. Ertapenem obtained to be the most sensitive marker to identify CRE. Along with CP-CRE, it might detect non-CP-CRE; which usually missed by other methods. Further studies should be conducted to detect the mechanism of resistance of these isolates. Hence, it should be taken as a useful marker for detection of CRE irrespective of the mechanisms.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trecarichi EM, Cauda R, Tumbarello M. Detecting risk and predicting patient mortality in patients with extended-spectrum β-lactamase-producing Enterobacteriaceae bloodstream infections. Future Microbiol. 2012;7:1173–89. doi: 10.2217/fmb.12.100. [DOI] [PubMed] [Google Scholar]
- 3.Tancrède CH, Andremont AO. Bacterial translocation and gram-negative bacteremia in patients with hematological malignancies. J Infect Dis. 1985;152:99–103. doi: 10.1093/infdis/152.1.99. [DOI] [PubMed] [Google Scholar]
- 4.Datta P, Gupta V, Singla N, Chander J. Asymptomatic colonization with Carbapenem Resistant Enterobacteriaceae (CRE) in ICU patients and its associated risk factors: Study from North India. Indian J Med Microbiol. 2015;33:612–3. doi: 10.4103/0255-0857.167316. [DOI] [PubMed] [Google Scholar]
- 5.Manoharan A, Barla GS, Peter R, Sugumar M, Mathai D. Multidrug resistance mediated by co-carriage of extended-spectrum beta-lactamases, AmpC and New Delhi metallo-beta-lactamase-1 genes among carbapenem-resistant Enterobacteriaceae at five Indian medical centres. Indian J Med Microbiol. 2016;34:359–61. doi: 10.4103/0255-0857.188350. [DOI] [PubMed] [Google Scholar]
- 6.Saseedharan S, Sahu M, Pathrose EJ, Shivdas S. Act fast as time is less: High faecal carriage of carbapenem-resistant Enterobacteriaceae in critical care patients. J Clin Diagn Res. 2016;10:DC01–5. doi: 10.7860/JCDR/2016/17638.8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC 2012 CRE Toolkit: Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE) [Last accessed on 2013 Jan 27]. Available from: http://www.cdc.gov/hai/organisms/cre/cre.toolkit/index.html .
- 8.Clinical and Laboratory Standards Institute. 26th ed. Wayne: CLSI supplement M100S; 2016. [Google Scholar]
- 9.Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. Wayne: National Committee for Clinical Laboratory Standards; 2014. Clinical and Laboratory Standards of Institute M100-S24. Clinical and Laboratory Standards of Institute; document no: M100.S24. [Google Scholar]
- 10.Thacker N, Pereira N, Banavali SD, Narula G, Vora T, Chinnaswamy G, et al. Alarming rise in the prevalence of community-acquired multidrug resistant organisms colonization in children with cancer and implications for therapy: A prospective study. Indian J Cancer. 2014;51:442–6. doi: 10.4103/0019-509X.175310. [DOI] [PubMed] [Google Scholar]
- 11.Teuber M. Veterinary use and antibiotic resistance. Curr Opin Microbiol. 2001;4:493–9. doi: 10.1016/s1369-5274(00)00241-1. [DOI] [PubMed] [Google Scholar]
- 12.Sawant AA, Hegde NV, Straley BA, Donaldson SC, Love BC, Knabel SJ, et al. Antimicrobial-resistant enteric bacteria from dairy cattle. Appl Environ Microbiol. 2007;73:156–63. doi: 10.1128/AEM.01551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer treatment and antimicrobial resistance. Lancet Oncol. 2013;14:265. doi: 10.1016/S1470-2045(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 14.Oteo J, Alcaraz R, Bou G, Conejo C, Díaz-Lamas AM, Fernández-Martínez M, et al. Rates of faecal colonization by carbapenemase-producing enterobacteriaceae among patients admitted to ICUs in Spain. J Antimicrob Chemother. 2015;70:2916–8. doi: 10.1093/jac/dkv187. [DOI] [PubMed] [Google Scholar]
- 15.Wozniak A, Villagra NA, Undabarrena A, Gallardo N, Keller N, Moraga M, et al. Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of Carbapenem resistance in Chile. J Med Microbiol. 2012;61:1270–9. doi: 10.1099/jmm.0.045799-0. [DOI] [PubMed] [Google Scholar]
- 16.El Din AA, Hameed Harfoush RA, Said Oksaha HA, Sayed Kohleif DA. Study of OmpK35 and OmpK36 expression in carbapenem resistant ESBL producing clinical isolates of Klebsiella pneumoniae. Adv Microbiol. 2016;6:662–70. [Google Scholar]
- 17.Schlesinger A, Paul M, Gafter-Gvili A, Rubinovitch B, Leibovici L. Infection-control interventions for cancer patients after chemotherapy: A systematic review and meta-analysis. Lancet Infect Dis. 2009;9:97–107. doi: 10.1016/S1473-3099(08)70284-6. [DOI] [PubMed] [Google Scholar]


